Abstract
Over the last five years, rapid advances have been made in our understanding of the location, function, and recently, organization of the central pattern generator (CPG) for locomotion. In the mammal, the use of the neonatal rat has largely contributed to these advances. Additionally, the use of the in vitro mouse spinal cord preparation is becoming more common, catalysed in part by the potential for the use of genetic approaches to study locomotor function. Although tempting, it is necessary to resist the a priori assumption that the organization of the spinal CPG is identical in the rat and mouse. This review will describe the development of locomotor-like behaviour in the mouse from embryonic day 12 to postnatal day 14. While there are still many gaps in our knowledge, compared with the rat, the in vitro mouse appears to follow a qualitatively similar course of locomotor development. The emphasis in this review is the use or potential use of the mouse as a complement to existing data using the neonatal rat preparation.
As early as 1911 it was recognized that the basic pattern of stepping could be generated by the spinal cord without the need for descending connections (Graham-Brown, 1911). Since that time, numerous studies have shown that the spinal cord contains sufficient circuitry to produce a rather sophisticated pattern of muscle activation (Barbeau & Rossignol, 1987; Edgerton et al. 1992; Rossignol, 1996; Dimitrijevic et al. 1998; Fedirchuk et al. 1998). The term central pattern generator (CPG) has been coined to describe the intrinsic spinal circuits that can generate rhythmic motor output.
Our knowledge of spinal CPG function in vertebrates is largely derived from work using the lamprey and Xenopus (Grillner et al. 1998; Roberts et al. 1998). These animals have relatively simple locomotor behaviours and small numbers of spinal neurons. Progress in understanding mammalian terrestrial locomotion has been more limited. Locomotion in mammals takes many different forms, such as walking, hopping, and running, requiring a more complex CPG. Commensurate with the variety of behaviours, there are greater numbers of neurons in the mammalian spinal cord. The main problem is ascribing a functional role to interneurons that are rhythmically active. Identification of neurons that form functional classes is difficult and typically involves stimulation of discrete groups of afferent fibres, along with intracellular recordings from interneurons or motoneurons (Jankowska, 1992; McCrea, 2001).
This problem has become more tractable with the development of in vitro neonatal rodent preparations of the spinal cord that allow more tools to be used in probing neuronal circuitry (Smith & Feldman 1985, 1987). First, the use of whole-cell patch-clamp techniques is greatly facilitated by access to the spinal cord. Second, pharmacology of receptor function is facilitated, since drugs do not need to cross a blood-brain barrier. Third, imaging of spinal cord networks can be accomplished using voltage- and calcium-sensitive dyes (Arai et al. 2002; Bonnot et al. 2002b; Nakayama et al. 2002). Finally, the use of split bath recording chambers allows drugs to be applied independently to two or more locations of the spinal cord (Cazalets et al. 1995; Kjaerulff & Kiehn, 1997), facilitating the exploration of the role of different regions of the spinal cord in generating rhythmic activity.
The in vitro mouse spinal cord preparation has emerged over the last few years as a complement to the existing rat model. The mouse has several advantages, including access to genetic tools, and smaller dimensions, which allows for greater in vitro spinal tissue viability across a wider range of ages. With these advantages in mind, the aim of this review is to examine the development of locomotor-like activity in the mouse across three stages of development (embryonic (E) days E12–18, postnatal (P) days P0–4, and late neonatal days P7–14).
Development of co-ordinated rhythmic patterns in embryonic rodents (E12–18)
During the last week of embryonic development, networks that produce behaviours such as locomotion start to produce rhythmic activity. By birth, the networks that produce patterned locomotor output have matured to the point that the output is similar to that observed in adults (see Fig. 3D). The study of the maturation of these circuits is of considerable interest, since it offers an opportunity to follow the evolution of a relatively simple network with a restricted repertoire of outputs, to the adult spinal network that must cope with the expression of multiple locomotor strategies.
Figure 3. Locomotor-like patterns produced by early neonatal mice.

A, schematic of an isolated spinal cord mouse preparation illustrating the recording arrangement used to record locomotor-like activity. B, locomotor-like bursting pattern recorded from the L2 and L5 ventral roots following bath application of α-methyl-5-HT (4 μm). C, schematic showing the recording arrangement from an in vitro leg attached mouse preparation. Electroneurogram recordings were obtained from the common peroneal (CP) and lateral gastrocnemius/soleus(LGS) nerve. D, top panel, flexor/extensorand left/rightalternating pattern recorded from the LGS and CP nerves following bath application of 5-HT/dopamine and NMA. Data modified from Whelan et al. (2000) with permission; bottom panel, EMG data recorded from the tibialis anterior (TA) and medial gastrocnemius (MG) muscles of an adult mouse walking on a treadmill. Data modified from Fortier et al. (1987) with permission.
Spontaneous activity
Spontaneous activity has been observed in many areas of the brain including the hippocampus (Ben Ari, 2001), spinal cord (O'Donovan, 1999), and retina (Penn & Shatz, 1999) and appears to be a characteristic of developing networks. In both the rat and mouse spinal cord preparation, spontaneous rhythmic activity can be observed early in embryogenesis (Nishimaru et al. 1996; Suzue, 1996; Branchereau et al. 2002; Hanson & Landmesser, 2003; Ren & Greer, 2003), before the innervation of muscles is complete (days E11-14). This early spontaneous activity differs from alternating locomotor-like activity in at least three respects: (1) early spontaneous activity is composed of episodic periods of bursts that are synchronized across the rostrocaudal extent of the spinal cord (Fig. 1B); (2) glutamate is not involved in generating spontaneous activity; rather, excitatory drive is supplied by GABAergic, glycinergic and cholinergic transmission (Nishimaru et al. 1996; Hanson & Landmesser, 2003; Ren & Greer, 2003); (3) chemical and electrical transmission are critical for generating spontaneous activity at early embryonic ages (E12.5) (Hanson & Landmesser, 2003), whereas in neonatal rodents chemical transmission appears to predominate (cf. Tresch & Kiehn, 2000).
Figure 1. Spontaneous network activity recorded from E12–14 mice.
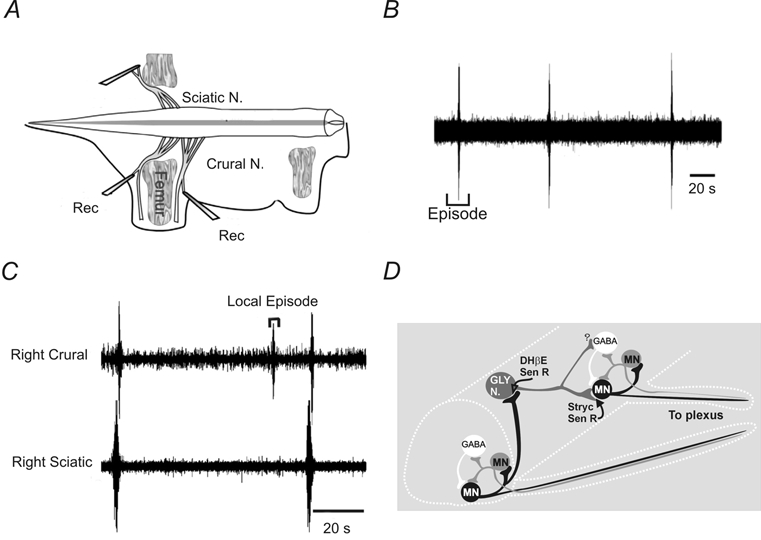
A, suction electrodes were placed on embryonic mouse motor nerves in an isolated spinal cord-hindlimb preparation. B, spontaneous activity consists of episodic synchronous bursts of activity separated by several minutes. C, on occasion, localized bursts could occur that were not propagated to different muscle nerves. D, circuit diagram showing the postulated local circuit consisting of GABAergic interneurons and motoneurons. Motoneurons contribute to the onset and reinforcement of network activity via excitatory recurrent collaterals onto motoneurons and interneurons in the network. A separate glycinergic circuit is responsible for the propagation of the bursts. Data adapted from Hanson & Landmesser (2003) with permission.
A circuit for spontaneous rhythm generation at E12–14 has been proposed (Fig. 1D) based on work using the mouse preparation (Hanson & Landmesser, 2003). At the segmental level, local circuits consisting of motoneurons and excitatory GABAergic interneurons are responsible for rhythm generation (Fig. 1D). Motoneurons excite each other, and may excite Renshaw-like interneurons, via recurrent collateral connections. Similarly, in the embryonic chick spinal network, motoneurons acting through Renshaw-like neurons are thought to play an important role in rhythm generation (Wenner & O'Donovan, 2001). The synchronization of local rhythm-generating nodes in the rostrocaudal direction is proposed to occur in the mouse via activation of functionally excitatory glycinergic interneurnons. Absent from the model is a mechanism to synchronize left-right bursts of activity, but functionally excitatory GABA/glycinergiccommissural interneurons are likely candidates.
The lack of GABA/glycinergicinhibition leads to a state of neural hyperexcitability, which provides an explanation for the presence of spontaneous activity but does not explain its episodic nature. From work in the embryonic chick spinal cord (Tabak et al. 2001), evidence suggests that activity-dependent depression is responsible for termination of episodic activity, and preliminary findings suggest similar mechanisms being responsible for episodic activity in the mouse (Hanson & Landmesser, 2003). Physiological mechanisms for activity-dependent depression are numerous and could include vesicle depletion and/ordepression of vesicle release.
Episodic spontaneous activity is observed from E12–18; however, by E18.5 the pattern becomes variable (Branchereau et al. 2002). During the perinatal period, episodes of spontaneous activity can lead to occasional bouts of co-ordinated locomotor-like activity being expressed (Bonnot et al. 1998; Whelan et al. 2000). The mechanisms underlying these changes are not determined, but it is likely that they represent a maturation of GABAergic/glycinergicinhibitory interneuronal connections. An interesting study in this regard is one by Branchereau et al. (2002). who have shown that organotypic cultures of embryonic mouse spinal cord show a normal maturation of GABA/ glycinergic inhibition as in normal mice. Using this culture system they show that blocking the synthesis of 5-HT can accelerate the maturation of inhibition, suggesting that the release of 5-HT by descending raphe terminals may delay this process.
Evoked rhythmic activity
In both the embryonic and neonatal mouse, bath application of monoamines to isolated spinal cord preparations can lead to co-ordinated rhythmic patterns being produced. Rhythmic bursting patterns can be chemically evoked from E12 onwards (Fig. 2) in the mouse by bath application of serotonin (5-HT) (Branchereau et al. 2000), well before the time where descending fibres can be detected in the mouse (E16) (Ballion et al. 2002). The segmental rhythmic pattern is coupled, as it is in the neonate, but the bursts are synchronized across segments. It is unclear whether evoked bursts are produced at early embryonic ages by a net depolarization of elements of existing spontaneous networks (Fig. 1D), or whether a separate locomotor network is being recruited. What we do know is that the cycle period of the 5-HT-evoked rhythm (≈4 s) is similar to locomotor-like activity evoked in neonates (2–4 s). In E15-17 embryos the evoked segmental rhythm becomes uncoupled and unstable, although the cycle period and burst duration remain in the same range as observed in E12–14 embryos (Fig. 2B). Preliminary evidence suggests that addition of strychnine converts this rhythm to a coupled synchronous rhythm, suggesting that functional inhibitory connections are in the process of developing. Finally, by E18, the rhythm is generally segmentally coupled and resembles the alternating left-right pattern observed in neonates (Fig. 2C and Fig. 3B). A gap in our knowledge is whether the ipsilateral pattern of alternation (i.e. between the L2-L5 ventral roots) exists at E18 or whether it develops later in embryonic development, as has been described in the rat (for review, see Nishimaru & Kudo, 2000).
Figure 2. Development of locomotor-like activity.
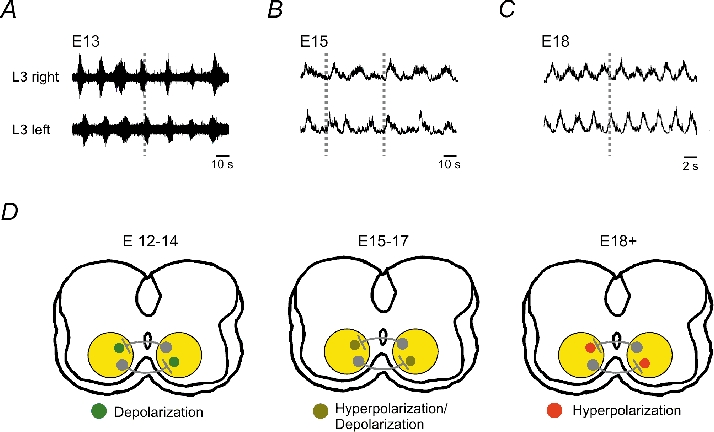
Rhythmic activity was evoked in all cases by bath application of 5-HT and recorded using suction electrodes placed on the L3 ventral roots. A, suction electrode recordings from embryonic mice show a coupled bursting pattern existing between segmental L3 ventral roots at E13. B, generally at E15, an unstable rhythm is evoked between the two L3 segmental roots with a complex coupling pattern. C, by E18, a coupled and alternating segmental pattern exists. Data in B and C were rectified. D, schematic showing the switch in GABAergic/glycinergicpostsynaptic conductances from excitatory at E13 to inhibitory at E18. Data adapted from Branchereau et al. (2000) with permission.
The transformation of the patterns from a coupled, synchronous pattern to an alternating pattern consistent with locomotion has been well described in the rat (Nishimaru & Kudo, 2000; Nakayama et al. 2001, 2002). Data support a role for commissural GABAergic interneurons in the coupling of the segmental synchronous rhythm in embryonic (E15) rats (Nakayama et al. 2002). Rather than new commissural connections being formed to mediate alternating activity, it is thought that a functional change in GABAA-mediated postsynaptic effects from excitatory to inhibitory occurs at E18 (Fig. 2D). The mechanisms underlying these shifts in function are unknown. However, work using the mouse preparation offers some possible insights. The cellular mechanisms leading to the change in the chloride reversal potential to more negative values is thought to be due to the development of a potassium chloride cotransporter (KCC2). Patch-clamp measurements of E18.5 spinal cord motoneurons demonstrated an excitatory GABA and glycine action in knockout mice lacking the KCC2 transporter and inhibitory actions in wild-type mice (Hubner et al. 2001). Although no functional assessment of the development of locomotion has used this knockout, it would be useful to do so, since these mice are viable up to P0. Given the importance of glycinergic/GABAergicsystems in early network function, the mouse may also offer interesting new opportunities for examining the function of these systems since mice with absent or altered receptor function are available (Kralic et al. 2002; Findlay et al. 2003).
Locomotor patterns produced during the postnatal period (P0–4)
Despite the fact that neonatal mice are ataxic at birth and ambulate by crawling with their forelimbs, the lumbar spinal networks (Fig. 3) can produce patterned locomotor-like output (Bonnot et al. 1998; Jiang et al. 1999a; Branchereau et al. 2000; Nishimaru et al. 2000; Whelan et al. 2000). Flexor-extensor and left-right alternating rhythmic patterns can be recorded from in vitro preparations with attached muscle nerves (Whelan et al. 2000) (Fig. 3C and D) and these patterns are similar to those recorded from hindlimb muscles of air-stepping neonatal mice in vivo (Hernandez et al. 1991). Similar to the rat preparation, the activity from flexor and extensor muscle nerves is correlated with bursts recorded from the L2 and the L5/L6 ventral root neurograms (Whelan et al. 2000). Therefore, the locomotor behaviour produced by isolated spinal cord preparations from mice and rats appear to be similar. These details have been previously reviewed for the mouse and will not be discussed here (Bonnot et al. 2002a).
CPG circuitry and function: a role for the neonatal mouse?
At the end of the last section, the maturation of GABAergic/glycinergiccommissural interneurons was mentioned as being important in the control of alternating locomotion. In the neonatal rat, the role of these commissural interneurons (CC-INs) in controlling alternating locomotor behaviour is much better understood (for review, see Butt et al. 2002b). Anatomical data suggest the existence of distinct populations of ventromedially located CC-INs that cross through the ventral commissure (Eide et al. 1999; Stokke et al. 2002; Birinyi et al. 2003). Some of these neurons project segmentally and probably mediate left-right alternation, as previously discussed (Nakayama et al. 2002). Last-order premotor CC-INs have been identified that project to motoneuronal pools and other identified CC-INs on the contralateral side (Birinyi et al. 2003). Other CC-INs cross and can project across several segments. Recent work from Ole Kiehn's group has examined the organization of CC-INs projecting to caudal segments and characterized interneurons that define activity in caudal motoneuronal pools (Butt & Kiehn, 2003). What is clear from this work is that the situation may be more complex than was initially appreciated. Several of the CC-INs identified can switch from inhibitory to excitatory actions once rhythmic activity is expressed. Interestingly, this switch occurred when 5-HT was added to the bath, suggesting a complex role for neuromodulators in reconfiguring vertebrate motor networks, similar to invertebrate systems (Marder & Thirumalai, 2002).
The mouse preparation has a complementary role to play, identifying populations of interneurons that contribute to rhythmogenesis. Preliminary labelling studies confirm that similar to the rat, populations of ventrally located cells in lamina VII, VIII and X project through the ventral commissure and these cells can be identified and patched using a spinal cord slice preparation (Carlin & Jordan, 2001). A recent study examined mice lacking either the EphA4 receptor or its ligand ephrinB3 and identified specific populations of interneurons that may contribute to the animal's characteristic deficit in gait (Kullander et al. 2003). Normally, neurons that possess the EphA4 receptor are repelled from the midline by EphrinB3. In EphA4−/− mice, there is a fivefold increase in the number of EphA4 neurons that cross the midline. These neurons appear to be excitatory, as many of the EphA4−/− neurons were also positive for markers of glutamatergic neurons. Increased glutamatergic projections may lead to the synchronization of the two oscillators, thus resulting in the characteristic hopping phenotype. However, further electro-physiological evidence will be needed to confirm this hypothesis.
There are several preliminary reports that use the mouse, suggesting the use of innovative approaches to dissect out populations of neurons involved in rhythmogenesis. Recent work has identified several distinct classes of progenitors in vertebrate embryos that ultimately go on to produce neurons in the ventral spinal cord (for review, see Jessell, 2000). These progenitors can be distinguished by the expression of homeobox transcription factors such as evx-1, En-1, Lim-3, Gsh-4, Lim-2 and Isl-1 (Davis & Joyner, 1988; Tsuchida et al. 1994; Li et al. 1994; Zhadanov et al. 1995; Moran-Rivard et al. 2001). The transcription factors listed are known to be required for the differentiation of specific subclasses of progenitors. The first approach involves the use of Cre-LoxP technology to label specific populations of neonatal interneurons. Cre-LoxP technology is a method used to introduce genetic alterations into specific genes at specific times during development (Nagy, 2000). Recently, adult mice have been generated in which Cre-LoxP technology has been exploited to drive permanent expression of a membrane-linked version of green fluorescent protein (GFP) and nuclear LacZ in neurons that transiently express Islet 1 (Isl-1) during development (Maxwell et al. 2002). In another study, similar technology was used to express eGFP in adult spinal cord interneurons that are probably derived from V1 progenitor populations that transiently express En-1 (Sapir et al. 2002). When these techniques are combined with electrophysiological or imaging tools, it may be possible to probe the functional characteristics of neurons that form specific circuits within the spinal cord. Another approach is to use activity-dependent neuronal markers, such as c–fos, to label neurons that are active during rhythmic activity. c–fos is now a well-established marker that has been used to identify neurons active during tasks such as mesencephalic locomotor region (MLR)-stimulated locomotion (Carr et al. 1995; Huang et al. 2000), walking on rotarods (Jasmin et al. 1994) or following sensory stimulation (Hunt et al. 1987). Instead of examining cellular properties post–hoc, transgenic eGFP-c–fos mice have been generated that allow the electrophysiological properties of tagged cells to be examined. In a preliminary study eGFP-c–fos mice were given an overground locomotor task, after which slices were prepared. Cells expressing c–fos could then be identified using fluorescence microscopy and targeted for intracellular recording using visually guided patch techniques (Brownstone et al. 2002).
Neurotransmitters involved in activation and modulation of the CPG
The ease of performing pharmacological experiments in vitro has led to a large volume of literature on the activation and modulation of CPG networks by bath-applied neurotransmitter and/orneuromodulator agonists in the neonatal rat. By comparison the data on this subject using the neonatal mouse is sparse (Table 1). Nevertheless, the available evidence suggests that substances that produce locomotor-like activity in the rat preparation can also effectively activate the spinal CPG in the mouse.
Table 1.
Activation of CPGs in the neonatal mouse
| Type of stimulation | Drug combination/stim. location | Publications | Rhythm stability |
|---|---|---|---|
| Bath applied drugs | NMDA/5-HT | Kullander et al. (2003), | *** |
| Whelan et al. (2000) | |||
| NMA/5-HT/dopamine | Whelan et al. (2000), | **** | |
| Jiang et al. (1999a) | |||
| 5-HT | Nishimaru et al. (2000) | *** | |
| 5-HT2 | Madriaga et al. (2002) | *** | |
| 5-HT/dopamine | Whelan et al. (2000) | **** | |
| Low Mg2+ | Bonnot et al. (1998) | ** | |
| Electrical stimulation | Lumbar dorsal roots | Whelan et al. (2000) | **(transient) |
| Cauda equina | Whelan et al. (2000) | ***(transient) | |
| Spontaneous activity | Bonnot et al. (1998), | * | |
| Whelan et al. (2000) |
As mentioned earlier, bath-applied 5-HT can effectively evoke bouts of rhythmicity in the embryonic and neonatal mouse preparation (Branchereau et al. 2000; Nishimaru et al. 2000), and appears to control the timing of the maturation of inhibitory GABA/glycinergicactions (Branchereau et al. 2002). As in the embryonic mouse, changing the endogenous production of 5-HT interferes with the development of postnatal CPG (Myoga et al. 1995; Branchereau et al. 2002). Therefore, 5-HT has long-term trophic effects on neural development and short-term neuromodulatory effects on neural excitability. The fact that 5-HT is an effective rhythmogenic agent in both the rat and mouse preparation, suggests that an important signalling pathway is activated that leads to sustained depolarization of elements of the CPG. A more likely scenario is that the net excitatory effect is due to the interaction of multiple signalling pathways, since so far seven families and 14 subtypes of receptors for 5-HT have been identified. Indeed, recordings from cells in vertebrates show that 5-HT can have differing effects on membrane potential, depending on the relative proportion of receptor subtypes present (for review, see Rekling et al. 2000; Schmidt & Jordan, 2000). For example, in some vertebrate motoneurons, 5-HT causes a net hyperpolarization, by acting through the 5-HT1 receptor family, while in other motoneurons, a net depolarization is produced by 5-HT binding to 5-HT2 receptor subtypes. The downstream effects on ion channels are predictably diverse. Increases in Ih conductances, decreases in K+ currents, reduction of afterhyperpolarization (AHP) amplitudes and an uncovering of Ca2+ currents have all been described for spinal neurons activated by 5-HT (for review, see Rekling et al. 2000). The two candidate receptor families for activating the CPG in the neonatal rodent are the 5-HT2 and 5-HT7 receptor families (see Schmidt & Jordan, 2000) that activate G-proteins coupled to a Gαq/11 and a Gαs pathway, respectively. Preliminary investigations in the mouse show that at concentrations of 5-HT (20–30 μm) that evoke locomotor-like activity, 5-HT2 or 5-HT7 receptor antagonists can abolish or disrupt the locomotor rhythm (Madriaga et al. 2002). The fact that the coupling pattern of the rhythm can be disrupted suggests that neurons comprising the CPG are being affected. Surprisingly, 5-HT also appears to interact with dopamine receptors, since blockade of D1 or D2 receptors also interferes with the 5-HT-evoked pattern (Madriaga et al. 2002). At present, only 5-HT2 receptor agonists have been shown to be sufficient (Fig. 3B) to evoke locomotor-like rhythms (5-HT7 agonists have not been tested in the mouse). Data from the neonatal rat preparation also suggest that 5-HT7 and 5-HT2 receptors contribute to 5-HT-evoked rhythmogenesis (Cina & Hochman, 1998; Schmidt & Jordan, 2000). The data suggest a rostrocaudal distribution of 5-HT7 and 5-HT2a receptors with 5-HT7 receptors concentrated in caudal thoracic and rostral lumbar segments, while 5-HT2a receptors are concentrated in lumbar sections below L3. This is interesting since in the rat and mouse rostrocaudal gradients of CPG excitability have been observed (Kjaerulff & Kiehn, 1996; Branchereau et al. 2000).
Although glutamate is not necessary for generating spontaneous rhythmic activity in early embryonic mice (E12–14) (Hanson & Landmesser, 2003), this changes through development and by E18, addition of kynurenate completely suppressed spontaneous activity in mice (Branchereau et al. 2002). In neonatal mice, activation of NMDA receptors, by reducing extracellular Mg2+, is sufficient to elicit a rhythmic locomotor pattern (Bonnot et al. 1998). Following activation of the rhythm by 5-HT and dopamine, application of the NMDA receptor antagonist, AP5, reduces the amplitude and increases the frequency of the rhythm (Whelan et al. 2000). In contrast, blockade of AMPA/kainatereceptors with CNQX reversibly blocks rhythmic activity (Nishimaru et al. 2000; Whelan et al. 2000). Thus it appears that while NMDA receptors are not essential for rhythmogenesis in the neonatal mouse, they clearly modulate the pattern. This conclusion is supported by a preliminary report suggesting that knockout mice lacking the NMDAR1 receptor subunit do not show functional deficits in locomotor behaviour (Smith et al. 1993). In the perinatal rat, several lines of evidence obtained from the slice and en bloc spinal cord preparations, suggest that NMDA receptor activation plays an important role in rhythmogenesis (for review, see Schmidt et al. 1998); however, not all reports agree that such activation is essential (Beato et al. 1997). The availability of NMDA and especially AMPA receptor knockout mice should be useful tools in the exploration of the role of these receptors in rhythmogenesis.
Peptides can modulate spinal motor circuits in many species of vertebrates, including the neonatal rat (Barthe & Clarac, 1997; Marchetti & Nistri, 2001). In the mouse, less work has been completed in this regard. However, a surprising new finding is that bath-applied peptides, such as arginine-vasopressin (AVP) or oxytocin (OXT), which are involved in regulating autonomic function in adult animals, can increase EMG responses in vitro and in some cases evoke bouts of rhythmic activity (Pearson et al. 2003). Interestingly, these peptides can interact with subthreshold (for evoking rhythmic activity) concentrations of 5-HT2 receptor agonists to produce long-lasting bouts of locomotor-like activity. This is of interest since the V1a (AVP receptors), oxytocin and 5-HT2 receptors are coupled to a Gαq/11 pathway, which activates a phospholipase C signalling cascade. Combined with the fact that suprathreshold concentrations of 5-HT2 can evoke sustained bouts of locomotor-like activity (see Fig. 3B), this points to an important role for this particular second messenger pathway. Using neonatal rat slices along with whole-cell patch techniques, AVP has been shown to depolarize the majority of ventral horn neurons tested, and in motoneurons this depolarization was associated with a 25 % reduction in a potassium-mediated conductance (Oz et al. 2001). This is different from brainstem motoneurons where AVP appears to act by opening of persistent sodium, voltage-dependent channels (Raggenbass et al. 1991; Palouzier-Paulignan et al. 1994). In ventral and also lateral horn interneurons, the situation was found to be more complex, where there is evidence for the activation of multiple conductances by AVP (Kolaj & Renaud, 1998; Oz et al. 2001). With regard to the functional role of AVP and oxytocin in vivo there are two clear questions. Firstly, what are the sources of endogenous ligands for the V1a and OXT receptors? and secondly, do they control locomotor behaviour in vivo? There is evidence for the presence of potential vasopressinergic fibres from the paraventricular nucleus of the hypothalamus to the lumbar spinal cord in the neonatal rat (Leong et al. 1984; Kudo et al. 1993; Lakke, 1997). This suggests that descending hypothalamic pathways could potentially drive spinal motor circuits early in development. We do not know the functional role of early vasopressinergic and oxytocinergic innervation of spinal cord neurons in vivo. Vasopressin has been shown to have a trophic, as well as a neuromodulatory function, suggesting a diverse role during spinal cord development (Iwasaki et al. 1991; Chevaleyre et al. 2002). An intriguing possibility is that during the period where descending tracts are not fully developed, peptidergic neuromodulatory systems may prime spinal neurons, allowing other descending excitatory inputs to have functional effects. Anatomical and electrophysiological evidence indirectly supports this idea in the neonatal rodent since V1a and oxytocin receptor expression have been shown to be transiently upregulated in the rat (Tribollet et al. 1989, 1991), and AVP and oxytocin can co-operate with 5-HT2 receptor agonists to induce locomotion in the mouse (Pearson et al. 2003).
Oxygenation of in vitro preparations
With the power to perform sophisticated experiments using in vitro preparations, it is often easy to lose sight of the fact that they are ‘brains’ in a dish devoid of their normal milieu. One issue with superfused in vitro preparations is that gas exchange occurs at the tissue surface. Since the tissue is metabolically active this creates an oxygen gradient from the surface to the centre of the preparation (Wilson et al. 2003). If the tissue is too thick, an anoxic core at the centre of the preparation will form. The detrimental effects of anoxia on neuronal activity are well documented, and include hyperpolarization, increased intracellular calcium and decreased adenosine triphosphate (ATP) (Luhmann et al. 1993; Ataka et al. 1996; Richter & Ballanyi, 1996; Krnjevic, 1999). The consequence of these events is a general cessation of neural activity. The suppression of spike activity is a hallmark of the effects of anoxia and probably serves a neuroprotective function. The neonatal mouse preparation, due to the small diameter of the spinal cord (≈1 mm), is well oxygenated up to P3 and possibly later, under quiescent conditions (Wilson et al. 2003). Bath application of drugs that induce locomotor-like activity (Fig. 4A) reduce the tissue PO2 at all ages (P0–3), but only induce an anoxic core in P3 mice. On average, this anoxic core extends from the centre of the preparation to within 400 μm of the ventral surface following bath application of rhythmogenic drugs in P3 mice (Fig. 4B).
Figure 4. Locomotor-like activity can persist despite large anoxic cores being present.
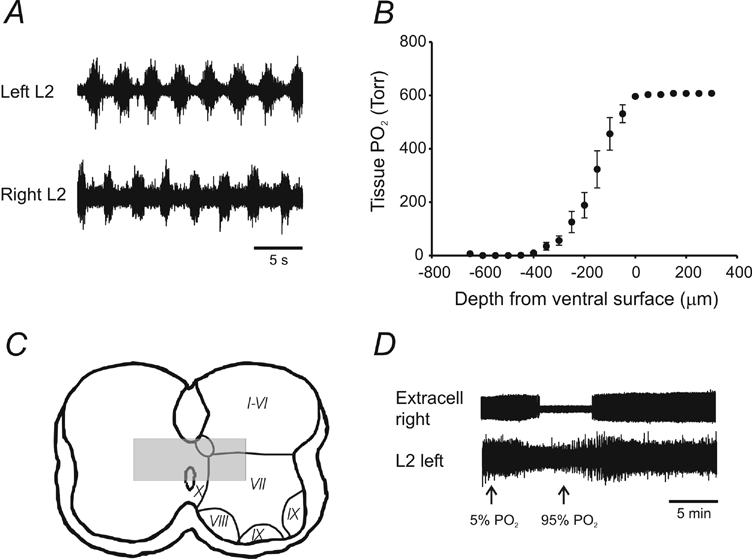
A, example of alternating rhythmic pattern evoked by the bath application of 5-HT (10 μm), NMA (5 μm) and dopamine (50 μm). B, depth profiles of the PO2 within the tissue show an anoxic core extending within 400 μm of the ventral surface (data from 3 animals, error bars represent s.e.m.). Depth profiles were generated by placing a Clarke-style PO2 microelectrode 300 μm above the ventral surface close to the midline. The electrode was advanced using a stepper motor in 50 μm steps until the PO2 started to increase. C, representation of the likely position of the anoxic core reconstructed from data in B. Rexed lamina positions are adapted from neonatal rat spinal cord data (Kiehn et al. 1996). The spinal cord schematic was obtained from tracing P2 mouse histological sections at L2. D, extracellular data showing the silencing of an active unit after the bath PO2 was changed from 95 to 5 %.
An important concern is whether neurons within the anoxic core continue spiking, and whether rhythmic activity can be compromised under severe hypoxic conditions. When severe hypoxic conditions are imposed on P0 mice (95 % to 5 % O2), the left-right alternating pattern is abolished, although a residual slow uncoupled rhythmic pattern can be recorded (Wilson et al. 2003). The residual rhythmogenic capacity under hypoxic conditions could be explained by activity of peripherally located neurons exposed to adequate oxygen tension. Extracellular recording from neurons deep in the tissue confirmed that the majority of neurons ceased to spike following the onset of hypoxic conditions (Fig. 4D). Interestingly, these results are similar to the respiratory system where anoxia-induced hyperpolarization causes a majority of cells in the medulla to stop spiking (Ballanyi et al. 1994), although an important difference is that the rhythmic respiratory pattern is not abolished.
Following bath application of rhythmogenic drugs, neurons within portions of lamina X and lamina VII (Fig. 4C) may be encompassed by an anoxic core in P3 mice (Wilson et al. 2003). Given the distributed nature of the CPG, it is likely that a considerable redundancy is built into the system, allowing operation even with relatively few neuronal elements. This point of view is supported by recent studies using activity-labelling dyes in which only 0.1 % of neurons were labelled during locomotor-like activity in the in vitro rat spinal cord preparation (Cina & Hochman, 2000; Hochman et al. 2001). However, several laboratories have reported that rhythmically active cells are located within lamina X and VII of neonatal rat in vitro preparations (MacLean et al. 1995; Raastad et al. 1997, 1998; Butt et al. 2002a; Raastad & Kiehn, 2000). One possible explanation is that in early studies, midsagittal hemisections were performed to gain access to interneurons (MacLean et al. 1995; Kiehn et al. 1996) in laminae VII and X. Since neurons can remain dormant for long periods of time in neonatal animals under anoxic conditions (Wilson et al. 2003), it is possible that the restoration of oxygen to the anoxic tissue following lesions allows these cells to become active. More recent studies that have used an intact rat spinal cord suggest that rhythmically active cells can be recorded in the ventromedial portions of the spinal cord (Tresch & Kiehn, 1999; Butt et al. 2002a). Data from the mouse do not conflict with these studies since much of the data in the rat were collected from cells located 350 μm below the ventral surface. Although the data from the mouse suggest that these regions may be well oxygenated, it would be desirable to measure the tissue oxygenation of neonatal rats during bouts of evoked locomotor-like activity so a direct comparison can be made.
Late neonatal-juvenile period (P7–14)
During the first two postnatal weeks, rodents show a gradual expression of motor behaviours such as weight-bearing locomotion and postural reflexes (Clarac et al. 1998). By P9 mice begin to support their weight, and by P14 many of the characteristics of their gait are qualitatively similar to those of the adult mouse (Jiang et al. 1999a; Breitling & Whelan, unpublished observations). Gait development is probably correlated with the maturation of descending pathways (vestibulospinal, reticulospinal) that control posture and locomotion (Clarac et al. 1998). Unlike the rat, locomotor-like activity can be recorded from functionally mature (P10-12) in vitro mouse preparations (Jiang et al. 1999a). In contrast to the perinatal mouse (Bonnot et al. 1998; Nishimaru et al. 2000; Whelan et al. 2000), only a combination of bath-applied 5-HT, dopamine and NMA can elicit rhythmicity in these older mouse preparations. This constraint may reflect the fact that many receptors are transiently overexpressed during the first week (GluR1, NMDA, AMPA, GABA, OXT and V1 (Tribollet et al. 1989, 1991; Kalb et al. 1992; Gonzalez et al. 1993; Watanabe et al. 1994; Jakowec et al. 1995; Stegenga & Kalb, 2001; Inglis et al. 2002)). The data from the functionally mature mouse (Jiang et al. 1999a) is also consistent with results using in vivo adult cat preparations in which 5-HT agonists can modulate ongoing locomotor rhythms, but do not appear to be capable of eliciting locomotor activity (Rossignol et al. 1998). However, the formation of an anoxic core may be an issue for these older preparations, although this remains to be determined.
An interesting developmental upregulation of L-type calcium channel expression has been observed from P2 to P14 (Jiang et al. 1999b; Carlin et al. 2000a, b). Blockade of L-type channels has no functional effect on a strychnine/NMDA-induced rhythm at P0–3, but starting at P7 the amplitude of the bursts and their frequency is markedly affected (Jiang et al. 1999b). The presence of L-type Ca2+ currents was confirmed by whole-cell recordings from P8-15 motoneurons using a slice preparation of the spinal cord (Carlin et al. 2000b). From a functional perspective, L-type Ca2+ channels appear to be essential for the production of plateau potentials in adult vertebrate motoneurons (Perrier et al. 2002), acting to amplify the response to a synaptic input (Kiehn, 1991). All things being equal, the expression of plateau potentials allow motoneurons to fire at sustained high rates and could allow muscles to generate greater contractile forces (Kiehn, 1991). In this context, it is interesting that mice begin to commence weight-bearing locomotion around the time when L-type channels are expressed (Jiang et al. 1999a).
Conclusions
This review has examined the development of locomotor activity in the mouse focusing on the isolated in vitro spinal cord preparation. Clearly, there are many gaps in our knowledge of mouse CPG function, especially compared with the rat. This reflects the relatively late adoption of the mouse by laboratories interested in CPG function. The data reviewed here suggest that the mouse is an excellent model for examining locomotor function. Firstly, the available data suggest that from a functional perspective the development of locomotion in the rat and mouse is similar. This suggests that we will observe similarities in rodent and mammalian CPG circuitry that parallel those observed in lower vertebrates (e.g. lamprey and Xenopus), allowing the rat and mouse to be used in a complementary fashion. Secondly, the genetic potential of the mouse is vast and currently underexploited in spinal CPG research. Over the next few years collaborations between molecular biologists and electrophysiologists will probably lead to new approaches being developed to examine CPG function. As we approach the centennial of the publication of Graham-Brown's half-centre model, we are finally starting to acquire the appropriate tools to identify the circuitry, and the function of central pattern generators.
Acknowledgments
I would like to acknowledge the technical assistance of Ms Michelle Madriaga. I am thankful for research support from the Alberta Heritage Foundation for Medical Research, Canadian Institutes of Health Research, Natural Sciences Engineering Research Council and the Heart and Stroke Foundation of Canada. I would like to thank Dr Ole Kiehn, Dr C. J. Heckman, and Dr Richard Wilson for helpful comments on an earlier version of this manuscript.
REFERENCES
- Arai Y, Mentis G, O'Donovan MJ. Voltage-sensitive dye analysis of locomotor-like network activity in neonatal mouse spinal cord slices. Soc Neurosci Abstr. 2002;32:65. [Google Scholar]
- Ataka H, Murakami M, Goto S, Moriya H, Hayashi F, Fukuda Y. Effects of hypoxia on the ventral root motor-evoked potential in the in vitro spinal cord preparation. Spine. 1996;21:2095–2100. doi: 10.1097/00007632-199609150-00007. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Volker A, Richter DW. Anoxia induced functional inactivation of neonatal respiratory neurones in vitro. NeuroReport. 1994;6:165–168. doi: 10.1097/00001756-199412300-00042. [DOI] [PubMed] [Google Scholar]
- Ballion B, Branchereau P, Chapron J, Viala D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res Dev Brain Res. 2002;137:81–88. doi: 10.1016/s0165-3806(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Barthe JY, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Exp Brain Res. 1997;115:485–492. doi: 10.1007/pl00005718. [DOI] [PubMed] [Google Scholar]
- Beato M, Bracci E, Nistri A. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proc R Soc Lond. 1997;B 264:877–884. doi: 10.1098/rspb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Weber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol. 2003;461:429–440. doi: 10.1002/cne.10696. [DOI] [PubMed] [Google Scholar]
- Bonnot A, Morin D, Viala D. Genesis of spontaneous rhythmic motor patterns in the lumbosacral spinal cord of neonate mouse. Brain Res Dev Brain Res. 1998;108:89–99. doi: 10.1016/s0165-3806(98)00033-9. [DOI] [PubMed] [Google Scholar]
- Bonnot A, Whelan PJ, Mentis GZ, O'Donovan MJ. Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Res Brain Res Rev. 2002a;40:141–151. doi: 10.1016/s0165-0173(02)00197-2. [DOI] [PubMed] [Google Scholar]
- Bonnot A, Whelan PJ, Mentis GZ, O'Donovan MJ. Spatiotemporal pattern of motoneuron activation in the rostral lumbar and the sacral segments during locomotor-like activity in the neonatal mouse spinal cord. J Neurosci. 2002b;22:RC203. doi: 10.1523/JNEUROSCI.22-03-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchereau P, Morin D, Bonnot A, Ballion B, Chapron J, Viala D. Development of lumbar rhythmic networks: from embryonic to neonate locomotor-like patterns in the mouse. Brain Res Bull. 2000;53:711–718. doi: 10.1016/s0361-9230(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Di Mauro M, Li Z, McMahon DG, Jordan LM. Whole cell patch clamp recordings from locomotor activity-labelled spinal cord neurones in c–fos-eGFP mice. Soc Neurosci Abstr. 2002;32:65. [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci. 2002a;22:9961–9971. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Lebret JM, Kiehn O. Organization of left-right coordination in the mammalian locomotor network. Brain Res Brain Res Rev. 2002b;40:107–117. doi: 10.1016/s0165-0173(02)00194-7. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jiang Z, Brownstone RM. Characterization of calcium currents in functionally mature mouse spinal motoneurons. Eur J Neurosci. 2000a;12:1624–1634. doi: 10.1046/j.1460-9568.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000b;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jordan LM. Identification of spinal commissural neurons for electrophysiological assessment in a mouse spinal cord slice preparation. Soc Neurosci Abstr. 2001;31:297. [Google Scholar]
- Carr PA, Huang A, Noga B, Jordan LM. Cytochemical characteristics of cat spinal neurons activated during fictive locomotion. Brain Res Bull. 1995;37:213–218. doi: 10.1016/0361-9230(94)00271-2. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Moos FC, Desarmenien MG. Interplay between presynaptic and postsynaptic activities is required for dendritic plasticity and synaptogenesis in the supraoptic nucleus. J Neurosci. 2002;22:265–273. doi: 10.1523/JNEUROSCI.22-01-00265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cina C, Hochman S. Serotonin receptor pharmacology of the mammalian locomotor CPG: Activation by a 5-HT7 receptor agonist in the rat spinal cord. Soc Neurosci Abstr. 1998;25:1669. [Google Scholar]
- Cina C, Hochman S. Diffuse distribution of sulforhodamine-labeled neurons during serotonin-evoked locomotion in the neonatal rat thoracolumbar spinal cord. J Comp Neurol. 2000;423:590–602. doi: 10.1002/1096-9861(20000807)423:4<590::aid-cne5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Clarac F, Vinay L, Cazalets JR, Fady JC, Jamon M. Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Res Brain Res Rev. 1998;28:35–43. doi: 10.1016/s0165-0173(98)00024-1. [DOI] [PubMed] [Google Scholar]
- Davis CA, Joyner AL. Expression patterns of the homeo-box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann NY Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, De Guzman CP, De Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J Neurotrauma. 1992;9:S119–128. [PubMed] [Google Scholar]
- Eide AL, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. [PubMed] [Google Scholar]
- Fedirchuk B, Nielsen J, Petersen N, Hultborn H. Pharmacologically evoked fictive motor patterns in the acutely spinalized marmoset monkey (Callithrix jacchus) Exp Brain Res. 1998;122:351–361. doi: 10.1007/s002210050523. [DOI] [PubMed] [Google Scholar]
- Findlay GS, Phelan R, Roberts MT, Homanics GE, Bergeson SE, Lopreato GF, Mihic SJ, Blednov YA, Harris RA. Glycine receptor knock-in mice and hyperekplexia-like phenotypes: comparisons with the null mutant. J Neurosci. 2003;23:8051–8059. doi: 10.1523/JNEUROSCI.23-22-08051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier PA, Smith AM, Rossignol S. Locomotor deficits in the mutant mouse, Lurcher. Exp Brain Res. 1987;66:271–286. doi: 10.1007/BF00243304. [DOI] [PubMed] [Google Scholar]
- Gonzalez DL, Fuchs JL, Droge MH. Distribution of NMDA receptor binding in developing mouse spinal cord. Neurosci Lett. 1993;151:134–137. doi: 10.1016/0304-3940(93)90004-5. [DOI] [PubMed] [Google Scholar]
- Graham-Brown T. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond. 1911;84:308–319. [Google Scholar]
- Grillner S, Parker D, El Manira A. Vertebrate locomotion - a lamprey perspective. Ann NY Acad Sci. 1998;860:1–18. doi: 10.1111/j.1749-6632.1998.tb09035.x. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P, Elbert K, Droge MH. Spontaneous and NMDA evoked motor rhythms in the neonatal mouse spinal cord: an in vitro study with comparisons to in situ activity. Exp Brain Res. 1991;85:66–74. doi: 10.1007/BF00229987. [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL. 5-HT receptors and the neuromodulatory control of spinal cord function. In: Cope TC, editor. Motor Neurobiology of the Spinal Cord. Blackwell Science Inc; 2001. pp. 47–87. [Google Scholar]
- Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c–fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Crockett R, Korada S, Abraham WC, Hollmann M, Kalb RG. The AMPA receptor subunit GluR1 regulates dendritic architecture of motor neurons. J Neurosci. 2002;22:8042–8051. doi: 10.1523/JNEUROSCI.22-18-08042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Kinoshita M, Ikeda K, Shiojima T, Kurihara T, Appel SH. Trophic effect of angiotensin II, vasopressin and other peptides on the cultured ventral spinal cord of rat embryo. J Neurol Sci. 1991;103:151–155. doi: 10.1016/0022-510x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Fox AJ, Martin LJ, Kalb RG. Quantitative and qualitative changes in AMPA receptor expression during spinal cord development. Neuroscience. 1995;67:893–907. doi: 10.1016/0306-4522(95)00026-f. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Gogas KR, Ahlgren SC, Levine JD, Basbaum AI. Walking evokes a distinctive pattern of Fos-like immunoreactivity in the caudal brainstem and spinal cord of the rat. Neuroscience. 1994;58:275–286. doi: 10.1016/0306-4522(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999a;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rempel J, Li J, Sawchuk MA, Carlin KP, Brownstone RM. Development of L-type calcium channels and a nifedipine-sensitive motor activity in the postnatal mouse spinal cord. Eur J Neurosci. 1999b;11:3481–3487. doi: 10.1046/j.1460-9568.1999.00765.x. [DOI] [PubMed] [Google Scholar]
- Kalb RG, Lidow MS, Halsted MJ, Hockfield S. N-methyl-D-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc Natl Acad Sci USA. 1992;89:8502–8506. doi: 10.1073/pnas.89.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Plateau potentials and active integration in the final common pathway. Trends Neurosci. 1991;14:68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience. 1996;75:263–273. doi: 10.1016/0306-4522(96)00250-3. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci. 1997;17:9433–9447. doi: 10.1523/JNEUROSCI.17-24-09433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Renaud LP. Vasopressin-induced currents in rat neonatal spinal lateral horn neurons are G-protein mediated and involve two conductances. J Neurophysiol. 1998;80:1900–1910. doi: 10.1152/jn.1998.80.4.1900. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Korpi ER, O'Buckley TK, Homanics GE, Morrow AL. Molecular and pharmacological characterization of GABA(A) receptor α1 subunit knockout mice. J Pharmacol Exp Ther. 2002;302:1037–1045. doi: 10.1124/jpet.102.036665. [DOI] [PubMed] [Google Scholar]
- Krnjevic K. Early effects of hypoxia on brain cell function. Croat Med J. 1999;40:375–380. [PubMed] [Google Scholar]
- Kudo N, Furukawa F, Okado N. Development of descending fibers to the rat embryonic spinal cord. Neurosci Res. 1993;16:131–141. doi: 10.1016/0168-0102(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lakke EA. The projections to the spinal cord of the rat during development: a timetable of descent. Adv Anat Embryol Cell Biol. 1997;135:I–143. doi: 10.1007/978-3-642-60601-4. [DOI] [PubMed] [Google Scholar]
- Leong SK, Shieh JY, Wong WC. Localizing spinal cord-projecting neurons in adult albino rats. J Comp Neurol. 1984;228:1–17. doi: 10.1002/cne.902280103. [DOI] [PubMed] [Google Scholar]
- Li H, Witte DP, Branford WW, Aronow BJ, Weinstein M, Kaur S, Wert S, Singh G, Schreiner CM, Whitsett JA, et al. Gsh-4 encodes a LIM-type homeodomain, is expressed in the developing central nervous system and is required for early postnatal survival. EMBO J. 1994;13:2876–2885. doi: 10.1002/j.1460-2075.1994.tb06582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Kral T, Heinemann U. Influence of hypoxia on excitation and GABAergic inhibition in mature and developing rat neocortex. Exp Brain Res. 1993;97:209–224. doi: 10.1007/BF00228690. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Hochman S, Magnuson DS. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neurosci Lett. 1995;197:9–12. doi: 10.1016/0304-3940(95)11882-w. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Whelan PJ. 5-HT-induced rhythmicity in the in vitro spinal cord of the neonatal mouse is partly dependent on D1 and D2 receptors. Soc Neurosci Abstr. 2002;65:21. [Google Scholar]
- Marchetti C, Nistri A. Neuronal bursting induced by NK3 receptor activation in the neonatal rat spinal cord in vitro. J Neurophysiol. 2001;86:2939–2950. doi: 10.1152/jn.2001.86.6.2939. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Hartley R, Todd AJ, Arber S, Kramer I, Jessell TM. Characterization of spinal interneuron subpopulations with green fluorescent protein by mouse genetics. Soc Neurosci Abstr. 2002;32:850. [Google Scholar]
- Moran-Rivard L, Kagawa T, Saueressig H, Gross MK, Burrill J, Goulding M. Evx1 is a postmitotic determinant of V0 interneuron identity in the spinal cord. Neuron. 2001;29:385–399. doi: 10.1016/s0896-6273(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Myoga H, Nonaka S, Matsuyama K, Mori S. Postnatal development of locomotor movements in normal and para- chlorophenylalanine-treated newborn rats. Neurosci Res. 1995;21:211–221. doi: 10.1016/0168-0102(94)00857-c. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Kudo N. Developmental changes in 5-hydroxytryptamine-induced rhythmic activity in the spinal cord of rat fetuses in vitro. Neurosci Lett. 2001;307:1–4. doi: 10.1016/s0304-3940(01)01913-9. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Kudo N. Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord. J Neurosci. 2002;22:10388–10398. doi: 10.1523/JNEUROSCI.22-23-10388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Iizuka M, Ozaki S, Kudo N. Spontaneous motoneuronal activity mediated by glycine and GABA in the spinal cord of rat fetuses in vitro. J Physiol. 1996;497:131–143. doi: 10.1113/jphysiol.1996.sp021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain Res Bull. 2000;53:661–669. doi: 10.1016/s0361-9230(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Oz M, Kolaj M, Renaud LP. Electrophysiological evidence for vasopressin V(1) receptors on neonatal motoneurons, premotor and other ventral horn neurons. J Neurophysiol. 2001;86:1202–1210. doi: 10.1152/jn.2001.86.3.1202. [DOI] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Dubois-Dauphin M, Tribollet E, Dreifuss JJ, Raggenbass M. Action of vasopressin on hypoglossal motoneurones of the rat: presynaptic and postsynaptic effects. Brain Res. 1994;650:117–126. doi: 10.1016/0006-8993(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Pearson SA, Mouihate A, Pittman QJ, Whelan PJ. Peptidergic activation of locomotor pattern generators in the neonatal spinal cord. J Neurosci. 2003 doi: 10.1523/JNEUROSCI.23-31-10154.2003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Shatz CJ. Brain waves and brain wiring: the role of endogenous and sensory-driven neural activity in development. Pediatr Res. 1999;45:447–458. doi: 10.1203/00006450-199904010-00001. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Raastad M, Enriquez-Denton M, Kiehn O. Synaptic signaling in an active central network only moderately changes passive membrane properties. Proc Natl Acad Sci USA. 1998;95:10251–10256. doi: 10.1073/pnas.95.17.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad M, Johnson BR, Kiehn O. Analysis of EPSCs and IPSCs carrying rhythmic, locomotor-related information in the isolated spinal cord of the neonatal rat. J Neurophysiol. 1997;78:1851–1859. doi: 10.1152/jn.1997.78.4.1851. [DOI] [PubMed] [Google Scholar]
- Raastad M, Kiehn O. Spike coding during locomotor network activity in ventrally located neurons in the isolated spinal cord from neonatal rat. J Neurophysiol. 2000;83:2825–2834. doi: 10.1152/jn.2000.83.5.2825. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Goumaz M, Sermasi E, Tribollet E, Dreifuss JJ. Vasopressin generates a persistent voltage-dependent sodium current in a mammalian motoneuron. J Neurosci. 1991;11:1609–1616. doi: 10.1523/JNEUROSCI.11-06-01609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J Neurophysiol. 2003;89:1187–1195. doi: 10.1152/jn.00539.2002. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballanyi K. Response of the medullary respiratory network to hypoxia. In: Haddad GG, Lister RE, editors. Tissue Oxygen Deprivation. New York: Blackwell Science Inc; 1996. pp. 751–777. [Google Scholar]
- Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao FY. Central circuits controlling locomotion in young frog tadpoles. Ann NY Acad Sci. 1998;860:19–34. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- Rossignol S. In: Neuronal Control of Stereotypic Limb Movements. Rowell L, Shepard J, editors. Bethesda, USA: Blackwell Science Inc; 1996. pp. 173–216. chapter 5, pp. [Google Scholar]
- Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci. 1998;860:346–359. doi: 10.1111/j.1749-6632.1998.tb09061.x. [DOI] [PubMed] [Google Scholar]
- Sapir T, Geiman EG, Velasquez T, Alvarez FJ, Goulding M. Pax6 and En1 regulate sequential steps in the development of Renshaw cells. Soc Neurosci Abstr. 2002;32:26. [Google Scholar]
- Schmidt BJ, Hochman S, MacLean JN. NMDA receptor-mediated oscillatory properties: potential role in rhythm generation in the mammalian spinal cord. Ann NY Acad Sci. 1998;860:189–202. doi: 10.1111/j.1749-6632.1998.tb09049.x. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. Motor patterns for respiratory and locomotion generated by an in vitro brainstem-spinal cord preparation. Soc Neurosci Abstr. 1985;24:11L. [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Meth. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Funk GD, Johnson SM, Dong XW, Lai J, Hsu S, Feldman JL. Functional networks for locomotion in spinal cord of neonatal mice lacking NMDA receptors. Soc Neurosci Abstr. 1993;19:270. [Google Scholar]
- Stegenga SL, Kalb RG. Developmental regulation of N-methyl-D-aspartate- and kainate-type glutamate receptor expression in the rat spinal cord. Neuroscience. 2001;105:499–507. doi: 10.1016/s0306-4522(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]
- Suzue T. Movements of mouse fetuses in early stages of neural development studied in vitro. Neurosci Lett. 1996;218:131–134. doi: 10.1016/s0304-3940(96)13141-4. [DOI] [PubMed] [Google Scholar]
- Tabak J, Rinzel J, O'Donovan MJ. The role of activity-dependent network depression in the expression and self-regulation of spontaneous activity in the developing spinal cord. J Neurosci. 2001;21:8966–8978. doi: 10.1523/JNEUROSCI.21-22-08966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. J Neurophysiol. 1999;82:3563–3574. doi: 10.1152/jn.1999.82.6.3563. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neurosci. 2000;3:593–599. doi: 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ. Appearance and transient expression of oxytocin receptors in fetal, infant, and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci. 1989;9:1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dubois-Dauphin M, Dreifuss JJ. Early appearance and transient expression of vasopressin receptors in the brain of rat fetus and infant. An autoradiographical and electrophysiological study. Brain Res Dev Brain Res. 1991;58:13–24. doi: 10.1016/0165-3806(91)90232-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal distributions of the N-methyl-D-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J Comp Neurol. 1994;345:314–319. doi: 10.1002/cne.903450212. [DOI] [PubMed] [Google Scholar]
- Wenner P, O'Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86:1481–1498. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Chersa T, Whelan PJ. Tissue PO2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience. 2003;117:183–196. doi: 10.1016/s0306-4522(02)00831-x. [DOI] [PubMed] [Google Scholar]
- Zhadanov AB, Bertuzzi S, Taira M, Dawid IB, Westphal H. Expression pattern of the murine LIM class homeobox gene Lhx3 in subsets of neural and neuroendocrine tissues. Dev Dyn. 1995;202:354–364. doi: 10.1002/aja.1002020405. [DOI] [PubMed] [Google Scholar]


