Abstract
We have investigated whether migrating motor complexes (MMCs) are impaired or absent in the small intestine of W/Wv mutant mice, which lack pacemaker interstitial cells of Cajal (ICC) and electrical slow waves. The intracellular electrical and mechanical activities of the small intestines of wild-type (+/+) and W/Wv mutant mice were recorded. Electrical recordings from circular muscle cells confirmed the absence of slow waves in W/Wv mice, whereas slow waves were always recorded from +/+ muscle cells. Spontaneous phasic contractions were recorded from W/Wv muscles in the absence of slow waves, but these events occurred at a lower frequency than in +/+ tissues. MMC activity was recorded consistently from the ileum of +/+ mice, and normal MMCs were also recorded from W/Wv mice. MMCs in both +/+ and W/Wv mice were abolished by tetrodotoxin (1 μm), hexamethonium (300 μm) or atropine (1 μm), suggesting that the neural control mechanisms responsible for MMCs in +/+ mice are intact and are responsible for MMCs in W/Wv mice. Transmural nerve stimulation demonstrated intact inhibitory and excitatory neural regulation of W/Wv intestinal muscles. Prolonged trains of cholinergic motor nerve stimulation failed to activate slow waves in the intestinal muscles of W/Wv mice. Our findings show that the generation and directional propagation of MMC activity in mouse small intestine does not require slow-wave activity or an intact network of myenteric ICC. The generation and propagation of MMCs appear to be an intrinsic capability of the enteric nervous system and are not related to slow waves or the gradient in slow-wave frequency.
Migrating motor complexes (MMCs) are cyclical contractions of the smooth muscle layers in the stomach, small intestine or colon that can propagate over large regions of the gastrointestinal tract. There has been much speculation about the exact role of the MMC, but it is thought to be (1) involved in the propulsion of gastric content, (2) for providing a largely aboral transit throughout the bowel and (3) for maintaining low bacterial counts within the intestinal lumen (Sarna, 1985; Nieuwenhuijs et al. 1998; Andrews et al. 2002). Szurszewski (1969) provided the first detailed description of the MMC in dog small bowel, and since his work, MMCs have been identified routinely from the stomach, small intestine and colon of a variety of species both in vitro and in vivo (Carlson et al. 1972; Marik & Code, 1975; Ruckebush & Bueno, 1977; Marlett & Code, 1979; Galligan et al. 1985; Sarna, 1985; Bywater et al. 1989; Fida et al. 1997; Spencer et al. 1998; Bush et al. 2000, 2001; Spencer, 2001).
Although the mechanisms underlying MMCs are unclear, the criteria used to classify them have remained consistent across species. The MMC is usually divided into three, and sometimes four, distinct phases that are defined in terms of the amount and regularity of contractile or electrical spiking activity (Carlson et al. 1972; Sarna, 1985; Huseby, 1999). Phase I has little or no contractile activity and is often called the quiescent phase. Phase II has intermittent or irregular spiking and both non-migrating and peristaltic contractions that propagate over short distances. Phase III, which is its most characteristic phase, consists of regular clusters of spikes or contractions of large amplitude that propagate over long lengths of the bowel. Phase III can be followed by a brief interval of intermittent contractions called phase IV. MMC-like activity can be recorded from in vitro segments of mouse small intestine (Bush et al. 2000, 2001). This activity resembles phase III since it is mediated by intrinsic nerves, occurs cyclically as a series of powerful phasic contractions and migrates distally over large regions of the small intestine. However, the role of slow waves during MMC activity is currently unclear (Sarna, 1985; Caenepeel et al. 1991).
Phasic contractions of the bowel are regulated by spontaneous membrane potential oscillations termed slow waves (for review see Sanders, 1996). Pacemaker cells, called interstitial cells of Cajal (ICC), that lie in the plane of the myenteric plexus (ICC-MY) are responsible for the generation of slow waves in gastric and intestinal smooth muscles (Smith et al. 1987; Ward et al. 1994; Huizinga et al. 1995; Dickens et al. 1999). ICC-MY are largely missing in the W/Wv mouse small intestine, and slow waves are consequently absent (Ward et al. 1994; Huizinga et al. 1995). Post-junctional neural responses to electrical field stimulation have been found to be normal in the mouse ileum of W/Wv mice (Ward et al. 1994), suggesting that neuromuscular inputs from the enteric nervous system are largely intact. These mice retain the mechanism for initiation of MMCs (i.e. enteric neuromuscular transmission) but lack slow-wave activity. Thus, the W/Wv mouse provides an excellent model with which to test whether slow waves are necessary for MMCs in the murine small intestine. Our results show that rhythmically occurring MMCs are not absent in the murine small intestine; however, the properties of these events are somewhat altered compared with the activity in wild-type (+/+) mice. Our findings suggest that neither slow waves nor an intact network of ICC-MY are required for the generation and propagation of MMCs.
METHODS
W/Wv mice and genetic controls (wild type: +/+ mice) were obtained from JAX Laboratories (Maine, USA). Mice of either sex were killed by inhalation anaesthetic (Isoflurane) in accordance with the animal ethics committee of the University of Nevada School of Medicine. Segments of terminal ileum (7 cm in length) were removed and placed immediately into a petri dish containing cold Krebs solution.
Recordings of circular muscle (CM) mechanical activity
For mechanical recordings, intact preparations of whole ileum were mounted into an organ bath using the protocol already described by our laboratory (Bush et al. 2001). In brief, the mechanical activity of the CM was monitored at three sites (proximal, mid and distal) along the terminal ileum using isometric tension transducers (Kent Scientific, Litchfield, CT, USA). These were connected by small stainless-steel clips (Micro-serrefines No. 18055–04; Fine Science Tools, Foster City, CA, USA) attached to the serosal surface. The initial tension of the CM was routinely set to 500 mg, and was readjusted back to this level when the resting tension decreased. Preparations were bathed in oxygenated Krebs solution at 35–37 °C. The Krebs solution was replaced approximately every 30 min.
Sharp intracellular recording technique
Intracellular microelectrode recordings were made from circular smooth muscle cells using conventional electrophysiological techniques. Two independent micromanipulators (model M3301L, World Precision Instruments, Sarasota, FL, USA) were used for simultaneous recording from two CM cells. Electrodes were filled with 1.5 m KCl solution and had resistances of about 100 MΩ. Electrical signals were amplified using a dual-input high-impedance amplifier (Axoprobe 1A; Axon Instruments, Foster City, CA, USA), using two Axon HS-2 headstages (gain 0.1L). Output signals from the amplifier were digitized on an A/Dconverter (Axon Instruments), and low-pass filtering frequencies ranging from 200 to 500 Hz were used. Recordings were visualized and recorded onto a PC running Axoscope (version 8.0; Axon Instruments) and onto a digital four-channel oscilloscope (Gould 1604, Ilford, Essex, UK).
Drugs and solutions
The following drugs were used throughout the study: hexamethonium, tetrodotoxin and atropine, all of which were obtained from Sigma (St Louis, MO, USA). The composition of the modified Krebs solution was (mm): NaCl 120.35, KCl 5.9, NaHCO3 15.5, NaH2PO4 1.2, MgSO4 1.2, CaCl2 2.5 and glucose 11.5. The Krebs solution in each chamber was gassed continuously with a mixture containing 3 % CO2-97 % O2 (v/v), pH 7.3-7.4.
Measurements and statistics
Paired or unpaired Student's t tests were used where appropriate. A minimum significance level of P < 0.05 was used throughout. The use of ‘n’ in the results section refers to the number of animals on which observations were made, and data are presented as means ±s.e.m. For intracellular recordings, typically more than one impalement was made in cells from the same animal, and this is stated as the number of cells recorded from. Measurements of amplitude, half-width and the interval between MMC contractions were all made using Acqknowledge 3.2.6 (BIOPAC Systems, Santa Barbara, CA, USA). The propagation velocity of MMCs was obtained by dividing the distance between the oral and anal recording sites (6 cm), over the time taken for MMCs to traverse the oral to anal recording sites (measured from 10 % peak amplitude, on the rising phase). Measurements of the intervals between slow waves were made from the half-peak amplitude on the rising phase. Similarly, the half-duration of individual slow waves was made at the half-peak amplitude on the rising phase to the same point of the repolarizing phase.
RESULTS
Mechanical recordings from the small intestine of +/+ mice
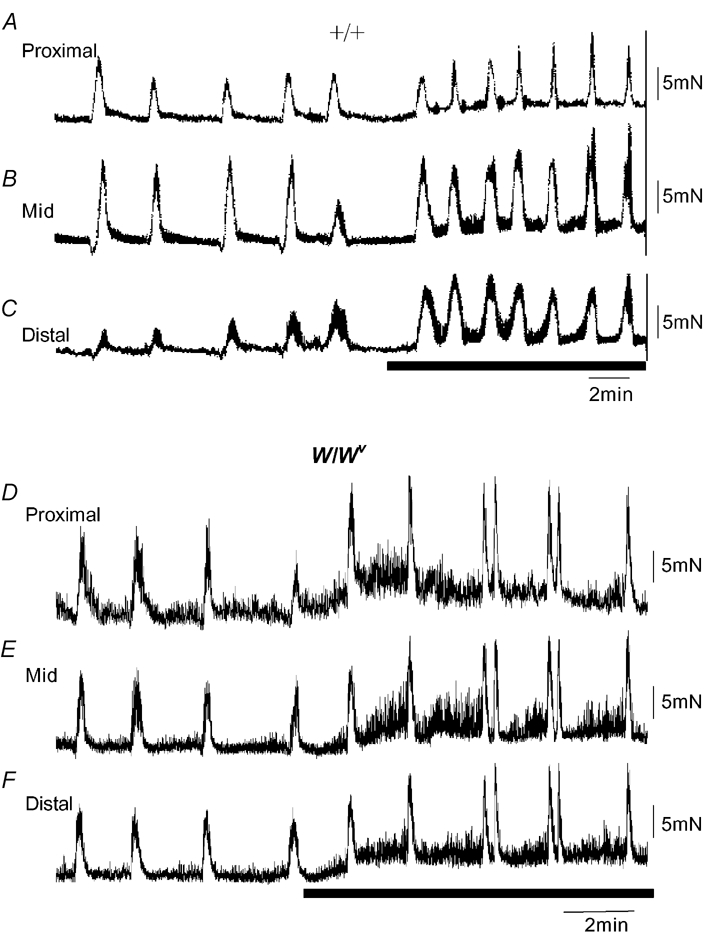
When mechanical recordings were made from the proximal, mid and distal regions of mouse ileum of +/+ mice, each region showed an ongoing discharge of phasic contractions (Fig. 1A–C). At the three recording sites, each phasic contraction was temporally uncoordinated. In the proximal, mid and distal regions of the ileum, ongoing phasic contractions had average amplitudes of 1.3 ± 0.2, 1.4 ± 0.3 and 1.2 ± 0.2 mN, respectively. These values were not significantly different between regions (n = 7; P > 0.05). The average interval between phasic contractions for the proximal, mid and distal regions was 2.3 ± 0.3, 2.0 ± 0.01 and 2.2 ± 0.3 s, respectively, and these average intervals were not significantly different (n = 7; P > 0.05).
Figure 1. Mechanical recordings made simultaneously from three sites of the ileum: proximal, mid and distal.
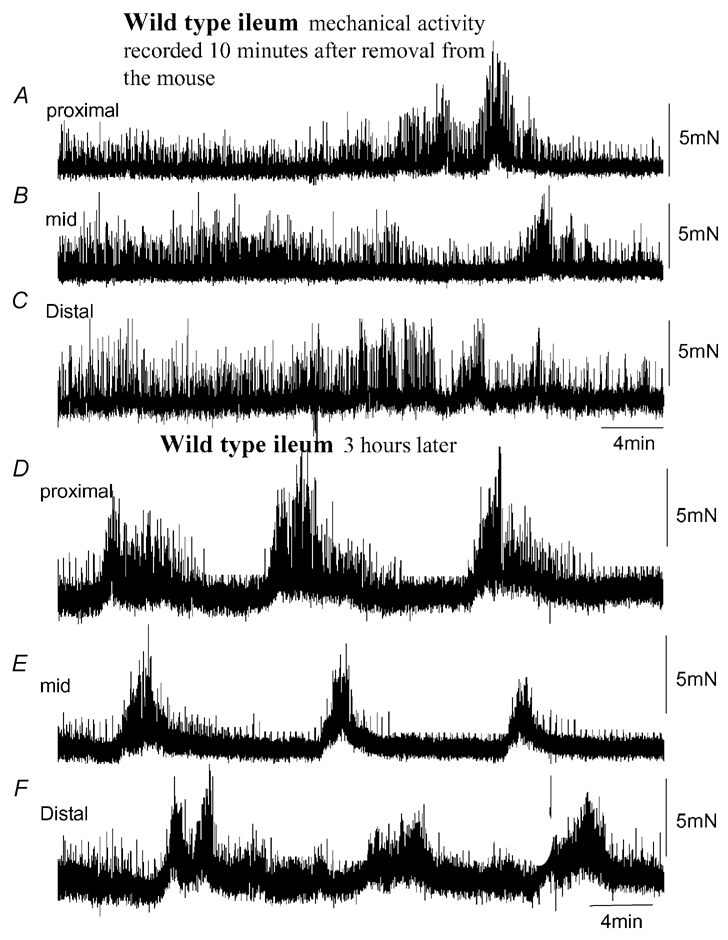
A, B and C, mechanical recordings made simultaneously from the ileum at three sites: proximal, mid and distal, respectively. Immediately upon removal from the animal, spontaneous phasic contractions were recorded that were temporally uncoordinated between each recording site. No clear MMCs were present. D, E and F, in the same preparation, approximately 3 h after the recordings in A–C were made, spontaneous MMCs were observed. MMCs originated in the proximal ileum and propagated to the terminal ileum.
After a period of equilibration lasting 1–3 h, spontaneous MMCs were recorded and became temporally coordinated between the proximal and distal regions of the ileum (Fig. 1D–F). The average interval between MMCs in +/+ mice was 5 ± 1 min (Fig. 2B), where the average amplitude of MMC contractions for the proximal, mid and distal regions of the ileum was 5.5 ± 3.2, 4.6 ± 0.9 and 4.5 ± 1. 6 mN, respectively (n = 7; taken between the troughs of phasic contractions). During MMCs, the amplitude of phasic contractions was found to increase significantly at each recording site (proximal: from 1.3 ± 0.3 mN between MMCs to 6.6 ± 1.5 mN during MMCs; mid: from 1.4 ± 0.3 mN between MMCs to 6.8 ± 1.7 mN during MMCs; distal: from 1.2 ± 0.2 mN between MMCs to 5.6 ± 1.1 mN during MMCs; Fig. 3B and E). However, despite this increase in phasic contraction amplitude during MMCs, the average interval between each phasic contraction remained constant with phasic contractions recorded during MMC intervals (proximal: P = 0.64; mid: P = 0.74; distal: P = 0.5; Fig. 3B and F).
Figure 2. Statistical comparison of the similarities of MMCs recorded from +/+ and W/Wv mouse ileum.
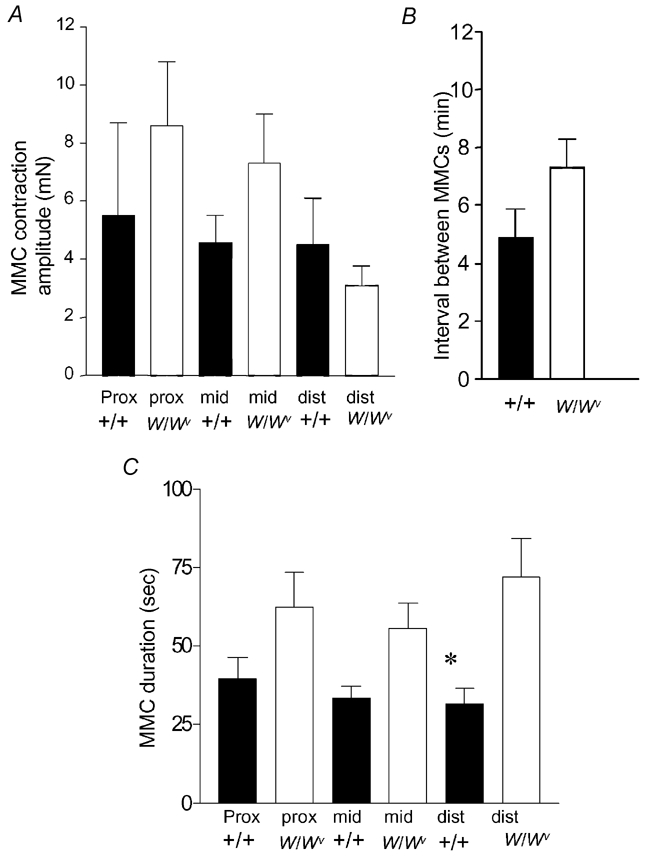
A, there was no significant difference in MMC peak contraction amplitudes in either the proximal (prox), mid or distal (dist) regions of ileum obtained from +/+ or W/Wv mice. B, no significant difference was noted in the interval between MMCs in +/+ or W/Wv mice. C, the mean half-durations between MMC contractions in the proximal and mid ileum were not significantly different between +/+ and W/Wv mice. The half-durations of MMC contractions in the distal ileum of W/Wv mice were found to be significantly longer than in +/+ controls.
Figure 3. Comparison of the amplitudes and intervals between phasic contractions in +/+ and W/Wv mice.
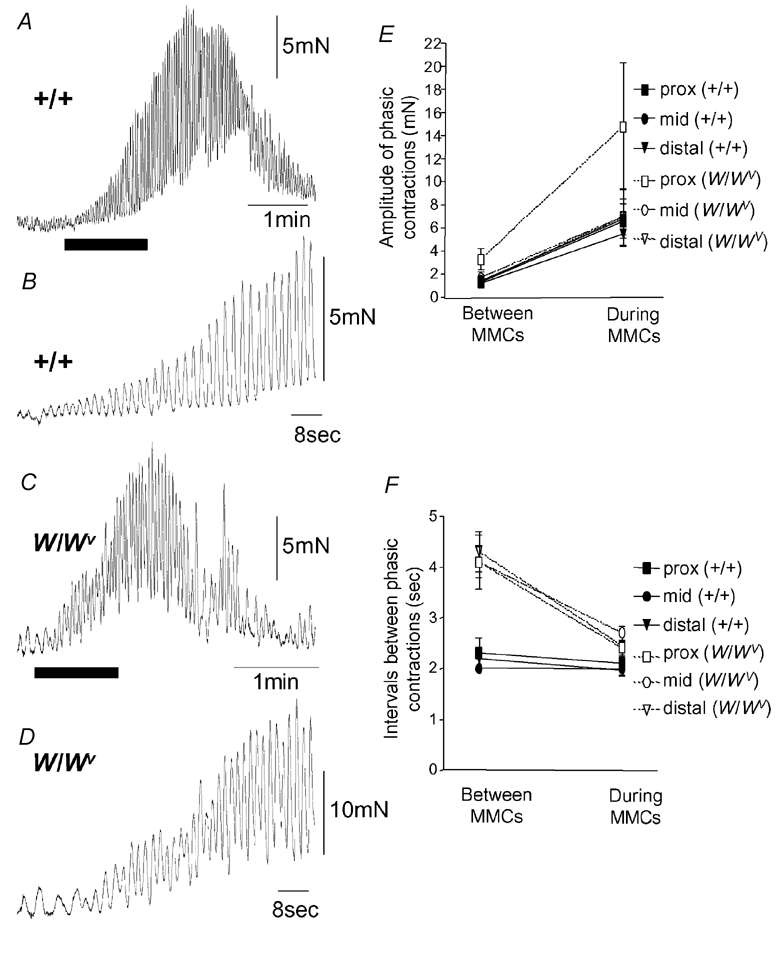
A, a single MMC contraction recorded from a +/+ mouse ileum. The recording represented by the black bar is shown on expanded time scale in B. B, the increase in amplitude of phasic contractions during the onset of the MMC. Note, the interval between phasic contractions does not change. C, a single MMC contraction recorded from a W/Wv mouse. The black bar is shown on an expanded time scale in D. D, the increase in amplitude and decrease in interval between phasic contractions during the onset of the MMC. E, a graphical representation of the changes in phasic contraction amplitude from the period between MMCs to the peak of the MMC contraction. Both +/+ and W/Wv mice exhibited a significant increase in amplitude of phasic contractions during the MMC in the proximal, mid and distal ileum. F, in the +/+ mouse, the intervals between phasic contractions remained relatively constant from the transition between MMCs to the peak of the MMC (every ≈2 s). These intervals were not significantly different from one another (proximal, P = 0.64; mid, P = 0.74; distal, P = 0.50). However, in the W/Wv mouse, the proximal, mid and distal ileum exhibited a significant decrease in phasic contraction intervals from the transition between MMCs to the peak of the MMC (P = 0.006, P < 0.001 and P < 0.001, respectively).
Mechanical activity of the small intestine of W/Wv mice
Mechanical recordings were made from the ilea of 15 W/Wv mice. In all preparations, an ongoing discharge of phasic contractions was also recorded from the CM layer of the proximal, mid and distal ileum, immediately upon removal from the animal (Fig. 4A–C). These contractions were similar to the spontaneous contractile activity seen in +/+ ilea (compare Fig. 1A and Fig. 4A). Despite the absence of slow waves in W/Wv mice, the average amplitude of phasic contractions was not significantly different in W/Wv and +/+ mice in each of the three regions of ileum studied (Fig. 3E). However, we noted that the average interval between spontaneous contractions was significantly longer in W/Wv ilea than in +/+ tissues (e.g. W/Wv proximal: 4.1 ± 0.2 s, P < 0.001; mid: 4.1 ± 0.5 s, P = 0.008; distal: 4.3 ± 0.4 s, P < 0.001; see Fig. 3F). The cumulative data from +/+ and W/Wv mice is compared in Fig. 3C and D.
Figure 4. Mechanical activity recorded from isolated W/Wv mouse ileum.
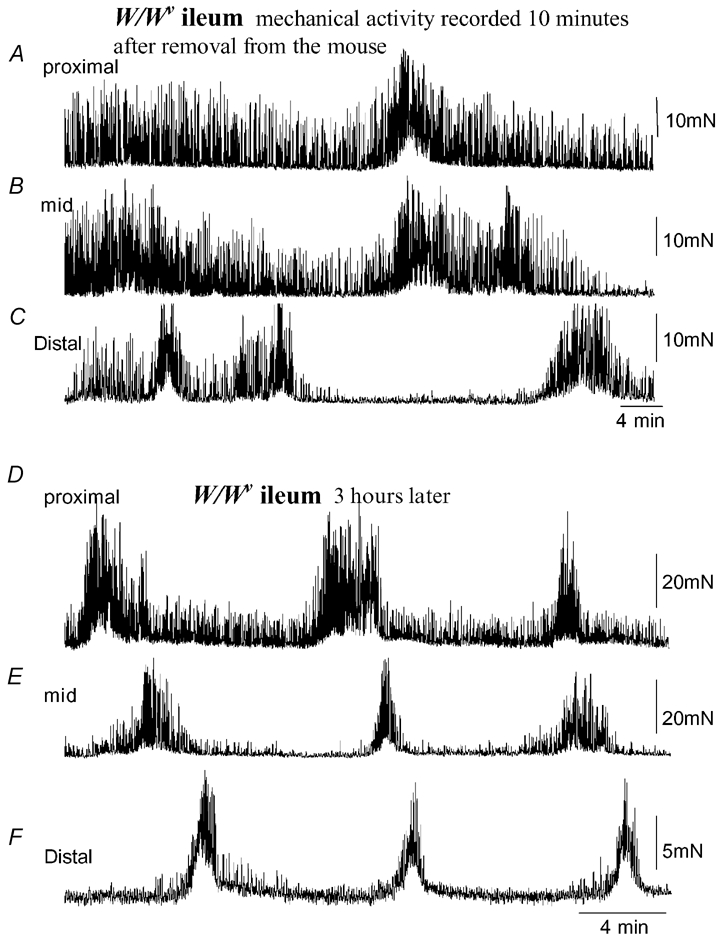
A, B and C, simultaneous recordings from the proximal (A), mid (B) and distal regions (C) of the W/Wv mouse ileum. Immediately upon removal from the mouse, spontaneous phasic contractions were recorded that were temporally uncoordinated between each recording site. No distinct MMC activity was present. D, E and F, approximately 3 h after the recording was made in A, B and C, spontaneous MMCs were observed, despite the absence of intestinal slow waves. MMCs in this example propagate from the proximal to distal ileum without slow waves.
Similar to +/+ mice, coordinated MMC activity was recorded from W/Wv mice typically within 1–3 h after removal from the animal (Fig. 4D–F). The mean interval between MMCs in W/Wv mice was 7 ± 1 min, which was not significantly different from MMCs recorded in +/+ mice (P = 0.294; n = 7, Fig. 2B). In the proximal and mid ileum, the half-durations of MMCs were not significantly different in +/+ and W/Wv mice (proximal: P = 0.26; mid: P = 0.12), but were found to be significantly longer in duration in the distal ileum of W/Wv mice (P = 0.04; see Fig. 2C). Similar to +/+ mice, the amplitude of each phasic contraction was found to increase significantly during each MMC (proximal: from 3.3 ± 0.9 mN between MMCs to 14.8. ± 5.5 mN during MMCs; mid: from 1.7 ± 0.5 mN between MMCs to 7.0 ± 1.6 mN during MMCs; distal: from 1.3 ± 0.4 mN between MMCs to 7.0 ± 2.5 mN during MMCs; Fig. 3E). Interestingly, in W/Wv mice, the mean interval between phasic contractions became significantly shorter during the MMC compared with those recorded during the intervals between MMCs (proximal: P < 0.001; mid: P = 0.008; distal P < 0.001; Fig. 3F).
Propagation velocities and direction of propagation of MMCs in +/+ and W/Wv mice
In both +/+ and W/Wv mice, the direction of propagation of MMCs was not constant between preparations, nor within recording periods in the same preparation. In both types of mice, the predominant direction of propagation of MMCs was from the oral to mid to distal region of ileum (+/+: 51 % of observations (55 out 141 MMCs); W/Wv: 39 % of observations (53 out of 103 MMCs)). Interestingly, in both +/+ and W/Wv mice, many MMCs propagated from the anal to oral regions of ileum (+/+:12 % of observations; W/Wv: 20 % of observations; Fig. 5A–E). Also, many MMCs occurred simultaneously at all three regions of the ileum (Fig. 5A–E). In +/+ mice, the mean propagation velocity of anally migrating MMCs was 0.9 ± 0.1 mm s−1 (range: 0.4-2.7 mm s−1). This value was not significantly different from the propagation velocity of anally migrating MMCs in W/Wv mice (mean: 1.3 ± 0.4 mm s−1, range: 0.13-5.0 mm s−1; P = 0.44). In +/+ mice, the mean propagation velocity for orally migrating MMCs was 1.6 ± 1.0 mm s−1 (range: 0.56-2.6 mm s−) and again this was not significantly different from the mean propagation velocity of orally migrating MMCs in W/Wv mice 0.72 ± 0.16 mm s−1 (range: 0.19-1.7 mm s−1; P = 0.36).
Figure 5. Comparison of the direction of propagation of MMCs recorded from the ileum of +/+ and W/Wv mice.
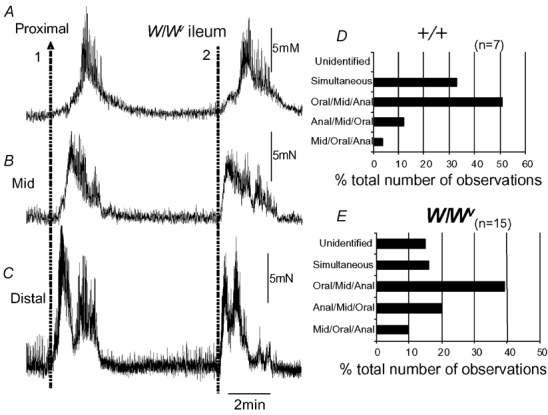
A, B and C, two MMC contractions, one that propagates from the anal to the oral region of the ileum (orally migrating, represented by arrow 1), while another (represented by the dotted line 2) appears to have occurred simultaneously in the proximal, mid and distal regions. D, the proportion of MMCs in +/+ mice where the direction of propagation was noted. E, shows the same graphical representation of MMCs in W/Wv mice and the direction of MMC propagation. In both types of mice, the predominant direction of propagation was from the proximal to mid to distal ileum.
Effects of nitric oxide (NO) synthesis inhibition, atropine and hexamethonium on MMC activity in the W/Wv mouse ileum
Hexamethonium was used to test the role of the enteric nerves in MMC generation in +/+ and W/Wv mice. Application of hexamethonium (100–300 μm) immediately abolished MMC activity without any detectable effect on resting tension. The phasic contractions observed between MMCs were unaffected by hexamethonium, suggesting that they are of myogenic origin. Similarly, when atropine (1 μm) was applied to the ileum, MMCs were abolished, while phasic contractions were unaffected (Fig. 6).
Figure 6. Effects of atropine on MMCs recorded from the W/Wv mouse ileum.
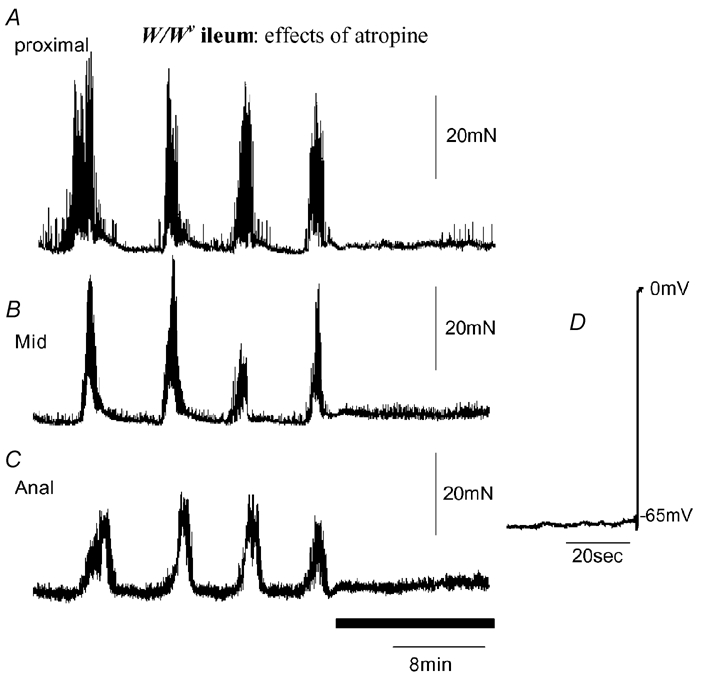
Anally migrating MMCs were recorded. Addition of atropine (1 μm; see black bar) immediately abolished all MMC activity. Intracellular recordings were made from CM at different regions of the ileum, proximal (A), mid (B) and distal (C) from the same preparation. Slow waves were confirmed to be absent from the preparation where MMCs were present. The electrical recording (D) shows withdrawal of the electrode.
Nitro-l-arginine (l-NA, 100 μm) was applied to six +/+ and six W/Wv mice to test the role of endogenous NO in MMC activity. In +/+ and W/Wv mice, l-NA increased the resting tension of each preparation by 1.3 ± 0.2 mN and 2.1 ± 0.6 mN, respectively (n = 6). Furthermore, in five out of six preparations from +/+ mice, the interval between MMCs significantly decreased from 4.7 ± 0.7 to 2.5 ± 0.3 min (P = 0.03; n = 5). In one +/+ animal, l-NA caused the appearance of uncoordinated MMCs, the contraction onset of which was not temporally correlated along the ileum. In these +/+ mice, the amplitude and half-duration of MMCs was not significantly altered (P = 0.47, P = 1.0, respectively, n = 5; Fig. 7A–C). When l-NA was applied to W/Wv mice, similar effects were noted as in +/+ animals. In three out of six W/Wv mice exposed to l-NA, a decrease in the interval between MMCs was also noted from 6.6 ± 1.1 to 3.6 ± 0.1 min (P = 0.03; n = 3; Fig. 7D–F). In the remaining three animals exposed to l-NA, the resting tone increased, but uncoordinated MMC activity was recorded, where no clear propagation between each recording site could be ascertained (Fig. 7D–F).
Figure 7. Effects of inhibition of NO synthesis on MMCs recorded from +/+ and W/Wv mouse ileum.
A, B and C, a recording of mechanical activity in the proximal, mid and distal ileum, respectively, in a +/+ mouse. MMCs occurred about every 4 min. The period represented by the hatched bar shows the point of application of l-NA (100 μm), where the resting tone increased and the interval between MMCs decreased at each point in the ileum. D, E and F, a typical example recording of mechanical activity from W/Wv mouse ileum. l-NA also increased the resting tension at each site along the ileum and decreased the interval between MMCs.
Intracellular electrical activity in the CM of +/+ and W/Wv mice
Intracellular electrical recordings made from CM cells of +/+ mice revealed normal slow-wave activity in the presence of nifedipine (1 μm), as reported previously (Ward et al. 1994; Huizinga et al. 1995). The mean interval between slow waves was 2.1 ± 0.1 s (range: 1.8-2.5 s, n = 5), while the mean amplitude and half-duration of slow waves was 16 ± 2 mV (range: 11–24 mV) and 947 ± 42 ms (range: 813–1134 ms), respectively (n = 5). The mean resting membrane potential, taken between the peak of slow-wave depolarizations, was −58 ± 1.5 mV (range: −50 to −70 mV; 13 cells, n = 5).
Intracellular recordings were made from a total of 18 W/Wv mice. Slow waves were absent in the ileal muscles of all of these animals (n = 18; Fig. 6B), consistent with previous observations (Ward et al. 1994). We also recorded electrical activity from CM cells of W/Wv intestines from the same preparations in which MMCs were recorded. In nine W/Wv ileum preparations where MMCs were recorded, intracellular recordings from these animals showed a total absence of slow waves. The mean resting membrane potential of CM cells in W/Wv mice was −51 ± 1 mV (range: −40 to −60 mV; 28 cells, n = 18). The resting membrane potentials of +/+ and W/Wv mice were significantly different; cells of W/Wv were significantly more depolarized (P = 0.004; unpaired Student's t test), as reported previously (see Ward et al. 1994).
Junction potentials evoked by transmural nerve stimuli in the CM of +/+ and W/Wv mouse small intestine
In five +/+ and six W/Wv mice, simultaneous recordings were made from two CM cells, located between 10 and 15 mm oral and anal to the transmural stimulating wires (Fig. 8). In all +/+ mice studied, a single pulse stimulus evoked an inhibitory junction potential (IJP) in CM cells both oral and anal to the stimulus site. The ongoing slow-wave activity in these animals precluded the identification of excitatory junction potentials (EJPs); only IJPs were observed (Fig. 8). IJPs had mean amplitudes of 5.8 ± 2.1 mV (half-duration; 970 ± 170 ms, n = 4) and 6 ± 1.6 mV (813 ± 100 ms; n = 5) oral and anal to the stimulus site, respectively. In W/Wv tissues, when single pulse stimuli (0.4 ms duration, supramaximal voltage) were applied, IJPs (mean amplitude: 3.7 ± 0.8 mV; half-duration: 1.1 ± 0.4 s; n = 3) were evoked oral to the stimulus site in three out of six preparations. In the other three animals, EJPs were the dominant response (mean amplitude: 5.3 ± 1.2 mV; half-duration: 1.0 ± 0.1 s; n = 3) oral to the stimulus site. Interestingly, anal to the stimulus site, IJPs (mean amplitude: 5.3 ± 0.7 mV; half-duration: 1.7 ± 0.6 s; n = 3) were evoked in three out of six animals, while in the other three animals EJPs were evoked (mean amplitude: 4.6 ± 1.4 mV; half-duration: 1.2 ± 0.1 s; n = 3; Fig. 8).
Figure 8. Intracellular electrical recordings from the CM and responses to transmural nerve stimulation in +/+ and W/Wv mouse ileum.
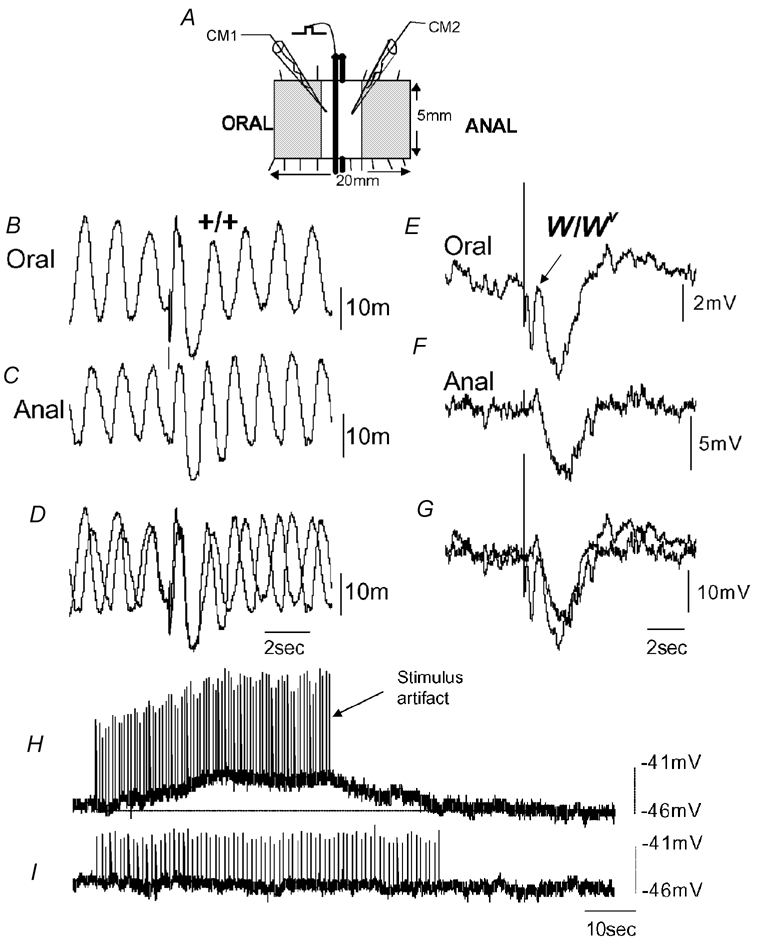
A, schematic of the flat-sheet preparation used. Two recording electrodes were impaled simultaneously in two CM cells, one oral and one anal of the transmural wires. Recordings were made 1.2 mm oral and anal from the stimulating wires. B and C, simultaneous recordings from two CM cells shows spontaneous slow-wave activity. A brief train of stimuli (10 Hz, 0.5 s, 50 V) was applied to the enteric nerves, and an IJP was evoked both oral and anal to the stimulating wires. D, the same simultaneous recordings shown in B and C superimposed. E and F, simultaneous recordings from two CM cells in the W/Wv mouse ileum. Note the absence of slow waves. A train of stimuli (10 Hz, 0.5 s, 50 V) delivered to the ileum also evoked slow IJPs both oral and anal to the stimulus. At the oral recording site, a small EJP can be seen to truncate the IJP (see arrow in E). G, recordings shown in E and F superimposed. H, in the presence of l-NA (100 μm) and apamin (500 nm) to block inhibitory neurotransmission, a prolonged train of nerve stimuli (2 Hz, 40 s, 0.4 ms, 40 V) evoked a slow membrane depolarization of the CM, but no slow waves were induced. I, the effects of atropine (1 μm) on the response to nerve stimulation in the same animal. It can be seen that the depolarization is abolished.
Cholinergic neurotransmission does not induce slow waves in W/Wv mice
MMCs were abolished by application of atropine in both +/+ and W/Wv intestines, suggesting that this activity is induced by acetylcholine released from the motor neurons. Intestinal muscles of W/Wv mice lack ICC-MY, but ICC in the deep muscular plexus (ICC-DMP) are present in these tissues (Ward et al. 1995). In larger mammals it has been shown that ICC-DMP can generate pacemaker activity (Jimenez et al. 1996). Thus, we tested whether cholinergic neurotransmission induces slow waves in W/Wv mice, possibly via ICC-DMP. Intracellular recordings were made from W/Wv intestines in the presence of l-NA (100 μm) and apamin (500 nm) to block the majority of inhibitory motor neurotransmission. Prolonged trains of nerve stimulation (2 and 5 Hz, 40 s, 0.4 ms, 40 V) depolarized tissues by an average of 5.0 ± 0.05 and 7.4 ± 0.5 mV, respectively (five preparations, n = 3; see Fig. 8), but failed to induce slow waves in any preparation. The time course of these depolarizations were 29 ± 4.5 and 37.2 ± 2.8 s, respectively, when measured at half-maximal amplitude, (five preparations, n = 3). Atropine (1–2 μm) abolished the depolarizations induced by 2 Hz stimulation and reduced the magnitude of the depolarizations induced at 5 Hz (i.e. from 7.4 ± 0.5 to 3.0 ± 1.0 mV; n = 3). Thus, cholinergic stimulation for periods simulating the MMC complex was not capable of activating slow waves in small intestinal tissues in the absence of ICC-MY.
DISCUSSION
The current study shows that cyclical MMCs occur in the isolated small intestine of W/Wv mutant mice despite the absence of ongoing slow-wave activity. Our results also show that phasic contractions can occur in the mouse ileum without slow waves. Although ICC-MY are clearly the pacemaker cell for intestinal (Ward et al. 1994) and gastric slow waves (Dickens et al. 1999), these cells do not appear to be necessary for the generation or propagation of MMC activity, at least in isolated preparations of mouse small intestine.
Neurally mediated MMCs in +/+ and W/Wv mouse small intestine
The amplitude, duration and interval between MMCs were not significantly different between +/+ and W/Wv mice. There was a tendency for longer intervals between MMCs in W/Wv mice (≈7 ± 1 min), compared with +/+ mice (≈5 ± 1 min), but these values did not reach statistical significance in our study. In both +/+ and W/Wv mice, MMC activity was abolished by hexamethonium or atropine. This is consistent with a neural origin for MMCs recorded from the small intestine of larger mammals such as dogs (Sarna et al. 1981), and the migrating myoelectric complexes (Bywater et al. 1989; Spencer et al. 1998; Spencer, 2001) and colonic MMCs recorded from the large intestine of rodents, such as mice (Fida et al. 1997; Bush et al. 2000; Spencer, 2001). The connectivity between the enteric nervous system and the muscularis required for the generation and propagation of MMCs appeared to be intact in the W/Wv mouse ileum because post-junctional neural responses, both inhibitory and excitatory, were observed in response to transmural nerve stimulation.
Phasic contractile activity in +/+ and W/Wv mice
In both W/Wv and +/+ mice, mechanical recordings from all preparations revealed ongoing phasic contractions immediately upon removal from the animal. Despite the absence of slow waves, W/Wv mice were mechanically active. In fact, during the intervals between MMCs and during the peak of the MMC contraction, there was no significant difference between the mean peak amplitude of the phasic contractions recorded in either +/+ or W/Wv mice. Interestingly, the mean interval between the phasic contractions of the W/Wv ileum were consistently longer (range: 4.1-4.3 s) compared to +/+ mice (range: 2.0-2.3 s). The intrinsic slow-wave frequency of the +/+ mouse ileum is similar to the frequency of the phasic contractions recorded in +/+ mice. During the periods between MMCs in W/Wv mice, the mean interval between phasic contractions ranged from 4.1 to 4.3 s. However, during the MMC, this value decreased to a range of 2.4-2.7 s, which was similar to the interval between phasic contractions in +/+ mice. During the MMC in both W/Wv and +/+ mice, the amplitude of phasic contractions increased approximately fivefold. Since the increase in contractions during MMCs was abolished by atropine in both groups of mice, release of acetylcholine from cholinergic motor neurons and stimulation of post-junctional muscarinic receptors is likely to generate MMCs. Hexamethonium abolished MMCs in both +/+ and W/Wv mice, suggesting that fast nicotinic synaptic potentials in enteric ganglia are also essential for MMC generation.
Recent studies have shown that particular classes of ICC are critically involved in neuromuscular transmission in gastric smooth muscles (Ward et al. 2000; Beckett et al. 2002). For example, intramuscular ICC appear to be essential for nitrergic and cholinergic neurotransmission in the gastric fundus of mice (Burns et al. 1996; Ward et al. 2000; Beckett et al. 2002). The extent of the role of intramuscular ICC in the transduction of neural inputs in the small intestine and colon is less well understood since this class of ICC is not lost in W/Wv and other mouse models with incomplete defects in the Kit signalling pathway. Intramuscular ICC in the small intestine are clustered into the region of the deep muscular plexus and are referred to as ICC-DMP. These cells are present in the ileum of W/Wv mice, and the fact that these cells are retained in W/Wv mice may explain why the neurotransmission underlying the MMC was conserved in these animals. It is also possible that enteric motor neurotransmission in the small intestine occurs via parallel innervation of ICC-DMP and smooth muscle cells. Studies in which ICC of all classes can be abolished in the small intestine are needed if we are to distinguish between these possibilities. Our study demonstrates that ICC-MY are not essential for motor neurotransmission and the integration of inputs to generate or propagate MMCs.
In a recent study, Der Silaphet et al. (1998) infused fluid into short isolated segments of duodenum and found pronounced differences between W/Wv and +/+ mice. They concluded that ‘… the net propulsive effect of contractile activity in W/Wv mutant mice was much weaker than in controls.‘. These findings appear to contrast with the results of the current study. It is important to identify the differences in methodology used these studies. In the study of Der Silaphet et al. (1998). fluid was infused into the lumen of the bowel to generate distension of the intestinal wall. Thus, propulsive contractions in that study occurred in response to distension. In our study the intestinal lumen was flushed free of its contents, and no obvious distension stimuli were applied to the bowel. The MMC activity described in our study represents a neural phenomenon, which occurs in the absence of ICC-MY and slow waves. As suggested by Der-Silaphet et al. (1998). distension responses appear to enhance slow-wave-driven motor patterns, and these contractions propagate over a very short length of bowel. In contrast, the MMCs recorded from our study readily propagated over the entire 6–7 cm of ileum and did not require distension stimuli for their generation. Furthermore, MMCs are highly sensitive to antagonists of neuronal transmission.
Conversion of ‘fed state’ to ‘fasted state’ motor patterns in vitro
MMCs were rarely observed in +/+ or W/Wv mouse ileum immediately upon removal from the animal. Typically, in the first 1–3 h after removal from the mouse, all preparations from either +/+ or W/Wv mice showed ongoing phasic contractions in the CM. This motor activity closely resembled the ‘fed state’ motor pattern (i.e. phase II) described in non-ruminant mammals in vivo. This ‘fed state’ motor pattern is often referred to as segmentation and is associated with digestion and absorption, which is largely non-propulsive. These segmental-type phasic contractions persisted on some occasions for up to 4 h prior to the onset of MMC activity. It is interesting to note that this apparent conversion from a ‘fed state’ to a ‘fasted state’ motor pattern occurred in vitro. Indeed, MMCs in the ileum were consistently abolished by hexamethonium or tetrodotoxin, confirming that the enteric nerves were essential for their generation. However, the phasic contractions were likely to be myogenic in origin, as they persisted in the presence of these antagonists. Although W/Wv small intestinal muscles lack the slow-wave mechanism expressed by ICC-MY, they clearly do not lack intrinsic excitability, and previous studies have demonstrated an ability of intestinal muscle to generate spontaneous action potentials that couple to contractions in the absence of slow waves (Ward et al. 1995; Malysz et al. 1996). Our data suggest that the excitability of smooth muscle cells in the absence of ICC is not due to tonic cholinergic input, as the spontaneous phasic contractions were not affected by atropine. The intrinsic activity of W/Wv muscles was, however, tonically suppressed by nitrergic neural inputs.
Role of NO in MMC activity
Blockade of NO synthesis decreased the interval between MMCs and increased the resting tension in the CM. These findings suggest that, similar to the mouse colon (Spencer, 2001), endogenous NO release normally suppresses MMC frequency and maintains the tonic inhibition of the smooth muscle during the intervals between MMCs. Similar findings were reported recently by Powell & Bywater (2003). when recordings were made from the longitudinal muscle of the isolated mouse ileum.
Is the aboral propagation of MMCs due to a slow-wave gradient along the small intestine?
In our study, only the terminal ileum (≈6 cm in length) was used, and in this length of preparation there is no gradient in the slow-wave frequency between the oral and anal regions. Yet, in these preparations the majority of MMCs were still found to propagate aborally. In addition, in the ileum of W/Wv mice, which lack slow waves, the characteristics of the MMC and the direction of MMC propagation were found to be no different from +/+ mice. These findings suggest that the aboral propagation of MMCs can be achieved in the absence of a slow-wave gradient and, in fact, in the absence of slow waves. These findings are consistent with the work of Galligan et al. (1985). who found that MMCs propagated anally along the guinea-pig small intestine, despite the fact that there was no gradient in slow-wave frequency along the intestine (duodenum: 26.2 ± 1.3 cycles min−1) compared with the ileum (24.7 ± 1.3 cycles min−1).
Orally migrating MMCs were recorded on a number of occasions during this study. This shows that the direction of propagation of MMCs is dynamic and clearly does not always occur in the oral-to-anal direction. These findings are similar to a recent study of human small intestine, where both antegrade (anally propagating) and retrograde (orally propagating) MMCs were recorded (Andrews et al. 2002). In the mouse colon, although most colonic MMCs propagate in a largely oral-to-anal (antegrade) direction, they can also propagate orally, in a retrograde fashion (Fida et al. 1997; Bush et al. 2000; Spencer & Bywater, 2002). Slow waves are rarely recorded from mouse colon (Bywater et al. 1989; Spencer et al. 1998; Spencer, 2001), yet colonic MMCs are consistently recorded from full-length segments of these preparations. In the mouse colon, the generation of colonic myoelectric complexes involves a process of disinhibition (Spencer et al. 1998; Spencer, 2001), rather than any obvious involvement of slow-wave activity.
Conclusion
Our findings show that in mouse small intestine, the generation and propagation of MMCs occurs in the absence of the ICC-MY network and electrical slow-wave activity. The data reaffirm the significance of the enteric nervous system in providing the timing and mechanism of propagation for MMCs, and show that the intrinsic pacemaker function of ICC-MY is unnecessary for this activity.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (POIDK41315 and USA; no. RO1 NIDDK 45713). We also wish to acknowledge Dr Sean Ward and Yulia Bayguinov for their expert assistance with confocal microscopy and immunohistochemistry, supported by core facilities of a program project grant PO1 DK41315.
REFERENCES
- Andrews JM, O'Donovan DG, Hebbard GS, Malbert CH, Doran SM, Dent J. Human duodenal phase III migrating motor complex activity is predominantly antegrade, as revealed by high-resolution manometry and colour pressure plots. Neurogastroenterol Motil. 2002;14:331–338. doi: 10.1046/j.1365-2982.2002.00337.x. [DOI] [PubMed] [Google Scholar]
- Beckett EAH, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J Physiol. 2002;543:859–858. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000;84:162–168. doi: 10.1016/S1566-0702(00)00201-0. [DOI] [PubMed] [Google Scholar]
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Effects of alosetron on spontaneous migrating motor complexes in the murine small and large bowel. Am J Physiol. 2001;281:G974–983. doi: 10.1152/ajpgi.2001.281.4.G974. [DOI] [PubMed] [Google Scholar]
- Bywater RAR, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol. 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caenepeel PJ, Janssens W, Accarino A, Janssens J, Vantrappen G, Eyssen H. Variation of slow wave frequency and locking during the migrating myoelectric complex in dogs. Am J Physiol. 1991;261:G1079–1084. doi: 10.1152/ajpgi.1991.261.6.G1079. [DOI] [PubMed] [Google Scholar]
- Carlson GM, Bedi BS, Code SF. Mechanism of propagation of intestinal interdigestive myoelectric complex. Am J Physiol. 1972;222:1027–1030. doi: 10.1152/ajplegacy.1972.222.4.1027. [DOI] [PubMed] [Google Scholar]
- Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–736. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- Dickens EM, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon) Neurogastroenterol Motil. 1997;9:99–107. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Costa M, Furness JB. Gastrointestinal myoelectric activity in conscious guinea pigs. Am J Physiol. 1985;249:G92–99. doi: 10.1152/ajpgi.1985.249.1.G92. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Huseby E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141–161. doi: 10.1046/j.1365-2982.1999.00147.x. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Cayabyab FS, Vergara P, Daniel EE. Heterogeneity in electrical activity of the canine ileal circular muscle: interaction of two pacemakers. Neurogastroenterol Motil. 1996;8:339–349. doi: 10.1111/j.1365-2982.1996.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W/Wv mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996;271:G387–399. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- Marik F, Code CF. Control of the interdigestive myoelectric activity in dogs by the vagus nerves and pentagastrin. Gastroenterology. 1975;679:387–395. [PubMed] [Google Scholar]
- Marlett JA, Code CF. Effects of celiac and superior mesenteric ganglionectomy on interdigestive myoelectric complex in dogs. Am J Physiol. 1979;237:E432–436. doi: 10.1152/ajpendo.1979.237.5.E432. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijs VB, Verheem A, Van Duijvenbode-Beumer H, Visser MR, Verhoef J, Gooszen HG, Akkermans LM. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg. 1998;228:188–193. doi: 10.1097/00000658-199808000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AK, Bywater RA. Murine intestinal migrating motor complexes: longitudinal components. Neurogastroenterol Motil. 2003;15:245–256. doi: 10.1046/j.1365-2982.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y, Bueno L. Origin of migrating myoelectric complex in sheep. Am J Physiol. 1977;233:E483–487. doi: 10.1152/ajpendo.1977.233.6.E483. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sarna S, Stoddard C, Belbeck L, McWade D. Intrinsic nervous control of migrating myoelectric complexes. Am J Physiol. 1981;241:G16–23. doi: 10.1152/ajpgi.1981.241.1.G16. [DOI] [PubMed] [Google Scholar]
- Sarna SK. Cyclic motor activity; migrating motor complex. Gastroenterology. 1985;89:894–913. doi: 10.1016/0016-5085(85)90589-x. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed B, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am J Physiol. 1987;252:C215–224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol. 2001;1:604–610. doi: 10.1016/s1471-4892(01)00103-5. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR. Enteric nerve stimulation evokes a premature colonic migrating motor complex in mouse. Neurogastroenterol Motil. 2002;14:657–665. doi: 10.1046/j.1365-2982.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Bywater RAR, Taylor GS. Disinhibition during myoelectric complexes in mouse colon. J Auton Nerv Syst. 1998;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-Kit blocks development of Interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]


