Abstract
Decreases in the activity of 5-HT-containing caudal raphe neurones during sleep are thought to be partially responsible for the resultant disfacilitation of hypoglossal motoneurones. Whilst 5-HT has a direct excitatory action on hypoglossal motoneurones as a result of activation of 5-HT2 receptors, microinjection of 5-HT2 antagonists into the hypoglossal nucleus reduces motor activity to a much lesser extent compared to the suppression observed during sleep suggesting other transmitters co-localised in caudal raphe neurones may also be involved. The aim of the present study was therefore to characterise raphe pallidus inputs to hypoglossal motoneurones. Whole cell recordings were made from hypoglossal motoneurones in vitro. 5-HT evoked a direct membrane depolarisation (8.45 ± 3.8 mV, P < 0.001) and increase in cell input resistance (53 ± 40 %, P < 0.001) which was blocked by the 5-HT2 antagonist, ritanserin (2.40 ± 2.7 vs. 7.04 ± 4.6 mV). Stimulation within the raphe pallidus evoked a monosynaptic EPSC that was significantly reduced by the AMPA/kainateantagonist, NBQX (22.8 ± 16 % of control, P < 0.001). In contrast, the 5-HT2 antagonist, ritanserin, had no effect on the amplitude of these EPSCs (106 ± 31 % of control, P = n.s.). 5-HT reduced these EPSCs to 50.0 ± 13 % of control (P < 0.001), as did the 5-HT1A agonist, 8-OH-DPAT (52.5 ± 17 %, P < 0.001) and the 5-HT1B agonist, CP 93129 (40.6 ± 29 %, P < 0.01). 8-OH-DPAT and CP 93129 increased the paired pulse ratio (1.38 ± 0.27 to 1.91 ± 0.54, P < 0.05 & 1.27 ± 0.08 to 1.44 ± 0.13, P < 0.01 respectively) but had no effect on the postsynaptic glutamate response (99 ± 4.4 % and 100 ± 2.5 %, P = n.s.). They also increased the frequency (P < 0.001), but not the amplitude, of miniature glutamatergic EPSCs in hypoglossal motoneurones. These data demonstrate that raphe pallidus inputs to hypoglossal motoneurones are predominantly glutamatergic in nature, with 5-HT decreasing the release of glutamate from these projections as a result of activation of 5-HT1A and/or5-HT1B receptors located on presynaptic terminals.
The activity of the subpopulation of hypoglossal motoneurones, which provide the motor innervation of the genioglossal muscle of the tongue (Dobbins & Feldman, 1995; Fay & Norgren, 1997) decreases during sleep (Sauerland & Harper, 1976). In individuals with an anatomically compromised airway this can lead to the occurrence of obstructive sleep apnoeas (Remmers et al. 1978). Animal models of sleep have demonstrated that this decrease in genioglossal motoneurone activity during sleep could be due to either direct postsynaptic inhibition (Yamuy et al. 1999) or as a result of a decrease in the activity of premotor neurones, that is a process of disfacilitation (Kubin et al. 1993: for review see Kubin et al. 1998). The caudal raphe nuclei (Heym et al. 1982; Veasey et al. 1995), the locus coeruleus/subcoeruleuscomplex (Aston-Jones & Bloom, 1981; Reiner, 1986) and the A5 group of cells (Fenik et al. 2002) show state-dependent decreases in activity during sleep, with these neurones projecting either directly or indirectly to the hypoglossal motonucleus (Dobbins & Feldman, 1995; Fay & Norgren, 1997). Thus, the observed disfacilitation of genioglossal motoneurones during sleep may be due to decreases in activity of first or second order premotor neurones within any or all of these latter regions.
The caudal raphe nuclei: the nucleus raphe magnus, obscurus and pallidus and the parapyramidal region, contain both serotonergic and non-serotonergic neurones (Kachidian et al. 1991; Henry & Manaker, 1998), with subpopulations of these neurones projecting directly to the hypoglossal motonucleus (Manaker & Tischler, 1993; Henry & Manaker, 1998). The activity of these caudal raphe neurones decreases in parallel with reductions in activity of genioglossal motoneurones in non-REM sleep, with further reductions in REM sleep (Heym et al. 1982; Megirian et al. 1985; Veasey et al. 1995; Woch et al. 1996). In animal models of sleep, this decrease in raphe neurone activity is associated with a decreased release of 5-HT within the hypoglossal nucleus during this period (Kubin et al. 1994; Lai et al. 2001). Microdialysis of 5-HT into the hypoglossal nucleus in freely moving animals increases genioglossal activity across all sleep-wake states (Jelev et al. 2001). Furthermore, anatomical and in vitro electrophysiological studies have demonstrated that 5-HT has a direct (Berger et al. 1992) 5-HT2 receptor-mediated (Al-Zubaidy et al. 1996; Bayliss et al. 1997; Fay & Kubin, 2000) excitatory action on hypoglossal motoneurones. Together these studies suggest that 5-HT released from the terminals of caudal raphe neurones, acts on 5-HT2 receptors to excite hypoglossal motoneurones, with decreased release during sleep accounting for all or part of the disfacilitation of these latter neurones during sleep. However, microinjection of 5-HT2 antagonists into the hypoglossal nucleus only reduces hypoglossal nerve activity to 50 % of that observed during animal models of sleep (Kubin et al. 1996, 1998). Similarly, in vitro studies have shown that the increase in hypoglossal nerve activity following activation of the raphe obscurus is only partially blocked by 5-HT2 antagonists (Al-Zubaidy et al. 1996). These latter data suggest that other neurotransmitters, in addition to 5-HT, are utilised in caudal raphe projections to hypoglossal motoneurones.
The aim of the present studies was therefore to characterise the transmitter(s) released from raphe pallidus projection to hypoglossal motoneurones and to determine the role of 5-HT in these pathways.
Some of these results have been presented in preliminary form (Bouryi & Lewis, 2001).
METHODS
Slice preparation
Forty-eight male Wistar rats (10–16 days) were deeply anaesthetised with pentobarbitone (60 mg kg−1i.p.) and cooled on ice. Following abolition of the pedal withdrawal reflex, the animals were decapitated, the brain rapidly removed and placed in ice-cold (4 °C) sucrose containing artificial cerebrospinal fluid (sACSF) equilibrated with 95 % O2/5 % CO2. The dura was removed, the medulla encased in agar (4.2 % w/vin ACSF), and 300 μm coronal slices of the appropriate region of the medulla were cut. These were transferred to an incubation chamber where they were stored in oxygenated normal ACSF at room temperature (21–23 °C) for 1–3 h prior to use. The above procedures were regulated by, and in accordance with, the UK Animals (Scientific Procedures) Act 1986; ethical review was also provided by the University of Leeds Ethical Review Committee.
Electrophysiological recordings
Slices were transferred, as required, to a recording chamber mounted on the stage of a microscope (Zeiss Axoscop) equipped with differential interference contrast (Nomarski) optics. The chamber was continually superfused with oxygenated ACSF at room temperature (21–23 °C, 2 ml min−1). Patch clamp electrodes (3–5 MΩ) were manufactured from borosilicate glass (Clarke Electromedical, GC150F-10). Whole cell voltage and current clamp recordings made from visualised motoneurones within the ventral subregion of the hypoglossal nucleus utilising an Axopatch-1D amplifier (Axon Instruments) interfaced to a PC computer via a Digidata 1200 A/Dconverter (Axon Instruments). Current and voltage steps, as well as data acquisition and analysis, were performed using pClamp 6 software (Axon Instruments). The current and voltage traces were filtered at 10 kHz before sampling and off-line analysis. The series resistance was always less than 15 MΩ and was compensated by 60–70 %. Liquid junction potentials were less than 2 mV and were not corrected.
Excitatory postsynaptic currents (EPSCs) were evoked in neurones under investigation by either single, pairs (stimulus interval 50 ms) or trains of four stimuli within the raphe pallidus (0.05-4 ms, 0–10 V, 0.05-1 Hz) utilising a bipolar stimulating electrode (SNE-100, Clarke Electromedical) placed on the surface of the slice in this latter region. In some studies, postsynaptic currents were also evoked by pressure ejection (WPI, PV800) of glutamate (10 mm) every 30 s from a glass micropipette (tip diameter 2–5 μm) positioned up to 50 μm from the neurone under investigation.
In studies to confirm that EPSCs evoked in hypoglossal motoneurones following stimulation within the raphe pallidus were the result of the activation of cell bodies within this latter nucleus rather than axons of passage, slices were prepared as above then transferred, as required, to an interface chamber. The chamber was inclined at an angle to the horizontal to ensure that the flow of ACSF within it was unidirectional. Slices were then orientated so that ACSF flowed directly from the dorsal to the ventral surface. A glutamate (10 mm)-containing pressure ejection electrode (tip diameter 2–5 μm) was placed on the surface of the slice within the confines of the raphe pallidus in order to activate cell bodies within this region, with recordings being made from neurones within the hypoglossal motonucleus. Pulses of glutamate were discharged from the pressure ejection electrode utilising a picopump (WPI, PV800), with the actual concentration of glutamate applied being varied by altering the duration of application (10–70 ms) or the pressures utilised (5–30 p.s.i. (34.5–207 kPa)).
Spontaneous (sEPSC) and miniature (mEPSC) excitatory postsynaptic currents were also recorded, the latter in the presence of 1 μm tetrodotoxin (TTX) in order to block action potential-evoked release.
Cell input resistance prior to and during drug applications was determined by measuring the amplitude of the voltage response during stepwise depolarisations and hyperpolarisations from the holding potential of −70 mV.
Data analysis
In order to determine the actions of an agonist or antagonist on evoked EPSCs, a minimum of 50 EPSCs were obtained and averaged, both before and during drug application. Drugs were applied for a sufficient time to ensure that averages were collected during steady state conditions. Each neurone then acted as its own control, i.e. the peak amplitude of the averaged EPSCs obtained during administration of a drug was compared to that before administration using a paired Student t test. Results are expressed as mean ±s.d., with statistical significance set at P < 0.05. Similarly, in investigations of the effects of agonists on the inward current evoked by glutamate applied by pressure ejection, averages of 15–20 such currents obtained prior to and during agonist application were compared.
The amplitude and frequency of sEPSCs and mEPSCs were analysed utilising MiniAnalysis software (Synaptosoft Inc., Decatur, GA, USA), with statistical comparisons of cumulative probability histograms made utilising a Kolmogorov-Smirnov test. Statistical significance was set at P < 0.005.
Solutions
The solution utilised for slicing (sACSF) had the following composition (mm): sucrose, 252; KCl, 3; CaCl2, 1.5; MgSO47H2O, 2; NaHCO3, 24; NaH2PO4, 1.4; glucose, 10 whilst the external solution utilised for incubation and recording comprised (mm): NaCl, 127; KCl, 1.9, CaCl2, 2; MgSO4, 1.3; NaHCO3, 26; KH2PO4, 1.2; glucose, 10. The solution within the patch electrodes contained (mm): KCl, 10; K-Gluconate, 130; CaCl2, 1; MgCl2, 2; Na2ATP, 2; EGTA, 11; NaOH, 30; KOH, 10; Hepes, 10 (pH 7.4). All compounds were purchased from Sigma.
Drugs
The following drugs were utilised: l-glutamic acid monosodium (Glu); 5-hydroxytryptamine creatinine sulphate (5-HT); (±)-8-hydroxy-2-9)di-n-propyl-amino)tetralin (8-OH-DPAT); 1,4-dihydro-3-(1,2,3,6-tetrahydro-4-pyridinyl)-5H-pyrrolo[3, 2-b]pyridin-5-one dihydrochloride (CP 93129); α-methyl-5-hydroxytryptamine maleate (α-Me-5HT); kynurenic acid; 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium (NBQX); ritanserin; pimozide; tetrodotoxin citrate (TTX). All drugs were purchased from Sigma with the exception of CP 93129 and NBQX, which were purchased from Tocris.
All compounds were prepared as stock solutions in distilled water and stored frozen until required. With the exception of glutamate, which was applied by pressure ejection from a micropipette, all other compounds were diluted in ACSF and applied in the superfusate at concentrations previously shown in the literature to be effective (Wang & Dun, 1990; Johnson, 1994a; Schmitz et al. 1998)
RESULTS
General properties of hypoglossal motoneurones
Whole cell recordings were made from 132 hypoglossal motoneurones within the ventral compartment of the hypoglossal motonucleus. The neurones had a mean resting membrane potential of −64.5 ± 3.9 mV (mean ±s.d.) and cell input resistance of 75.9 ± 23 MΩ.
Actions of 5-HT and selective 5-HT agonists on hypoglossal motoneurones
5-HT (5–30 μm) evoked a membrane depolarisation in all neurones tested, with 10 μm 5-HT evoking a mean depolarisation of 8.45 ± 3.8 mV (P < 0.001, n = 28) at a holding potential of −70 mV (Fig. 1A and C). This depolarisation was associated with an increase in cell input resistance (53 ± 40 %, P < 0.001, n = 28), measured during voltage clamp studies, in all cells tested (Fig. 1B and D). A membrane depolarisation (3.3 ± 3 mV, n = 2) and increase in cell input resistance (25 ± 16 %) were also observed during superfusion of the 5-HT uptake inhibitor, fluoxetine (50 μm), suggesting that spontaneous release of 5-HT was occurring. The 5-HT-evoked depolarisations persisted during co-application of TTX (1 μm) in the superfusate, indicating a direct action of the agonist on postsynaptic receptors.
Figure 1. The postsynaptic actions of 5-hydroxytryptamine are mediated via 5-HT2 receptors.
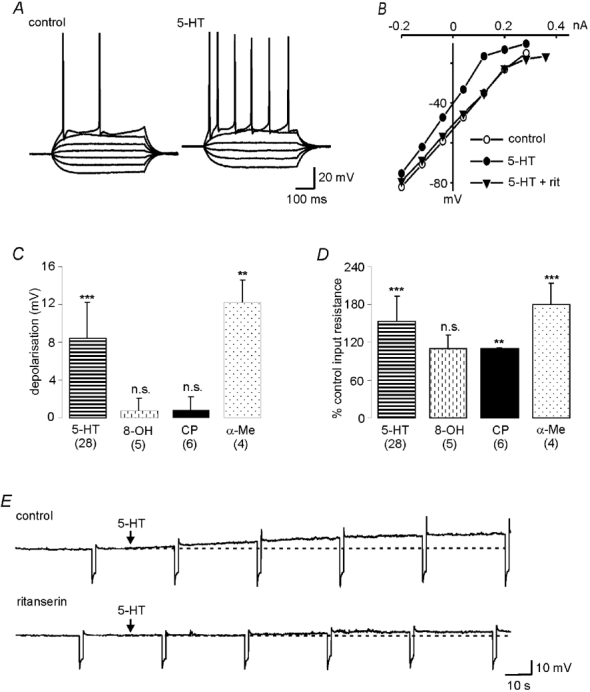
A, current clamp records showing membrane reponses to hyperpolarising and depolarising current pulses in the absence and presence of 5-HT. 5-HT evokes a membrane depolarisation and increase in cell input resistance. B, current–voltage relationships generated by step depolarisations from a holding potential of −70 mV in the absence (○) and presence (•) of 10 μm 5-HT. The 5-HT-mediated increase in cell input resistance is abolished by concurrent administration of the 5-HT2 antagonist, ritanserin (Rit, 10 μm, ▾). C, the 5-HT2 agonist, α-Me-5-HT (α-Me, 20 μm), but not the 5-HT1A agonist, 8-OH-DPAT (8-OH, 50 μm) or the 5-HT1B agonist, CP 93129 (CP, 10 μm), also evoked a membrane depolarisation. ***P < 0.001; **P < 0.01. D, α-Me-5-HT and CP 93129, but not 8-OH-DPAT, evoked increases in cell input resistance. E, current clamp records showing a 5-HT (10 μm) -evoked depolarisation (upper trace) that was markedly reduced in the presence of ritanserin (10 μm, lower trace). Both traces were obtained in the same neurone.
The excitatory action of 5-HT was mimicked by the 5-HT2 receptor agonist, α-Me-5-HT (10–50 μm), with 20 μmα-Me-5-HT evoking a mean depolarisation of 12.2 ± 2.4 mV (P < 0.01, n = 4) and increase in cell input resistance of 79 ± 35 % (P < 0.001) at a holding potential of −70 mV (Fig. 1C and D). In contrast, both the 5-HT1A agonist, 8OH-DPAT (5–50 μm, n = 5), and the 5-HT1B agonist, CP 93129 (5–10 μm, n = 6), had no significant effect on membrane potential (Fig. 1C). However, CP 93129 did evoke a significant increase in cell input resistance (10 ± 1 %, P < 0.01, n = 6, Fig. 1D).
The membrane depolarisation evoked by 5-HT (10 μm) could be markedly reduced by the concurrent application of the 5-HT2 receptor antagonist ritanserin (10 μm, 2.40 ± 2.7 vs. 7.04 ± 4.6 mV, ritanserin + 5-HT vs. 5-HT, P < 0.01, n = 10, Fig. 1E). Recovery of these 5-HT-evoked responses was achieved following washout of the antagonist.
Together, these data suggest that the predominant effect of 5-HT on the postsynaptic membrane of hypoglossal motoneurones is a membrane depolarisation as a result of activation of 5-HT2 receptors confirming previous studies (Berger et al. 1992; Bayliss et al. 1997).
Pharmacological characterisation of EPSCs in hypoglossal motoneurones
Having demonstrated the above 5-HT2 receptor-mediated excitatory action of 5-HT on the postsynaptic membrane of hypoglossal motoneurones, we then addressed the question of the origin of this input, that is, whether these receptors are utilised following activation of serotonergic pathways from the caudal raphe and in particular, the raphe pallidus. In order to do this, excitatory postsynaptic currents (eEPSCs) evoked following electrical stimulation within the raphe pallidus were examined and pharmacologically characterised. In those neurones in which spontaneous EPSCs (sEPSCs) were observed, these were also recorded and characterised.
Focal electrical stimulation (1 Hz) of the raphe pallidus evoked EPSCs in hypoglossal motoneurones with a mean latency to onset of 5.87 ± 1.31 ms (n = 58). These eEPSCs were characterised as being monosynaptic utilising standard criteria (Li & Bayliss, 1998) namely: they occurred at a constant latency in individual neurones; exhibited an all or none increase in amplitude as the stimulus intensity increased; and had the ability to follow high frequency stimulation (>20 Hz). They decreased in amplitude on membrane depolarisation and had a mean reversal potential of −10.4 ± 0.4 mV (data not shown).
At a holding potential of −70 mV, superfusion of the 5-HT2 -receptor antagonist, ritanserin, had no effect on the amplitude of the eEPSC (10 μm, 106 ± 31 % of control, P = n.s., n = 9, Fig. 2A). Blockade of NMDA receptors with the NMDA receptor antagonist, d-APV, (50 μm) had no effect on the EPSC (Fig. 2B) demonstrating the lack of involvement of NMDA receptors at this holding potential. However, the selective AMPA/kainateantagonist, NBQX, reduced the eEPSC to 39.8 ± 18 % (10 μm, P < 0.001, n = 4) of control, with recovery occurring on washout of the antagonist. A similar involvement of AMPA/kainate, but not NMDA receptors, at this potential has been described for the glutamatergic synaptic projections between adjacent caudal raphe neurones (Li & Bayliss, 1998).
Figure 2. Stimulation of the raphe pallidus evokes glutamatergic excitatory postsynaptic currents in hypoglossal motoneurones.
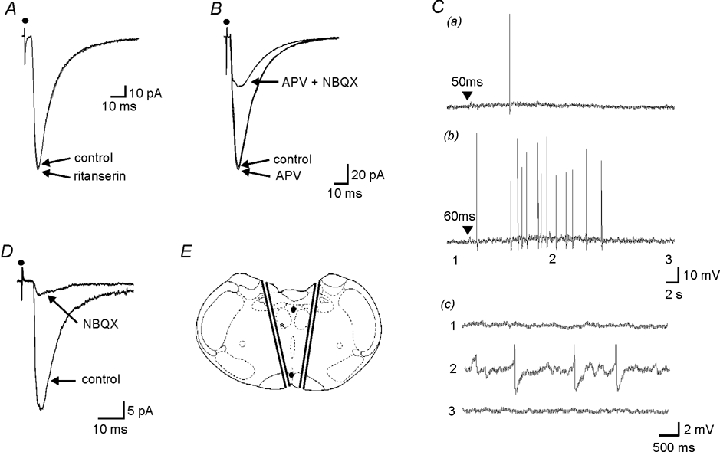
A. EPSCs were evoked in hypoglossal motoneurones by stimulation within the raphe pallidus, these EPSCs were unaffected by the 5-HT2 antagonist, ritanserin (10 μm). Superimposed averages of 50 consecutive EPSCs in the presence and absence of ritanserin, with the motoneurone being held at a holding potential of −70 mV. B, at this holding potential, the NMDA antagonist, APV (50 μm), had no effect on the EPSC. In contrast, it was markedly reduced by the AMPA/kainateantagonist, NBQX (20 μm). C, these EPSCs were the result of activation of the cell bodies of neurones within the raphe pallidus. Pressure ejection of glutamate (▾, 10 mm, 20 p.s.i. (138 kPa)), for a, 50 ms and b, 60 ms, within the confines of the raphe pallidus evoked a concentration-dependent increase in synaptic activity and the induction of neuronal firing in a hypoglossal motoneurone. Action potential amplitudes have been truncated. c, membrane potential before (1), during (2), and after (3) the glutamate-evoked response. Same response as b at increased gain and faster time base. D, EPSCs with the same electrophysiological and pharmacological characteristics could still be evoked in a reduced slice preparation. E, schematic showing cuts made to reduce the coronal slice to a triangle of tissue and location of recording (*) and stimulating (•) electrodes. Adapted from Paxinos & Watson, 1998.
With supramaximal concentrations of NBQX (20 μm), a residual component of the EPSC remained (22.8 ± 16 % of control, P < 0.001, n = 4, Fig. 2B) suggesting that this residual component was not mediated via glutamate receptors. The receptor(s) and transmitter(s) underlying this other component of raphe pallidus-evoked EPSCs in hypoglossal motoneurones was not investigated further.
It could be argued that these glutamatergic eEPSCs were the result of activation of fibres of passage through the raphe pallidus rather than raphe pallidus neurones themselves, or alternatively, activation of glutamatergic inputs to hypoglossal motoneurones from regions external to the raphe pallidus (Funk et al. 1993; Bellingham & Berger, 1994; Lipski et al. 1994; Singer et al. 1996). In order to clearly demonstrate that these eEPSCs were due to the direct activation of raphe pallidus neurones, studies were undertaken in which the brain slices were placed on an inclined plane within an interface chamber and orientated such that the superfusing ACSF flowed from the dorsal to the ventral surface of the slice. A pressure ejection electrode containing glutamate (10 mm) was placed on the surface of the slice within the raphe pallidus and recordings made from hypoglossal motoneurones. Pressure ejection of glutamate within the confines of the raphe pallidus resulted in an increase in synaptic activity and/ora membrane depolarisation in hypoglossal motoneurones (n = 8). These responses were not the result of direct activation of hypoglossal motoneurones brought about following glutamate transfer across the surface of the slice, because superfusate flow within the chamber was unidirectional and from the dorsal to the ventral surface of the slice, the responses were also abolished in the presence of TTX (1 μm). In the example in Fig. 2C, pressure ejection of glutamate within the raphe pallidus resulted in an concentration-dependent increase in synaptic activity and the induction of action potentials in a hypoglossal motoneurone. Placement of the pressure ejection electrode outside the confines of the raphe pallidus resulted in the inability to evoke synaptic potentials or generate action potentials in the same neurone.
Other glutamatergic inputs to hypoglossal motoneurones have previously been described, with these inputs originating from neurones located dorsolateral to the hypoglossal nucleus (Bellingham & Berger, 1994; Singer et al. 1996) and from the ventral respiratory group (VRG, Funk et al. 1993; Lipski et al. 1994). Whilst the above microinjection studies strongly suggest that the glutamatergic eEPSCs evoked in the present studies were not due to the activation of neurones within these two regions, additional studies were performed to further rule out these possibilities. A reduced slice preparation consisting of a triangle of tissue, with the raphe pallidus at the apex and the dorsally running cut edges going through or at the lateral edge of the hypoglossal nucleus was prepared (Fig. 2E). In this preparation, both tissue lateral to the hypoglossal nucleus and the region containing the VRG were specifically ablated. EPSCs with the same latency (5.92 ± 1.15, n = 6 from three different slices, P = n.s, unpaired student t test), electrophysiological and pharmacological characteristics (Fig. 2D) as those in the intact slice preparation could still be evoked following stimulation within the raphe pallidus.
Together, this combination of electrical and chemical stimulation studies clearly demonstrates that monosynaptic glutamatergic EPSCs can be evoked in hypoglossal motoneurones following activation of cell bodies specifically within the raphe pallidus and that these eEPSCs are not the result of activation of fibres of passage through this latter region or other, previously described, glutamatergic inputs to these motoneurones.
In addition to eEPSCs evoked in hypoglossal motoneurones following stimulation within the raphe pallidus, spontaneous EPSCs (sEPSCs) were observed in the majority of neurones. These sEPSCs were also reduced in amplitude or abolished by the AMPA/kainateantagonist, NBQX (10–20 μm, P < 0.05, n = 6, Fig. 3A) but were unaffected by the 5-HT2 receptor antagonist, ritanserin (n = 2, Fig. 3B). Ritanserin had no effect on the frequency of the sEPSCs. In the presence of TTX, the majority of these sEPSCs were abolished indicating that they consist of both EPSCs arising from activity in neurones presynaptic to the hypoglossal motoneurones and also activity-independent, spontaneous release from presynaptic terminals i.e. miniature EPSCs (mESPCs).
Figure 3. Spontaneous excitatory postsynaptic currents in hypoglossal motoneurones are glutamatergic.
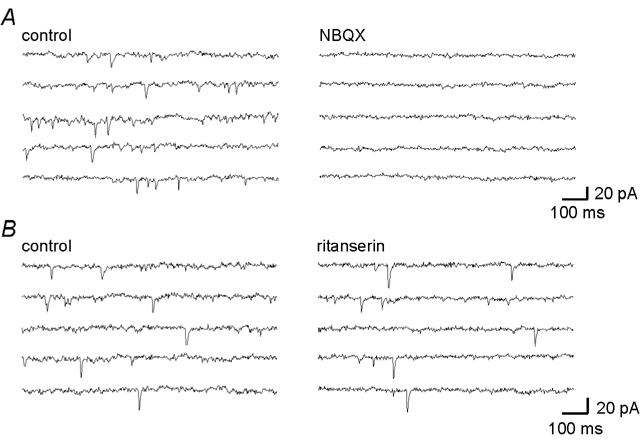
Current records showing spontaneous EPSCs in a hypoglossal motoneurone and their abolition by the AMPA/kainateantagonist, NBQX (20 μm, A) but not by the 5-HT2 antagonist, ritanserin (10 μm, B).
Together, these data demonstrate that, in addition to the previously described glutamatergic input to these neurones from neurones located lateral to the hypoglossal motonucleus (Bellingham & Berger, 1994; Singer et al. 1996) and from the VRG (Funk et al. 1993; Lipski et al. 1994), hypoglossal motoneurones also receive a glutamatergic input from the nucleus raphe pallidus
5-HT modulation of glutamatergic inputs to hypoglossal motoneurones
The majority of raphe pallidus neurones which project to the hypoglossal nucleus are immunoreactive for 5-HT (Henry & Manaker, 1998). However, the above studies have demonstrated that the principal transmitter underlying raphe pallidus-evoked EPSCs in hypoglossal motoneurones is glutamate. Thus, the following studies were undertaken to determine whether 5-HT modulates the glutamatergic EPSC via an action at either a pre- or postsynaptic site.
In the presence of the 5-HT uptake inhibitor fluoxetine (50 μm), the amplitude of the eEPSC was reduced (n = 2, 75.6 % and 79.3 % of control, neurones from different animals) suggesting that 5-HT, released in the vicinity of the neurone, is modulating the ESPC. This inhibition of the EPSCs by fluoxetine was mimicked by 5-HT in all neurones investigated, with 10 μm 5-HT reducing the amplitude of the EPSCs to 50.0 ± 13 % of control at a holding potential of −70 mV (P < 0.001, n = 7, Fig. 4A and B). Recovery of the eEPSCs was observed on washout of the 5-HT. Similarly, the 5-HT1A agonist, 8-OH-DPAT (50 μm, 52.5 ± 17 % of control, P < 0.001, n = 13), and the 5-HT1B agonist, CP 93129 (10 μm, 40.6 ± 29 % of control, P < 0.01, n = 8), but not the 5-HT2 agonist α-Me-5-HT (94.1 ± 7.5 % of control, P = n.s., n = 6), also reversibly inhibited the eEPSC (Fig. 4B). The 5-HT-mediated inhibition of the EPSC was also not reduced in the presence of the 5-HT2 antagonist, ritanserin (49.2 ± 8.4 % of control vs. 52.1 ± 11 %, 5-HT vs. 5-HT and ritanserin, P = n.s, n = 3). Since, in these neurones, 8-OH-DPAT and CP 93129 had negligible effects on resting membrane potential and cell input resistance (see earlier), it is highly likely that the inhibition of the EPSC by these two agonists is due to an action at a presynaptic site.
Figure 4. 5-HT decreased the amplitude of EPSCs following activation of presynaptic 5-HT1A and 5-HT1B receptors.
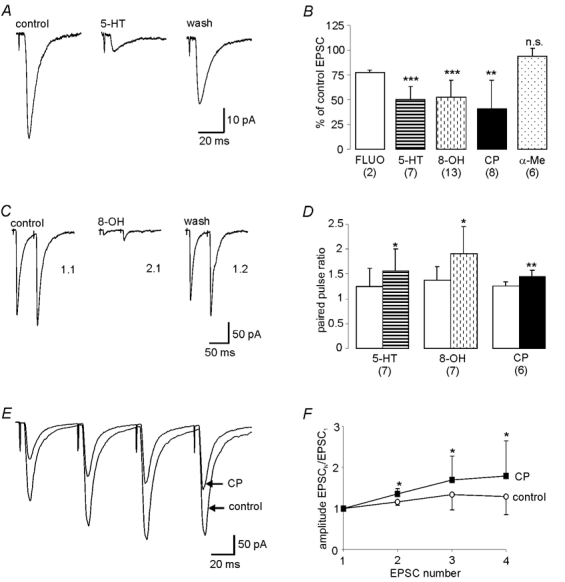
A. 5-HT (20 μm) decreases, in a reversible manner, the amplitude of EPSCs evoked in hypoglossal motoneurones following stimulation within the raphe pallidus. B, the 5-HT-evoked decreases in EPSC amplitude were mimicked by the 5-HT uptake inhibitor, fluoxetine (Fluo, 50 μm), the 5-HT1A agonist, 8-OH-DPAT (8-OH, 50 μm) and the 5-HT1B agonist CP 93129 (CP, 10 μm), but not the 5-HT2 agonist, α-Me-5-HT (α-Me, 50 μm). The amplitude of the average of 50 consecutive EPSCs obtained during the peak agonist response is expressed as a percentage of the averaged EPSC obtained in normal aCSF prior to agonist application. C and D, 5-HT and its selective agonists also evoked significant increases in the paired pulse ratio. C, twin pulse stimulation within the raphe pallidus (stimulus interval 50 ms) evoked pairs of EPSCs in hypoglossal motoneurones. The amplitude of the second EPSC was compared to the first to give the paired pulse ratio (PPR). In this neurone, whilst the PPR in normal aCSF was 1.1, it was increased to 2.1 in the presence of 8-OH-DPAT (8-OH, 50 μm) suggesting that the agonist was having its effects via an action at presynaptic terminals. Recovery of the PPR was observed on washout. D, mean data showing increases in the PPR in the presence of 5-HT (10 μm), 8-OH-DPAT (50 μm) and CP 93129 (10 μm). E, CP 93129 evokes progressively less inhibition of sequential eEPSCs in a train. Superimposed averages of EPSCs evoked by a train of four pulses (stimulus interval = 50 ms, train frequency = 0.02 Hz) in normal ACSF and in the presence of CP 93129. Data are averages from 64 trials. F, CP 93129 increases the amplitude ratio of successive EPSCs in a train. Mean data of amplitude ratio (Amplitude EPSCn/EPSC1) of each EPSC in train of four relative to the first in normal ACSF (○) and in the presence of CP 93129 (▪). Data are averages from 5 neurones of 64 trials in each neurone. * denotes significance of amplitude ratio for each EPSC in the train in the presence of CP 93129 compared to the corresponding ratio obtained in normal ACSF (*P < 0.05).
In order to conclusively show that the 5-HT-evoked reduction in the eEPSC was in fact due to a presynaptic action three methods, which are commonly utilised to demonstrate such an action, were employed (Bertollino et al. 1997; Li & Bayliss, 1998). Thus, the effects of 5-HT and its selective agonists on the eEPSC paired pulse ratio (PPR), the response to glutamate applied directly to the postsynaptic cell membrane and the amplitude and frequency of miniature EPSCs (mEPSCs) were investigated. The PPR is the ratio of the amplitude of two EPSCs evoked in quick succession (amplitude EPSC2/EPSC1). A ratio of greater than one, that is the second EPSC is greater than the first (paired pulse facilitation, PPF), is thought to reflect a low probability of synaptic release. On the other hand, if the first EPSC is greater than the second (paired pulse depression, PPD) giving a ratio <1, this is thought to be indicative of a high probability of synaptic release (Manabe et al. 1993; Debanne et al. 1996). Therefore, if 5-HT is modulating presynaptic release, it will alter the PPR, either increasing (presynaptic inhibition) or decreasing (presynaptic excitation) the ratio. Whilst it will modulate the amplitude of the glutamatergic eEPSCs and hence the PPR, it should have no effect on the inward currents evoked when glutamate is applied directly in the vicinity of the postsynaptic cell membrane. However, if 5-HT is acting postsynaptically, it will modulate both the glutamate-evoked inward currents and the glutamatergic EPSCs. Furthermore, in this instance, since this modulation of the EPSCs would be in proportion to their initial amplitude, the PPR would remain unchanged. The final evidence for 5-HT acting presynaptically would be if it modulated activity-independent release from the presynaptic terminals, that is, altered the frequency but not the amplitude of mEPSCs observed in hypoglossal motoneurones.
When the raphe pallidus was stimulated utilising a train of paired stimuli (stimulus interval = 50 ms, 0.05 Hz), paired pulse facilitation was observed (PPR = 1.46 ± 0.33, n = 10) in all hypoglossal motoneurones except one, this latter neurone exhibiting paired pulse depression. When 5-HT (10 μm) was applied during this paired pulse stimulation, an increase in the ratio was observed in the majority of neurones (1.25 ± 0.36 to 1.56 ± 0.44, P < 0.05, n = 7, Fig. 4D). This included the single neurone which showed PPD under control conditions switching to exhibiting PPF. These data suggest that 5-HT is acting at the presynaptic terminals, decreasing glutamate release in the majority of neurones. This effect of 5-HT was mimicked by both the 5-HT1A agonist 8-OH-DPAT (Fig. 4C) and the 5-HT1B agonist CP 93129, resulting in increases in the PPR from 1.38 ± 0.27 to 1.91 ± 0.54 with 8-OH-DPAT (50 μm, P < 0.05, n = 7/10) and from 1.27 ± 0.08 to 1.44 ± 0.13 with CP 93129 (10 μm, P < 0.01, n = 6/8)(Fig. 4D). Similarly, when the more physiological stimulus of a train of four pulses was utilised, a similar facilitation of the amplitude ratio of successive EPSCs relative to the first in the train (amplitude EPSCN EPSC1) was observed in the presence of either 5-HT (n = 2), 8-OH-DPAT (n = 3) or CP 93129 (P < 0.05, n = 5). In these studies, trains of four stimuli (stimulus interval = 50 ms) were delivered every 5 s and the average amplitude of each EPSC in the train over 64 trials in the absence and presence of either 5-HT, 8-OH-DPAT or CP 93129 determined. In the example in Fig. 4E, under control conditions there was an increase in amplitude of sequential EPSCs in the train when compared to the first. Whilst application of CP 93129 resulted in inhibition of the amplitude of these EPSCs (P < 0.05), this inhibition was less marked with later EPSCs in the train. When the amplitude ratio for each EPSC in the train in the presence of CP 93129 was compared to the ratio for the same EPSC obtained under control conditions, a progressive increase in paired pulse facilitation was observed. Figure 4F shows the mean data taken from five neurones, with CP 93129 evoking a significant increase in the amplitude ratios of each EPSC in the train (P < 0.05).
In addition to 8-OH-DPAT and CP 93129 increasing the PPR or the amplitude ratio when multiple EPSCs were evoked, further evidence for 5-HT activating presynaptic 5-HT1A and/or5-HT1B receptors to inhibit glutamate release from raphe pallidus inputs was obtained by demonstrating that these agonists could inhibit the glutamatergic EPSCs, increasing the PPR, whilst having no effect on the current evoked by glutamate directly applied to the postsynaptic membrane. Pulses of glutamate were pressure ejected every 30 s from a micropipette placed in the immediate vicinity of the neurone under investigation and the resultant inward current recorded. In the same neurone, immediately after each glutamate response, EPSCs were evoked by paired pulse electrical stimulation within the raphe pallidus. Averages of 15–20 consecutive trials before and during either CP 93129 or 8-OH-DPAT application were compared. The two agonists had no effect on the glutamate-evoked inward currents; the mean amplitudes of these currents evoked in the presence of CP 93129 and 8-OH-DPAT compared to those evoked in normal aCSF were 100 ± 2.5 % (P = n.s., n = 3) and 99.0 ± 4.4 % (P = n.s., n = 3) respectively. In contrast, in the same neurones, the PPR was increased from 1.32 ± 0.06 to 1.58 ± 0.08 in the presence of CP 93129 and from 1.57 ± 0.35 to 2.31 ± 0.55 in the presence of 8-OH-DPAT.
Finally, mEPSCs were recorded in the presence of TTX (1 μm, n = 6), with EPSCs considered as ‘minis’ after abolition by TTX of electrically evoked synaptic potentials and action potentials during depolarising current steps. Both 8-OH-DPAT (n = 3) and CP 93129 (n = 3, Fig. 5) markedly reduced the frequency (P < 0.001) of mEPSCs but had no effect on their amplitude (P = n.s.). The actions of 8-OH-DPAT and CP 93129 on mEPSC amplitudes and frequencies were also investigated in other hypoglossal motoneurones. However, in these latter neurones, these agonists increased membrane noise and therefore their actions on mEPSCs could not be clearly evaluated. These data provide further evidence that 5-HT can inhibit glutamate release via an action at presynaptic terminals.
Figure 5. CP 93129 decreases the frequency, but not the amplitude, of miniature excitatory postsynaptic currents.
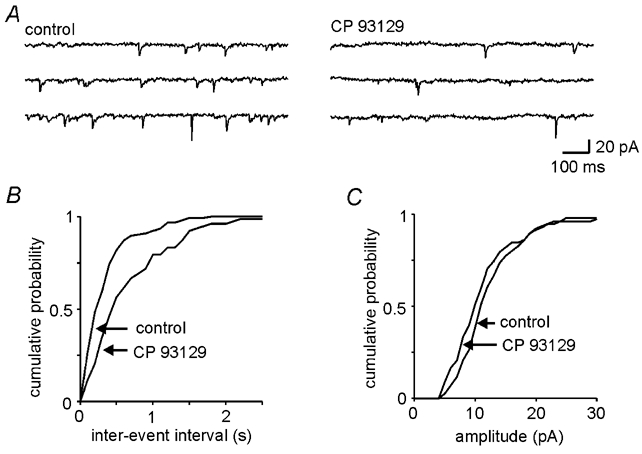
A, voltage clamp records showing miniature EPSCs recorded in the absence and presence of the 5-HT1B agonist, CP 93129 (10 μm). B, C, cumulative probability histograms of the data from A showing that CP 93129 application resulted in a significant reduction in the frequency of mESPCs (B, P < 0.001) but had no effect on their amplitude (C, P = n.s.)
Together, these data demonstrate that 5-HT, acting via 5-HT1A and/or5-HT1B receptors located on presynaptic terminals of raphe pallidus projections to hypoglossal motoneurones, inhibits the release of glutamate from these projections.
DISCUSSION
The main findings of this study were that the principal excitatory transmitter underlying EPSCs in hypoglossal motoneurones following activation of projections from the raphe pallidus was glutamate and that these EPSCs could be decreased in amplitude as a result of 5-HT activating 5-HT1A and/or5-HT1B receptors located on the presynaptic terminals of these inputs.
Given the wealth of data from anatomical and in vivo studies suggesting that raphe pallidus inputs to hypoglossal motoneurones are 5-hydroxytryptaminergic (Kubin et al. 1994; Henry & Manaker, 1998; Jelev et al. 2001), the observation from the present study that the resultant EPSC is mediated principally by glutamate may appear surprising. However, data from other studies support the present findings. Anatomical studies have demonstrated that all 5-HT-immunoreactive neurones within the caudal raphe nuclei are also immunoreactive for glutamate (Nicholas et al. 1992). They are also all immunopositive for glutaminase (Kaneko et al. 1990), the synthesising enzyme for glutamate and GABA, yet few of these are immunopositive for GABA (Milhorn et al. 1988), glutamic acid decarboxylase (GAD) (Bellin et al. 1983; Milhorn et al. 1987; Stamp & Semba, 1995) or contain GAD mRNA (Stornetta & Guyenet, 1999), suggesting that 5-HT is co-localised with glutamate rather than GABA in virtually all of these neurones. These 5-HT/glutamate-immunopositive caudal raphe neurones project to many different regions of the neuroaxis including the spinal cord (Manaker et al. 1992; Nicholas et al. 1992; For review see Jacobs & Azmitia, 1992). Activation of neurones within one of these caudal raphe nuclei, the raphe pallidus, results in an increase in firing rate of phrenic motoneurones, both during the inspiratory phase but also during the normally quiescent excitatory phase (Lalley, 1986). 5-HT antagonists reduce this tonic increase in firing rate, re-establishing the phasic nature of phrenic discharge. However, in reserpinised animals, activation of the raphe pallidus still results in an increase in neuronal firing rate during the inspiratory phase which is unaffected by 5-HT2 antagonists, suggesting that raphe pallidus modulation of phrenic motoneurones involves both serotonergic and non-serotonergic mechanisms (Lalley, 1986).
In addition to caudal raphe neurones modulating the activity in other brain regions by these dual mechanisms, there is also evidence to suggest that single raphe neurones modulate the activity of both themselves and other caudal raphe neurones in a similar fashion. Caudal raphe neurones have axon collaterals that remain within the nuclei (Gao & Mason, 1997; Li & Bayliss; 1998), with activation of individual caudal raphe neurones eliciting a glutamatergic EPSP in adjacent serotonergic neurones within the nucleus (Li & Bayliss, 1998). Similarly, in cultures of single serotonergic raphe neurones, activation of these single neurones results in the generation of a glutamatergic EPSP in the neurone (Johnson, 1994b). In a minority of these cultured single neurones, co-release of glutamate and 5-HT could be demonstrated, with the fast glutamatergic EPSP being followed by a slow 5-HT1A receptor-mediated IPSP. Together, the data from these in vivo and in vitro studies suggest that a population of caudal raphe neurones modulate the activity of themselves, adjacent caudal raphe neurones and projection neurones external to the nuclei by dual glutamatergic and 5-HT receptor-mediated mechanisms. The data from the present study are consistent with this mode of interaction between glutamatergic and serotonergic transmission.
Whilst we have shown that the principal transmitter underlying raphe pallidus-evoked EPSCs in hypoglossal motoneurones was glutamate, we could find no evidence of 5-HT-mediated excitatory synaptic potentials. This is in keeping with studies in other brain regions which, whilst providing numerous examples of 5-HT-mediated IPSCs (Pan et al. 1989; Bobker & Williams, 1990; Piguet et al. 2000), have demonstrated few examples of 5-HT receptor-mediated EPSCs (Wang & Dun, 1990; Bobker, 1994).
The question then arises as to whether 5-HT is being released from these caudal raphe projections to hypoglossal motoneurones, and if so, by what mechanisms does it modulate the postsynaptic neurone. Application of the 5-HT uptake inhibitor, fluoxetine, resulted in a membrane depolarisation and increase in cell input resistance in hypoglossal motoneurones, an action which mirrored that of 5-HT applied directly to the postsynaptic membrane, implying that 5-HT is being released within the hypoglossal nucleus. Since the caudal raphe provides the only source of 5-HT innervation of these neurones (Manaker & Tischler, 1993), these data suggest that whilst 5-HT is being released from raphe pallidus projections to hypoglossal motoneurones, the 5-HT2 receptors on the postsynaptic cell must be extrasynaptic, with 5-HT acting in a paracrine fashion (Bunin & Wightman, 1999). Again, the observation from anatomical studies that 68 % of 5-HT-containing axon terminals and varicosities within the hypoglossal nucleus do not appose any synaptic specialisation in the postsynaptic cell supports this suggestion (Aldes et al. 1989). Activation of these extrasynaptic receptors by 5-HT would result in the facilitation of excitatory synaptic inputs, for example glutamate, as has been described for other populations of motoneurones (Lewis & Coote, 1996).
The present studies do not enable us to determine whether 5-HT and glutamate are released from the same or separate populations of raphe pallidus neurones. The former is more likely since all 5-HT-containing caudal raphe neurones contain co-localised glutamate (Nicholas et al. 1992), glutamate and 5-HT are co-released from cultured caudal raphe neurones (Johnson, 1994b) whilst, in other neurones, 5-HT synaptic potentials are also always preceded by fast glutamatergic EPSCs (Pan et al. 1989; Bobker & Williams, 1990; Wang & Dun, 1990; Bobker, 1994; Piguet et al. 2000). However, this release of glutamate or 5-HT from a single neurone may well be dependent on the level of ongoing activity and pattern in that neurone. (Iverfeldt et al. 1989; Franck et al. 1993). Spinally projecting caudal raphe neurones release different complements of transmitters depending on the frequency of activation, with the neurone functioning purely as a 5-HT neurone at low frequencies of activation, releasing both 5-HT and TRH at intermediate frequencies, with 5-HT and substance P being released at higher frequencies (Iverfeldt et al. 1989). Similarly, at the same frequency of activation, intermittent rather than continuous stimulation results in greater 5-HT release (Franck et al. 1993).
In addition to a postsynaptic excitatory action of 5-HT, the present study also suggests that 5-HT inhibits glutamate release from raphe pallidus terminals onto hypoglossal motoneurones following activation of both presynaptic 5-HT1A and 5-HT1B receptors. A 5-HT1B receptor-mediated presynaptic inhibition of glutamate release is in keeping with previous studies which have demonstrated a similar 5-HT1B-mediated inhibition for glutamatergic projections between adjacent neurones within the caudal raphe nuclei (Li & Bayliss, 1998) and for glutamatergic inputs to hypoglossal motoneurones from neurones located lateral to the hypoglossal nucleus (Singer et al. 1996). However, in contrast to the present study, no involvement of 5-HT1A receptors was noted in these latter studies. Whilst PCR studies have demonstrated the existence of mRNA for 5-HT2 and 5-HT1B receptors in the hypoglossal nucleus of rats of ages greater than 25 days, mRNA for 5-HT1A receptors could not be identified (Okabe et al. 1997). However, in neonatal rats, autoradiographical and in situ hybridisation studies have demonstrated the existence of 5-HT1A receptors themselves within the hypoglossal nucleus, the levels of which peak at postnatal day 7 (P7) before declining to low levels by P28 (Talley et al. 1997). Similarly, there is a 5-HT1A receptor-mediated inhibition of the afterhyperpolarisation in neonatal hypoglossal motoneurones, an action that is absent in juvenile or adult animals (Talley et al. 1997). Since the present study was undertaken in animals aged between P10 and P14, the dual 5-HT1A and 5-HT1B receptor-mediated presynaptic inhibition of raphe pallidus inputs to hypoglossal motoneurones observed in this study is highly likely to be a developmental phenomena, with a 5-HT1B receptor-mediated inhibition being predominant in adult animals. A similar dual receptor-mediated inhibition has been described for the 5-HT-mediated inhibition of glycinergic inputs to hypoglossal motoneurones in neonatal rats (Umemiya & Berger, 1995).
In summary, these studies have described two mechanisms by which inputs from the raphe pallidus may modulate hypoglossal motoneurones; a glutamatergic fast EPSC and a 5-HT-mediated modulation of the postsynaptic cell following activation of presumably extrasynaptic 5-HT2 receptors. The EPSCs could also be inhibited by co-released 5-HT acting on 5-HT1A and/or5-HT1B receptors located on the presynaptic terminals of the raphe pallidus projection neurones.
Functional relevance
Caudal raphe neurones exhibit state-dependent alterations in activity, with the firing rates of these neurones decreasing during sleep but increasing during feeding or locomotion (Heym et al. 1982; Veasey et al. 1995; Woch et al. 1996). Decreases in their activity during sleep result in a decreased release of 5-HT within the hypoglossal motonucleus (Kubin et al. 1994; Lai et al. 2001), a decreased activation of presumed extrasynaptic 5-HT2 receptors and therefore disfacilitation of hypoglossal motoneurones during this period. The neurone will be maintained at more negative membrane potentials diminishing the effectiveness of incoming EPSCs, for example respiratory-related inputs from the ventral respiratory group (Withington-Wray et al. 1998), a principal source of excitation in these neurones, or glutamate-mediated EPSCs from the caudal raphe neurones themselves. This diminution of the effectiveness of incoming inputs will be exacerbated by the decrease in cell input resistance of the motoneurones as a result of decreased 5-HT2 receptor activation, with the same magnitude of EPSC producing a smaller voltage response in the neurone (Bayliss et al. 1997).
However, this direct modulation of hypoglossal motoneurones is only one of many mechanisms by which caudal raphe neurones could modulate activity in hypoglossal motoneurones. The motoneurones could also be modulated indirectly as a result of the 5-HT-mediated presynaptic inhibition of afferent inputs (Singer et al. 1996) or glycinergic hypoglossal interneurones (Umemiya & Berger, 1995). Alternatively, the frequency of inspiratory-related activity in hypoglossal motoneurones could be altered as a result of 5-HT modulating the activity of select populations of inspiratory neurones within the ventral respiratory group (Holtman et al. 1990; Arita & Ochiishi, 1991; Al-Zubaidy et al. 1996). Thus, in vivo, caudal raphe modulation of hypoglossal motoneurones will be a summation of both direct and indirect pathways, with the potential for different pathways to predominate depending on behavioural state or activity.
Acknowledgments
This work was funded by the Wellcome trust whose support is gratefully acknowledged.
REFERENCES
- Aldes LD, Marco LA, Chronister RB. Serotonin-containing axon terminals in the hypoglossal nucleus of the rat. An immuno-electronmicroscopic study. Brain Res Bull. 1989;23:249–256. doi: 10.1016/0361-9230(89)90154-8. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Eur J Physiol. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Arita H, Ochiishi M. Opposing effects of 5-HT on two types of medullary inspiratory neurones with distinct firing patterns. J Neurophysiol. 1991;66:285–292. doi: 10.1152/jn.1991.66.1.285. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Viana F, Talley EM, Berger AJ. Neuromodulation of hypoglossal motoneurons: cellular and developmental mechanisms. Respir Physiol. 1997;110:139–150. doi: 10.1016/s0034-5687(97)00079-0. [DOI] [PubMed] [Google Scholar]
- Bellin MF, Nanopoulos D, Didier D, Aguera M, Steinbusch H, Verhofstad A, Maitre M, Pujol JF. Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res. 1983;275:329–339. doi: 10.1016/0006-8993(83)90994-0. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Adenosine suppresses excitatory glutamatergic inputs to rat hypoglossal motoneurons in vitro. Neurosci Lett. 1994;177:143–146. doi: 10.1016/0304-3940(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Bertollino M, Vicini S, Gillis R, Travagli A. Presynaptic α2-adrenoceptors inhibit excitatory synaptic transmission in rat brainstem. Am J Physiol. 1997;272:G654–661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- Bobker DH. A slow EPSP mediated by 5-HT2 receptors in the nucleus prepositus hypoglossi. J Neurosci. 1994;14:2428–2434. doi: 10.1523/JNEUROSCI.14-04-02428.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin mediated IPSPs in guinea-pig prepositus hypoglossi and feedback inhibition by serotonin. J Physiol. 1990;422:447–462. doi: 10.1113/jphysiol.1990.sp017994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouryi VA, Lewis DI. The modulation by 5-hydroxytryptamine of excitatory inputs to rat hypoglossal motoneurones in-vitro. J Physiol. 2001;533:84P. doi: 10.1113/jphysiol.2003.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Wightman RM. Paracrine neurotransmission in the CNS: involvement of 5-HT. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comp Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–45. [PubMed] [Google Scholar]
- Fay RA, Norgren R. Identification of rat brain multisynaptic connections to the oral motor nuclei using pseudorabies virus III. Lingual muscle motor systems. Brain Res Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Fenik V, Marchenko V, Janssen P, Davies RO, Kubin L. A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J Appl Physiol. 2002;93:1448–56. doi: 10.1152/japplphysiol.00225.2002. [DOI] [PubMed] [Google Scholar]
- Franck J, Brodin E, Fried G. Differential release of endogenous 5-HT substance P and neurokinin A from rat ventral spinal cord in response to electrical stimulation. J Neurochem. 1993;61:704–711. doi: 10.1111/j.1471-4159.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gao K, Mason P. Somatodendritic and axonal anatomy of intracellularly labelled serotonergic neurones in the rat medulla. J Comp Neurol. 1997;389:309–328. [PubMed] [Google Scholar]
- Henry JN, Manaker S. Co-localisation of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1998;391:491–505. [PubMed] [Google Scholar]
- Heym J, Steinfles GF, Jacobs BL. Activation of serotonin containing neurones in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Marion LJ, Specks DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neurosci. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Arnesto LD, Bartfai T. Differential release of co-existing neurotransmitters- frequency dependence of the efflux of substance P, TRH and 5-HT from tissue slices of rat ventral spinal cord. Acta Physiol Scand. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD. Electrophysiological and histochemical properties of postnatal rat serotonergic neurons in dissociated cell culture. Neurosci. 1994a;63:775–788. doi: 10.1016/0306-4522(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurones in microculture. Neuron. 1994b;12:433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the co-existence of substance P, thyrotopin releasing hormone, GABA, met-enkephalin and leu-enkephalin in serotonergic neurones of the caudal raphe nuclei - a dual labelling study in the rat. J Neurosci Res. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Immunohistochemical demonstration of glutaminase in catecholaminergic and serotonergic neurones of rat brain. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack AI. Control of upper airways motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kiumura H, Tojima H, Davis RO, Pack AL. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Kubin L, Reigner C, Tojima H, Taguchi O, Pack AL, Davies RO. Changes in serotonin levels in the hypoglossal nucleus region during carbachol-induced atonia. Brain Res. 1994;645:291–302. doi: 10.1016/0006-8993(94)91663-2. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Reigner C, Pack AI, Davies RO. Interaction of serotonergic excitatory drive to hypoglossal motoneurons with carbachol-induced REM sleep. Sleep. 1996;19:187–195. [PubMed] [Google Scholar]
- Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. Serotonergic and non-serotonergic responses of phrenic motoneurones to raphe stimulation in the cat. J Physiol. 1986;380:373–385. doi: 10.1113/jphysiol.1986.sp016291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DI, Coote JH. Evidence that the firing pattern of sympathetic preganglionic neurones is determined by an interaction between amines and an excitatory amino acid. J Biol Res. 1996;72:279–294. [PubMed] [Google Scholar]
- Li YW, Bayliss DA. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurons in rat. J Physiol. 1998;510:121–134. doi: 10.1111/j.1469-7793.1998.121bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurones in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Manabe T, Wyllie DJ, Perkel DJ, Nicoll RA. Modulation of synaptic transmission and long-term potentiation: effects on paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J Neurophysiol. 1993;70:1451–1459. doi: 10.1152/jn.1993.70.4.1451. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotonergic afferents to the hypoglossal nucleus in the rat. J Comp Neurol. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ, Morrison AR. Raphe spinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- Megirian D, Hinrichsen CF, Sherry JH. Respiratory roles of genioglossus, sternothyroid and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hokfelt T, Seroogy K, Oertel W, Verhofstad AAJ, Wu JY. Immunohistochemical evidence for the co-localisation of γ-aminobutyric acid and serotonin in neurones of the ventral medulla oblongata projecting to the spinal cord. Brain Res. 1987;410:179–185. doi: 10.1016/s0006-8993(87)80043-4. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Hokfelt T, Seroogy K, Verhofstad AAJ. Extent of co-localisation of serotonin and GABA in neurones of the ventral medulla oblongata in rat. Brain Res. 1988;461:169–174. doi: 10.1016/0006-8993(88)90736-6. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Arvidsson U, Hokfelt T. Serotonin, substance P and glutamate/aspartate-like immunoreactivities in medullo-spinal pathways of rat and primate. Neurosci. 1992;48:545–559. doi: 10.1016/0306-4522(92)90401-m. [DOI] [PubMed] [Google Scholar]
- Okabe S, Mackiewicz M, Kubin L. Serotonin receptor mRNA expression in the hypoglossal motor nucleus. Respir Physiol. 1997;110:151–160. doi: 10.1016/s0034-5687(97)00080-7. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Colmers WF, Williams JT. 5-HT mediated synaptic potentials in the dorsal raphe nucleus: Interactions with excitatory amino acid and GABA neurotransmission. J Neurophysiol. 1989;62:481–486. doi: 10.1152/jn.1989.62.2.481. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Blackwell Science Inc; 1998. [Google Scholar]
- Piguet P, Stoeckel ME, Schlichter R. Synaptically released 5-HT modulates the activity of tonically discharging neuronal populations in the rostral ventral medulla. Eur J Neurosci. 2000;12:2662–2675. doi: 10.1046/j.1460-9568.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- Reiner PB. Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Brain Res. 1986;378:86–96. doi: 10.1016/0006-8993(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Degroot WJ, Sauerland ek, Anch MA. Pathogenesis of upper airways occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Gloveli T, Empson RM, Draguhn A, Heinemann U. Serotonin reduces synaptic excitation in the superficial medial entorhinal cortex of the rat via a presynaptic mechanism. J Physiol. 1998;508:119–129. doi: 10.1111/j.1469-7793.1998.119br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Belingham MC, Berger AJ. Presynaptic inhibition of glutamatergic synaptic transmission to rat motoneurons by serotonin. J Neurophysiol. 1996;76:799–807. doi: 10.1152/jn.1996.76.2.799. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Semba K. Extent of co-localisation of serotonin and GABA in neurones of the rat raphe nuclei. Brain Res. 1995;677:39–49. doi: 10.1016/0006-8993(95)00119-b. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Guyenet PG. Distribution of glutamic acid decarboxylase mRNA-containing neurones in rat medulla projecting to rat thoracic spinal cord in relation to monoaminergic brainstem neurones. J Comp Neurol. 1999;407:367–380. [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brainstem. J Neurophysiol. 1995;73:1192–1200. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Responses of serotonergic caudal raphe neurones in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5340–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Intracellular analysis of respiratory modulated hypoglossal motoneurons in the cat. Neurosci. 1988;25:1041–1051. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Woch G, Davies RO, Pack AI, Kubin L. Behaviour of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol. 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurones are postsynaptically inhibited during carbachol induced rapid eye movement sleep. Neurosci. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]


