Abstract
This study examined enteric neural reflexes activating submucosal cholinergic vasodilator motoneurons, which innervate the final resistance vessels regulating mucosal blood flow. Videomicroscopy was employed to monitor dilatation of submucosal arterioles in in vitro preparations from guinea-pig ileum. Balloon distension of intact lumen evoked reflex vasodilatation and flat sheet preparations were employed to separate mucosal mechanical stimulation from intestinal distension. Mucosal stroking and balloon distension of the orad segment evoked vasodilatations > 1.5 cm from the stimulating site. Mucosal stimulation was blocked by combined 5-HT3/5HT4 antagonists but distension-evoked responses were unaffected. Distension-evoked responses were also unaffected by nifedipine (5 μm) or nifedipine (1 μm) and wortmannin (300 nm), suggesting stretch activation rather than stretch-activated contraction was involved. Mucosal and distension-evoked responses were completely blocked when the myenteric plexus was surgically lesioned and were significantly inhibited by hexamethonium. The muscarinic antagonist 4-DAMP, which inhibits vasodilatations evoked by submucosal cholinergic vasodilator neurons, blocked dilatations elicited by mucosal stimulation and balloon distension. Maximal dilatations evoked with either sensory modality could be further enhanced when stimulated with the second modality. Dilatations evoked by stimulation of the aborad segment were similar to those elicited in the orad segment. In conclusion, sensory mechanisms in the mucosa and muscularis propria activate vasodilator pathways in the myenteric plexus which project for significant distances in both ascending and descending directions before innervating submucosal arterioles. These reflexes could co-ordinate mucosal blood flow during multiple motor events such as peristalsis and intestinal mixing between propulsive events.
Whole animal studies have demonstrated that enteric neural reflexes are important mechanisms regulating mucosal blood flow in the intestine (Biber et al. 1971, 1973, 1974). In these studies, mechanical stimulation of the mucosa induced a 30–140 % increase in intestinal blood flow. These vasodilatations were blocked by the neurotoxin, tetrodotoxin and mimicked by transmural electrical stimulation, but were not affected by extrinsic denervation. Together, these findings established that the vasodilatation was mediated by enteric nerves. In the ensuing years, much has been learned about cellular mechanisms underlying sensory transduction and related enteric neural reflexes mediating motor and secretory responses, in large measure due to the development of novel approaches using in vitro models (Hirst et al. 1975; Smith & Furness, 1988; Smith et al. 1990, 1991, 1992; Bornstein et al. 1991; Kunze et al. 1997; Neunlist et al. 1998; Kunze et al. 1999; Weber et al. 2001). While these enteric reflexes may be linked to those mediating intestinal blood flow, the inaccessibility of intestinal resistance vessels has limited parallel studies.
Studies employing in vitro videomicroscopy techniques (Neild et al. 1990) have established that cholinergic vasodilator neurons in the guinea-pig submucosal plexus provide the ‘final common pathway’ to submucosal arterioles (see however Galligan et al. 1990). Submucosal arterioles are the final resistance vessels of the splanchnic circulation and therefore understanding the nature of the enteric reflex pathways and their interconnections would be of considerable interest given that regulation of mucosal blood flow by these vessels is essential to the function and integrity of this tissue (Granger et al. 1989). Specifically, these vasodilator neurons could be activated by localized reflexes confined to the mucosa and submucosa, as suggested by recent studies (Vanner et al. 1993), or also by long polysynaptic pathways in the myenteric plexus. This latter possibility would not only provide a wider range of control but could enable co-ordination of blood flow with complex motility patterns generated in the myenteric plexus. In the present study, our aim was to determine whether such pathways may exist by examining if distension of the whole intestine stimulated neural reflexes dilating submucosal arterioles. We found evidence for such a response and describe studies designed to characterize the sensory modalities involved and the neural pathways which mediate the reflex.
METHODS
Adult Hartley guinea-pigs (150–250 g) of either sex were anaesthetized with isoflurane inhalation and killed by cervical transection and exsanguination, a method approved by the Animal Care Committee of Queen's University. The abdomen was opened, and 30–40 mm segments of ileum were excised approximately 10 cm from the ileocaecal junction and flushed of their contents. Segments were pinned in a Sylgard-lined (Dow Corning Corp., Midland, MI, USA) Petri dish containing a physiological saline solution (mm: 126 NaCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 5 KCl, 25 NaHCO3, and 11 glucose) for tissue dissection.
Intact and flat sheet in vitro ileal preparations
A 10 mm portion of the ileal segment was opened along the mesenteric border starting at the aboral extremity, leaving the remaining lumen intact. The aboral section was pinned open flat with mucosa facing upwards and the mucosa was stripped off by gentle dissection with blunt jeweller's forceps exposing the submucosa and arterioles, as previously described (Moore & Vanner, 2000). The entire segment was pinned in an organ bath (volume 3–4 ml) with the submucosa facing upwards.
The flat sheet ileal preparations were dissected by opening segments along the entire mesenteric border and pinning them flat with the mucosa facing upwards. The submucosa was exposed in the distal 5–10 mm of the aboral or oral portion by dissecting off the overlying mucosa. The preparation was then pinned in a small organ bath (3–4 ml) with the exposed submucosa facing upwards.
Both the intact and flat sheet preparations were continuously superfused with a physiological saline solution solution (mm: 126 NaCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 5 KCl, 25 NaHCO3, and 11 glucose) which was gassed with 95 % O2-5 % CO2 and maintained at 35–36 °C. Nifedipine (1 μm) was added to the solution to suppress muscle movement. In a small series of experiments, higher concentrations of nifedipine, nicardipine, and wortmannin were substituted for nifedipine (1 μm) to examine whether muscle stretch or tension was important in balloon distension evoked responses.
Surgical denervations
Circumferential surgical lesions were made by cutting the tissue at positions approximately equidistant between the stimulating and recording sites (i.e. 5–7 mm). A dissecting microscope (magnification × 70) was employed which allowed tissue planes to be clearly recognized. The submucosal plexus was severed by cutting through the submucosa to the underlying circular muscle layer. The myenteric plexus was lesioned by pinning the preparation serosal side up and cutting through the longitudinal muscle into the circular muscle layer.
Stimulation and recording of vasodilatations
The intestine was stimulated by distending the intestinal wall with a balloon catheter or by mechanically distorting the mucosal villi with a hand-held painter's brush, as previously described (Smith & Furness, 1988; Vanner et al. 1993). The balloon tipped catheter (Wilson-Cook) was positioned within the intact lumen or beneath the tissue in flat tissue preparations, 1–2 cm from the recording site. Air was injected into the catheter (volumes of 0.4-0.6 ml, duration ≈0.5 s) producing a balloon diameter of ≈8 mm. Distances recorded for balloon distension were measured from the recording site to the mid-point of the uninflated balloon. The tip width of the brush used for mucosal stroking was 5 mm. Stroking was carried out by directing the brush away from the site of recording (1 Hz, duration ≈0.5 s). The lead point from the onset of the stroke to the recording site was the distance measured between the two sites.
The outside diameter of individual submucosal arterioles was monitored using a computer-assisted videomicroscopy system (Diamtrak), as previously described (Neild, 1989). Briefly, this system uses an Imaging Technology PCVision frame-grabber board in an IBM PC/ATcomputer to digitize television images of the arteriole. This is converted to an analog signal and stored on a chart recorder. The resolution of the system is less than 1 μm, and the sampling rate was 15 Hz. Vasodilator responses were monitored by first preconstricting arterioles 80–95 % of the maximum they can constrict from their resting diameter with 9,11-dideoxy-11α9α-epoxy-methanoprostaglandin F2α (PGF2α). Constriction occurred in the presence of L-type calcium channel blockers. Calcium channel blockers do not alter vasodilator responses mediated by activation of submucosal neurons (Vanner, 2000) and do not appear to alter synaptic neurotransmission in the enteric nervous system (Bornstein et al. 1991). The magnitude of the response was quantified by measuring the peak amplitude of the response and expressing it as a percentage of the maximal response to muscarine, as previously described (Vanner & Bolton, 1996).
Drugs
The following drugs were used: hexamethonium, nifedipine, nicardipine, tetrodotoxin (TTX), tropisetron, SDZ-205,577, wortmannin (all from Sigma-Aldrich), 4-diphenylacetoxy-N-(2-chloroethyl)-piperdine hydrochloride (4-DAMP) (Research Biochemicals International), and ondansetron (GlaxoSmithKline). All drugs were added to the bath by superfusion.
Statistics
Data are expressed as mean ±s.e.m. A Fisher's exact test or a two-tailed Student's t test were used to compare data for significant differences. P < 0.05 was considered significant.
RESULTS
General observations
The resting outside diameter of submucosal arterioles ranged between 40 and 90 μm (n = 186). In most studies, nifedipine (1 μm) was added to the bath to limit contraction of the muscularis externa. Following a 15 min equilibration period, these contractions were largely suppressed and stable recordings were obtained. Previous studies have shown that submucosal cholinergic vasodilator neurons are the ‘final common pathway’ innervating submucosal arterioles (Neild et al. 1990).
All vasodilator responses were studied by first constricting arterioles 80–95 % of the maximum they can constrict from their resting diameter with PGF2α (400 nm), as previously described (Neild et al. 1990). One or two preparations were dissected from each animal and studied within 30 min of the dissection. Consistent vasodilator responses were observed during the ≈30 min duration of each experiment. The orad ends of each ileal segment were marked when removed from the animal to allow the orad and aborad orientation to be determined in subsequent studies. All experiments were conducted by stimulating in the orad segment and recording in the distal segment, except in a small series where responses obtained by stimulating in orad and aborad segments were compared.
Dilatations evoked by distension of the intact lumen
To establish that long (i.e. centimetres) enteric vasodilator reflexes exist, we first examined the effects of balloon distension of the intact intestinal lumen (Fig. 1A). With the balloon positioned in the orad segment of the preparation 1–2 cm from the recording site, balloon distension consistently evoked large dilatations of submucosal arterioles (n = 11 of 13 preparations) (Fig. 1B). Single balloon distension evoked a mean dilatation of 31.0 ± 4.7 % (n = 4). When TTX (1 μm) was applied to the bath for 3 min prior to balloon distension, subsequent stimulation was blocked (mean dilatation = 1.7 ± 1.7 %, n = 7) (Fig. 1C). Following a washout of TTX, balloon-evoked responses returned to control levels (mean dilatation = 36 ± 8.9 %; P < 0.01; n = 7), demonstrating that distension-evoked responses were mediated by enteric nerves. The magnitude of the dilatations increased with consecutive distensions (≈1 Hz, duration ≈0.5 s) (Fig. 1D).
Figure 1. Vasodilatation evoked by luminal distensions.
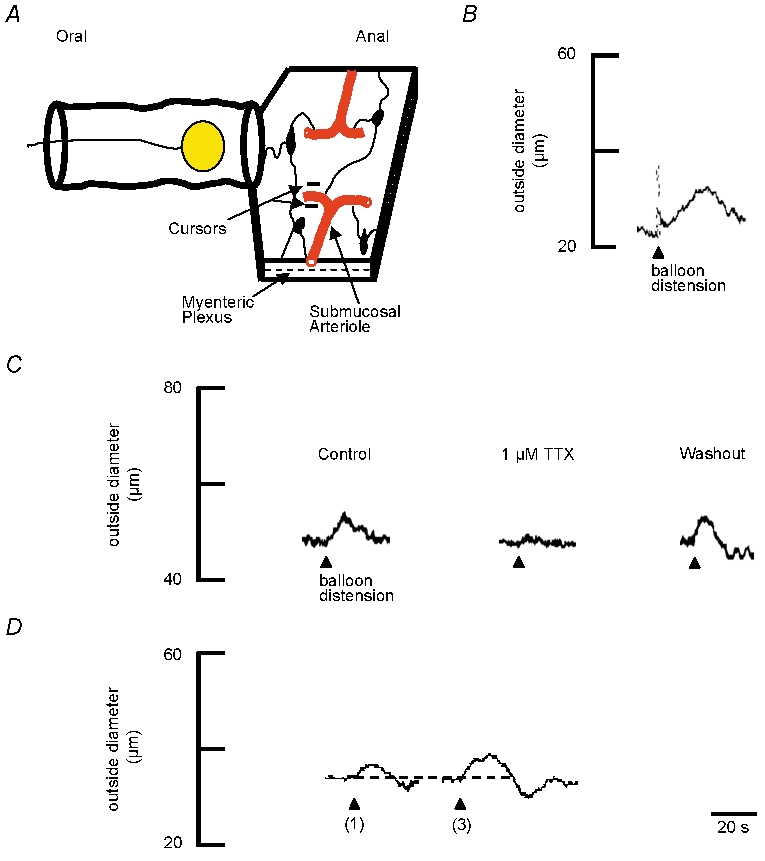
A, schematic representation of ileal preparation. The mucosa was removed from the aborad segment to expose submucosal arterioles and submucosal ganglia (dark elliptical shapes). Changes in outside diameter of the arteriole were monitored at a single site (cursors) by videomicroscopy. Arterioles were preconstricted 80–95 % of maximum with the prostaglandin analogue PGF2α (400 nm; present in the superfusate for the duration of the experiment). The intestine was distended with a balloon placed within the lumen. The distance from the centre of the balloon to the recording site on the arteriole was 1–2 cm. B, representative trace showing that one distension (arrowhead) evokes dilatation of the arteriole. The resting outside diameter was 44 μm. C, representative traces showing TTX (1 μm) blocks the distension-induced vasodilatation compared to control. The resting outside diameter was 48 μm. A 15 min washout period restores the vasodilatation evoked by distensions. D, representative traces showing that increasing the number of balloon distensions from one distension to three distensions (≈1 Hz) increases the size of dilatations. Dotted line indicates the baseline prior to distension. The resting outside diameter was 46 μm.
Stimulus-evoked dilatation of flat-sheet preparations
Flat-sheet preparations were employed to determine whether dilatation evoked by distension of the intact lumen was mediated by sensory transduction mechanisms in the mucosa and/orthe muscularis externa. The flat-sheet preparation enabled the stimulus site (orad or aborad) to be activated by balloon distension from the serosal side or by stroking the mucosa with a fine painter's brush (Fig. 2A).
Figure 2. Vasodilatation evoked by balloon distension or mucosal stroking.
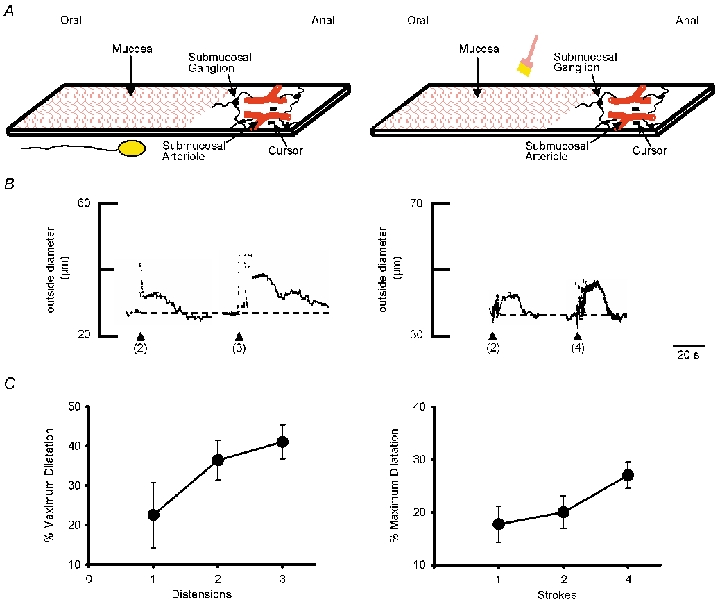
A, schematic representations of ileal preparations. The ileum was opened along the mesenteric border and pinned flat. The mucosa was removed from the anal segment to expose submucosal arterioles and submucosal ganglia (dark elliptical shapes). Changes in outside diameter of the arteriole were monitored at a single site (cursors) using a videomicroscopy system. The oral segment was stimulated by distending the balloon from beneath the preparation (left) or by stroking the mucosa with a fine painter's brush (right). The distance from recording to the stimulus site was 1–1.5 cm. Arterioles were preconstricted with PGF2α, as described in Fig. 1. B, representative traces showing that increasing the number of balloon distensions (≈1 Hz; left) or the number of mucosal brush-strokes (≈1 Hz; right) increases the magnitude of the vasodilatation. The dotted line indicates the baseline prior to stimulation. Resting outside diameters were 50 μm (left) and 68 μm (right). C, summary of the mean increase in peak dilatations evoked by increasing numbers of distensions (left) (≈1 Hz; n = 4–14 preparations for each point) and increasing numbers of mucosal brush-strokes (right) (≈1 Hz; n = 3 preparations for each point).
Both balloon distension and mucosal stroking evoked dilatations when positioned 1–1.5 cm orad to the arteriolar recording site (Fig. 2B). The effects of repetitive stimulation with either balloon distension or mucosal stroking were examined by comparing one distension or mucosal stroke to a progressive increase in the number of stimuli (≈1 Hz, duration ≈0.5 s). These studies showed that responses to both distension and mucosal stroking were greater with increasing number of stimuli (Fig. 2B and C). Maximal responses occurred after three to four successive stimuli. Mean dilatation evoked by balloon distension was 36.5 ± 3.7 % (3 distensions at ≈1 Hz; n = 13) and the mean dilatation evoked by mucosal stroking was 25.4 ± 1.1 % (4 strokes at ≈1 Hz; n = 7). These stimulus parameters provided a consistent and robust response and were therefore applied in all subsequent experiments.
Influence of mucosa on distension-induced vasodilatation in flat sheet preparations
The effect of the mucosa on distension-evoked vasodilatations was examined by comparing responses from preparations with intact mucosa to separate preparations where the mucosa had been removed. Preparations were studied in alternating sequence i.e. the first preparation was a ‘mucosa-off’ preparation (n = 13) and the second preparation was a ‘mucosa-on’ preparation (n = 12). Figure 3A and C illustrates that two types of responses were recorded from ‘mucosa-off’ preparations. In 46 % of preparations distension evoked vasodilatations whereas in 54 % of preparations responses were absent. In contrast, balloon-evoked vasodilatations were consistently recorded in ‘mucosa-on’ preparations (Fig. 3B and C). These data are summarized in Fig. 3C, demonstrating that removing the mucosa significantly decreases the number of preparations in which a vasodilatation is evoked in response to balloon distension.
Figure 3. Effect of mucosa on distension-induced vasodilatations.
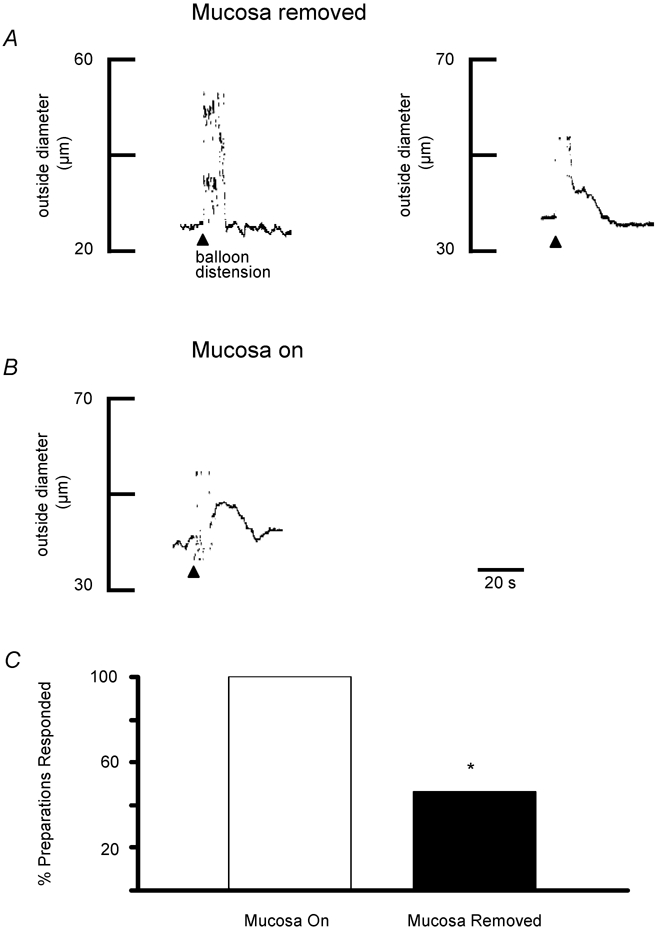
A, representative traces of preconstricted arterioles from two separate preparations illustrating that varying responses were obtained when the mucosa was removed from the entire preparation. In the preparation on the left, balloon distension (arrowhead, 3 distensions) failed to elicit a dilatation whereas a vasodilatation was elicited in the preparation shown on the right. Resting outside diameters were 50 μm (left) and 57 μm (right). B, when the mucosa was not removed from the stimulating site, balloon distensions consistently dilated arterioles, as shown in this representative trace (arrowhead, 3 distensions). Resting outside diameter was 60 μm. C, summary of the percentage of preparations in which balloon distension evoked a dilatation with the mucosa intact (n = 12 preparations) compared to those where it had been removed (n = 13 preparations). *P = 0.005
Effect of muscle tension on distension-induced vasodilatation
Previous studies have shown that mechanosensory myenteric AH neurons in the small intestine are activated by stretch or stretch-activated muscular contraction (Smith et al. 1990, 1992; Kunze et al. 1999; Spencer et al. 2001, 2003). This latter mechanism has been blocked by antagonists which cause muscle paralysis, such as L-type calcium channel blockers. We observed that small muscle contractions were occasionally visible with 1 μm nifedipine in the bath and therefore we examined the effects of increasing concentrations of calcium channel blockers or the addition of wortmannin to determine if mechanosensory neurons were activated by stretch or stretch-activated muscle contraction.
When nicardipine (3 μm) was substituted for nifedipine (1 μm) for 3 min, distension-evoked dilatations were almost completely blocked (n = 5; Fig. 4A). When 300 nm nicardipine (n = 3) was applied, distension evoked consistent vasodilatations (Fig. 4A). However, previous studies have suggested that higher doses of nicardipine may non-selectively block muscarinic responses (Cousins et al. 1995) and therefore the effects of higher concentrations of nifedipine or nifedipine (1 μm) combined with wortmannin (300 nm) were examined. Higher concentrations of nifedipine were studied by comparing the magnitude of distension-evoked dilatations obtained following superfusion of 1 μm nifedipine for 3 min to those obtained in 5 μm nifedipine, following a 10 min washout. There was no difference in the magnitude of the responses at the two different concentrations (17.3 ± 6.8 vs. 24.0 ± 7.5 % respectively, n = 3; Fig. 4B). The effects of wortmannin were studied in a similar fashion by comparing the magnitude of distension-evoked dilatations in 1 μm nifedipine alone to 1 μm nifedipine plus wortmannin (300 nm), following a 10 min washout. There also was no difference if the magnitude of the dilatations between the two groups (22 ± 6.9 vs. 21.4 ± 6.5 % respectively, n = 3; Fig. 4C).
Figure 4. Effects of the Ca2+ channel inhibition on distension-induced vasodilatation.
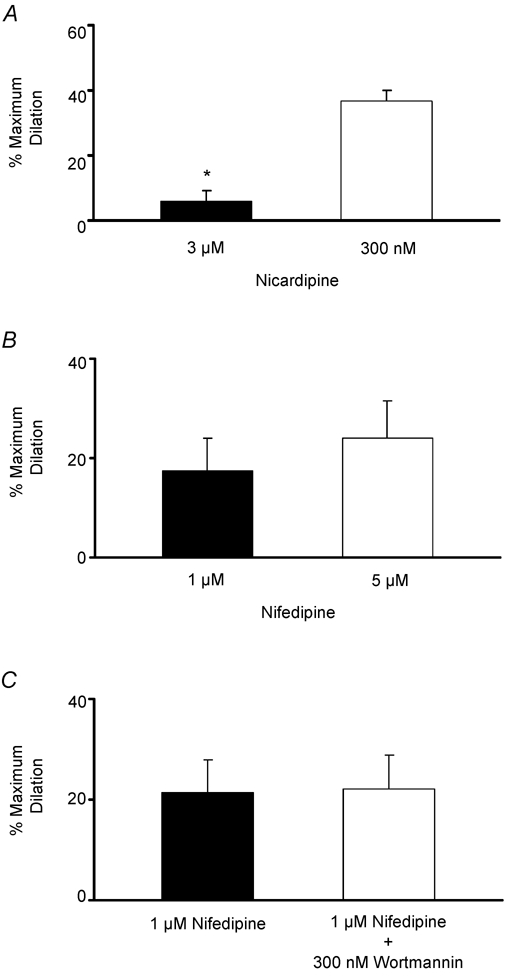
A, summary of the mean dilatations evoked by balloon distension (3 distensions, 1 Hz, duration ≈0.5 s) in the presence of nicardipine (3 μm) compared to those obtained in a separate group of preparations receiving nicardipine (300 nm), demonstrating that responses were inhibited by the higher concentration (n = 9 for each concentration, *P < 0.01). B, summary of mean dilatations evoked by balloon distension (3 distensions, 1 Hz, duration ≈0.5 s) in the presence of 1 μm nifedipine. Following a 10 min washout, responses were re-examined in the same preparations in the presence of 5 μm nifedipine and similar responses were obtained (n = 5). C, summary of mean dilatations evoked by balloon distension (3 distensions, 1 Hz, duration ≈0.5 s) in the presence of 1 μm nifedipine. Following a 10 min washout, responses were re-examined in the same preparations in the presence of nifedipine (1 μm) and wortmannin (300 nm) and similar responses were also obtained (n = 5). Arterioles were preconstricted with PGF2α, as described in Fig. 1.
Effect of 5-HT3 and 5-HT4 antagonists
5-HT released from enterochromaffin cells has been shown to trigger the peristaltic reflex (Foxx-Orenstein et al. 1996) and the secretory reflex (Cooke et al. 1997) in the guinea-pig. These studies suggest 5-HT acts on 5-HT3, 5-HT4, and/or5-HT1P receptors on intrinsic afferent nerve terminals of AH neurons. Therefore, the combined effects of the 5-HT3/4 antagonists, SDZ-205,577 (10 μm) and tropisetron (1 μm), on both balloon distension and mucosal stroking was studied.
Balloon distension responses in preparations receiving both antagonists compared to controls were not significantly different (Fig. 5A and B) (mean dilatation = 52.3 ± 9.8 % vs. mean dilatation = 49 ± 8.1 % respectively; P > 0.05; n = 3). In contrast, the combined antagonists almost completely blocked dilatations evoked by mucosal stroking (Fig. 5A and B) (mean dilatation with antagonist = 4.6 ± 1.4 % vs. mean dilatation in controls = 21.3 ± 4.2 %; P < 0.01; n = 11). In a separate series of experiments (Fig. 5C) the effects of tropisetron (1 μm) or ondansetron (1 μm) on mucosal stroking were examined. Compared to control values, tropisetron inhibited responses by 71 % (P = 0.01; n = 4) and ondansetron caused a 29 % inhibition (P = 0.02; n = 6).
Figure 5. Effects of 5-HT antagonists on distension-induced and stroking-induced vasodilatation.
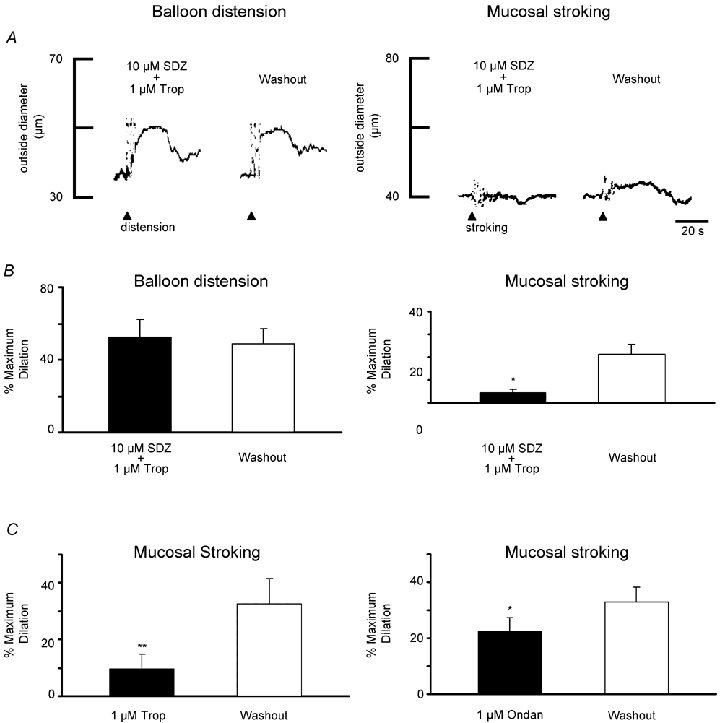
A, representative traces of preconstricted arterioles showing responses to balloon distension (left) or mucosal stroking (right) in the presence and absence of the 5-HT4 antagonist SDZ-205,577 (SDZ, 10 μm) plus the 5-HT3 antagonist tropisetron (Trop, 1 μm). Balloon distension (arrowhead; 3 distensions, ≈1 Hz) evoked a vasodilatation in the presence of the antagonists that was similar in amplitude to the distension-induced vasodilatation following washout (15 min) of the antagonists. Mucosal stroking (arrowhead; 4 strokes, ≈1 Hz) did not elicit a dilatation in the presence of the antagonists. Following a 15 min washout of the antagonists, repeat mucosal stroking elicited a dilatation. Resting outside diameters were 75 μm (left) and 56 μm (right). B, summary of the percentage of mean peak dilatation in the presence of the antagonists and following washout for balloon distensions (left, n = 3) and brush-stroking the mucosa (right, n = 11) *P < 0.01. C, summary of percentage of maximum dilatation in the presence of tropisetron alone (Trop, 1 μm) and washout for mucosal stroking (left, n = 4) and in the presence of ondansetron (Ondan, 1 μm) and washout for mucosal stroking (right, n = 6) *P = 0.02, **P = 0.01.
Effects of surgical lesioning of submucosal and myenteric plexi
The effects of surgical lesioning of the myenteric or submucosal plexus were studied to determine whether the vasodilator neural reflex initiated by distension or mucosal stroking projected through neural pathways in the myenteric plexus and/orthe submucosal plexus. Lesions (see Methods) completely severed the submucosa or myenteric plexus, typically 5–7 mm from the recording site.
When the myenteric plexus was severed, dilatations evoked by distension and mucosal stroking were completely blocked in six of seven preparations (Fig. 6). In one preparation, a lesion was performed closer to the recording site (≈3 mm) and distension evoked a dilator response (31 %). This may reflect projections from the myenteric plexus to the submucosal plexus which then travel for several millimetres within the submucosal plexus before innervating submucosal vasodilator neurons. Lesioning the submucosal plexus had no effect on dilatations evoked by either modality (Fig 6; n = 3 balloon distension, n = 5 mucosal stroking).
Figure 6. Effects of lesioning the submucosal plexus or myenteric plexus on vasodilatation.
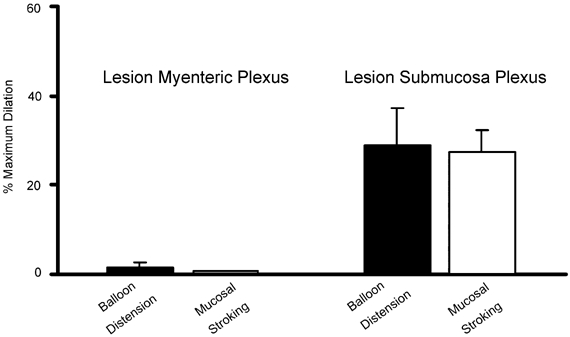
Summary of the percentage of mean peak dilatations evoked by balloon distension (3 distensions, ≈1 Hz) or mucosal stroking (4 strokes, ≈1 Hz) after the myenteric plexus or submucosal plexus was severed using surgical lesioning techniques. Lesions were made approximately equidistant from the stimulating and recording sites (n = 3–7 arterioles for each group).
Effect of muscarinic and nicotinic blockade
Previous studies have demonstrated that submucosal cholinergic vasodilator neurons provide the ‘final common pathway’ (Neild et al. 1990) mediating vasodilator reflexes in guinea-pig ileum. These neurons release acetylcholine which acts on M3 receptors on the submucosal arteriole, an action which is blocked by the muscarinic antagonist 4-DAMP (1 μm). Anatomical studies have also described a population of vasoactive intestinal peptide (VIP)-immunoreactive neurons which project to the arterioles (Jiang et al. 1993) but functional studies have not identified a non-cholinergic component to the nerve-evoked responses in the guinea-pig submucosa (Neild et al. 1990). We studied the effects of muscarinic antagonist 4-DAMP (1 μm) on vasodilatations evoked by both distension and muscosal stroking by comparing dilatations in the presence and absence of the antagonist in the same preparation. In each case, dilatations were virtually abolished by the antagonist (Fig. 7A and B).
Figure 7. Effect of the muscarinic antagonist 4-DAMP and hexamethonium on vasodilatation evoked by balloon distension or mucosal stroking.
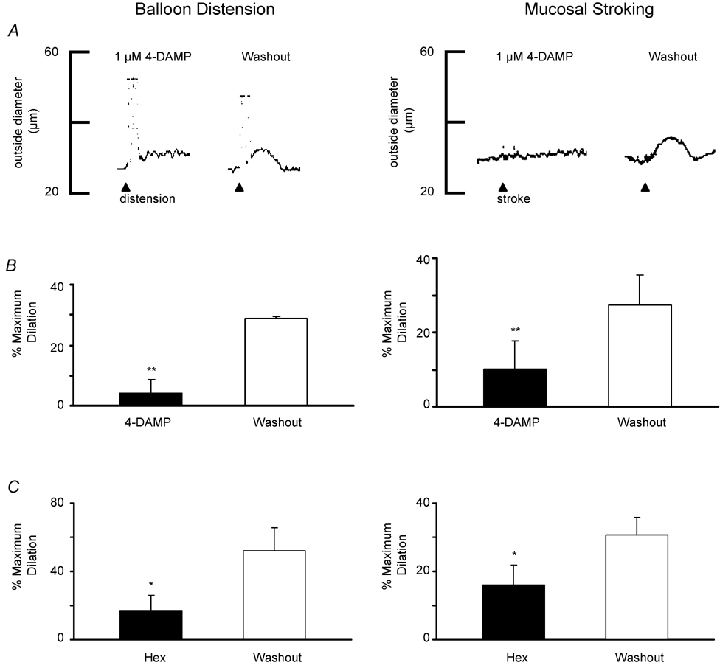
A, left, representative trace of preconstsricted arteriole showing balloon distension (arrowhead; 3 distensions, ≈1 Hz) in the presence of 4-DAMP (1 μm) does not evoke vasodilatation. Following a 10 min washout of 4-DAMP, dilatation was evoked by repeat distension. Right, a representative trace of a preconstricted arteriole from a separate preparation showing vasodilatation in response to mucosal stroking (arrowhead; 4 strokes, ≈1 Hz) is blocked by 4-DAMP and that following washout, stroking can elicit a dilatation. Resting outside diameters were 46 μm (left) and 54 μm (right) **P = 0.02. B, summary of the percentage of mean peak dilatations in the presence of 4-DAMP and following washout for balloon distensions (left, n = 3) and mucosal stroking (right, n = 5). C, summary of the percentage of mean peak dilatations in the presence of hexamathonium (Hex, 300 μm) and following washout for balloon distension (left, n = 6) and mucosal stroking (right, n = 9) *P < 0.05.
Our previous studies suggest that vasodilator pathways mediated by myenteric neurons involve nicotinic synapses in both the myenteric plexus and the submucosal plexus (Vanner, 2000). The effects of hexamethonium (300 μm) on dilatations evoked by mucosal stroking or balloon distension were examined and following a 10 min washout period were compared to controls. Dilatations evoked by mucosal stroking were completely blocked in four of nine preparations and hexamethonium caused a mean inhibition of almost 50 % (P < 0.05, Fig. 7C). Mean dilatations evoked by balloon distension were inhibited by 68 % (P < 0.02, Fig. 7C).
Effects of consecutive stimulation with distension and mucosal stroking
To examine possible interactions between the neural pathways mediating vasodilatations evoked by balloon distension and mucosal stroking, the effect of consecutive stimulation with the two sensory modalities was examined. We first established a stimulus paradigm for each sensory modality which produced a maximal peak amplitude, by examining the effects of additional stimulation at the peak amplitude of the response. Dilatations evoked by three balloon distensions (≈1 Hz) were not increased further by a second stimulation (3 distensions; ≈1 Hz) (mean dilatation = 36.7 ± 3.8 % vs. 35.7 ± 7.4 %, respectively; n = 4). Dilatations evoked by mucosal stroking (4 strokes; ≈1 Hz) demonstrated a minor increase in amplitude of the response when a second series of stimuli were applied (4 strokes; ≈1 Hz) (mean dilatation = 16.5 ± 3.6 % vs. 22 ± 3.4 % respectively, n = 9). A third series of stimuli always resulted in a smaller amplitude. The mean decrease in peak amplitude compared to the peak amplitude following the second series of strokes was 10 %. Therefore, in subsequent experiments interactions between the two sensory modalities were studied by applying either one series of three balloon distensions or one or two series of four mucosal strokings. Subsequent stimulation with the second modality was always applied at the peak of the response to the first stimulus.
When balloon distensions were initiated first, subsequent mucosal stroking increased the peak amplitude of dilatations in three of seven preparations (Fig. 8A) (range in interval between stimuli = 19.2-26.4 s, range in total duration of dilatation = 45–52.8 s). In the other four preparations, the peak dilatation persisted at the same amplitude following the second stimulus in one preparation (interval between stimuli = 9.6 s, total duration of dilatation = 28.8 s) whereas in the remaining three preparations, the peak amplitude returned to baseline during or immediately following the second stimulus (range in interval between stimuli = 10.8-20.4 s, range in total duration of dilatation = 24–40.8 s). In those preparations where an increase was observed, mean dilatation increased from 38.7 ± 13.0 % to 53.3 ± 8.2 % (P < 0.05). When mucosal stroking was applied first, subsequent balloon distension enhanced peak dilatations in 4 of 11 vessels (Fig. 8B) (range in interval between stimuli = 10.8-12.0 s, range in total duration of dilatation = 38.4-45.6 s). In the other seven preparations, the peak dilatation persisted at the same peak amplitude following the second stimulus in four preparations (range in interval between stimuli = 14.4-16.8 s, range in total duration of response = 36–55.2 s) whereas in the remaining three preparations, the peak amplitude returned to baseline during or immediately following the second stimulus (range in interval between stimuli = 14.4-21.6 s, range in total duration of dilatations = 30–43.4 s). In those preparations where responses were enhanced (n = 4/11), mean dilatations increased from 20.8 ± 6.3 % after mucosal stroking to 45.8 ± 10.0 % following balloon distensions (P < 0.05).
Figure 8. Responses to successive stimulation with balloon distension and mucosal stroking.
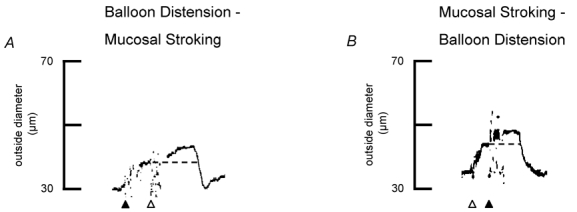
A, representative trace of preconstricted arteriole showing that maximal stimulation with balloon distension (black arrowhead; 3 distensions, ≈1 Hz) evokes vasodilatation of the arteriole. At the peak of the dilatation, the mucosa was stroked (white arrowhead; 4 strokes, ≈1 Hz) at the same stimulus site, evoking a further increase in the amplitude of the response. Resting outside diameter was 50 μm. B, a representative trace of a preconstricted arteriole showing that maximal stimulation with mucosal stroking (white arrowhead; 4 strokes, ≈1 Hz) causes vasodilatation of the arteriole. At peak dilatation, the balloon was distended (black arrowhead; 3 distensions, ≈1 Hz) at the same stimulus site and a further increase in the amplitude of the response was also obtained. Resting outside diameter was 63 μm. The dotted line indicates the peak dilatation evoked by the first stimulus.
Orientation of vasodilator reflexes
To determine whether vasodilator reflexes also project in an aborad to orad direction, the effects of stimulation in the aborad segment were compared to stimulation in the orad segment in separate flat sheet preparations. Recording distances were 1.0-1.5 cm. Balloon distension consistently evoked dilatations in the aborad to orad direction and they did not differ in magnitude to those projecting distally (Fig. 9A). Similarly, mucosal stroking evoked dilator reflexes in the aborad to orad direction and these were similar in magnitude to those evoked in the opposite direction (Fig. 9B).
Figure 9. Comparison of responses evoked by stimulation in orad and aborad segments.
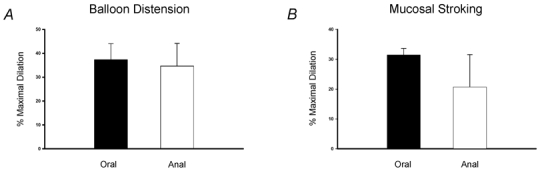
A, summary of the mean dilatations evoked by balloon distension (3 distensions, ≈1 Hz) in the orad and aborad segment showing responses were similar in magnitude (n≥ 6 preparations). B, summary of the mean dilatations (n≥ 7 preparations) evoked by mucosal stroking (4 strokes, ≈1 Hz) also showing responses were similar in magnitude when elicited by stimulation in oral and anal segments.
DISCUSSION
This study examined the properties of enteric vasodilator reflexes dilating submucosal arterioles, the major resistance vessels in the gastrointestinal tract. In the guinea-pig ileum, previous studies have shown that submucosal cholinergic vasodilator neurons are the ‘final common pathway’ to these vessels (Neild et al. 1990). The current study has demonstrated that these motoneurons are innervated by long vasodilator pathways projecting through the myenteric plexus. Furthermore, these pathways are activated by distinct sensory mechanisms, involving both intestinal distension and mucosal mechanical stimulation. In contrast to previously described short vasodilator reflexes (i.e. millimetres) which are confined within the mucosa and submucosa (Vanner et al. 1993), the current findings demonstrate that myenteric neural reflexes also exist and project over much greater distances within the intestine.
Sensory mechanisms activating vasodilator pathways
Intrinsic primary afferent neurons in the intestine are morphologically classified as Dogiel type II neurons and belong to an electrophysiological class known as AH neurons (Song et al. 1996; Furness et al. 1998). Their cell bodies are found in the myenteric plexus and to a lesser degree in the submucosal plexus of the small intestine. They possess two or more axons, some of which project to the mucosa (Furness et al. 1998). Axons of neurons projecting to the mucosa have been shown to be chemosensitive and those within the muscle respond to changes in muscle stretch and/ortension (Kunze et al. 1995; Bertrand et al. 1997; Kunze et al. 1999; Kunze et al. 2000). Neurons responding to mucosal distortion have been described in the submucosal plexus and in some (Kirchgessner et al. 1992, 1996) but not all studies in the myenteric plexus (Kunze et al. 1995; Bertrand et al. 1997). Balloon distension of the intact intestine provides a robust in vitro stimulus for studying enteric reflexes and we used this technique in initial studies to establish the presence of distension-induced vasodilatation of submucosal arterioles and their dependence on enteric nerves (see Fig. 1). Interpretation of the mechanisms underlying this response however was limited because balloon distension of the intact intestine could stimulate sensory mechanisms located in the mucosa and/ormuscle (Smith et al. 1990). We therefore developed in vitro flat sheet models which enabled mucosal mechanical stimulation to be isolated from distension of the muscle.
Mucosal mechanical stimulation was performed by gently stroking the mucosa in a flat sheet of intestine (Fig. 2), as described by others (Smith & Furness, 1988; Vanner et al. 1993). This stimulus evoked vasodilator responses which extended at least 1–1.5 cm. Numerous studies have shown that mechanical distortion of the villi releases 5-HT from mucosal enterochromaffin cells which in turn activates 5-HT receptors on afferent axons. These studies suggest 5-HT4 and/or5-HT1P, and possibly 5-HT3 receptors are involved (Foxx-Orenstein et al. 1996; Cooke et al. 1997; Grider et al. 1998; Pan & Gershon, 2000). We tested whether 5-HT mediated the mechanically evoked dilatations observed in our studies by combining 5-HT3 and 5-HT4 antagonists and found responses were blocked. These findings also parallel those of previous in vivo studies where increased intestinal blood flow evoked by mechanical stimulation of the mucosa was blocked by intra-arterial injection of a non-selective 5-HT blocker (Biber et al. 1974). There is some controversy concerning 5-HT receptor subtypes and their distribution, particularly on submucosal neurons. There is relatively strong evidence that 5-HT3 but not 5-HT4 receptors are located on mucosal terminals of myenteric neurons (Bertrand et al. 2000). Submucosal AH neurons are reported to contain 5-HT4 and/or5-HT1P receptors but not 5-HT3 receptors (Cooke et al. 1997; Pan & Gershon, 2000). The 5-HT1P receptor however, has yet to be cloned. Our data demonstrated that a small component of the mucosal stroking-evoked dilatation was mediated by 5-HT3 receptors (ie. inhibited by 1 μm ondansetron) and hence there was a significant non-5-HT3 component. Combined 5-HT3 and 5-HT4/1P blockade with tropisetron (1 μm) produced a large inhibition. Although this pharmacological data is limited, it is consistent with the view that the majority of the response occurs following activation of 5-HT4 and/or5-HT1P receptors on the axon terminals of submucosal AH neurons. The small 5-HT3 component may be mediated by axon terminals of myenteric AH neurons although not all studies have found that mucosal distortion can activate myenteric AH neurons (Kunze et al. 1995; Bertrand et al. 1997).
Mechanosensory mechanisms underlying muscle distension (Smith et al. 1990, 1991) were examined by distending a balloon positioned beneath the flat intestinal preparation (Fig. 2) and this stimulus also evoked vasodilator responses extending at least 1–1.5 cm. Previous studies of mechanosensory transduction mechanisms in the guinea-pig small intestine indicate that stretch or the development of active tension by the muscle cells following stretch can activate myenteric afferent neurons to trigger myenteric neural pathways (Smith et al. 1990, 1992; Kunze et al. 1999; Spencer et al. 2001, 2003). We tested which of these mechanisms may underlie the balloon distension responses observed in this study by examining whether inhibiting the ability of smooth muscle to develop tension with increasing concentrations of Ca2+ channel blockers or wortmannin affected responses. The results demonstrated that increasing the concentration of nifedipine to 5 μm or combining nifedipine (1 μm) with wortmannin (300 nm) had no effect on the stretch-induced responses. We did find that nicardipine (3 μm) inhibited the responses but this is probably due to non-specific muscarinic blockade rather than an effect on muscle tension induced by stretch, given the findings of others (Cousins et al. 1995) and the absence of an effect with high concentrations of nifedipine or the addition of wortmannin. Although there may be a mechanosensitive component originating from the submucosa (Weber et al. 2001), when our findings are taken together with previous studies (Smith et al. 1990, 1992; Cousins et al. 1995; Kunze et al. 1999; Spencer et al. 2001, 2003) they suggest that distension-evoked vasodilator responses result predominantly from stretch-activated signal transduction in myenteric afferent neurons rather than from tension generated by smooth muscle in the muscularis propria in response to stretch. This contention is also supported by the stimulus parameters used in the present study, as previous studies have shown that relatively brief distensions trigger stretch-activated mechanisms whereas sustained distension over a number of seconds activates tension-induced responses (Smith et al. 1990, 1992; Kunze et al. 1999).
We also observed that the proportion of preparations responding to balloon distension was significantly greater when the mucosa remained intact with the underlying submucosal and myenteric plexus. Previous electrophysiological studies (Kunze et al. 1997) have compared the excitability of myenteric neurons in in vitro preparations with and without mucosa to determine if the mucosa may alter the excitability of these neurons. They found that when the mucosa was intact there was an increased background firing of AH neurons which in turn increased excitability of S-type neurons (presumably interneurons and motoneurons). Therefore it seems likely that the intact mucosa also provides tonic signalling to AH neurons in our preparation and this in turn increases excitability of these neurons and the reflex vasodilator pathway they innervate.
Neurally evoked vasodilator responses in submucosal arterioles are also mediated by axon reflexes in nerve terminals of capsaicin-sensitive extrinsic afferent nerves (Vanner & Surprenant, 1996). A role for these nerves in the responses described in the current study was not examined because previous studies provide compelling evidence they are not involved in these reflexes (Biber, 1973; Furness et al. 1995; Vanner & Bolton, 1996).
Organization of the vasodilator pathways
In the current study, we established that both mucosal and muscle-activated vasodilator responses projected through the myenteric plexus, by performing surgical lesioning studies. When the myenteric plexus was lesioned more than 5 mm from the recording site, dilatations were completely blocked, whereas severing the submucosal plexus had no effect. In vitro studies in the myenteric plexus have identified long polysynaptic pathways (Smith & Furness, 1988; Smith et al. 1990) and single myenteric interneurons projecting for distances as great as 10 cm (Song et al. 1996) whereas submucosal polysynaptic pathways appear to be limited to a few millimetres (Hirst & McKirdy, 1975; Surprenant, 1984; Moore & Vanner, 1998). In addition, previous studies have shown that myenteric neurons projecting to the submucosal plexus densely innervate submucosal neurons (Bornstein et al. 1987; Moore & Vanner, 2000) but do not innervate submucosal arterioles (Galligan et al. 1990; Neild et al. 1990). In the current study, the nicotinic blocker, hexamethonium, caused significant but incomplete inhibition suggesting fast nicotinic EPSPs are important mediators but also that mediators of non-cholinergic fast EPSPs and/orcholinergic and non-cholinergic slow EPSPs may be involved. Guinea-pig submucosal vasodilator neurons also release acetylcholine which acts on arteriolar M3 receptors (Neild et al. 1990), although species differences exist (Sjoqvist et al. 1983). In the present study, we found that the muscarinic antagonist 4-DAMP completely blocked both mucosal and muscle-activated responses, consistent with this action resulting from the inhibition of acetylcholine from vasodilator neurons acting on the submucosal arteriole.
Given that previous studies in the myenteric plexus (Smith et al. 1992) suggest that distinct populations of afferent neurons mediate the mucosal and muscle-activated reflexes, we attempted to examine whether these findings pertain to the vasodilator reflexes described in this study. We found that the magnitude of vasodilator responses evoked by maximal stimulation paradigms using either sensory modality could be enhanced when stimulated with the alternate modality (see Fig. 8). Although the interpretation of these data is limited because not all preparations responded in this fashion and there are multiple potential sites of interaction in polysynaptic pathways, this finding is consistent with the notion that one population of afferent neurons desensitized to a specific stimulus and that a separate group of afferent neurons was recruited by the other sensory modality, giving a blended response. Previous studies which suggested that two populations of afferent neurons were involved, employed intracellular recording techniques to record from myenteric neurons in response to mucosal mechanical stimulation and muscle distension (Smith et al. 1992). These studies demonstrated that deforming the mucosa evoked a synaptic response in neurons when the synaptic responses to repeated distensions had disappeared. This study also provided evidence that these afferent pathways converge onto common interneurons and motoneurons, because the majority of these S-type neurons responded to both types of stimulation.
Our previous studies of myenteric neural projections to submucosal vasodilator neurons suggested that these inputs occurred in both orad and aborad directions (Vanner, 2000), a finding that was somewhat unexpected in view of the polarized nature of the peristaltic reflex. The findings of the current study however, also support this conclusion, given that both the mucosal and muscle distension stimuli evoked both descending and ascending responses. Although previous studies of the peristaltic reflex have demonstrated that the major components of these reflexes are mediated by ascending excitation and descending inhibition, there is also evidence for descending excitation and ascending inhibitory pathways (Smith et al. 1992; Spencer et al. 1999, 2000).
Conclusions
Mucosal blood flow is critical for the maintenance of the integrity and function of the mucosal lining of the intestine (Granger et al. 1989). Whole animal studies have shown that mechanical stimulation of the mucosa can increase mucosal blood flow over 100 % by activating enteric nerves (Biber et al. 1971, 1973). The current study has described two separate afferent pathways which activate neural pathways in the myenteric plexus which in turn innervate submucosal vasodilator neurons. These pathways are poised to co-ordinate mucosal blood flow in response to specific motor and secretory patterns in the intestine. Both ascending and descending pathways exist and it is possible that these pathways are activated during more complex motor patterns such as the mixing behaviour which occurs between bouts of propulsive activity in vivo (Cannon, 1912).
Acknowledgments
S.V. is supported by the Canadian Institute of Health Research (CIHR). D.R. is supported by a Studentship from Canadian Digestive Health Foundation (CDHF)/CIHR. The authors wish to thank Ms Margaret O'Reilly for her excellent technical support
REFERENCES
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5–hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- Biber B. Vasodilator mechanisms in the small intestine. Acta Physiol Scand Suppl. 1973;401:1–31. [PubMed] [Google Scholar]
- Biber B, Fara J, Lundgren O. Intestinal vasodilatation in response to transmural electrical field stimulation. Acta Physiol Scand. 1973;87:277–282. doi: 10.1111/j.1748-1716.1973.tb05391.x. [DOI] [PubMed] [Google Scholar]
- Biber B, Fara J, Lundgren O. A pharmacological study of intestinal vasodilator mechanisms in the cat. Acta Physiol Scand. 1974;90:673–683. doi: 10.1111/j.1748-1716.1974.tb05635.x. [DOI] [PubMed] [Google Scholar]
- Biber B, Lundgren O, Svanvik J. Studies on the intestinal vasodilatation observed after mechanical stimulation of the mucosa of the gut. Acta Physiol Scand. 1971;82:177–190. doi: 10.1111/j.1748-1716.1971.tb04957.x. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Costa M. Sources of excitatory synaptic inputs to neurochemically identified submucous neurons of guinea-pig small intestine. J Auton Nerv Syst. 1987;18:83–91. doi: 10.1016/0165-1838(87)90137-8. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J Neurosci. 1991;11:505–518. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Peristalsis, segmentation and the myenteric reflex. Am J Physiol. 1912;30:114–128. [Google Scholar]
- Cooke HJ, Sidhu M, Wang YZ. Activation of 5-HT1P receptors on submucosal afferents subsequently triggers VIP neurons and chloride secretion in the guinea-pig colon. J Auton Nerv Syst. 1997;66:105–110. doi: 10.1016/s0165-1838(97)00075-1. [DOI] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hirst GD. Neuronally released and applied acetylcholine on the longitudinal muscle of the guinea-pig ileum. Neuroscience. 1995;65:193–207. doi: 10.1016/0306-4522(94)00466-i. [DOI] [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Kuemmerle JF, Grider JR. Distinct 5-HT receptors mediate the peristaltic reflex induced by mucosal stimuli in human and guinea pig intestine. Gastroenterology. 1996;111:1281–1290. doi: 10.1053/gast.1996.v111.pm8898642. [DOI] [PubMed] [Google Scholar]
- Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Jiang MM, Shen KZ, Surprenant A. Substance P mediates neurogenic vasodilatation in extrinsically denervated guinea-pig submucosal arterioles. J Physiol. 1990;420:267–280. doi: 10.1113/jphysiol.1990.sp017911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DN, Kvietys PR, Korthuis RJ, Preman AJ. Microcirculation of the Intestinal Mucosa. Bethesda, MD: Blackwell Science Inc; 1989. pp. 1405–1474. [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, McKirdy HC. Two descending nerve pathways activated by distension of guinea-pig small intestine. J Physiol. 1975;244:113–127. doi: 10.1113/jphysiol.1975.sp010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, McKirdy HC. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975;249:369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MM, Kirchgessner A, Gershon MD, Surprenant A. Cholera toxin-sensitive neurons in guinea pig submucosal plexus. Am J Physiol. 1993;264:G86–94. doi: 10.1152/ajpgi.1993.264.1.G86. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. J Comp Neurol. 1996;371:270–286. doi: 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci. 1992;12:235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Bertrand PP, Furness JB, Bornstein JC. Influence of the mucosa on the excitability of myenteric neurons. Neuroscience. 1997;76:619–634. doi: 10.1016/s0306-4522(96)00408-3. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Bertrand PP, Furness JB. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J Physiol. 1999;517:547–561. doi: 10.1111/j.1469-7793.1999.0547t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Vanner S. Organization of intrinsic cholinergic neurons projecting within submucosal plexus of guinea pig ileum. Am J Physiol. 1998;275:G490–497. doi: 10.1152/ajpgi.1998.275.3.G490. [DOI] [PubMed] [Google Scholar]
- Moore BA, Vanner S. Properties of synaptic inputs from myenteric neurons innervating submucosal S neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G273–280. doi: 10.1152/ajpgi.2000.278.2.G273. [DOI] [PubMed] [Google Scholar]
- Neild TO. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- Neild TO, Shen KZ, Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol. 1990;420:247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Frieling T, Rupprecht C, Schemann M. Polarized enteric submucosal circuits involved in secretory responses of the guinea-pig proximal colon. J Physiol. 1998;506:539–550. doi: 10.1111/j.1469-7793.1998.539bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Gershon MD. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J Neurosci. 2000;20:3295–3309. doi: 10.1523/JNEUROSCI.20-09-03295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoqvist A, Fahrenkrug J, Jodal M, Lundgren O. Effect of apamin on release of vasoactive intestinal polypeptide (VIP) from the cat intestines. Acta Physiol Scand. 1983;119:69–76. doi: 10.1111/j.1748-1716.1983.tb07307.x. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J Auton Nerv Syst. 1990;29:203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Interactions between reflexes evoked by distension and mucosal stimulation: electrophysiological studies of guinea-pig ileum. J Auton Nerv Syst. 1991;34:69–75. doi: 10.1016/0165-1838(91)90009-r. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J Neurosci. 1992;12:1502–1510. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Furness JB. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J Auton Nerv Syst. 1988;25:205–218. doi: 10.1016/0165-1838(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Song Z, Brookes SJ, Costa M. Projections of specific morphological types of neurons within the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res. 1996;285:149–156. doi: 10.1007/s004410050630. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G231–241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith CB, Smith TK. Role of muscle tone in peristalsis in guinea-pig small intestine. J Physiol. 2001;530:295–306. doi: 10.1111/j.1469-7793.2001.0295l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine. J Physiol. 1999;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanner S. Myenteric neurons activate submucosal vasodilator neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G380–387. doi: 10.1152/ajpgi.2000.279.2.G380. [DOI] [PubMed] [Google Scholar]
- Vanner S, Bolton M. Neural circuitry of capsaicin-sensitive afferents innervating submucosal arterioles in guinea pig ileum. Am J Physiol. 1996;270:G948–955. doi: 10.1152/ajpgi.1996.270.6.G948. [DOI] [PubMed] [Google Scholar]
- Vanner S, Jiang MM, Surprenant A. Mucosal stimulation evokes vasodilation in submucosal arterioles by neuronal and nonneuronal mechanisms. Am J Physiol. 1993;264:G202–212. doi: 10.1152/ajpgi.1993.264.2.G202. [DOI] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Neural reflexes controlling intestinal microcirculation. Am J Physiol. 1996;271:G223–230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J Physiol. 2001;536:741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


