Abstract
Isometric handgrip exercise (IHG) increases sweating rate without changing core or skin temperatures. The contribution of central command resulting in increases in sweating rate during IHG is unknown. To investigate this question, seven subjects performed IHG (35 % maximum voluntary contraction (MVC) for 2 min) followed by 2-min of post-exercise ischaemia (PEI), with and without partial neuromuscular blockade (PNB). PNB was performed to augment central command during the IHG bout. These trials were conducted while the subject was normothermic, mildly heated, and moderately heated. On the non-exercising arm, forearm sweating rate was monitored over a microdialysis membrane perfused with neostigmine (acetylcholinesterase inhibitor), and at an adjacent untreated site. In normothermia with PNB, despite reduced force production during IHG (17 ± 9 versus 157 ± 13 N; P < 0.001), the elevation in sweating rate at the neostigmine-treated site was greater relative to the control IHG bout (P < 0.05). During subsequent PEI, for the PNB trial mean arterial blood pressure (MAP) and sweating rate returned towards pre-IHG levels, while during the control trial these variables remained elevated. During IHG while mildly heated, the elevation in sweating rate was greater during the PNB trial relative to the control trial. In contrast, during moderate heating sweating increased during IHG for both trials, however the elevation in sweating rate during the PNB trial was not greater than during the control trial. These results suggest that central command is capable of modulating sweating rate in all thermal conditions, however its effect is reduced when body temperatures and/or sweating rate are substantially elevated.
Isometric exercise elevates heart rate (HR) and mean arterial pressure (MAP) and increases muscle and skin sympathetic nerve activities (Mitchell, 1985; Secher, 1985; Victor et al. 1989; Vissing et al. 1991). Sweating rate also increases during isometric handgrip exercise (IHG) without changes in core or skin temperatures (van Beaumont & Bullard, 1966; Crandall et al. 1995; Kondo et al. 1999; Shibasaki et al. 2001). An increase in sweating rate during IHG in the absence of changes in core and skin temperatures, suggests that sweating can be modulated by non-thermal factors such as central command, stimulation of muscle afferents (mechano- and metabo-sensitive receptors), and perhaps baroreceptor loading. Sweating rate remains elevated during post isometric exercise ischaemia (PEI), even when MAP is returned to pre-exercise levels via administration of a depressor agent during the ischaemic period, while sweating rate returns to pre-IHG levels upon release of PEI (Shibasaki et al. 2001). These findings provide evidence that muscle metaboreceptor stimulation is capable of modulating sweating rate, while increases in MAP during IHG are unlikely to contribute.
In addition to muscle metabo/mechanoreceptors, central command is an important mechanism by which HR and MAP increase during exercise (Mitchell, 1990). Although central command may contribute to the modulation of sweating rate during exercise (van Beaumont & Bullard, 1966; Yamazaki et al. 1994), this hypothesis has not been tested. Partial neuromuscular blockade, using agents such as curare derivatives, augment central command during exercise at a given workload resulting in greater increases in HR and MAP (Leonard et al. 1985, Mitchell, 1985; Iwamoto et al. 1987). Using a similar protocol, Vissing & Hjortsø (1996) showed greater increases in skin sympathetic nerve activity during IHG when central command was augmented, but sweating responses were not measured in that study. Given multiple signals in the integrated skin nerve recording (i.e. cutaneous vasoconstrictor, sudomotor, and possibly cutaneous vasodilator), it remains unanswered whether central command is capable of modulating sweating.
The purpose of this study was to test the hypothesis that central command is capable of modulating sweating in humans, and to identify whether the level of heat stress affects the contribution of central command in modulating sweating. These objectives were accomplished by assessing sweating responses during IHG with and without partial neuromuscular blockade under normothermic conditions, when sweating responses were sensitized via local acetylcholinesterase inhibition (Shibasaki et al. 2001), as well as during mild and moderate heat stress.
METHODS
Seven healthy subjects aged 21–39 years participated in this study. All subjects were of normal weight (74 ± 1 kg) and height (182 ± 3 cm), and each subject was informed of the purpose and risks of this study before providing their written consent. An approved informed consent document, by the Ethical Committee of Copenhagen, Denmark (KF01-080100), was reviewed and signed by all participants. All experiments were performed in accordance with the Declaration of Helsinki.
Upon entering the laboratory (room temperature: 22–23 °C), each subject swallowed a telemetry pill for measurement of core (intestinal) temperature, and was instrumented for the measurement of mean skin temperature, from the weighted electrical average of six thermocouples attached to the skin (Taylor et al. 1989), and electrocardiogram. The subject was then dressed in a tube-lined suit that permitted the control of mean skin temperature. The suit covered the entire body surface except for the head, feet, and forearms. A thermistor was inserted in the oesophagus from the nose at a distance equal to one-quarter of the subject's height, except for two subjects who were unable to insert the oesophageal temperature probe. Reported internal temperature data are from the oesophageal probe with the exception of the aforementioned two subjects in whom internal temperature was indexed from the telemetry pill.
Each subject rested in the supine position, while a microdialysis probe (BAS, West Layfette, IN, USA) was placed in the dermal space of dorsal forearm skin. The purpose of this microdialysis probe was to locally administer the acetylcholinesterase inhibitor neostigmine, which permits the assessment of sweating responses at this site in normothermia (Shibasaki et al. 2001). The semi-permeable cellulose membrane window for the probe was 10 mm in length. The probe was placed by piercing a 25-gauge needle in the dermal space without anaaesthesia, and then having the needle exit 20≈25 mm away from the point of entry. The microdialysis probe was inserted through the lumen of the needle and the needle withdrawn, leaving the probe in place. After placement, the probes were perfused with Ringer's solution at a rate of 2 μl min−1. Chambers having a small window (10 × 5 mm, i.e. surface area of 0.5 cm2) were positioned over the membrane. A compressed dry gas mixture (90 % of N2 and 10 % of O2) was perfused through the capsules at a rate of 150 ml min−1. Absolute humidity was calculated from relative humidity and temperature of the effluent gas exiting the chambers (HMP 233, Vaisala Inc.), with the detector positioned 1 m from the capsule on the skin. Sweating rate (in mg cm−2 min−1) was calculated as: sweating rate (mg cm−2 min−1) = absolute humidity (mg m−3) × gas flow (m3 min−1)/chamber window (cm2). Location of capsule placement was aided through the use of markings on the tubing that indicated the centre of the membrane portion of the microdialysis probe. Another sweat capsule (surface area = 2.83 cm2) was attached at least 3 cm away from the aforementioned capsule. This distance is sufficient that the effects of the drug infused through the microdialysis membrane do not affect the response at the adjacent sweat capsule (Shibasaki & Crandall, 2001). Capsules were attached on the skin by using circular double sided tape.
The MAP was recorded from the integrated signal obtained from a finger (Finapres, Amsterdam, The Netherlands) of the hand not performing exercise. The HR was obtained from the electrocardiogram signal, and respiratory frequency was monitored via piezoelectric pneumography to confirm the absence of Valsalva manoeuvres during IHG. Data collection did not begin until a minimum of 60 min after microdialysis probe placement to allow for the hyperaemic response associated with probe placement to subside. Following this period, neostigmine (100 μm), was administered through the microdialysis membrane at a rate of 2 μl min−1. Ten minutes following the administration of neostigmine, subjects performed two maximum voluntary handgrip contractions (MVC) using the contralateral arm relative to the arm from which sweating rate and MAP were obtained. The higher of these two values was used to calculate the workload to be performed during all ensuing IHG bouts.
Following a brief rest period, subjects performed IHG at 35 % MVC for 2 min. During the final 5 s of the 2 min exercise bout, a cuff around the upper portion of the exercising arm was inflated to 350 mmHg and remained inflated for 2 min. The IHG + PEI protocol was performed twice in normothermia separated by a minimum of 5 min. The first bout of IHG + PEI was to familiarize the subject with the protocol, while responses from the second bout were analysed. The subjects were then heated by the water-perfused suit to two levels of heat stress, first mild heating (increased core temperature ≈0.5 °C) followed by moderate heating (increased core temperature ≈1.0 °C). Subjects repeated the IHG + PEI protocol in both of these heated conditions.
Using a randomized crossover design, on a different day, but within a week of the prior test, the aforementioned protocol was repeated. However, prior to each bout of exercise the non-depolarizing neuromuscular blocking agent cisatracurium besylate (Nimbex; GlaxoSmithKline) was administered intravenously using the following dosing regimen. Initially, a 2 mg ml−1 bolus of cisatracurium was administered. Supplemental doses were administered until MVC was reduced to less than 50 % of the force obtained prior to receiving the first dose of cisatracurium in normothermia. Five minutes after the appropriate dose was administered and subsequent identification of reduced strength, each subject performed the IHG + PEI protocol. As the recovery period following cisatracurium administration was 15–20 min, dosing of the drug and subsequent confirmation of reduced strength was repeated prior to each IHG bout for both heating stages. At all times an Ambu-E (Copenhagen, Denmark) resuscitator apparatus, neostigmine, and atropine were available; however it was not necessary to use these in the present protocol. The order of the first test performed (i.e. control day or PNB day) was randomised.
For both trials, subjects were given verbal feedback as to the force necessary to maintain 35 % MVC. If, during the PNB trials the subject was unable to maintain the prescribed force, the subjects were encouraged to maintain whatever force they could produce.
Statistical analysis
Data were recorded at 200 Hz via a 16-bit A/Dconverter (Biopac, Santa Barbara, CA, USA) and stored as 20 s averages. Data represent an average of the final 20 s from each of the following stages: a pre-exercise period, minutes 1 and 2 of IHG, minute 2 of PEI, and recovery.
To assess sweating responses in normothermia, sweating rate at the neostigmine-treated sites was compared between control and PNB trials, since IHG does not increase forearm sweating rate at untreated sites in normothermia (Shibasaki et al. 2001). In contrast, sweating occurred as a result of mild and moderate heating at the untreated sites, and thus sweating responses to IHG between control and PNB trials were statistically analysed at these sites.
Data obtained within each IHG bout and subsequent PEI were compared by one-way repeated measures ANOVA followed by a Dunnett's test when the significant main factor (time) was identified. Differences in response (i.e. Δ) between the resting period and the end of exercise were compared using Student's paired t test. Two-way repeated measures ANOVA, followed by Sheffe's post hoc test, was used to identify the drug and thermal effect on responses such as HR, MAP, and sweating rate prior to exercise. In addition, a two-way repeated measures ANOVA was used to identify the drug and thermal effect on the increase (i.e. Δ) of the variables and force due to IHG. All data are expressed as means ±s.e.m. The level of statistical significance was set at P < 0.05.
RESULTS
The following doses of cisatracurium were administrated prior to IHG: normothermia: 1.39 ± 0.06 mg; mild heating: 0.79 ± 0.06 mg, and moderate heating: 0.80 ± 0.05 mg. These doses were sufficient to cause at least a 50 % reduction in MVC and significantly reduced force production during the IHG bouts.
For the normothermic IHG bouts, sweating was observed at the site in which acetylcholinesterase was inhibited during both the control and PNB trials, while sweating was not observed at the untreated site with the exception of one subject. Importantly, the increase in sweating at the neostigmine-treated site during the normothermic PNB trial occurred earlier and was significantly greater relative to the normothermic control trial (Table 1). These augmented sweating responses occurred despite reduced force production throughout the PNB trial, such that IHG force was close to zero at the end of the bout. During PEI of the PNB trial, sweating rate and MAP returned towards pre-IHG baseline, while these responses remained elevated during PEI for the control trial. These results suggest that during the PNB trial insufficient metabolites were produced during IHG to stimulate metabosensitive receptors during IHG exercise and PEI. Core temperature did not change during this bout of exercise, whereas subtle increases in mean skin temperature (≈0.1 °C) were observed. However, it is unlikely that the large elevation in sweating rate observed during the normothermic PNB trial was due to this small increase in skin temperature. Regardless of PNB, HR increased during IHG, followed by a return to baseline during PEI. Respiratory frequency during IHG and PEI remained normal regardless of whether cisatracurium was administered.
Table 1.
Thermal and haemodynamic responses during isometric handgrip exercise (IHG) and subsequent post-exercise ischaemia (PEI) during control and partial neuromuscular blockade (PNB) conditions in normothermia
| Rest | IHG 1 | IHG 2 | PEI | Recovery | ||
|---|---|---|---|---|---|---|
| Core temperature (°C) | PNB | 36.77 ± 0.09 | 36.78 ± 0.09 | 36.76 ± 0.09 | 36.76 ± 0.09 | 36.76 ± 0.09 |
| Control | 36.68 ± 0.09 | 36.69 ± 0.09 | 36.68 ± 0.09 | 36.67 ± 0.10 | 36.66 ± 0.10 | |
| Mean skin temperature (°C) | PNB | 34.55 ± 0.19 | 34.60 ± 0.18 | 34.66 ± 0.18* | 34.64 ± 0.19* | 34.66 ± 0.17* |
| Control | 34.49 ± 0.19 | 34.49 ± 0.19 | 34.50 ± 0.19 | 34.52 ± 0.20 | 34.52 ± 0.20 | |
| Heart rate (beats min−1) | PNB | 66.1 ± 4.4 | 88.0 ± 4.3*† | 85.7±3.6* | 63.0 ± 4.0 | 64.2 ± 3.8 |
| Control | 63.4 ± 3.5 | 72.7 ± 3.3* | 79.0 ± 2.8* | 62.6 ± 3.8 | 62.3 ± 3.6 | |
| Mean arterial pressure (mmHg) | PNB | 84.9 ± 2.6 | 108.0 ± 3.3* | 109.6 ± 2.7* | 86.4 ± 2.8† | 80.6 ± 3.0 |
| Control | 84.2 ± 3.0 | 98.5 ± 2.5* | 114.5 ± 5.0* | 104.8 ± 3.3* | 82.8 ± 2.5 | |
| Force (N) | PNB | 0 ± 0 | 53 ± 22*† | 17 ± 9† | 0 ± 0 | 0 ± 0 |
| Control | 0 ± 0 | 157 ± 12*† | 157 ± 13* | 0 ± 0 | 0 ± 0 | |
| Sweat rate at untreated site | PNB | 0.0 ± 0.0 | 0.003 ± 0.004 | 0.009 ± 0.009 | 0.005 ± 0.005 | 0.001 ± 0.002 |
| (mg cm−2 min−1) | Control | 0.0 ± 0.0 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Sweat rate at neostigmine site | PNB | 0.0 ± 0.0 | 0.063 ± 0.020*† | 0.119 10.019*† | 0.028 ± 0.015 | 0.000 ± 0.019 |
| (mg cm−2 min−1) | Control | 0.0 ± 0.0 | 0.002 ± 0.001 | 0.024 ± 0.008* | 0.047 ± 0.008* | 0.022 ± 0.007 |
Rest: period prior to the onset of isometric exercise; IHG 1 and IHG 2: minutes 1 and 2 of isometric handgrip exercise; Recovery: period following PEI.
Significant differences relative to the resting period, P < 0.05;
significant differences relative to the control trial, P < 0.05.
Prior to IHG, at both mild and moderate heating stages, there were no differences in skin and core temperatures, sweating rate, blood pressure, or HR between control and PNB trials (Fig. 1). Force production during IHG was dramatically reduced at all thermal levels for the PNB trial (Fig. 2). Although there was a tendency for HR to be elevated during IHG with PNB, relative to the control trial, these differences were not significant. In contrast, the increase in MAP during IHG was greater during the control trial relative to the PNB trial at each thermal stage. During mild and moderate heating trials, IHG did not significantly alter core or skin temperatures.
Figure 1. Average thermal and haemodynamic responses during each thermal condition prior to isometric handgrip exercise.
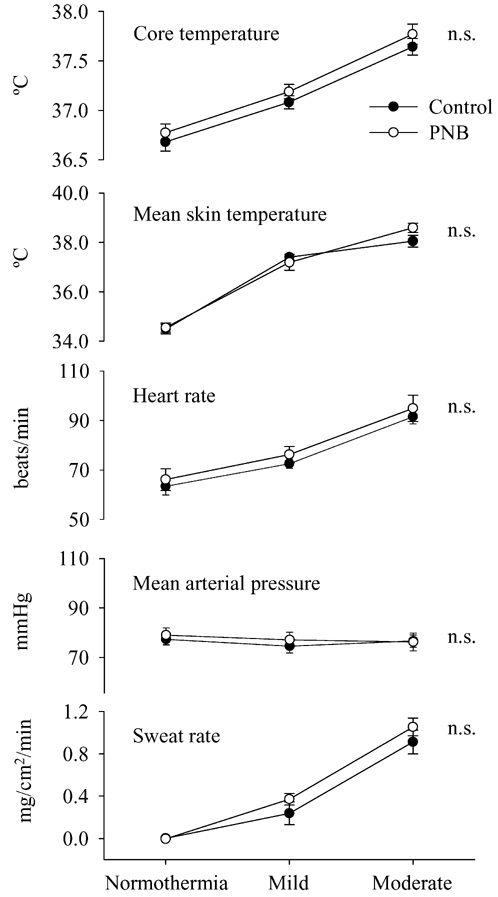
For both the control (•) and the cisatracurium (○) trials, whole-body heating, at both mild and moderate levels, significantly increased core and mean skin temperature, heart rate, and sweating rate. n.s.: Not significantly different between control and PNB main effects from the ANOVA.
Figure 2. Changes in thermal and haemodynamic responses due to isometric handgrip exercise (IHG) across differing thermal loads.
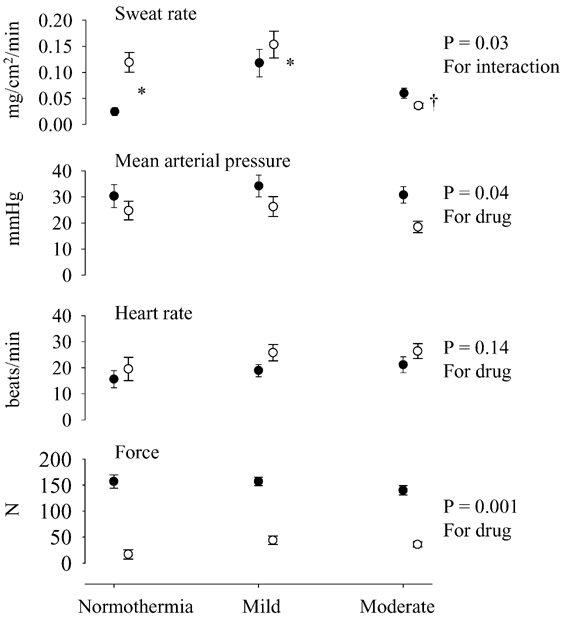
For both the control (•) and the cisatracurium (○) trials, data were obtained by calculating the increase in the response between pre-exercise levels relative to the last minute of IHG. A significant interactive effect was identified for sweating rate from the ANOVA, while a significant main factor effect (control versus partial neuromuscular blockade trials) was identified for blood pressure, and force. During IHG with partial neuromuscular blockade, the increase in sweating rate was greater during the normothermic and mild heated conditions despite a significantly lower IHG force. However, while moderately heated, the increase in sweating during IHG was attenuated for both trials relative to the mild heating condition. * Significantly greater than the control trial, P < 0.05. † Significantly lower than the control trial, P < 0.05.
For the mild heating period, despite considerably reduced force production during the PNB trial, sweating rate was significantly elevated relative to the control trial (Fig. 2). However, during the moderate heating IHG bout, the elevation in sweating rate was slightly greater during the control trial relative to the PNB trial. Nevertheless, increases in sweating rate occurred during the moderate heating PNB trial even though IHG force production was very low relative to the control IHG bout.
DISCUSSION
A primary finding of the present study is that, despite reduced force production, when central command during IHG exercise was augmented by PNB, the increase in sweating rate during the normothermic and mild heat trials was greater compared to the control trials. In contrast, during moderate heating the increase in sweating rate during IHG was slightly lower during the PNB trial relative to the control trial, although this slightly lower sweating rate occurred in response to dramatically reduced force production. These data suggest that central command is capable of modulating cholinergic nerve activity governing sweating rate from non-glabrous skin in all thermal conditions tested.
During the normothermic control trial, sweating rate at the neostigmine-treated site, as well as HR and MAP, increased during IHG. During subsequent PEI, sweating rate and MAP remained elevated relative to pre-IHG levels, while HR return to the baseline; consistent with our prior observations (Crandall et al. 1995; Kondo et al. 1999; Shibasaki et al. 2001). Conversely, in the normothermic PNB trial, during PEI sweating rate and MAP decreased to a level not different from pre-IHG levels. Presumably, during the PNB trial accumulation of exercise-induced metabolites was insufficient to engage the metaboreflex during IHG due to attenuated force production (Table 1 and Fig. 2). However, subjects continued to attempt to exercise throughout the entire 2 min IHG bout. Despite reduced force production during the PNB trials, sweating rates for the normothermic and mild heating bouts were greater than in the respective control bouts. Taken together, these data suggest that the elevation in sweating rate during IHG with partial neuromuscular blockade was primarily due to central command with little or no influence from muscle metaboreceptors.
Previously Vissing & Hjortsø (1996) and Vissing et al. (1991) reported that central command increases integrated skin sympathetic nerve activity. Although this finding is consistent with the present findings, variations exist. For example, in normothermic subjects, despite central command-induced increases in skin sympathetic nerve activity, neither cutaneous vasoconstriction nor sweating responses were observed at skin sites within the field of innervation of the recorded nerve. In addition, during PEI Vissing et al. (1991) report that integrated skin sympathetic nerve activity returned to pre-exercise baseline, whereas we showed that sweating remains elevated in PEI during heat stress and in normothermia at neostigmine-treated sites (Crandall et al. 1995; Kondo et al. 1999; Shibasaki et al. 2001). Thus, there is an apparent disconnection between the integrated neural signal and the associated efferent response that may be related to multiple signals within integrated microneuographic recordings, such as cutaneous vasoconstrictor, sudomotor, piloerector, and possibly cutaneous vasodilator signals. Nevertheless, the present data reveal that central command is capable of modulating sweating, however the effects of central command in modulating this response may be dependent on thermal load and/orthe level of sweating.
IHG increased sweating rate during mild and moderate heating, and at the neostigmine-treated site in normothermia, and this increase occurred without appreciable changes in core or skin temperatures. However, the magnitude of the increase in sweating rate appeared to depend on the thermal status, as the largest increase in sweating during IHG occurred during mild heating stages when core temperature and sweating responses were relatively low (Fig. 2); which is consistent with prior observations (Kondo et al. 2002). The increase in sweating rate was greater when central command was augmented via PNB under normothermic and mild heating conditions when compared to non-PNB trials. Interestingly, in the moderately heated condition the increase in sweating rate during IHG with PNB was slightly less than during the control trial. The mechanism resulting in this observation is unclear, but two possible hypotheses are raised. First, the influence of central command in modulating sweating may be attenuated when body temperature and/orsweating rate is substantially increased (i.e. reduced sweating responsiveness to a given level of central command). Another possibility is that as core temperature rises, activation of central command is attenuated during an exercise bout resulting in attenuated modulation of sweating rate (i.e. reduced central command during exercise while moderately heated). However, if the latter is correct, during IHG with PNB the elevation in HR should also be attenuated, which was not the case.
Limitations of the interpretation of the findings
During the final minute of PEI for the mild and moderate heating trials, slight increases in core temperature were observed (0.03 °C to 0.15 °C). Given this rise in core temperature, we chose to not report these data because during this period the thermal contribution leading to changes in sweating could not be separated from the potential effects of non-thermal factors. Nevertheless, during PEI for the mild and moderate heating PNB trials, sweating rate decreased relative to the last minute of IHG despite the slight increase in core temperature. However, sweating rate at the end of PEI remained greater than pre-IHG baseline. Thus, it is likely that a component of the increase in sweating during PEI was due to slight increases in core temperature notwithstanding our attempts to clamp core temperature during IHG and subsequent PEI. Nevertheless, the observation of greater increases in sweating during the normothermic and mild heating PNB trials, when force production was very low, coupled with the absence of detectable changes in core temperature, suggests that a major component of the increase in sweating rate during IHG was due to central command.
Neostigmine was locally administered to inhibit the breakdown of acetylcholine at one of the sites where sweating was measured. Our prior work demonstrates that this technique sensitizes the sweating response such that changes in sweating rate are observed due to perturbations that otherwise would not results in measurable increases in sweating (Shibasaki & Crandall, 2001; Shibasaki et al. 2001). These prior observations also indicate that as sweating rate increases, the effectiveness of neostigmine in sensitizing the sweating response is diminished. Given this, in some subjects the monitoring of sweating rate from the neostimine-treated sites was discontinued during the mild and moderate heating bouts. Thus, reported sweating responses during the heated conditions are solely from the untreated site. It is important to emphasize that, in contrast to normothermic conditions, analysis of sweating rate at the neostigmine-treated site during mild and moderate heating was not necessary to identify whether central command is capable of modulating sweating rate.
In conclusion, a number of studies have shown that central command is capable of modulating variables such as heart rate, blood pressure, and muscle and skin nerve activities. Results from the present study add to that list by demonstrating that central command is capable of modulating sweating rate. In addition, sweating throughout PEI during the control IHG bouts supports our previous findings suggesting that metaboreflexes are also capable of modulating sweating rate. Finally, the contribution of central command to increasing sweating rate during IHG is minimized when body temperature and/orsweating rate is substantially elevated.
Acknowledgments
Appreciation is expressed to the subjects for their assistance and participation in the study. The research project was funded in part by grants from the National Institutes of Health - National Heart, Lung, and Blood Institute (Crandall: HL-61388 and HL-67422), Human Frontier Science Program (Crandall: ST00066/2001C), Grant-in-Aid for the Encouragement of Young Scientists (Shibasaki: 14704020) and the Danish National Research Foundation (Secher: 504–4).
REFERENCES
- Crandall CG, Musick J, Hatch JP, Kellogg DL, Jr, Johnson JM. Cutaneous vascular and sudomotor responses to isometric exercise in humans. J Appl Physiol. 1995;79:1946–1950. doi: 10.1152/jappl.1995.79.6.1946. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Mitchell JH, Mizuno M, Secher NH. Cardiovascular responses at the onset of exercise with partial neuromuscular blockade in cat and man. J Physiol. 1987;384:39–47. doi: 10.1113/jphysiol.1987.sp016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Horikawa N, Aoki K, Shibasaki M, Inoue Y, Nishiyasu T, Crandall CG. Sweating responses to a sustained static exercise is dependent on thermal load in humans. Acta Physiol Scand. 2002;175:289–295. doi: 10.1046/j.1365-201X.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Tominaga H, Shibasaki M, Aoki K, Koga S, Nishiyasu T. Modulation of the thermoregulatory sweating response to mild hyperthermia during activation of the muscle metaboreflex in humans. J Physiol. 1999;515:591–598. doi: 10.1111/j.1469-7793.1999.591ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Mitchell JH, Mizuno M, Rube N, Saltin B, Secher NH. Partial neuromuscular blockade and cardiovascular responses to static exercise in man. J Physiol. 1985;359:365–379. doi: 10.1113/jphysiol.1985.sp015590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH. Cardiovascular control during exercise: central and reflex neural mechanisms. Am J Cardiol. 1985;55:34–41D. doi: 10.1016/0002-9149(85)91053-7. [DOI] [PubMed] [Google Scholar]
- Mitchell JH. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. [PubMed] [Google Scholar]
- Secher NH. Heart rate at the onset of static exercise in man with partial neuromuscular blockade. J Physiol. 1985;368:481–490. doi: 10.1113/jphysiol.1985.sp015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol. 2001;90:757–762. doi: 10.1152/jappl.2001.90.3.757. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Kondo N, Crandall CG. Evidence for metaboreceptor stimulation of sweating in normothermic and heat-stressed humans. J Physiol. 2001;534:605–611. doi: 10.1111/j.1469-7793.2001.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- van Beaumont W, Bullard RW. Sweating exercise stimulation during circulatory arrest. Science. 1966;152:1521–1523. doi: 10.1126/science.152.3728.1521. [DOI] [PubMed] [Google Scholar]
- Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.res.65.2.468. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Hjortsø EM. Central motor command activates sympathetic outflow to the cutaneous circulation in humans. J Physiol. 1996;492:931–939. doi: 10.1113/jphysiol.1996.sp021359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Stimulation of skin sympathetic nerve discharge by central command. Circ Res. 1991;69:228–238. doi: 10.1161/01.res.69.1.228. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Sone R, Ikegami H. Responses of sweating and body temperature to sinusoidal exercise. J Appl Physiol. 1994;76:2541–2545. doi: 10.1152/jappl.1994.76.6.2541. [DOI] [PubMed] [Google Scholar]


