Abstract
The detection of focal Ca2+ transients (called neuroeffector Ca2+ transients, or NCTs) in smooth muscle of the mouse isolated vas deferens has been used to detect the packeted release of ATP from nerve terminal varicosities acting at postjunctional P2X receptors. The present study investigates the sources and sequestration of Ca2+ in NCTs. Smooth muscle cells in whole mouse deferens were loaded with the Ca2+ indicator Oregon Green 488 BAPTA-1 AM and viewed with a confocal microscope. Ryanodine (10 µm) decreased the amplitude of NCTs by 45 ± 6 %. Cyclopiazonic acid slowed the recovery of NCTs (from a time course of 200 ± 10 ms to 800 ± 100 ms). Caffeine (3 mm) induced spontaneous focal smooth muscle Ca2+ transients (sparks). Neither of the T-type Ca2+ channel blockers NiCl2 (50 µm) or mibefradil dihydrochloride (10 µm) affected the amplitude of excitatory junction potentials (2 ± 5 % and −3 ± 10 %) or NCTs (−20 ± 36 % and 3 ± 13 %). In about 20 % of cells, NCTs were associated with a local, subcellular twitch that remained in the presence of the α1-adrenoceptor antagonist prazosin (100 nm), showing that NCTs can initiate local contractions. Slow (5.8 ± 0.4 µm s−1), spontaneous smooth muscle Ca2+ waves were occasionally observed. Thus, Ca2+ stores initially amplify and then sequester the Ca2+ that enters through P2X receptors and there is no amplification by local voltage-gated Ca2+ channels.
The neurotransmitter ATP initiates excitatory junction potentials (EJPs) and a fast component of contraction by acting at P2X1 receptors on smooth muscle cells of the rodent vas deferens (Sneddon & Burnstock, 1984; Sneddon & Westfall, 1984; Morris & Gibbins, 1992; Mulryan et al. 2000). Recently, we reported that nerve stimulation intermittently initiates focal increases in intracellular Ca2+ concentration ([Ca2+]i) in those parts of smooth muscle cells that are closely apposed to nerve terminal varicosities, even though there is no intermittence of the action potential in the terminal; these focal Ca2+ transients have been termed NCTs (Brain et al. 2002). NCTs are abolished at most junctions by α,β-methylene ATP, which desensitises P2X1 receptors, suggesting that they are initiated by the activation of P2X receptors (Brain et al. 2002). NCTs are of interest as a tool for monitoring neurotransmitter release, on an impulse-to-impulse basis, simultaneously from a large number of varicosities including sequential varicosities on the same nerve terminal branch. The existence of such focal Ca2+ transients may have important implications for the contraction and subcellular regulation of smooth muscle cells.
While P2X receptor activation is obligatory for NCT initiation, the source of Ca2+ and the mechanisms terminating these Ca2+ transients have not yet been determined. The most obvious source of Ca2+ following the activation of P2X receptors is the influx of Ca2+ through the P2X receptor itself, which is known to have a high Ca2+ permeability in smooth muscle cells (Benham & Tsien, 1987). However, it is also possible that local Na+ influx following P2X receptor activation could cause sufficient local depolarisation to open either high-voltage activated (L-type) or low-voltage activated (T-type) Ca2+ channels. It has previously been shown that L-type Ca2+ channels do not contribute to NCTs (Brain et al. 2002), but there has been no previous investigation of T-type channels.
Intracellular Ca2+ stores have, for some time, been implicated in the intermittent, subcellular increases in Ca2+ concentration known as sparks or quarks in excitable cells. Such events were first studied in cardiac and skeletal muscle cells, but they also occur in many smooth muscle cells (for reviews, see Jaggar et al. 2000; Pabelick et al. 2001), including myocytes isolated from the guinea-pig vas deferens (Ohi et al. 2001; White & McGeown, 2003). Any small local Ca2+ transient, such as that following the influx of Ca2+ through P2X1 receptors, could potentially trigger Ca2+-induced Ca2+-release (CICR). The present work explores the role of intracellular Ca2+ stores and T-type voltage-gated Ca2+ channels in the genesis and termination of NCTs.
Methods
Ca2+ indicator loading
Eight- to twelve-week-old Balb/c mice (Harlan, UK) were killed by cervical fracture and both vasa deferentia removed. Efforts were made to minimise the number of animals used; all experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986. The connective tissue around each vas deferens was carefully dissected in order to obtain clear images of smooth muscle cells and to remove any ganglia or isolated nerve cell bodies located close to the vas deferens. Each vas deferens was then exposed to 10 µm Oregon Green 488 BAPTA-1 AM (Oregon-BAPTA; Molecular Probes) in 1 % DMSO/0.2 % pluronic F-127 in physiological salt solution (PSS) for 2 h at 36 °C. Each vas deferens was then rinsed in bubbled PSS for at least 10 min prior to transfer to an organ bath mounted on the stage of a confocal microscope. The PSS contained (mm): NaCl 118.4, NaHCO3 25.0, NaH2PO4 1.13, KCl 4.7, CaCl2 1.8, MgCl2 1.3 and glucose 11.1. The pH was maintained at 7.4, and the solution oxygenated, by continuously bubbling with 95 % O2/5 % CO2.
Confocal microscopy
The vas deferens was placed in a chamber that was continuously perfused with the standard PSS (bath temperature 31–32 °C). The base of the chamber was a coverslip; images were acquired with a Leica NT inverted confocal microscope. The effective confocal pinhole was set to 3.65 Airy discs; this gives a large depth of field. Field stimuli (pulse width 0.6 ms; amplitude 10 V) were applied with parallel Ag/AgCl electrodes located near the prostatic end of the aganglionic vas deferens; stimuli were synchronised with the start of image acquisition, so that the time between each stimulus and recording was fixed. Unless stated otherwise, images were captured at twice the stimulus frequency (1–2 Hz), so that a stimulus occurred on every second frame. This protocol provided a control image prior to every stimulus and hence allowed consecutive [Ca2+] transients from the same location to be distinguished. Confocal images were continuously acquired for 30 s to generate one image set. Such sets were acquired every 2 min. Eight such sets were acquired for each preparation in order to measure the control response at each neuroeffector junction. Preparations were then exposed to drugs for either 90 min (ryanodine) or 20 min (all other drugs) to ensure adequate equilibration, and then a further eight sets of images were acquired. During incubation in ryanodine (and in the corresponding control), field stimuli of 10 impulses at 10 Hz were applied every 30 s because the action of ryanodine is use dependent (see, for example, Kano et al. 1995; Smith & Cunnane, 1996).
Image analysis
Image analysis was performed with NIH Image version 1.62 (from http://rsb.info.nih.gov/nih-image/) or Image SXM version 1.68 (from http://reg.ssci.liv.ac.uk). Adjacent cells were distinguished from one another by step-like changes in the fluorescent signal at cell boundaries, as even adjacent cells were differentially loaded with Ca2+ indicator. In some cases the raw images were too dim to confidently distinguish cell boundaries. In such cases an average from 100 consecutive frames was calculated and this averaged image (with its greater signal to noise ratio) was used to count cells. Local, discrete changes in [Ca2+]i were detected using a modification of previously-described custom-written macros (Brain et al. 2002). Raw images were convolved with a Gaussian filter of half-width 1.5 µm. The ratio of the fluorescent intensities on consecutive images was calculated on a pixel-by-pixel basis. The Particle Analysis algorithm of NIH Image was used to detect focal changes in the ratio of fluorescent intensities that were greater than one s.d. above the noise in the control images over a continuous area of at least 2.5 µm2. A threshold was set, equal to the mean background signal of the image, below which the algorithm did not search for events.
When measuring the kinetics of NCTs, the effects of movement were reduced with the ‘Auto Register’ facility of Image SXM (i.e. the use of a cross-correlation algorithm, based on the fast Fourier transform of each image, to align all images in a set). The change in fluorescent signal was measured within a rectangular region (area 20–60 µm2) that completely enclosed the [Ca2+]i transient. The measurement box was larger than the area of the [Ca2+]i transient so that the entire change in fluorescent signal was measured. The time course of recovery (the time taken to fall to e−1) of focal [Ca2+]i transients was calculated using an exponential curve fit to the average response (over the first 1 s after the point of first detection) at a given junction. Least squares curve fitting was performed with either Excel (Microsoft) or GraphPad Prism 3.0a (GraphPad Software Inc.).
Electrophysiology
Conventional intracellular recording techniques were used to record excitatory junction potentials (EJPs) in smooth muscle cells (see Brock & Cunnane, 1992). Each vas deferens was superfused with PSS and drugs were applied by swapping the perfusion solution to one containing the drug at the required final bath concentration. Stimuli (rectangular pulses, 0.1 ms, amplitude 10 V) were delivered through Ag/AgCl electrodes positioned around the proximal end of the vas deferens. Microelectrodes were filled with 5 m potassium acetate and had tip resistances of 40–80 MΩ. The membrane potential was measured with an Axoclamp 2A (Axon Instruments, California, USA) in bridge mode, then digitised with a PowerLab system and Chart 4.2 (both from ADInstruments, Chalgrove, UK). The time course of repolarisation (decay time constant) was the time taken to fall from 0.90 to 0.33 of the peak amplitude (i.e. the time taken to fall by e−1).
Drug sources and preparation
Cyclopiazonic acid (CPA; Tocris, Avonmouth, UK) and ryanodine (Sigma-Aldrich, Poole, Dorset, UK) were stored frozen (−20 °C) in aliquots at a concentration of 10 mm in DMSO; NiCl2 was stored frozen at a concentration of 1 m in distilled water. Caffeine (Sigma-Aldrich, Dorset, England) solutions were prepared on the day of the experiment at the working concentration. Mibefradil dihydrochloride (donated by Dr E. V. Gutknecht and P. Weber, Hoffmann-La Roche, Basel, Switzerland) stock solutions had a concentration of 10 mm in distilled water; aliquots were frozen until required.
When comparing the amplitudes of NCTs it was noted that the amplitude distribution is skewed and so a non-parametric test (Wilcoxon matched pairs test) was applied. All other statistical tests were two-tailed Student's t tests; the results are reported as significant when P < 0.05. In the confocal imaging experiments, the assumption was made that each junction behaves independently and hence the n-number for the statistical test is the number of junctions studied (nj).
Results
In each vas deferens the Ca2+ indicator Oregon-BAPTA filled the cytoplasm of smooth muscle cells within one to two cell layers of the surface. Field stimulation (1 Hz) of vasa deferentia induced highly intermittent, focal [Ca2+]i transients in Oregon-BAPTA-filled smooth muscle cells (Fig. 1A). These events occurred in characteristic locations (or clusters) within the cells (Fig. 1B) at sites attributed to single varicosity – smooth muscle cell neuroeffector junctions; these focal [Ca2+]i transients are referred to as NCTs (Brain et al. 2002). Within each cluster, the mean probability that a stimulus evoked an NCT was 0.020 ± 0.002 (nj = 53 junctions; number of smooth muscle cells (nsm) = 10; number of preparations (np) = 5; mean ± s.e.m.). When α,β-methylene ATP (10 µm), which desensitises P2X1 receptors, was bath applied for 30 min, NCTs were abolished at the majority of junctions (64/68). At the remaining junctions (4/68) the amplitude of the evoked Ca2+ transient was reduced (by >40 % at each junction), and the probability of detecting an NCT was reduced by 60 ± 20 %.
Figure 1. Purinergic neuroeffector Ca2+ transients (NCTs).
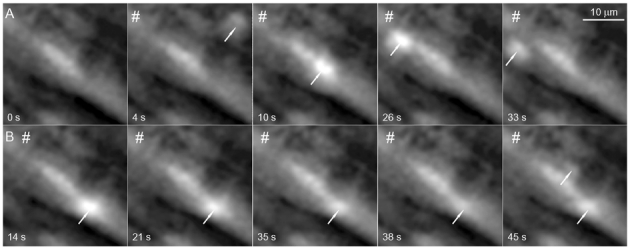
A small region close to the surface of the mouse vas deferens is shown during field stimulation (#) at 1 Hz (while recording images at 2 frames s−1). Smooth muscle cells are heterogeneously loaded with the Ca2+ indicator Oregon-BAPTA. A, a set of selected frames to demonstrate that NCTs (arrows) are intermittently evoked at several sites within smooth muscle cells. The time at which the image was acquired is marked on each frame. B, another selection of images, from the same recording, showing all NCTs arising at a given site. Each frame should be compared with the control frame (A, frame 1). B, frame 5, shows synchronous events at two sites within the same smooth muscle cell.
About 20 % of smooth muscle cells (7 of 35 cells) contained at least one junction where NCTs were immediately followed by a local twitch (commencing within 0.5 s of the NCT). In such cases the smooth muscle cell deformed towards the site of the NCT (see the supplementary material), although not every NCT in these cells induced a contraction. It appeared, subjectively, that a contraction was more likely when the local Ca2+ transient was of greatest amplitude. No distinguishing feature was identified that could predict which cells would locally contract. In all three preparations in which the α1-adrenoceptor antagonist prazosin (100 nm) was applied, the local contractions associated with NCTs remained after at least 20 min of exposure.
Ca2+-induced Ca2+ release
While the activation of P2X receptors is necessary for the generation of NCTs, the potential role of intracellular Ca2+ stores in amplifying the local [Ca2+]i change, or in sequestering the remaining Ca2+, is unknown. After incubating vasa deferentia in ryanodine (10 µm) for 90 min, the amplitude of NCTs (during field stimulation at a frequency of 2 Hz) was reduced by 45 ± 6 % (nj = 42; np = 7; Fig. 2). In the continued presence of ryanodine, the application of caffeine (3 mm) did not elicit a detectable change in the smooth muscle Ca2+ concentration (np = 3 of 3). In control preparations (when only the vehicle was present for 90 min) there was no significant change in the amplitude of NCTs (+9 ± 10 %; nj = 13; np = 3).
Figure 2. Intracellular Ca2+ stores and NCTs.
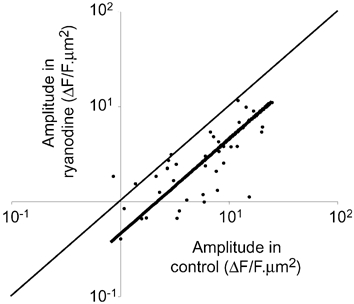
The amplitudes of NCTs under control conditions and in the presence of ryanodine (10 µm) are shown. Each point represents the amplitude of NCTs occurring at a single neuroeffector junction. The thin line is a line of equivalence (where the amplitudes are unchanged), while the thick line is a linear curve fit (assuming that the curve passes through 0,0). F is the fluorescent intensity, in arbitrary units; ΔF is the change in F from its resting level.
During field stimulation (2 Hz), the sarcoplasmic reticulum Ca2+-ATPase inhibitor cyclopiazonic acid (CPA) had no significant effect on the amplitude of NCTs (3 ± 12 %; nj = 12; nsm = 4; np = 3; Fig. 3). The rate of recovery of the Ca2+ concentration slowed upon exposure to CPA, from a time constant of 200 ± 10 ms to 800 ± 100 ms (nj = 12; nsm = 4; np = 3).
Figure 3. The rate of recovery of NCTs is slowed by CPA.
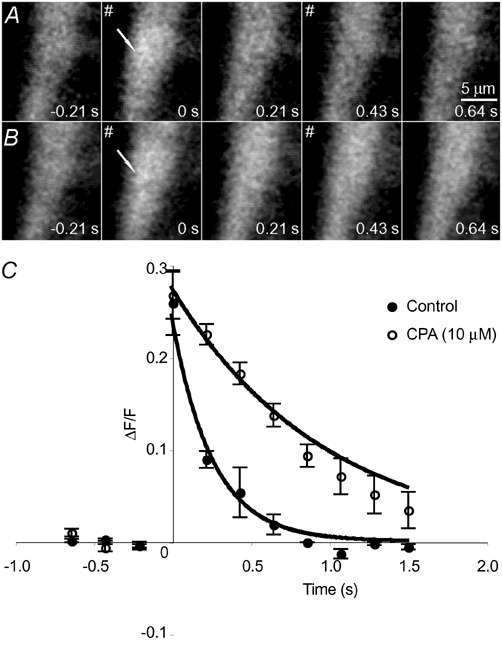
A, a control recording from a smooth muscle cell during field stimulation (#). In this cell, NCTs arose at the location marked with an arrow with a probability of 0.06 per field stimulus. B, the same region in the presence of CPA (10 µm). Field stimuli still evoked NCTs with a similar probability (0.05 at this site), but the rate of the recovery of Ca2+ to its resting concentration is significantly slower. This is quantified for this junction in C. Points plotted are means ± s.e.m.
Thapsigargin (1 µm), another sarcoplasmic reticulum Ca2+-ATPase inhibitor, also had no detectable effect on the amplitude of NCTs (−20 ± 10 %; nj = 9; nsm = 5; np = 4), and slowed the time course of recovery (by 12 ± 6 %; nj = 9; nsm = 5; np = 4).
When exposed to 3 mm caffeine there was a 16-fold increase in the frequency of spontaneous focal [Ca2+]i transients (Fig. 4; nsm = 5; np = 3), which were not necessarily grouped in NCT clusters (Fig. 4).
Figure 4. Caffeine evokes focal Ca2+ transients.
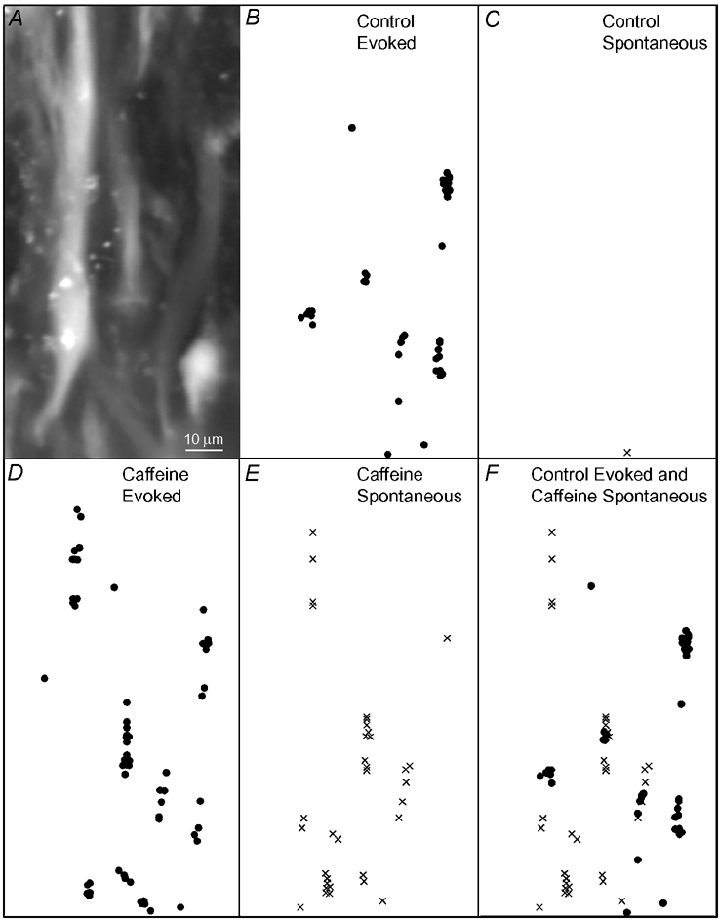
A, images of several smooth muscle cells loaded with the Ca2+ indicator Oregon-BAPTA. B-F show maps (drawn to the same scale and position as the image in A) of the location of focal Ca2+ transients occurring immediately after field stimuli (dots; B, D and F), or without stimuli (crosses; frames C, E and F), at some time over 20 min of recording in control (B, C and F) or in the presence of caffeine (3mM; D, E and F). Note that the evoked focal Ca2+ transients (NCTs) cluster in characteristic locations within parts of each cell (B). Caffeine induces spontaneous focal Ca2+ transients at additional locations (E; F, a composite of B and E).
T-Type Ca2+ channels
In some smooth muscle cells, T-type Ca2+ channels contribute to the EJP and the opening of these channels may produce local changes in [Ca2+]i. However, during stimulation at 0.33 Hz, the T-type Ca2+ channel blocker Ni2+ (at 30 µm, 50 µm or 300 µm) did not significantly change the amplitude of EJPs (5 ± 7 %; 4 ± 3 %; 7 ± 30 %, respectively; np = 4) or the rate of repolarisation (0 ± 7 %; 9 ± 14 %; 8 ± 12 %, respectively; np = 4). At a high concentration (300 µm), Ni2+ shifted the resting membrane potential towards more positive values (from −4 ± 3 mV to −69 ± 2 mV; np = 4; P≤ 0.05; Fig. 5A). Another T-type Ca2+ channel blocker (mibefradil; 10 µm) had no significant effect on the amplitude of EJPs (−3 ± 10 %, np = 6; Fig. 5B), but did slightly slow the rate of repolarisation of the EJP (by 14 ± 6 %). At a higher concentration (20 µm), mibefradil reduced the amplitude of excitatory junction potentials (by 37 ± 10 %; np = 6), slowed the rate of repolarisation (by 44 ± 16 %) and caused a significant membrane potential depolarisation (from −83 ± 1 mV to −71 ± 5 mV). Given that the higher concentrations of Ni2+ (300 µm) or mibefradil (20 µm) caused effects that could not readily be attributed to T-type Ca2+ channel inhibition, lower concentrations were used in confocal imaging experiments.
Figure 5. The effects of T-type Ca2+ channel blockers on EJPs and NCTs.
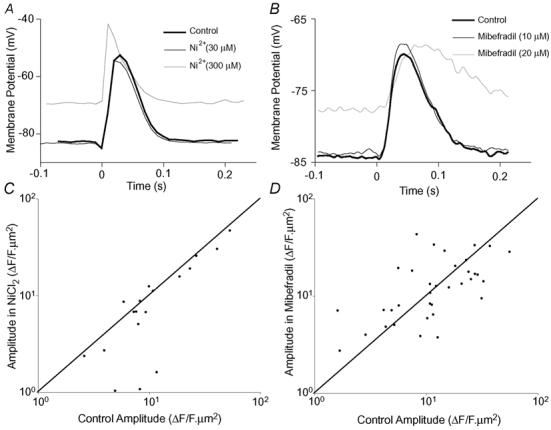
A and B show the effect of Ni2+ or mibefradil (respectively) on EJPs during low frequency (0.33 Hz) stimulation. The recordings were zeroed with respect to the potential recorded by the microelectrode outside the cell at the end of each experiment. The sampling frequency in A is 100 Hz, while that in B is 4 kHz. The amplitudes of NCTs under control conditions and in the presence of either NiCl2 (100 µm; C) or mibefradil (10 µm; D) are also shown. Each point represents the amplitude of NCTs occurring at a single neuroeffector junction. A line of equivalence (where the amplitudes are unchanged) is marked on each graph. At concentrations specific for T-type Ca2+ channel block, neither of these drugs significantly affects the amplitude of EJPs or NCTs.
Another measure of neurotransmitter release is the probability of detecting an NCT at a given junction following a stimulus (PNCT). Neither Ni2+ (100 µm) nor mibefradil (10 µm) had any significant effect on PNCT (for Ni2+ experiments, control, 0.018 ± 0.004; Ni2+, 0.021 ± 0.005; nj = 18; nsm = 6; np = 3; for mibefradil experiments, control, 0.014 ± 0.003; mibefradil, 0.017 ± 0.003; nj = 35; nsm = 5; np = 3).
It is possible that Na+ influx through P2X1 receptors could activate local voltage-gated Ca2+ channels. However, neither Ni2+ (100 µm; Fig. 5C) nor mibefradil (10 µm; Fig. 5D) affected the amplitude of NCTs (for Ni2+: −20 ± 36 %; for mibefradil: 3 ± 13 %).
Smooth muscle Ca2+ waves
Ca2+ waves arose spontaneously in a small number of smooth muscle cells (see Fig. 6 for an example). These arose from characteristic locations within the cell and propagated bidirectionally at an average speed of 5.8 ± 0.4 µm s−1 (nsm = 3; np = 3). They travelled for 5–50 µm within the cells before collapsing. The frequency at which these waves occurred varied over time, and hence they could not be well characterised. In one cell only, waves arose from the same location as a cluster of NCTs, and were initiated upon nerve stimulation even in the presence of the α1-adrenoceptor antagonist prazosin (100 nm). No intercellular Ca2+ waves were observed (np = 12).
Figure 6. Spontaneous Ca2+ waves in a smooth muscle cell.
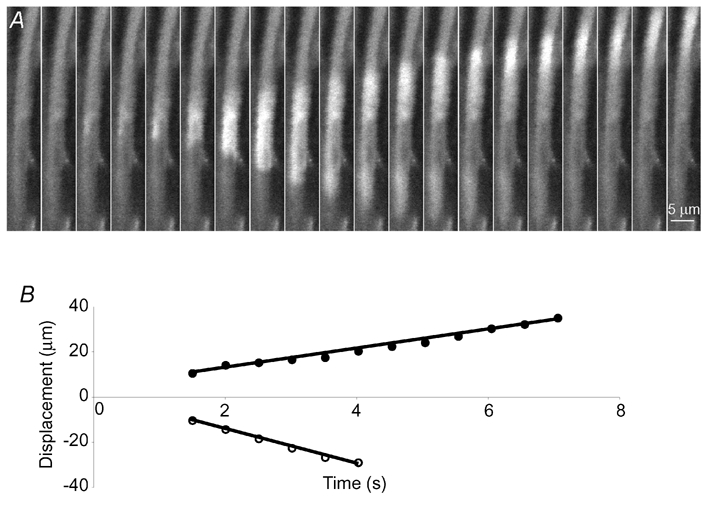
A, an example of a cell in which Ca2+ waves arose spontaneously and propagated bidirectionally. The locations of the wave fronts are plotted in B. In this cell the average speeds of the waves were 4.4 µm s−1 (•) and 7.4 µm s−1 (^). A and B have the same time scale.
Discussion
Purinergic NCTs have now been identified in both the mouse vas deferens (Brain et al. 2002) and rat mesenteric arteries (Lamont & Wier, 2002; Lamont et al. 2003). NCTs are of physiological interest because of their potential role in excitation-contraction coupling and as an optical tool for detecting the packeted release of neurotransmitters.
The initial observations of NCTs were made in smooth muscle cells filled with the dextran conjugate of the Ca2+ indicator Oregon Green 488 BAPTA-1. The present work confirms that the acetoxymethyl ester of the indicator can be loaded into these smooth muscle cells, and detects NCTs with a similar frequency to those reported with the dextran conjugate of the indicator (0.020 ± 0.002 compared to 0.019 ± 0.002 per junction; Brain et al. 2002).
In some smooth muscle cells from the mouse vas deferens, excitatory junction potentials (or currents) are not abolished by 10–20 µmα,β-methylene ATP (Allcorn et al. 1986; Liang et al. 2000), a P2X1 receptor agonist which causes desensitisation and internalisation of P2X1 receptors (Ennion & Evans, 2001). Given that EJPs do not occur in P2X1 receptor knockout mice (Mulryan et al. 2000), it may be that α,β-methylene ATP achieves poor access to some junctions and hence does not abolish all NCTs.
It has previously been noted that NCTs precede local contraction of smooth muscle cells (Brain et al. 2002). That these local contractions remain in the presence of prazosin (100 nm) shows that the contractions do not require α1-adrenoceptor activation and are generated by purinergic receptor activation. It is known that the L-type Ca2+ channel blocker nifedipine (1–10 µm) abolishes the purinergic component of contraction in the mouse vas deferens (Rae & Calixto, 1989; Cleary et al. 2003), suggesting that NCTs alone are not sufficient for coordinated contraction of the longitudinal layer of the mouse vas deferens. However, in rat mesenteric arteries NCTs contribute to the purinergic component of contraction (Lamont et al. 2003). Whether the local contraction subserves a physiological role in the mouse vas deferens, or whether the local [Ca2+]i transient has a local regulatory function (such as coordinating the assembly of P2X1 receptor clusters) are matters for further investigation.
The present results indicate that the local Ca2+ influx through P2X receptor clusters is amplified by CICR, as blocking CICR with ryanodine reduced the amplitude of NCTs. This assertion is plausible given that the Ca2+ permeability of P2X1 receptors is large (Evans et al. 1996) and that the sarcoplasmic reticulum in many smooth muscle cells is closely associated with the plasma membrane (Devine et al. 1972; Gollasch et al. 1998; Lesh et al. 1998). CICR has previously been shown to augment the purinergic component of contraction in the rat vas deferens (Bourreau et al. 1991) and guinea-pig urinary bladder (without affecting the EJP; Hashitani et al. 2000), and to amplify whole-cell Ca2+ transients that follow the focal application of ATP to dissociated rat portal vein smooth muscle cells (Mironneau et al. 2001). It has recently been reported that NCTs in rat mesenteric artery smooth muscle cells are ‘largely unaffected’ by ryanodine, although a small but significant reduction (about 13 %) in the amplitude of NCTs was noted (Lamont & Wier, 2002). Hence, the extent to which intracellular Ca2+ stores contribute Ca2+ to NCTs may vary among different tissues.
Ryanodine can prevent CICR by preventing high conductance Ca2+ flux though ryanodine (RYR) receptors (Meissner, 1986), while CPA and thapsigargin are endoplasmic reticulum (ER) Ca2+-ATPase inhibitors (for review, see Treiman et al. 1998) and may not prevent CICR if Ca2+ depletion in the ER is incomplete. Both CPA and thapsigargin slowed the rate at which Ca2+ returned to its resting concentration, implying that intracellular Ca2+ stores sequester at least some of the Ca2+ in NCTs. This effect was more pronounced with CPA. While thapsigargin is widely used as a Ca2+-ATPase inhibitor, there are Ca2+-ATPases that fill stores responsible for CICR that are resistant to thapsigargin (Bian et al. 1991; Tanaka & Tashjian, 1993). Furthermore, ER stores located close to the plasma membrane of glial cells and triggered by exogenously applied ATP are particularly sensitive to cyclopiazonic acid (Golovina & Blaustein, 2000). Such sub-plasmalemmal (‘junctional’) ER (Blaustein & Golovina, 2001) may be functionally adapted to link local neurotransmitter action with a local increase in Ca2+ concentration.
The observation that focal [Ca2+]i transients can be induced by caffeine demonstrates that Ca2+‘sparks’ can occur in mouse vas deferens, as in other (Bolton & Imaizumi, 1996; Kirber et al. 2001; Ohi et al. 2001) smooth muscle cells. Such Ca2+ sparks can be induced both at locations where NCTs could be evoked and at other sites, suggesting that not all intracellular Ca2+ stores are associated with functional P2X receptors. It is unlikely that caffeine increased the frequency of spontaneous transmitter release from otherwise silent junctions, as caffeine (10 mm) has no effect of the frequency of spontaneous EJPs in the rodent (guinea-pig) vas deferens (Ziogas et al. 1995).
In the present study mibefradil (10 µm) had no effect on the amplitude of excitatory junction potentials or on PNCT, implying that T-type Ca2+ channels do not contribute to neurotransmitter release from nerve terminals or act postjunctionally to affect EJPs in the mouse vas deferens. This finding is consistent with the observations that neither mibefradil (up to 30 µm) nor Ni2+ (300 µm) had a significant effect on nerve stimulation-evoked contraction in the rat vas deferens (Xi & Angus, 2001; Xi et al. 2002). It is known that T-type Ca2+ channels contribute to EJPs in rat mesenteric arteries (Xi et al. 2002). It may be that this difference between tissues arises because of the relatively negative resting membrane potential in the mouse vas deferens (about −85 mV, compared to −60 mV in mesenteric arteries), so that a small depolarisation will not reach the threshold for activation of T-type Ca2+ channels. At a higher mibefradil concentration (20 µm) there was significant membrane potential depolarisation and a slowing of repolarisation. These actions of mibefradil are consistent with K+ channel inhibition, which has been shown to occur in human myoblasts and other cells (Liu et al. 1999).
The observation that neither Ni+ (100 µm) nor mibefradil (10 µm) affected the amplitude of NCTs implies that T-type Ca2+ channels do not contribute to the local Ca2+ influx that follows P2X receptor activation.
It is known that α1-adrenoceptor agonists and noradrenergic transmission can induce or increase the frequency of Ca2+ waves in many smooth muscle cells (for examples, see Iino et al. 1994; Ruehlmann et al. 2000; Lee et al. 2001). The wave speed range varied from 20–90 µm s−1 in isolated smooth muscle cells of the rabbit inferior vena cava and was increased by exposure to an α-adrenergic receptor agonist (Ruehlmann et al. 2000). In the present study, spontaneous waves occurred even in the presence of prazosin, suggesting that they did not require the activation of α1-adrenoceptors. The waves were also slower (at about 5 µm s−1) than those previously reported.
The proposed model for the genesis and sequestration of NCTs is summarised in Fig. 7. It may be that the sequestration of Ca2+ by intracellular Ca2+ stores, following its entry through P2X receptors, is important in terminating the purinergic twitch. Ca2+ influx through P2X receptors may also charge intracellular Ca2+ stores for subsequent Ca2+ release, hence providing a mechanism for purinergic augmentation of noradrenergic neurotransmission.
Figure 7. A model for the generation and sequestration of NCTs.
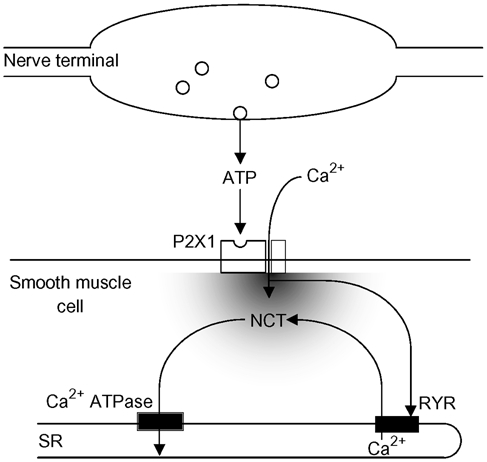
Following a nerve terminal action potential there is only a small probability (about 0.02) that a packet of ATP will be released. This ATP acts on postjunctional P2X1 receptors. The influx of Ca2+ through the P2X receptors is sufficient to cause a significant change in the local Ca2+ concentration, which is amplified by CICR from the RYR receptor. At least some of this Ca2+ is sequestered by the sarcoplasmic reticulum (SR) Ca2+-ATPase.
Acknowledgments
K.L.B. was supported with an Oxford Nuffield Medical Fellowship. Mibefradil was kindly donated by Dr E.-V. Gutknecht and P. Weber (Hoffmann-La Roche, Basel, Switzerland).
Supplementary material
The online version of this paper can be found at: DOI: 10.1113/jphysiol.2003.049734 and contains material entitled: Local twitches are associated with NCTs.
In memory of Dr S. J. Trout.
References
- Allcorn RJ, Cunnane TC, Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: support for cotransmission. Br J Pharmacol. 1986;89:647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham CD, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Bian JH, Ghosh TK, Wang JC, Gill DL. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991;266:8801–8806. [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca2+ stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- Bourreau JP, Zhang ZD, Low AM, Kwan CY, Daniel EE. Ryanodine and the adrenergic, purinergic stimulation in the rat vas deferens smooth muscle: functional and radioligand binding studies. J Pharm Exp Ther. 1991;256:1063–1071. [PubMed] [Google Scholar]
- Brain KL, Jackson VM, Trout SJ, Cunnane TC. Intermittent ATP release from nerve terminals elicits focal smooth muscle Ca2+ transients in mouse vas deferens. J Physiol. 2002;541:849–862. doi: 10.1113/jphysiol.2002.019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Impulse conduction in sympathetic nerve terminals in the guinea-pig vas deferens and the role of the pelvic ganglia. Neuroscience. 1992;47:185–196. doi: 10.1016/0306-4522(92)90131-k. [DOI] [PubMed] [Google Scholar]
- Cleary L, Vandeputte C, Docherty JR. Investigation of postjunctional α1- and α2-adrenoceptor subtypes in vas deferens from wild-type and α2A/D-adrenoceptor knockout mice. Br J Pharmacol. 2003;138:1069–1076. doi: 10.1038/sj.bjp.0705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine CE, Somlyo AV, Somlyo AP. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972;52:690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. Agonist-stimulated internalisation of the ligand-gated ion channel P2X1 in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/s0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res. 1998;83:1104–1114. doi: 10.1161/01.res.83.11.1104. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Blaustein MP. Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca2+ stores in astrocytes. Glia. 2000;31:15–28. doi: 10.1002/(sici)1098-1136(200007)31:1<15::aid-glia20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M, Kasai H, Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994;13:5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Kano M, Garaschuk O, Verkhratsky A, Konnerth A. Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones. J Physiol. 1995;487:1–16. doi: 10.1113/jphysiol.1995.sp020857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirber MT, Etter EF, Bellve KA, Lifshitz LM, Tuft RA, Fay FS, Walsh JV, Fogarty KE. Relationship of Ca2+ sparks to STOCs studied with 2D and 3D imaging in feline oesophageal smooth muscle cells. J Physiol. 2001;531:315–327. doi: 10.1111/j.1469-7793.2001.0315i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol. 2003;549:801–808. doi: 10.1113/jphysiol.2003.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont C, Wier WG. Evoked and Spontaneous Purinergic Junctional Ca2+ Transients (jCaTs) in rat small arteries. Circ Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- Lee CH, Poburko D, Sahota P, Sandhu J, Ruehlmann DO, Van Breemen C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh RE, Nixon GF, Fleischer S, Airey JA, Somlyo AP, Somlyo AV. Localization of ryanodine receptors in smooth muscle. Circ Res. 1998;82:175–185. doi: 10.1161/01.res.82.2.175. [DOI] [PubMed] [Google Scholar]
- Liang SX, d'Arbe M, Phillips WD, Lavidis NA. Development of fast purinergic transmission in the mouse vas deferens. Synapse. 2000;37:283–291. doi: 10.1002/1098-2396(20000915)37:4<283::AID-SYN5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Liu JH, Bijlenga P, Occhiodoro T, Fischer-Lougheed J, Bader CR, Bernheim L. Mibefradil (Ro 40–5967) inhibits several Ca2+ and K+ currents in human fusion-competent myoblasts. Br J Pharmacol. 1999;126:245–250. doi: 10.1038/sj.bjp.0702321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- Mironneau J, Coussin F, Morel JL, Barbot C, Jeyakumar LH, Fleischer S, Mironneau C. Calcium signalling through nucleotide receptor P2X1 in rat portal vein myocytes. J Physiol. 2001;536:339–350. doi: 10.1111/j.1469-7793.2001.0339c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Gibbins IL. Co-transmission and neuromodulation. In: Burnstock G, Hoyle CHV, editors. Autonomic Neuroeffector Mechanisms. Switzerland: Harwood Academic; 1992. pp. 33–119. [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Ohi Y, Yamamura H, Nagano N, Ohya S, Muraki K, Watanabe M, Imaizumi Y. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabelick CM, Sieck GC, Prakash YS. Significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol. 2001;91:488–496. doi: 10.1152/jappl.2001.91.1.488. [DOI] [PubMed] [Google Scholar]
- Rae GA, Calixto JB. Interactions of calcium antagonists and the calcium channel agonist Bay K 8644 on neurotransmission of the mouse isolated vas deferens. Br J Pharmacol. 1989;96:333–340. doi: 10.1111/j.1476-5381.1989.tb11822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehlmann DO, Lee CH, Poburko D, van Breemen C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ Res. 2000;86:E72–79. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P, Burnstock G. ATP as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- Sneddon P, Westfall DP. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tashjian AH., JR Functional identification and quantitation of three intracellular calcium pools in GH4C1 cells: evidence that the caffeine-responsive pool is coupled to a thapsigargin-resistant, ATP-dependent process. Biochemistry. 1993;32:12062–12073. doi: 10.1021/bi00096a017. [DOI] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- White C, McGeown JG. Inositol 1, 4, 5-trisphosphate receptors modulate Ca2+-sparks and Ca2+-store content in vas deferens myocytes. Am J Physiol Cell Physiol. 2003;285:C195–C204. doi: 10.1152/ajpcell.00374.2002. [DOI] [PubMed] [Google Scholar]
- Xi Q, Angus JA. Evidence against an action of mibefradil at N-type voltage-operated calcium channels. Naunyn Schmiedeberg's Arch Pharmacol. 2001;364:430–436. doi: 10.1007/s002100100470. [DOI] [PubMed] [Google Scholar]
- Xi Q, Ziogas J, Roberts JA, Evans RJ, Angus JA. Involvement of T-type calcium channels in excitatory junction potentials in rat resistance mesenteric arteries. Br J Pharmacol. 2002;137:805–812. doi: 10.1038/sj.bjp.0704943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziogas J, Ofarrell M, Slaughter M. Caffeine enhances sympathetic purinergic and noradrenergic transmission in the guinea-pig isolated vas deferens. Naunyn Schmiedeberg's Arch Pharmacol. 1995;352:497–505. doi: 10.1007/BF00169383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


