Abstract
Evidence suggests that chromaffin cells employ separate mechanisms for evoked endocytosis and granule recycling when stimulated at basal (∼0.5 Hz) and stress-activated (∼15 Hz) rates. Previous studies have focused mainly on elucidating the cellular mechanisms responsible for membrane recycling under conditions similar to the stress-activated state and indicate a clathrin/dephosphin-mediated retrieval via coated pits. However, the mechanism for membrane internalisation at basal stimulus intensity remains largely unexplored. We electrically stimulated chromaffin cells in adrenal tissue slices at the sympathetic basal firing rate and measured cell capacitance in the perforated voltage clamp configuration. A new method for the separation of non-secretory from secretory cell capacitance signals is presented. Simultaneous catecholamine release was measured electrochemically to isolate the exocytic from endocytic components of the capacitance responses. Using this approach we demonstrate that firing patterns that mimic basal sympathetic input results in rapid and graded membrane retrieval. We show that block of the calcium-mediated protein phosphatase 2B, a common step in clathrin-mediated processes, did not alter endocytosis elicited at basal firing levels. We further blocked clathrin-mediated retrieval with a clathrin/dephosphin-disrupting peptide (PP-19) and found endocytosis to be blocked at 15 Hz stimulation but complete and indistinguishable from control cells at 0.5 Hz stimulation. Lastly, pharmacological treatments show that conventional isoforms of protein kinase C (cPKC) are required for the 0.5 Hz-evoked retrieval mechanism. From these data we conclude that unlike endocytosis evoked under stress conditions, basal firing activity results in a clathrin-independent rapid membrane retrieval mediated through conventional isoforms of PKC.
The splanchnic nerve forms a cholinergic chemical synapse on chromaffin cells of the adrenal medulla. Acetylcholine is released from the splanchnic nerve at a rate governed by sympathetic nervous system activity and excites the postsynaptic adrenal chromaffin cell. Upon stimulation, the chromaffin cells fire Na+-based action potentials that open voltage-gated Ca2+ channels and result in Ca2+-dependent fusion of large catecholamine-containing dense-core secretory granules with the plasma membrane. Under most instances chromaffin cell firing is dictated by the sympathetic tone, and they release catecholamine at a moderate rate (Brandt et al. 1976; Kidokoro, 1980; Wallace et al. 2002). Under this basal chromaffin cell firing state catecholamines affect multiple homeostatic functions that help define the ‘breed-and-feed’ condition, setting the organism for optimised energy storage and reproduction. In contrast, under stress conditions heightened sympathetic activity readies the organism for energy expenditure. This ‘fight-or-flight’ response increases chromaffin cell firing by up to 30-fold, causing a proportional increase in catecholamine output (Watkinson et al. 1990), which prepares the organism to meet higher metabolic demands associated with physical exertion during escape or defence. Thus, regulated and sustained catecholamine release from the chromaffin cells is a vital component of an adaptive mechanism that must respond to two disparate physiological demands depending on the environment.
Sustained catecholamine release under either the basal firing (‘breed-and-feed’) or stress-activated (‘fight-or-flight’) state requires continual endocytosis to maintain the cell surface area and to supply new releasable granules. Previous studies using cultured cells have provided a range of cellular mechanisms that result in exocytosis-coupled internalisation of surface membrane. In a kinetic context, there is an activity-enhanced process that accelerates with increased cell stimulation (Burgoyne, 1995; Smith & Neher, 1997; Engisch & Nowycky, 1998), ultimately resulting in a rapid excess endocytosis (Artalejo et al. 1995). These endocytic processes differ from a molecular and pharmacological perspective. One study provides evidence that under light intermittent stimulation, the endocytic process depends upon the GTP-ase dynamin but functions independently of clathrin (Artalejo et al. 2002). However, at moderate to high cell activity, membrane retrieval involves the protein phosphatase calcineurin (Artalejo et al. 1996; Engisch & Nowycky, 1998; Chan & Smith, 2001) as well as dynamin, a GTPase that has been shown to act in concert with clathrin to effect membrane retrieval in multiple systems (Marks & McMahon, 1998; Slepnev et al. 1998; Sever et al. 2000; Cousin & Robinson, 2001; Miwako et al. 2003). Thus, by kinetic, pharmacological, and molecular indicators, the cellular mechanisms for membrane retrieval at low and high stimulation rates are separate. Hypothetically, these discrepancies in endocytic behaviour in vitro may underlie corresponding differences in granule recycling mechanisms in situ under physiological stimulation. For example, membrane recycling events during low-intensity stimulation may mimic those during sustained homeostatic release of catecholamine in response to basal sympathetic activity. In this study, we tested this hypothesis by analysing membrane endocytosis in mouse adrenal chromaffin cells in situ under conditions that match the ‘breed-and-feed’ state. We minimised experimental perturbations of normal cell physiology by using an adrenal tissue slice preparation to preserve cyto-architecture, which plays a role in the regulation and segregation of endocytic processes in other cell types (Eker et al. 1994; Gad et al. 1998). Additionally, we recorded cell activity in the perforated patch voltage clamp configuration to preserve cytoplasmic content and to maintain nominally normal cell function. Our results demonstrate that basal sympathetic firing leads to an exocytosis-coupled endocytosis that internalises membrane through a rapid, clathrin-independent mechanism. This process depends upon the activation of conventional isoforms of protein kinase C. These data help define the cellular mechanism responsible for granule recycling under basal rates of exocytosis of the ‘breed-and-feed’ sympathetic state.
METHODS
Adrenal slice preparation
Adult C57/BL6 mice (4 to 8 weeks old) acquired from Jackson Laboratories (Bar Harbor, ME, USA) were used in this study. All anaesthesia and killing protocols were reviewed and approved by the institutional animal care and use committee (IACUC) of Case Western Reserve University, an accredited oversight body (Federal animal welfare assurance no. A3145-01). Animals were deeply anesthetised by halothane (Sigma, St Louis, MO, USA) inhalation and killed by decapitation. Adrenal glands were immediately removed and immersed in ice-cold, low calcium bicarbonate buffered saline (BBS) containing (mm): 140 NaCl, 2 KCl2, 0.1 CaCl2, 5 MgCl2, 26 NaHCO3, 10 glucose) that was gassed with 95 %O2-5 %CO2 (all chemicals were acquired from Fisher Scientific, Cleveland, OH, USA unless otherwise noted). Osmolarity was approximately 320 mosm l−1. Glands were trimmed of excess fat and embedded in 3 % low gelling point agarose (Sigma) that was prepared beforehand by melting agar in low calcium BBS at 110 °C followed by equilibration to 35 °C. The agarose was gelled on ice following embedding. The agarose block was trimmed to approximately 3 mm cubes that contained a single gland each and glued to the tissue stand of a vibrotome (WPI, Sarasota, FL, USA). The tissue stand was then placed in a slicing chamber filled with ice-cold, bubbled, low Ca2+ BBS. Adrenal glands were cut into 200 μm thick sections. The slices were collected and kept in the low calcium BBS first at 35 °C for 30 min, then at 25 °C until recording. Experiments were performed 2 to 8 h after slice preparation.
Electrophysiological recording
Tissue slices were constantly superfused with normal Ca2+ BBS during recording (adapted from Barbara et al. 1998) containing (mm): 140 NaCl, 2 KCl2, 3 CaCl2, 2 MgCl2, 26 NaHCO3, 10 glucose and gassed with 95 %O2-5 %CO2. The osmolarity was approximately 310 mosm l−1. Following stimulation in normal BBS, cells were washed with a Ca2+-block BBS and re-stimulated to isolate Ca2+-independent capacitance signals. The Ca2+-block BBS was of the following composition (mm): 140 NaCl, 2 KCl2, 0.5 CaCl2, 5 MgCl2, 0.2 CdCl2, 26 NaHCO3, 10 glucose and was gassed with 95 %O2-5 %CO2. The osmolarity was approximately 310 mosm l−1. The recording chamber volume was about 1.5 ml and the buffer flow was set at 1 ml min−1. Tissue slices were held in place by silver wires placed over the agar margin and visualised using an upright microscope (Olympus, Melville, NY, USA) equipped with a ×40 water-immersion objective.
For cell electrophysiology patch pipettes were pulled from borosilicate glass (4–5 MΩ). They were partially coated with molten dental wax and lightly polished using a microforge (Narishige, Tokyo, Japan). All recordings in this study were performed in the perforated patch configuration, unless specifically noted. The perforated patch pipette solution contained (mm); 135 caesium glutamate, 10 HEPES-H, 9.5 NaCl, 0.5 TEA-Cl and 0.53 amphotericin B. pH was adjusted to 7.3 and osmolarity was 310 mosm l−1. Amphotericin B was prepared as a 100× stock solution in DMSO (tissue culture-tested, Sigma) twice daily and diluted into internal pipette solution every 2 h. The internal solution was back-filled into patch pipettes without tip-dipping. For the whole-cell recording presented in Fig. 1A, the pipette solution contained (mm): 135 caesium glutamate, 10 Hepes-H, 9.5 NaCl, 0.5 TEA-Cl, 2 MgATP, 1 Lucifer Yellow, 0.002 NaGTP, and 0.001 EGTA. pH was adjusted to 7.3 and osmolarity was approximately 310 mosm l−1. The liquid junction potential between extracellular saline and the pipette internal solution was measured to be approximately −13 mV for the caesium glutamate-based solution, and all potentials reported are adjusted accordingly. Cells were chosen for patching based on their appearance and physical orientation within the slice. Preference was given to cells that exhibited a homogeneous cytosol (un-mottled) and a clean surface. An orientation was considered preferable if it left access for the carbon fibre on the capillary pole. Cells were perforated to a series resistance of no more than 30 M Ω (mean = 22.7 ± 3.18 MΩ, n = 68 and held at −83 mV. Leak currents were not corrected. Voltage clamp records were acquired through an EPC-9 (HEKA Elektronik, Lambrecht, Germany) amplifier under control of ‘Pulse’ software (v8.40; HEKA Elektronik). Cell capacitance (Cm) was estimated using a software lock-in module based on the Lindau-Neher technique (Gillis, 1995) implemented as the ‘Sine + D.C.’ mode. A 323 Hz, 25–35 mV peak amplitude sine wave was applied to the holding potential, and the reversal potential of the lock-in module was set to 0 mV. Membrane current was sampled at 20 kHz, and Cm was calculated at 323 Hz from the average value of 62 points per sine cycle. Only cells with less than −60 pA leak current were analysed for this study (leak current was −32.4 ± 5.68 pA; pre-stimulus cell capacitance = 9.27 ± 1.5 pF, n = 68 cells).
Figure 1. The rate of membrane retrieval is slowed by increased Ca2+ influx.

A, a chromaffin cell was patch clamped in the whole cell voltage clamp condition and the internal pipette solution contained 1 mm Lucifer Yellow. The image on the left shows the orientation of the cells as utilised in this study with the patch pipette visible on the left and the carbon fibre on the right. The cell was imaged for 45 min following whole cell rupture and no dye spreading was observed. Scale bars = 10 μm. B, the cell was voltage clamped in the perforated patch configuration and stimulated with action potential wave forms at 0.5 Hz to simulate basal sympathetic activity. The cell was stimulated with 100 action potentials in a normal BBS and then again in a Ca2+-block BBS that contained 200 μm Cd2+, elevated Mg2+ and lowed Ca2+ (see Materials sections for composition). The evoked currents in both solution conditions were signal-averaged with respect to the stimulus and are plotted. C, evoked cell capacitance was measured during the protocol and signal-averaged with respect to the stimulus. The averaged responses are plotted for the normal and Ca2+-block conditions. D, simultaneous catecholamine release was determined by carbon fibre amperometry, signal-averaged with respect to the stimulus and is plotted for each condition. The strong stimulus-coupled signal in the normal condition and lack of signal in Ca2+-block BBS confirm that no catecholamine exocytosis was elicited in conditions designed to block Ca2+ entry, thus indicating that capacitance signals in these conditions represent non-secretory events. E, the Ca2+-dependent capacitance component was resolved by subtracting the Ca2+-block response from the normal response and is plotted. The rate of membrane retrieval was determined by fitting the post-pulse capacitance response to a mono-exponential decay (grey trace). F, the protocol presented in panels B–E was repeated on 17 separate cells. Due to cell-to-cell variability the evoked Ca2+ influx spanned a range between 0.45 and 1.8 pC. Cells that exhibited higher levels of Ca2+ influx also showed the slowest rate of membrane internalisation. A linear fit to this trend indicates that the rate of endocytosis followed Ca2+ influx at a rate of −1.8 fF s−1 pC−1.
Peptide transfection protocol
The PP-19 peptide was synthesised by Sigma Genosys (The Woodlands, TX, USA) after a previously published sequence (Gad et al. 2000). Cells were transfected by using the Chariot (Active Motif, Carlsbad, CA, USA) protein transfection method. The transfection reaction was altered from the manufacture's suggested protocol to increase efficiency in the adrenal slice preparation (determined empirically). The reactions were prepared as follows: 2 ng Chariot reagent was dissolved in 50 μl distilled water, the peptide was prepared as a solution of 500 ng protein in 50 μl normal BBS. These solutions were mixed, sonicated and kept at room temperature for 30 min to allow the Chariot-peptide complex to form. The Chariot-peptide mixture was then diluted to a final volume of 5 ml, and bubbled with 95 %O2-5 %CO2. Slices were incubated in this mixture for at least 1 h prior to recording.
Electrochemical catecholamine detection
Commercially available carbon fibre electrodes (ALA Scientific, Longneck, NY, USA) of 5 μm tip diameter were used for amperometric catecholamine detection (Wightman et al. 1991). Carbon fibres were cut prior to each recording. A +650 mV potential was placed on the carbon fibre once it was in the bath, and the background current was allowed to relax to a steady value. If the resting current was larger than 15 pA or was unstable, the fibre was re-cut or replaced. Amperometric currents were recorded through a dedicated amplifier (VA-10, ALA Scientific). The head stages for the VA-10 and EPC-9 amplifiers shared a common Ag-AgCl bath ground. Also, to minimise cross-talk between them a 10 Ω resistor was placed into the ground wire of the VA-10 to separate the ground planes. During recordings the fibre was placed as close to the capillary pole of the patched cell as possible without physically distorting the cell. The fibre was placed by the capillary pole because it is here that the majority of the catecholamine is thought to be released by cells in situ (Carmichael, 1986). Consistent with this, we noted that signals were most robust when the fibre was placed directly against the capillary pole and were elusive when the fibre was placed at other regions. Oxidative amperometric currents, indicating catecholamine release, were passed through an analog 1 kHz Bessel filter and sampled at 20 kHz simultaneously to the capacitance measures in the Pulse software.
Voltage-clamped chromaffin cells were stimulated by trains of 100 simulated action potential waveforms. Simulated action potentials were composed of a 3-step ramp as follows (start potential (mV), end potential (mV), duration (ms)): −80, 50, 2.5; 50, −90, 2.5; −90, −80, 2.5. This wave form evoked Na+ and Ca2+ currents statistically identical to native action potentials (Chan & Smith, 2001) and are, therefore, considered equivalent. Gap-junction electrical coupling between rat chromaffin cells in situ has been reported under conditions of denervation (Martin et al. 2003). We tested for the potential electrical coupling in mouse cells by loading with the low molecular weight dye Lucifer Yellow and looking for dye spreading, a diagnostic tool used to indicate gap junctions between cells (Martin et al. 2001). In no instance did we observe dye spreading (Fig. 1A). We also looked for electrical coupling in slice recordings (Moser, 1998) and did not detect any. The apparent diversity in electrical coupling between the mouse system used in this study and the rat system studied by Martin and colleagues (2001, 2003) may be due to preparation and/orspecies differences. All experiments were carried out at 23–25 ° C, and data analysis was performed with IGOR Pro software (WaveMetrics Inc., Lake Oswego, OR, USA). Data are presented as means ± s.d.
RESULTS
Separation of secretory from non-secretory capacitance signals
We measured cell surface area as electrical cell capacitance. Changes in capacitance serve as an index of exocytic granule fusion and endosomal fission. However, in addition to measuring changes in cell surface area, capacitance measures are influenced by other electrical events. In excitable cells, depolarising electrical pulses evoke a transient capacitance signal that is non-secretory in nature; it does not reflect the addition or retrieval of secretory granule membrane, rather it represents the transient membrane current generated by the movement of voltage-sensitive ion channel gating charges. This signal has been termed ΔCmt and is quite modest and fast in bovine adrenal chromaffin cells (≈1 fF, τ = 16 ms) (Chow et al. 1996) but can be significantly larger and longer-lasting in rodent cells (≈30 fF, τ = 240 ms) (Horrigan & Bookman, 1993; Moser & Neher, 1997b). For this reason we developed a protocol to separate secretory from non-secretory capacitance signals; an example is provided in Fig. 1.
Comparing cell capacitance signals in the presence and absence of Ca2+ allows for the isolation of the Ca2+-dependent components. In order to accomplish this separation in this initial series of experiments the cell was stimulated in Ca2+-containing normal BBS as well as a ‘Ca2+-block’ BBS that contained lowered Ca2+, elevated Mg2+ and 200 μm CdCl2 in order to block all Ca2+ influx. A train of 100 action potential waveforms was delivered to the cell at 0.5 Hz, and membrane capacitance and amperometric currents were measured and signal averaged in each condition with respect to the stimulus (Fig. 1C). Only one Ca2+/Cd2+ paired protocol was performed on each cell, in that slices did not recover fully from Cd2+ treatment. Calcium currents were measured and found to be nearly totally abolished in the Ca2+-block BBS n = 17; mean = 558 ± 251 pA in control versus 15.8 ± 10.5 pA in Ca2+-block BBS (Fig. 1B)). Average sodium currents across 17 cells were unchanged in normal and Ca2+-block BBS (peak currents of 1.46 ± 0.48 nA and 1.34 ± 0.52 nA, respectively) indicating that the Ca2+-block BBS was specific for Ca2+ influx and did not inhibit general cell excitability. Additionally, evoked capacitance jumps were always larger in the Ca2+-containing BBS than in the Ca2+-block solution (Fig. 1C) as would be expected if it were simply eliminating a Ca2+-dependent component. Lastly, the Ca2+-independent capacitance response was similar in both magnitude and kinetics to that previously published in mouse chromaffin cells as measured under multiple stimulus protocols (Moser & Neher, 1997b).
In addition to measuring membrane currents and cell capacitance, we confirmed that the Ca2+-block BBS eliminated Ca2+-dependent processes. Amperometric recordings were also signal-averaged with respect to the stimulus and show that catecholamine was released in a highly synchronised manner in the control condition but was absent in the Ca2+-block BBS (Fig. 1D). This confirms that the Ca2+-blocking solutions eliminated granule fusion and catecholamine exocytosis. The subtraction of the Ca2+-block capacitance record from the control BBS capacitance trace furnishes the evoked Ca2+-dependent component of the capacitance signal. An example of a Ca2+-dependent capacitance response is presented in Fig. 1E (peak post-pulse Cm = 12.85 fF). The average response measured over 17 cells was 9.72 ± 3.26 fF. Single granule capacitance has been quantified in isolated mouse cells and averages 1.3 fF per quantum (Moser & Neher, 1997a). From these data we predict that the average quantal content on average represents the release of 6.7 granules in situ. This value is lower than previously reported in mouse slices (Moser & Neher, 1997b) and may reflect differences in basal Ca2+ and post-stimulus Ca2+ clearance in the perforated patch configuration, both of which affect secretory output (Smith et al. 1998).
We conclude that serial stimulus trains delivered in normal BBS followed by Ca2+-block BBS enables the isolation of Ca2+-dependent capacitance changes. Given the high temporal correlation between stimulus and amperometric current and that the amperometric current is of brief duration, we also conclude that the post-pulse capacitance record provides an accurate measure of endocytic membrane retrieval. From these data we determined the rate of membrane retrieval from a mono-exponential fit to the post-stimulus capacitance decay (Fig. 1E). Endocytosis has been shown to be regulated by intracellular Ca2+ in many systems. One of the best characterised roles for Ca2+ is in the activation of calmodulin leading to an up-regulation in the activity of the protein phosphatase calcineurin. Calcineurin, in turn, activates the dephosphins and initiates clathrin-dependent membrane retrieval (De Camilli et al. 1995; Cousin & Robinson, 2001; Takei & Haucke, 2001). This mechanism exhibits a positive dependence on cytosolic Ca2+, and thus on cell activity. We quantified the rate of membrane internalisation as a function of stimulus strength in our records. Due to an unfavourable signal-to-noise ratio we were not able to analyse this function across multiple stimuli within a record but rather had to compare averaged signals between cells that exhibited varied Ca2+ influx. Calcium currents were integrated to quantify stimulus strength and ranged by a factor of approximately 4 across 17 experiments due to cell-to-cell variability. Plotting the rate of membrane retrieval against the evoked Ca2+ influx revealed a strong negative dependence (Fig. 1F).
Endocytic activity at basal firing rates is not sensitive to calcineurin blockers
The negative relationship between the rate of membrane retrieval and Ca2+ influx shown in Fig. 1 is inconsistent with a mechanism that includes a limiting Ca2+-dependent step such as activation of calcineurin. We wanted to test whether endocytosis at basal firing rates exhibited a sensitivity to calcineurin blockers as has been found under stronger stimulation in chromaffin (Artalejo et al. 1995; Engisch & Nowycky, 1998; Chan & Smith, 2001) as well as other cell types (Marks & McMahon, 1998; Slepnev et al. 1998; Lai et al. 2000; Cousin & Robinson, 2001; Takei & Haucke, 2001). Adrenal slices were pre-treated with 1 μm cyclosporin A (Calbiochem, San Diego, CA, USA), a common calcineurin-blocking agent, for 10 min after patching and prior to stimulation. We have previously shown that this treatment effectively blocks endocytosis under 15 Hz stimulation in isolated chromaffin cells (Chan & Smith, 2001). Cyclosporin A-treated cells were subjected to the same serial stimulus protocol presented in Fig. 1, first in Ca2+-containing BBS and then in Ca2+-block BBS (Fig. 2A). We observed a decreased Ca2+ influx following cyclosporin A treatment (0.98 ± 0.33 pC, n = 10versus 1.17 ± 0.38 pC in 17 control cells). Although not statistically significant, this trend is consistent with previous studies in which isolated bovine cells were pre-treated with cyclosporin A (Engisch & Nowycky, 1998). As in controls, a robust capacitance increase was evident following cyclosporin A treatment (Fig. 2A). Additionally, as in the control case, highly synchronous Ca2+-dependent amperometric currents were observed (Fig. 2B). Calcium-dependent exocytosis of approximately the same magnitude as in controls was also measured (Fig. 2C). Evoked exocytosis was followed by a rapid internalisation of membrane that was equal in size to that added by the stimulus. Quantification of the rate of retrieval in cyclosporin A revealed an identical kinetic and stimulus-dependent modulation as compared to control conditions (Fig. 2D). These results indicate that, in chromaffin cells, there are no cyclosporin A-sensitive steps in endocytosis at basal sympathetic activity.
Figure 2. Cyclosporin A does not block evoked membrane retrieval.
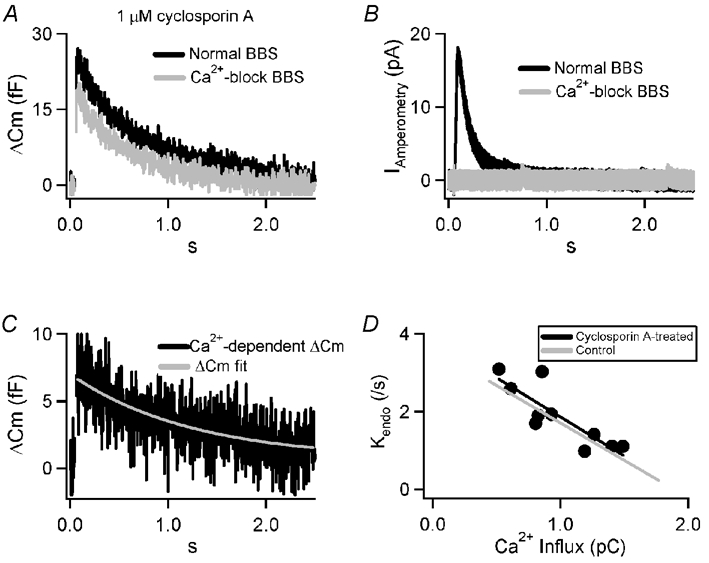
A, a protocol identical to that pictured in Fig. 1 was repeated on a cell pre-treated for 10 min with 1 μm cyclosporin A. The averaged capacitance shifts are plotted for the normal (black) and Ca2+-block (grey) conditions. B, amperometric currents measured from the same cell as in A show a highly synchronous catecholamine release in normal BBS. A record from the same in Ca2+ current-blocking BBS shows no catecholamine release. C, the Ca2+-dependent secretory component of the evoked capacitance jump in panel A is plotted along with the best-fit mono-exponential decay (grey). D, the same protocol as in panels A–C was repeated in 10 cells that were pre-treated with cyclosporin A and the Ca2+-dependent capacitance response was fitted with a mono-exponential decay (black line). For comparison, the same analysis from untreated cells (Fig. 1E) is plotted (grey line).
Endocytosis at basal firing rates does not proceed through a clathrin/dephosphin-mediated mechanism
The preceding data suggest that endocytosis during the basal firing rate in chromaffin cells is not affected by blockers of calcineurin, a critical step in clathrin-mediated processes (Cousin & Robinson, 2001). To further test this possibility we directly perturbed critical steps of the clathrin/dephosphinpathway. We utilised the acute peptide transfection system Chariot (Active Motif) to introduce specific blocking peptides into cells in the tissue slices. Chariot is a 21 amino acid peptide that forms complexes with other peptides or macro-molecules and is actively taken up into cells where it dissociates and releases its cargo (Morris et al. 2001). We used the Chariot system to introduce a peptide (PP-19) that has been shown to block clathrin-mediated endocytosis by disrupting dephosphin function (Gad et al. 2000). We then measured exocytic and endocytic activity in transfected cells stimulated at basal firing rates (0.5 Hz; Fig. 3B) and at stress-activated firing rates (15 Hz; Fig. 3A). Endocytosis is expected to occur via a clathrin-mediated mechanism in the stress-activated condition and thus 15 Hz stimulation serves as a positive control for PP-19 action. Both Ca2+ influx and quantal content were found to be equivalent in control and PP-19 transfected cells at each frequency (Table 1). Total endocytosis over the stimulus train was calculated as the difference between exocytosis (quantal content × number of stimuli) minus the final ΔCm (the sum of evoked exocytosis plus endocytosis). In order to correct for differing exocytic rates between frequencies, endocytic efficiency was calculated as the ratio of endocytosis to exocytosis. These data are summarised in Table 1. Transfection with PP-19 and stimulation at 15 Hz resulted in an equivalent quantal content as compared to control, but a significantly increased ΔCm over control. This was not the case under 0.5 Hz stimulation; quantal content and ΔCm were equivalent in control and PP-19 transfected cells. These data show that endocytosis evoked at basal firing rates is not affected by PP-19 transfection and is unlikely to proceed via a clathrin/dephosphin-mediated mechanism. Cell stimulation at stress-activated rates evoked an endocytosis that was nearly completely abolished by PP-19 transfection, as expected of a clathrin/dephosphin-dependent process.
Figure 3. PP-19 blocks stress-associated but not basal-firing endocytosis.

A, chromaffin cells were stimulated with action potentials at 15 Hz and cell capacitance was measured. Cells transfected with the PP-19 peptide exhibited a much larger net capacitance increase during the recording than did control cells. The quantal content (exocytosis) was not significantly altered from control (see Table 1), thus the divergence of the ΔCm traces indicates that PP-19 treatment inhibits endocytosis. B, control and PP-19 transfected cells stimulated at 0.5 Hz resulted in nearly identical measured exocytosis (Table 1) as well as ΔCm traces indicating no effect on evoked endocytosis.
Table 1.
PP-19 disrupts stress-evoked but not basal-firing endocytosis.
| Evoked ICa | Rate of granule fusion | ΔCmAP−1 | Endocytic efficiency | |
|---|---|---|---|---|
| (pA) | (fF s−1) | (fF) | ||
| 15 Hz Control (n = 5) | 315 ± 91.1 | 38.55 ± 7.35 | 2.57 ± 0.49 | 0.81 ± 0.01 |
| 15 Hz PP-19 (n = 7) | 292 ± 55.5 | 33.75 ± 7.20 | 2.25 ± 0.48 | 0.33 ± 0.08 |
| 0.5 Hz Control (n = 17) | 572 ± 29.8 | 4.86 ± 1.63 | 9.72 ± 3.26 | 0.84 ± 0.18 |
| 0.5 Hz PP-19 (n = 8) | 590 ± 92.4 | 4.58 ± 0.56 | 9.15 ± 1.12 | 0.77 ± 0.26 |
AP, action potential. Evoked ICa is lower at 15 Hz than at 0.5 Hz presumably due to activity dependent inhibition of voltage-gated Ca2+ channels (Currie & Fox, 1996, 1997, 2000)
Endocytosis at basal firing is dependent upon conventional isoforms of PKC
We have demonstrated that basal-firing endocytosis does not depend on the action of the calcium-dependent protein phosphatase calcineurin and does not depend on clathrin-mediated processes. In order to better characterise the cellular mechanism of endocytosis at basal activity levels, we tested the ability of other cellular perturbations to block membrane retrieval in chromaffin cells. Protein phosphorylation/dephosphorylationcycles represent common regulatory steps in membrane retrieval and intracellular cycling (Turner et al. 1999). Examples in chromaffin cells include the actions of both tyrosine kinases (Nucifora & Fox, 1999) and protein kinase C (Graham et al. 2002), as well as adaptor associated kinase (AAK1) in neuronal cells (Conner & Schmid, 2002). Utilising the same protocol as outlined in Fig. 1 we probed known kinase/phosphatasepaths that may represent molecular steps through which endocytosis occurs. Preliminary experiments with the broad-spectrum kinase inhibitor, staurosporine (40 nm) did not affect capacitance increases or amperometric exocytic signals (Fig. 4A and B) but did abolish post-stimulus membrane retrieval (Fig. 4C) (see also Henkel et al. 2000). At 40 nm, staurosporine blocks CaM kinase (IC50 = 20 nm), myosin light chain kinase (IC50 = 1.3 nm), protein kinase A (IC50 = 7 nm), protein kinase C (IC50 = 0.7 nm), and protein kinase G (IC50 = 8.5 nm), some of which have already been shown to play a role in regulated endocytosis in other systems (Eker et al. 1994; Gekle et al. 1997). Utilising more targeted inhibitors of each of these kinase families (Fig. 5A), we found that that only Gö 6976 and Ro-31-8220 (Calbiochem) blocked endocytosis as was observed with staurosporine treatment. Endocytic activities under the different pharmacological conditions are quantified and summarised in Fig. 5B. This array of kinase inhibitors eliminates all the potential staurosporine targets (at 40 nm) except conventional isoforms of protein kinase C (cPKC). To further investigate the role of PKC we also tested whether phorbol ester activation would enhance endocytic activity. Pre-treatment of cells with 100 nm phorbol-12-myristate-13-acetate (PMA) is expected to maximally activate PKC in chromaffin cells (Gillis et al. 1996; Smith et al. 1998). However our data show that PMA treatment did not affect endocytic membrane retrieval during basal-rate stimulation (Fig. 5). This may be the result of a limited potential for further PKC activation. Stimulation at 0.5 Hz is expected to raise intracellular calcium to approximately 600 nm immediately beneath the plasma membrane (Chan et al. 2003), a concentration shown to activate PKC in chromaffin cells (Smith et al. 1998; Smith, 1999). We turned to the measured quantal content to determine whether this may be the case. PMA treatment has been shown to facilitate chromaffin cell exocytosis by nearly a factor of 2 by increasing the rate at which granules are recruited to the releasable state (Gillis et al. 1996; Smith et al. 1998). If PKC were activated in the control condition then PMA treatment should not be able to further increase the quantal content. Indeed the measured quantal content after PMA treatment did not significantly differ from control levels (11.8 ± 2.01 fF in PMA versus 9.72 ± 3.26 fF in control) indicating that PKC may already have been activated.
Figure 4. Staurosporine pre-treatment abolishes evoked endocytosis.

A, a protocol identical to that introduced in Fig. 1 was repeated on cells pre-treated with 40 nm staurosporine for 10 min prior to recording. The averaged capacitance shifts are plotted for the control (black) and Ca2+-block (grey) conditions. B, amperometric currents measured from the same cell as in A show evoked catecholamine release in normal BBS but none in the Ca2+-block condition again indicating isolation of Ca2+-dependent from Ca2+-independent capacitance signals. C, the Ca2+-dependent secretory component of the evoked capacitance jump in panel A is plotted and reveals no post-pulse decay, indicating no membrane internalisation took place.
Figure 5. Endocytosis elicited at basal firing rates is dependent on conventional isoforms of PKC.

A, in order to isolate the substrate of the staurosporine, the same protocol as introduced in Fig. 1 was repeated on cells in the presence of a variety of protein kinase/phosphatase inhibitors as well as PMA, a phorbol ester that activates PKCs. KT 5720 blocks protein kinase A, Gö 6976 inhibits PKC, PMA activates PKC and Ro-31-8220 blocks conventional isoforms of PKC. B, endocytosis was calculated with respect to exocytosis and is supplied for each pharmacological condition. Asterisks mark data sets that were determined to be significantly different from the control condition. Significance was assessed by a student's t test with P < 0.02. Numbers of cells are as follows: control = 19, cyclosporin A = 10, PMA = 5, KT 5720 = 6, Gö 6976 = 5 and Ro-31-8220 = 6.
DISCUSSION
Using an adrenal slice chromaffin cell preparation, we have demonstrated that the mechanism of membrane cycling under low frequency stimulation is highly regulated by the magnitude of Ca2+ influx. We also show that this mechanism is not sensitive to cyclosporin A and does not include a clathrin/dephosphin-dependent mechanism. We also identify a necessary role for conventional isoforms of protein kinase C in endocytosis elicited under basal firing rates. We consider the significance of these findings.
Calcium influx initiates the exocytic process, evoking membrane fusion and leading to catecholamine release. Thus Ca2+ is present at the appropriate time and in a stimulus-dependent concentration, and may be a regulatory signal for the endocytic process. For this reason it has been postulated that Ca2+ represents a critical and necessary trigger for recycling of secretory membrane. Indeed careful biochemical characterisation strongly supports a Ca2+-dependent activation of membrane retrieval through the calcium/calmodulin-dependent activation of calcineurin. Calcineurin in turn activates the dephosphins, a class of molecules that are involved in membrane retrieval (Cousin & Robinson, 2001), and leads to the clathrin-mediated internalisation of surface membrane. This path is positively regulated by cytosolic Ca2+ and is, as such, positively regulated by cell activity (Marks & McMahon, 1998; Lai et al. 2000). However, several studies have also demonstrated that endocytic activity may diminish in response to elevated cell firing or stimulus strength. For example, post-fusion membrane recycling in rat pituitary peptidergic nerve terminals is slowed with increased stimuli (Hsu & Jackson, 1996). The same scenario is found in retinal bipolar terminals of the goldfish (von Gersdorff & Matthews, 1994) where extended exocytosis strands membrane that must eventually be compensated via a dramatic ‘avalanche’ retrieval after prolonged secretion (von Gersdorff & Matthews, 1999). Others have shown that Sr2+ and Ba2+ are also capable of supporting endocytosis (Nucifora & Fox, 1998; Neves et al. 2001) although they are extremely inefficient at activating calmodulin/calcineurinactivity (Gu & Cooper, 2000). Thus, identifying the calcium-dependency of endocytosis at basal firing rates allows for an initial identification or elimination of some of the potential cellular mechanisms that are responsible for membrane cycling in chromaffin cells. We show in this study a clear Ca2+-dependent decrease in the kinetics of membrane retrieval at 0.5 Hz stimulation. We also show that this retrieval is insensitive to transfection with PP-19 peptide, a blocker of clathrin/dephosphin-mediated endocytosis (Fig. 3A; Gad et al. 2000). Thus it seems that Ca2+ sensitivity may represent a fundamental diagnostic tool for determining the mode and mechanism of exocytosis-coupled endocytosis. Calcium-dependent facilitation of membrane retrieval may indicate clathrin-mediated processes while a Ca2+-dependent decrease, or even lack of Ca2+ sensitivity (see Wu & Betz, 1996) may implicate clathrin-independent processes.
What then is the cellular mechanism for basal-activity endocytosis in chromaffin cells? A recent study performed in calf adrenal chromaffin cells supplies biochemical and physiological data indicating that early in a stimulus train, and thus at relatively low Ca2+ concentrations, membrane endocytosis is achieved via a dynamin-dependent but clathrin-independent mechanism. Only later in a train does endocytosis become sensitive to blockers of clathrin and dynamin II (Artalejo et al. 2002). Our data are consistent with this scenario in that we failed to detect any characteristics of the calcineurin/clathrin/dephosphin-mediated pathway under basal stimulation. Furthermore, the study by Artalejo et al. (2002) is consistent with a previous study showing that conditions matching stress-activated stimulation evoke a calcium-activated, calcineurin-dependent process (Chan & Smith, 2001), and may be similar to the parallel paths found in the neuromuscular junction (Heuser & Reese, 1973; Ceccarelli & Hurlbut, 1980; Koenig & Ikeda, 1996; Richards et al. 2000). Thus, taken together, these studies begin to form a scenario where the predominant endocytic mechanism under the basal sympathetic tone is not achieved through clathrin/dephosphin-mediated processes and may be of the dynamin-dependent ‘kiss and run’ variety (as defined by Ryan, 2003). One might predict that this cellular process is triggered by exocytosis and is modulated by calcium, perhaps through conventional isoforms of PKC (Figs. 4 and 5 of this study but see also Graham et al. 2002). However, calcium alone can not be the initial trigger for endocytosis; if it were then it seems that sub-micromolar elevations in calcium would evoke endocytosis even in the absence of exocytosis. Further investigation will be required to fully elucidate the trigger for clathrin-independent endocytosis.
Acknowledgments
We would like to thank Drs Lynn Landmesser and Luis Polo-Parada for valuable discussions and Drs Dean Smith and Iain Robinson and Stephen Radabaugh for their helpful comments in the preparation of this manuscript. This work was supported by grants from the Alfred P. Sloan foundation (CS) and the National Science Foundation (CS; IBN-0196136).
REFERENCES
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci U S A. 2002;99:6358–6363. doi: 10.1073/pnas.082658499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG, Lemos VS, Takeda K. Pre- and post-synaptic muscarinic receptors in thin slices of rat adrenal gland. Eur J Neurosci. 1998;10:3535–3545. doi: 10.1046/j.1460-9568.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- Brandt B, Hagiwara S, Kidokoro Y, Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976;263:417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD. Fast exocytosis and endocytosis triggered by depolarisation in single adrenal chromaffin cells before rapid Ca2+ current run-down. Pflugers Arch. 1995;430:213–219. doi: 10.1007/BF00374652. [DOI] [PubMed] [Google Scholar]
- Carmichael SW. Morphology and innervation of the adrenal medulla. In: Lelkes P, editor. Stimulus-Secretion Coupling. Vol. 1. Boca Raton, Florida, USA: Blackwell Science Inc; 1986. pp. 40–49. [Google Scholar]
- Ceccarelli B, Hurlbut WP. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980;60:396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- Chan SA, Chow R, Smith C. Calcium dependence of action potential-induced endocytosis in chromaffin cells. Pflugers Arch. 2003;445:540–546. doi: 10.1007/s00424-002-0966-y. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RH, Klingauf J, Heinemann C, Zucker RS, Neher E. Mechanisms determining the time course of secretion in neuroendocrine cells. Neuron. 1996;16:369–376. doi: 10.1016/s0896-6273(00)80054-9. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156:921–929. doi: 10.1083/jcb.200108123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Currie KP, Fox AP. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron. 1996;16:1027–1036. doi: 10.1016/s0896-6273(00)80126-9. [DOI] [PubMed] [Google Scholar]
- Currie KP, Fox AP. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J Neurosci. 1997;17:4570–4579. doi: 10.1523/JNEUROSCI.17-12-04570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Fox AP. Voltage-dependent, pertussis toxin insensitive inhibition of calcium currents by histamine in bovine adrenal chromaffin cells. J Neurophysiol. 2000;83:1435–1442. doi: 10.1152/jn.2000.83.3.1435. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Takei K, McPherson PS. The function of dynamin in endocytosis. Curr Opin Neurobiol. 1995;5:559–565. doi: 10.1016/0959-4388(95)80059-x. [DOI] [PubMed] [Google Scholar]
- Eker P, Holm PK, Van Deurs B, Sandvig K. Selective regulation of apical endocytosis in polarized Madin-Darby canine kidney cells by mastoparan and cAMP. J Biol Chem. 1994;269:18607–18615. [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J Physiol. 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, Brodin L. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Schwerdt G, Silbernagl S. Albumin endocytosis in OK cells: dependence on actin and microtubules and regulation by protein kinases. Am J Physiol. 1997;272:F668–677. doi: 10.1152/ajprenal.1997.272.5.F668. [DOI] [PubMed] [Google Scholar]
- Gillis KD. Techniques for membrane capacitance measurements. In: Neher E, editor. Single-Channel Recording. 2. New York: Blackwell Science Inc; 1995. pp. 155–198. chap. 7. [Google Scholar]
- Gillis KD, Mossner R, Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996;16:1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci U S A. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Cooper DM. Ca(2+), Sr(2+), and Ba(2+) identify distinct regulatory sites on adenylyl cyclase (AC) types VI and VIII and consolidate the apposition of capacitative cation entry channels and Ca(2+)-sensitive ACs. J Biol Chem. 2000;275:6980–6986. doi: 10.1074/jbc.275.10.6980. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W. Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. Embo J. 2000;19:84–93. doi: 10.1093/emboj/19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Bookman RJ. Na channel gating charge movement is responsible for the transient capacitance increase evoked by depolarization in rat adrenal chromaffin cells. Biophys J. 1993;64:101A. [Google Scholar]
- Hsu SF, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J Physiol. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Role of action potentials in hormone secretion. Biochem Res. 1980;1:117–123. [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MM, Luo HR, Burnett PE, Hong JJ, Snyder SH. The calcineurin-binding protein cain is a negative regulator of synaptic vesicle endocytosis. J Biol Chem. 2000;275:34017–34020. doi: 10.1074/jbc.C000429200. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Chevillard C, Guerineau NC. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: a role in catecholamine release. J Neurosci. 2001;21:5397–5405. doi: 10.1523/JNEUROSCI.21-15-05397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO, Mathieu MN, Guerineau NC. Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J Neurosci. 2003;23:3669–3678. doi: 10.1523/JNEUROSCI.23-09-03669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwako I, Schroter T, Schmid SL. Clathrin- and dynamin-dependent coated vesicle formation from isolated plasma membranes. Traffic. 2003;4:376–389. [PubMed] [Google Scholar]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- Moser T. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol. 1998;506:195–205. doi: 10.1111/j.1469-7793.1998.195bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Neher E. Estimation of mean exocytic vesicle capacitance in mouse adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1997a;94:6735–6740. doi: 10.1073/pnas.94.13.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Neher E. Rapid exocytosis in single chromaffin cells recorded from mouse adrenal slices. J Neurosci. 1997b;17:2314–2323. doi: 10.1523/JNEUROSCI.17-07-02314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Neef A, Lagnado L. The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J Physiol. 2001;535:809–824. doi: 10.1111/j.1469-7793.2001.t01-1-00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora PG, Fox AP. Barium triggers rapid endocytosis in calf adrenal chromaffin cells. J Physiol. 1998;508:483–494. doi: 10.1111/j.1469-7793.1998.483bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora PG, Fox AP. Tyrosine phosphorylation regulates rapid endocytosis in adrenal chromaffin cells. J Neurosci. 1999;19:9739–9746. doi: 10.1523/JNEUROSCI.19-22-09739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Ryan TA. Kiss-and-run, fuse-pinch-and-linger, fuse-and-collapse: the life and times of a neurosecretory granule. Proc Natl Acad Sci U S A. 2003;100:2171–2173. doi: 10.1073/pnas.0530260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever S, Damke H, Schmid SL. Dynamin: GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis. J Cell Biol. 2000;150:1137–1148. doi: 10.1083/jcb.150.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepnev VI, Ochoa GC, Butler MH, Grabs D, Camilli PD. Role of phosphorylation in regulation of the assembly of endocytic coat complexes. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- Smith C. A persistent activity-dependent facilitation in chromaffin cells is caused by Ca2+ activation of protein kinase C. J Neurosci. 1999;19:589–598. doi: 10.1523/JNEUROSCI.19-02-00589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Haucke V. Clathrin-mediated endocytosis: membrane factors pull the trigger. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- Turner KM, Burgoyne RD, Morgan A. Protein phosphorylation and the regulation of synaptic membrane traffic. Trends Neurosci. 1999;22:459–464. doi: 10.1016/s0166-2236(99)01436-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Electrophysiology of synaptic vesicle cycling. Annu Rev Physiol. 1999;61:725–752. doi: 10.1146/annurev.physiol.61.1.725. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Chen C, Marley PD. Histamine promotes excitability in bovine adrenal chromaffin cells by inhibiting an M-current. J Physiol. 2002;540:921–939. doi: 10.1113/jphysiol.2001.013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson A, O'Sullivan AJ, Burgoyne RD, Dockray GJ. Differential accumulation of catecholamines, proenkephalin- and chromogranin A-derived peptides in the medium after chronic nicotine stimulation of cultured bovine adrenal chromaffin cells. Peptides. 1990;11:435–441. doi: 10.1016/0196-9781(90)90039-8. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Jankowski JA, Kennedy RT, Kawagoe KT, Schroeder TJ, Leszczyszyn DJ, Near JA, Diliberto EJ, Viveros OH. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc Natl Acad Sci U S A. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]


