Abstract
Hepatic fibrosis represents the generalized response of the liver to injury and is characterized by excessive deposition of extracellular matrix. The cellular basis of this process is complex and involves interplay of many factors, of which cytokines are prominent. We have identified divergent fibrosing responses to injury among mouse strains and taken advantage of these differences to examine and contrast T helper (Th)-derived cytokines during fibrogenesis. Liver injury was induced with carbon tetrachloride, fibrosis was quantitated, and Th1/Th2 cytokine mRNAs measured. Liver injury in BALB/c mice resulted in severe fibrosis, whereas C57BL/6 mice developed comparatively minimal fibrosis. Fibrogenesis was significantly modified in T and B cell-deficient BALB/c and C57BL/6 severe combined immunodeficient (SCID) mice compared with wild-type counterparts, suggesting a role of Th subsets. Fibrogenic BALB/c mice exhibited a Th2 response during the wounding response, whereas C57BL/6 mice displayed a Th1 response, suggesting that hepatic fibrosis is influenced by different T helper subsets. Moreover, mice lacking interferon γ, which default to the Th2 cytokine pathway, exhibited more pronounced fibrotic lesions than did wild-type animals. Finally, shifting of the Th2 response toward a Th1 response by treatment with neutralizing anti-interleukin 4 or with interferon γ itself ameliorated fibrosis in BALB/c mice. These data support a role for immune modulation of hepatic fibrosis and suggest that Th cytokine subsets can modulate the fibrotic response to injury.
The result of injury to the liver, regardless of type (i.e., virus, toxin, or biliary obstruction), is fibrosis. During the fibrogenic response to injury, stellate cells (also known as lipocytes, Ito, or perisinusoidal cells) differentiate into matrix-producing cells, which in large part are responsible for hepatic fibrosis (reviewed in ref. 1). The fibrotic response is complex and involves interplay between cytokines and extracellular matrix, each of which are modulated during the inflammatory and reparative stages of injury. A number of cytokines have been implicated in the pathogenesis of hepatic fibrosis via direct or indirect effects on stellate cells and include transforming growth factor β (TGF-β), tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interferon γ (IFN-γ)(2–5). Although available evidence suggests these cytokines are important in the modulation of stellate cell phenotype and hepatic wounding response, other, as yet unidentified, cytokines undoubtedly play a role.
Potential sources of cytokines in the hepatic wounding response include macrophages (hepatic Kupffer cells), hepatocytes, stellate cells, and natural killer (NK) cells. In addition, lymphocytes, including CD4+ T helper (Th) cells reside in the liver (6, 7) and represent a further potential source of cytokines. Th lymphocytes help orchestrate the host-response via cytokine production and can differentiate into Th1 and Th2 subsets, a classification that is based on the pattern of cytokines produced (reviewed in refs. 8 and 9). In general, Th1 cells produce cytokines that promote cell-mediated immunity including IFN-γ, tumor necrosis factor α, and IL-2. Th2 cells produce IL-4, IL-5, IL-6, and IL-13 and promote humoral immunity (including IgE production) and eosinophilia. Th1 cytokines inhibit the development of Th2 cells and vice versa. Thus, the host-response to infection or injury frequently polarizes to either a Th1 or Th2 response, but not both. Polarized Th1 and Th2 responses have been implicated in a variety of infectious and inflammatory conditions, including mycobacterial infections, asthma, inflammatory bowel disease, and leishmaniasis (8, 9).
Support for a role of Th-derived cytokines in determining the immune response is evident in many experimental models. The polarization of the immune response is enhanced when chronic exposure to an agent occurs, such as with persistent infections or exposure to environmental toxins. Further, polarization of the immune response to Th1 or Th2 cytokines is under genetic control, as demonstrated by divergent responses of different inbred strains of mice to experimental murine leishmaniasis (10, 11). Genetically resistant mice, such as C57BL/6 mice exhibit an expansion of IFN-γ-producing Th1 cells and control the infection, whereas susceptible strains such as BALB/c mice develop an IL-4 mediated Th2 response (10, 12, 13).
Exogenous IFN-γ protects against fibrosis in several models of wounding, including in the liver (5, 14). In the context of these data, as well as that indicating that IFN-γ is a key component of the Th1 cytokine pathway (10, 12), we hypothesized that the immune response and specifically Th1 and Th2 cytokines could be involved in the fibrogenic response to hepatic injury. To examine the role of Th1 and Th2 cytokines in hepatic fibrosis, we analyzed the fibrogenic responses of BALB/c and C57BL/6 mice in toxin-induced hepatic injury. We show that these two strains of mice exhibit divergent fibrogenesis after injury. Moreover, minimal fibrosis in C57BL/6 mice is associated with elaboration of Th1 cytokines, whereas severe fibrosis in BALB/c mice is associated with a Th2 cytokine response. We also demonstrate that fibrosis is significantly modulated in severe combined immunodeficient (SCID) BALB/c and C57BL/6 mice, implicating a role for T and/or B cells. Finally, we show that neutralizing antibody to IL-4 or exogenous IFN-γ, results in attenuation of the fibrogenic response, presumably by modulating Th2 and Th1 responses. These data provide evidence that the host immune response is an important component of the fibrogenic response to toxin-induced liver injury.
MATERIALS AND METHODS
Animals and Model of Hepatic Fibrosis.
BALB/c and C57BL/6 mice (wild type and SCID) were purchased from The Jackson Laboratories. SCID mice were housed in a barrier facility. IFN-γ-deficient (−/−) mice were provided by Timothy Stewart (Genentech, South San Francisco, CA) and bred and maintained under standard conditions. IFN-γ genotype determination was as described (15). Carbon tetrachloride (CCl4; 3.5 ml/kg) in a 1:1 mixture with corn oil (Mazola, Inglewood Cliffs, NJ) was administered to lightly sedated (ether) male mice weighing 25–30 g by gavage at 7-day intervals for four doses. Livers and cells were harvested 7 days after the final dose of CCl4. Animals received care according to National Institutes of Health guidelines.
Histological Assessment of Liver Injury.
Liver specimens were fixed in 10% neutral buffered formalin. Sections (15 μm) were stained in 0.1% Sirius Red F3B in saturated picric acid (both from Sigma), and collagen was extracted with 0.1 M NaOH/methanol (1:1) and quantitated spectrophotometrically as described (16).
Cell Isolation.
Nonparenchymal cells were isolated from mice with modifications of procedures previously described (17, 18). In brief, after in situ perfusion of the liver with 0.016 mg/dl collagenase (Boehringer Mannheim), dispersed cells were fractionated on a discontinuous nycodenz (Accurate Chemicals) density gradient. The entire population of nonparenchymal cells was isolated from the lower layer of the discontinuous density gradient. Phase-contrast microscopy and immunohistochemical staining confirmed the presence of a heterogeneous population of cells (including small mononuclear, desmin-negative, vimentin-negative cells consistent with lymphocytes), which did not include hepatocytes.
RNA Isolation and Extracellular Matrix mRNA Detection.
Total liver or nonparenchymal cell RNA was extracted using guanidinium isothiocyanate, the concentration of RNA was determined spectrophotometrically, and the integrity of all samples was documented by visualization of 18S and 28S ribosomal bands after electrophoresis through an 0.8% formaldehyde/agarose minigel stained with ethidium bromide.
Radiolabeled cRNA coding for type I collagen and for S-14 was generated by transcription with T7 polymerase using [α-32P]CTP (19–21) by standard methods. Specific activity of all radiolabeled transcripts was approximately 0.5 × 109 cpm per microgram.
Total RNA was incubated with 0.5–1.0 × 106 Cerenkov cpm of 32P-labeled cRNA, denatured at 78°C, and hybridized in solution for 16 h at a temperature established as optimal in preliminary experiments (range, 55–65°C). Following hybridization, T2 RNase (GIBCO/BRL) was added to digest unbound label and unprotected mRNA. The protected hybrids were denatured and separated by electrophoresis through a 5% polyacrylamide/urea sequencing gel. Dried gels were applied to x-ray film (Kodak X-OMat AR-5) for 12–24 h. Bands corresponding to the protected labeled fragment were quantitated by scanning densitometry (Hoefer Scientific Instruments), and where statistical data are given, normalized to the level of S-14 mRNA.
Quantitative Cytokine Measurement.
Quantitative competitive PCR with polycompetitor cDNA coding for hypoxanthine-guanine phosphoribosyltransferase (HPRT), IL-4, IFN-γ, as well as TGF-β was performed as described (22). Briefly, total RNA was reverse transcribed in a reaction containing 1–5 μg RNA dissolved in DEPC-H2O, specific oligo(dt)12–18 primers (GIBCO/BRL), RNasin, and Moloney murine leukemia virus reverse transcriptase (Promega). PCR was performed in a reaction containing 10× buffer, 25 mM MgCl2, DNTPs, specific cytokine or HPRT primers, polycompetitor, and Taq polymerase (GIBCO/BRL). Cycling conditions were 94°C for 40 s, 60°C for 20 s, 72°C for 40 s, and a final extension of 72°C for 10 min. Titration of polycompetitor cDNA to ensure quantitative detection of mRNA was as described (22). Bands corresponding to the wild type mRNA were quantitated by scanning densitometry (Hoefer Scientific Instruments), and where statistical data are given, normalized to HPRT mRNA.
IgE Quantitation.
Total serum IgE was quantitated using a sandwich ELISA as described (13). Briefly, sera were added to wells that had been previously coated with 0.1 mg/ml primary antibody (B1E3). After blocking with PBS containing 5% BSA and washing with PBS containing 0.05% Tween 20, wells were incubated with biotinylated murine anti-IgG, washed, and developed using horseradish peroxidase-streptavidin with o-phenylenediamine.
Statistics.
ANOVA or the Student’s t test were used for statistical comparisons among groups. For calculation of mean values and statistical variation, n refers to the number of separate experiments each with an individual animal or cell preparation. Error bars depict the standard error of the mean (SEM); absence of error bars indicates that the SEM was <1%, unless stated otherwise.
RESULTS
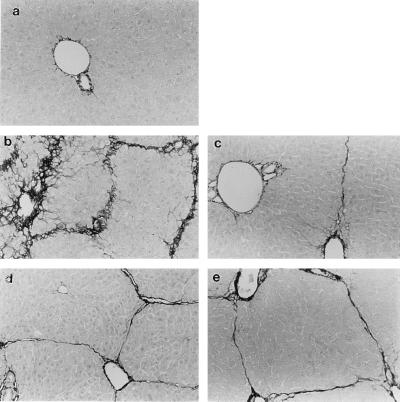
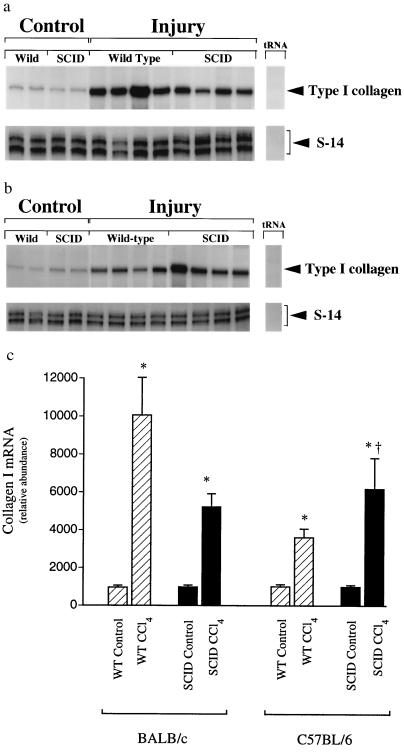
Fibrosing liver injury was induced by recurrent administration of CCl4, which causes acute injury and inflammation followed by fibrosis (23). Histologic examination of liver specimens demonstrated that wild-type BALB/c mice developed substantially more fibrosis than C57BL/6 animals (Fig. 1). The fibrosing response to injury was assessed by measuring whole liver type I collagen mRNA and collagen content (Fig. 2 and Table 1) and paralleled the morphometric findings. Uninjured liver contained little type I collagen mRNA whereas after chronic CCl4 administration, type I collagen mRNA was up-regulated in both BALB/c and C57BL/6 wild-type mice. However, there was significantly more type I collagen mRNA in the livers of BALB/c mice than of C57BL/6 mice. Quantitation of total hepatic collagen (using Sirius Red) revealed findings analogous to the histologic and collagen I mRNA analysis (Fig. 2). To investigate the possibility that the immune response was involved in the differential response to fibrosis revealed in BALB/c and C57BL/6 mice, we investigated mice with SCID, which lack T and B cells. The fibrosing response was distinctly different in SCID than in wild-type mice. Total hepatic collagen content was nearly equivalent in the two strains of SCID mice; BALB/c SCID mice developed less fibrosis than their wild-type counterparts, and fibrosis in C57BL/6 SCID mice was more severe than C57BL/6 wild-type animals, suggesting the presence of modulatory effects by the immune system (i.e., T and/or B cells).
Figure 1.
Hepatic extracellular matrix expression is differentially regulated in wild-type and SCID mice BALB/c and C57BL/6 mice. Liver fibrosis was induced by gavage with CCl4 (3.5 ml/kg, mixed 1:1 with corn oil administered at 7 day intervals). Seven days after the 4th dose of CCl4, animals were killed, and livers were fixed in 10% formalin and stained with Sirius Red as described. Collagen is stained by Sirius Red and appears black. (a) Normal control BALB/c receiving corn oil vehicle alone (identical to C57BL/6; data not shown). (b) Wild-type BALB/c. (c) Wild-type C57BL/6. (d) SCID BALB/c. (e) SCID C57BL/6. Representative photomicrographs are shown. (×90.)
Figure 2.
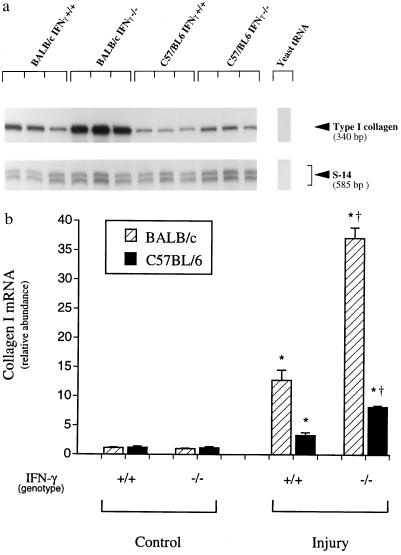
Type I collagen mRNA expression in wild-type and SCID mice BALB/c and C57BL/6 mice. Liver fibrosis was induced by gavage with CCl4 (3.5 ml/kg, mixed 1:1 with corn oil, was administered at 7 day intervals by gavage). Seven days after the 4th dose of CCl4, animals were killed and total cellular RNA extracted as described. RNase protection assay with probes complimentary to type I collagen and S-14 (an internal standard mRNA) using 10 μg of RNA was performed. Representative RNase protection assays are shown in a and b [BALB/c and C57BL/6 mice, respectively]. Specific bands were quantitated and normalized to the signal for S-14 in c. (n = 4, ∗, P < 0.05 for CCl4 compared with control for each individual strain, †, P < 0.05 for SCID compared with wild type.
Table 1.
Total hepatic collagen content after liver injury
| Mouse strain | Control | Wild type | SCID |
|---|---|---|---|
| BALB/c | 1.0 ± 0.05 | 2.98 ± 0.16*‡ | 2.21 ± 0.22*‡ |
| C57BL/6 | 1.1 ± 0.06 | 1.52 ± 0.03* | 2.12 ± 0.14*‡ |
Liver fibrosis was induced by gavage with CCl4 (3.5 ml/kg, mixed 1:1 with corn oil, was administered at 7-day intervals) in wild-type and SCID BALB/c and C57BL/6 mice. Seven days after the 4th dose of CCl4, animals were killed and livers fixed in 10% formalin. Liver tissue sections were stained with Sirius Red, and collagen was extracted and quantitated spectrophotometrically as described. Control mice received corn oil alone on the same schedule as animals receiving CCl4. The value for normal BALB/c mice was arbitrarily set at 1; other values were normalized.
P < 0.05 compared to control.
P < 0.05 for BALB/c wild-type compared to C57BL/6 wild-type mice.
P < 0.05 compared to normal or wild type (n = 4 for each group; values shown are mean ± SD).
We postulated that variability in the degree of acute hepatocellular injury could lead to differences in the chronic response to injury. Therefore, we measured aminotransferase levels (as markers for acute hepatocellular necrosis) and histologic abnormalities 24 h after the initial exposure to CCl4. Liver aminotransferases (AST/ALT) levels were similar in all animals, except for SCID C57BL/6 mice, which displayed lower aminotransferase levels than wild-type SCID C57BL/6 or BALB/c animals (Table 2). Inspection of histologic specimens revealed that there were no notable differences between wild-type C57BL/6 and BALB/c animals, but that acute hepatocellular necrosis was slightly less in C57BL/6 SCID than BALB/c SCID mice, consistent with aminotransferase values.
Table 2.
Aminotransferases 24 h after toxin administration
| Mouse strain | AST, units/liter | ALT, units/liter |
|---|---|---|
| BALB/c WT | 20,476 ± 2,583 | 4,554 ± 726 |
| BALB/c SCID | 17,787 ± 3,032 | 4,256 ± 3,762 |
| C57BL/6 WT | 26,620 ± 4,841 | 4,667 ± 1,503 |
| C57BL/6 SCID | 4,002 ± 916* | 2,225 ± 2,439 |
Liver injury was induced by administration of CCl4 by gavage (3.5 ml/kg, mixed 1:1 with corn oil). Twenty-four hours after gavage, serum aminotransferase levels were measured. WT, wild type; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
P < 0.05 SCID C57BL/6 vs. SCID BALB/c mice (n = 4 for each group).
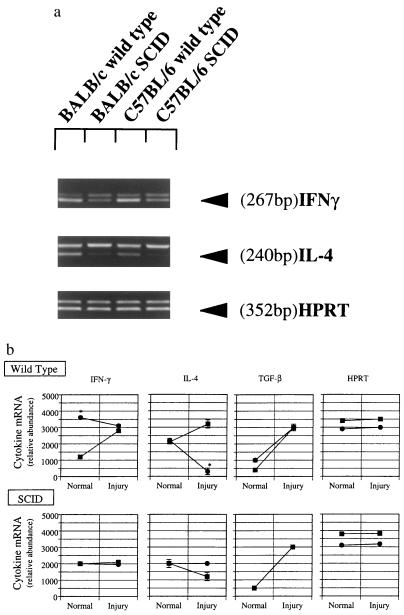
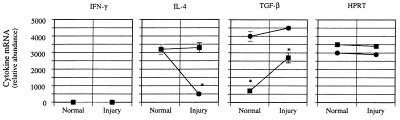
To define the cytokines present in the hepatic wound-healing environment, we quantitated IFN-γ, IL-4, and TGF-β mRNA levels in crude nonparenchymal cell preparations (Fig. 3). In normal wild-type mice, IFN-γ mRNA levels were significantly higher in C57BL/6 mice than in BALB/c mice, but the level of IL-4 was similar in both strains. Following liver injury and fibrogenesis, IFN-γ and IL-4 mRNA levels increased in BALB/c mice. In contrast, in C57BL/6 mice, IFN-γ remained essentially unchanged whereas IL-4 mRNA levels decreased dramatically. The response of IL-10 to liver injury and fibrosis was similar to that of IL-4 (data not shown). In contrast to both strains of wild-type mice, mRNA levels for IFN-γ and IL-4 in SCID mice did not change significantly with injury (Fig. 3). TGF-β mRNA levels were increased in all animals after liver injury.
Figure 3.
Hepatic cytokine mRNA expression in fibrotic liver injury in SCID mice. Liver fibrosis was induced as in Fig. 1. Seven days after the 4th dose of CCl4, animals were killed, total cellular RNA extracted, and competitive PCR using primers for IFN-γ, IL-4, TGF-β, and HPRT performed as described. A representative gel is shown in a. Scanned, quantitated, and normalized data are presented graphically in b. Circles represent C57BL/6 mice, and squares represent BALB/c animals. ∗, P < 0.05 for C57BL/6 compared with BALB/c mice (n = 4 for group).
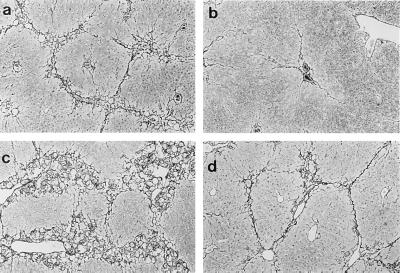
We next examined fibrogenesis in IFN-γ−/− mice, which we hypothesized would default to a Th2 response. Morphometrically, both strains displayed greater fibrosis than wild-type animals (Fig. 4). Quantitative analysis of type I collagen mRNA levels and whole liver collagen further demonstrated enhanced fibrogenesis in both strains in IFN-γ−/− animals compared with IFN-γ+/+ mice [Fig. 5 and data not shown (Sirius Red)]. Similar quantitative differences were detected for the extracellular matrix mRNA, cellular fibronectin (data not shown). Similar qualitative differences have been detected in IFN-γ−/− animals after recurrent administration of dimethylnitrosamine, a hepatotoxin that also induces a mechanistically different fibrosing response in the liver after chronic administration (data not shown). Aminotransferase levels in IFN-γ−/− mice were as follows: AST: BALB/c, 28,482 ± 8,659; C57BL/6, 36,461 ± 4,709; ALT: BALB/c, 2,665 ± 607; C57BL/6, 1,122 ± 466. There were no significant differences compared with wild-type animals.
Figure 4.
Hepatic extracellular matrix production in IFN-γ-deficient mice after liver injury. Liver fibrosis was induced by gavage with CCl4 (3.5 ml/kg, mixed 1:1 with corn oil, was administered at 7 day intervals by gavage). Seven days after the 4th dose of CCl4, animals were killed, and livers were fixed in 10% formalin and Sirius Red as described. Collagen is stained with Sirius Red and appears black. (a) Wild-type BALB/c. (b) Wild-type C57BL/6. (c) IFN-γ−/− BALB/c. (d) IFN-γ−/− C57BL/6. Representative photomicrographs are shown. (×60.)
Figure 5.
Type I collagen mRNA expression after toxin-induced fibrosis in IFN-γ-deficient BALB/c and C57BL/6 mice. Liver fibrosis was induced by gavage with CCl4 (3.5 ml/kg, mixed 1:1 with corn oil, was administered at 7 day intervals by gavage). Seven days after the 4th dose of CCl4, animals were killed and total cellular RNA extracted as described. RNase protection assay with probes complimentary to type I collagen, and S-14 (an internal standard mRNA) using 10 μg of RNA was performed. A representative RNase protection assay is shown in a. Specific bands were quantitated, normalized to the signal for S-14, and displayed graphically in b. The value for type I collagen mRNA in normal BALB/c mice was arbitrarily set to 1. ∗, P < 0.05 compared with control (either IFN-γ+/+ or IFN-γ−/−). †, P < 0.05 for IFN-γ−/− compared with IFN-γ+/+ mice receiving CCl4.
Cytokine mRNA levels were also investigated in IFN-γ−/− mice. As expected, IFN-γ mRNA was undetectable in IFN-γ−/− mice (Fig. 6). In BALB/c IFN-γ−/− animals, IL-4 mRNA levels increased slightly after wounding; however, levels of this cytokine mRNA decreased in C57BL/6 IFN-γ−/− animals. In both strains of IFN-γ−/− mice, TGF-β mRNA levels rose during injury and wound healing.
Figure 6.
Hepatic cytokine mRNA expression in fibrotic liver injury in IFN-γ-deficient mice. Liver fibrosis was induced as in Fig. 1. Seven days after the 4th dose of CCl4, animals were killed, total cellular RNA extracted, and competitive PCR using primers for IFN-γ, IL-4, TGF-β, and HPRT performed as described. Scanned, quantitated, and normalized data are presented graphically. Circles represent C57BL/6 mice and squares BALB/c animals. ∗, P < 0.05 for C57BL/6 compared with BALB/c mice (n = 3 for each group).
To further investigate Th cytokine subset responses in mice after toxin-induced liver fibrosis, serum IgE levels were quantitated. Wild-type BALB/c mice displayed striking (nearly 8-fold) increases in serum IgE values, whereas C57BL/6 mice exhibited a significantly less pronounced change (Table 3). Both strains of IFN-γ-deficient mice displayed significant increases in serum IgE values, in particular the BALB/c strain.
Table 3.
Serum IgE levels in BALB/c and C57BL/6 wild-type and IFN-γ-deficient mice
| Mouse strain | IFN-γ phenotype | Basal (normal) | CCl4 |
|---|---|---|---|
| BALB/c | ++ | 0.22 ± 0.03 | 1.73 ± 0.33* |
| C57BL/6 | ++ | 0.33 ± 0.04 | 0.98 ± 0.23*† |
| BALB/c | −− | 0.17 ± 0.01 | 2.60 ± 0.32*‡ |
| C57BL/6 | −− | 0.15 ± 0.06 | 1.47 ± 0.48*‡ |
Units of IgE are mg/nl. Liver injury and fibrosis were as in Table 1. Seven days after the final dose of CCl4, total serum IgE was quantitated as described.
P < 0.05 compared to basal levels.
P < 0.05 for C57BL/6 IFN-γ+/+ compared to BALB/c IFN γ+/+.
P < 0.05 for BALB/c or C57BL/6−/− compared to wild-type animals of each strain, respectively (n = 4 for each group).
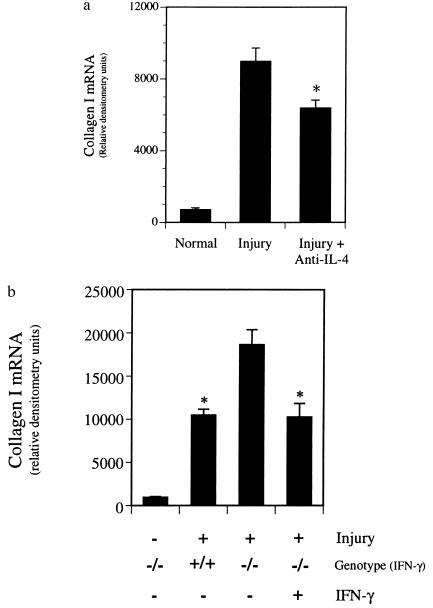
Finally, we examined the role of Th cytokine subsets in the hepatic wounding response by blocking development of the Th2 response with neutralizing antibody to IL-4 (24) or by enhancing the Th1 pathway via administration of IFN-γ in wild-type and IFN-γ−/− BALB/c animals, respectively. Neutralizing anti-IL-4 reduced extracellular matrix deposition by morphometric examination (Sirius Red, as described in Materials and Methods, data not shown) as well as type I collagen mRNA expression by ≈30% (Fig. 7a), whereas control IgG had no effect on type I collagen mRNA expression (data not shown). In addition, exogenous IFN-γ administered to IFN-γ−/− animals reduced the level of fibrosis to that of wild-type animals as evidenced by both morphometric analysis (Sirius Red, as described in Materials and Methods, data not shown) and type I collagen mRNA expression (Fig. 7b).
Figure 7.
Effect of anti-IL-4 or IFN-γ on hepatic fibrogenesis. Liver injury and fibrosis were induced with CCl4 as in Fig. 1. (a) Anti-IL-4 (5 mg) [hybridoma cells producing neutralizing anti-IL-4 (clone 11B11) were a kind gift of B. Paul (National Institutes of Health)] was administered intraperitoneally 48 h after each dose of CCl4 to wild-type BALB/c mice. Livers were harvested and RNase protection assay for type I collagen and S-14 mRNAs was performed as described. Data presented represent those after quantitation of specific bands and normalization as in Fig. 2. ∗, P < 0.05 compared with CCl4 alone (n = 4). (b) Recombinant mouse IFN-γ (25,000 units; Genentech) was begun 48 h after the initial dose of CCl4 and continued daily in IFN-γ−/− BALB/c mice. After four doses of CCl4, type I collagen and S-14 mRNAs were detected and quantitated, and type I collagen mRNA abundance expressed graphically. ∗, P < 0.05 compared with IFN-γ−/− animals not receiving IFN-γ (n = 3).
DISCUSSION
We have demonstrated that C57BL/6 mice developed significantly less fibrosis following CCl4-induced liver injury than BALB/c mice. In addition, C57BL/6 mice exhibited an elevated ratio of hepatic IFN-γ to IL-4 mRNA levels during the fibrogenic response, whereas BALB/c mice displayed elevated IL-4 message and IgE levels; the former finding is consistent with a Th1 response and the latter result is consistent with a Th2 response. Many infectious disease models demonstrate that host phenotype-resistance or susceptibility is dependent on cytokines produced by specific CD4+ T cell subsets (reviewed in refs. 8 and 25). This paradigm has also been extended to noninfectious models such as asthma and autoimmune disease (26–28). Our findings raise the possibility that Th1/Th2 cytokine subsets can modulate fibrosis in a noninfectious model of hepatic fibrosis.
We considered the possibility that the degree of acute hepatocellular injury after toxin exposure influenced the observed fibrogenic response. However, aminotransferases 24 h after acute exposure to CCl4 were identical in wild-type BALB/c and C57BL/6 mice, yet fibrogenesis was significantly greater in BALB/c mice. We also found that acute hepatocellular necrosis was similar in wild-type BALB/c and in SCID BALB/c mice, but that late fibrosis was significantly more pronounced in the former animals. Thus, the degree of acute toxicity (with accompanying hepatocellular destruction dependent production and/or release of profibrogenic factors) itself does not explain the differential fibrogenic response. These data further support the contention that host factors including the host immune response (and cytokine production) are involved in tissue repair mechanisms and contribute to observed differences in fibrosis.
Although there was a significant difference in the extent of hepatic fibrosis in wild-type BALB/c and C57BL/6 mice, it was nearly identical in each strain of SCID mice. In addition, cytokine mRNA expression varied in wild-type mice but was similar in the SCID strains. Given the lack of T and B cells in SCID mice, these data strongly support a role for immune modulation of liver fibrosis. However, because SCID mice exhibited a fibrotic response to injury, albeit different than that of wild-type mice, the data also emphasize that other factors, including, but not limited to, T or B lymphocyte-derived cytokines, play a role in fibrogenesis. For example, we found that the mRNA for TGF-β, a known profibrogenic cytokine, was increased in all animals. Thus, in our models, this factor may contribute at least to a baseline level of fibrogenesis.
An important finding of the cytokine analyses was that levels of IL-4 mRNA in nonparenchymal cells from livers of wild-type BALB/c were elevated relative to those for C57BL/6 mice after the induction of injury. Though serum IgE was also elevated after injury in both strains of mice, the magnitude of the response was much greater in BALB/c than C57BL/6 mice. Together, these findings implicate a role for the lymphocyte-derived cytokine IL-4 in the fibrogenic response and imply a Th2 response in wild-type BALB/c mice. The ability of IL-4 to promote collagen synthesis has been reported in previous studies (29, 30), although its mechanism remains unclear. The finding that anti-IL-4 diminished hepatic fibrosis further implicates Th2 differentiation in (this model of) hepatic wounding. Such data are consistent with other systems in which manipulating IL-4 alters the host phenotype, as seen in leishmaniasis (24, 31) schistosomiasis (32), and asthma (26). These data lead to the postulate that genetic host factors may be important in determining differential fibrotic responses to injury. It should be emphasized, however, that the interrelationships between immune modulatory cells (i.e., T, NK cells) themselves as well as the cytokines they produce, is complex. Thus, ongoing experiments to isolate and/or deplete specific lymphocyte cell subsets are expected to provide further insight into the diverse fibrosing responses elucidated here.
Both strains of IFN-γ−/− mice developed more severe fibrosis than their wild-type counterparts. These data support an important role for IFN-γ, a Th1 cytokine, in regulating the development of hepatic fibrosis and are consistent with previous studies in liver and in extra-hepatic organs (33–35). Interestingly, fibrosis was more prominent in IFN-γ−/− BALB/c than IFN-γ−/− C57BL/6 mice. The mechanism of differential fibrogenesis in these animals is open to speculation, but could be related to the enhanced production of IL-4 during injury in BALB/c compared to C57BL/6 mice. Alternatively, the degree and severity of fibrosis may be influenced by the balance between IL-4 and IFN-γ production. Finally, we cannot exclude the possibility that other cytokines or factors play a role in the observed response in IFN-γ-deficient animals. For example, TGF-β levels were elevated in both strains of IFN-γ deficient mice, and given previous data (36), this compound is likely to play a role in fibrogenesis.
Further support for the postulate that the Th phenotype helps determine the extent of fibrogenesis in our model of liver injury was provided by demonstrating that skewing the host Th response toward a Th1 pathway, either with anti-IL-4 or with IFN-γ, ameliorated fibrosis. Thus, not only are host factors such as the immune response important components of fibrogenesis, but they may be critical in explaining divergent wounding responses of individuals exposed to identical injurious agents. Because genetic background influences T lymphocyte development and therefore the specific immune response, the data raise the possibility that the fibrogenic response to injury may be in part genetically dependent. The data presented in this study elucidate a paradigm for the fibrogenic response to liver injury, and they raise the possibility that analogous responses are present in other organs. In addition, the findings of this study have important implications for treatment of patients with liver disease. If future studies corroborate similar paradigms in human fibrosing liver disease, modulation of the Th response within the liver may represent a novel and beneficial approach for treatment of patients with liver injury.
Acknowledgments
We thank Genentech and Dr. Timothy A. Stewart (South San Francisco, CA) for supplying IFN-γ-deficient mice, Dr. Richard Locksley for providing polycompetitor cDNA, and Dr. William Paul for providing 11B11 hybridoma cells (anti-IL-4). We thank Drs. Monty Bissell, John Imboden, and Jay Ryan for review the manuscript and helpful discussions. Z.S. was supported initially by the University of California, San Francisco, Cheng Scholarship program. A.E.W. was supported by the Crohn’s and Colitis Foundation of America and the Technical Training Foundation of North Andover. D.C.R. was supported by the American Liver Foundation and the National Institutes of Health (Grants DK 02124 and DK 50574).
ABBREVIATIONS
- IFN-γ
interferon γ
- IL
interleukin
- TGF-β
transforming growth factor β
- Th
T helper
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- CCl4
carbon tetrachloride
- SCID
severe combined immunodeficient
References
- 1.Friedman S L. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka M, Pham N T, Tsukamoto H. Liver. 1989;9:71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakatsukasa H, Nagy P, Evarts R P, Hsia C C, Marsden E, Thorgeirsson S S. J Clin Invest. 1990;85:1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czaja M J, Weiner F R, Flanders K C, Giambrone M A, Wind R, Biempica L, Zern M A. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czaja M J, Weiner F R, Takahashi S, Giambrone M A, van der Meide P H, Schellekens H, Biempica L, Zern M A. Hepatology. 1989;10:795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- 6.Winnock M, Garcia Barcina M, Lukomska B, Huet S, Saric J, Balabaud C, Bioulac-Sage P. J Gastroenterol Hepatol. 1995;10:S43–S46. doi: 10.1111/j.1440-1746.1995.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 7.Tiegs G, Hentschel J, Wendel A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 10.Reiner S L, Locksley R M. J Cell Biochem. 1993;53:323–328. doi: 10.1002/jcb.240530409. [DOI] [PubMed] [Google Scholar]
- 11.Guler M L, Gorham J D, Hsieh C S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 12.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granstein R D, Murphy G F, Margolis R J, Byrne M H, Amento E P. J Clin Invest. 1987;79:1254–1258. doi: 10.1172/JCI112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 16.Yamane M, Tanaka Y, Marumo F, Sato C. Liver. 1993;13:282–287. doi: 10.1111/j.1600-0676.1993.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 17.de Leeuw A M, McCarthy S P, Geerts A, Knook D L. Hepatology. 1984;4:392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]
- 18.Bissell D M, Hammaker L E, Meyer U A. J Cell Biol. 1973;59:722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher J J, McGuire R F. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarnagin W R, Rockey D C, Koteliansky V E, Wang S S, Bissell D M. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton D A, Krieg P A, Rebagliati M R, Maniatis T, Zinn K, Green M R. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiner S L, Zheng S, Corry D B, Locksley R M. J Immunol Methods. 1993;165:133. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 23.Proctor E, Chatamra K. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- 24.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locksley R M, Wakil A E, Corry D B, Pingel S, Bix M, Fowell D J. Ciba Found Symp. 1995;195:110–117. doi: 10.1002/9780470514849.ch8. (discussion, 117–122). [DOI] [PubMed] [Google Scholar]
- 26.Corry D B, Folkesson H G, Warnock M L, Erle D J, Matthay M A, Wiener-Kronish J P, Locksley R M. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott B, Liblau R, Degermann S, Marconi L A, Ogata L, Caton A J, McDevitt H O, Lo D. Immunity. 1994;1:73–83. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 28.Katz J D, Benoist C, Mathis D. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 29.Postlethwaite A E, Holness M A, Katai H, Raghow R. J Clin Invest. 1992;90:1479–1485. doi: 10.1172/JCI116015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sempowski G D, Derdak S, Phipps R P. J Cell Physiol. 1996;167:290–296. doi: 10.1002/(SICI)1097-4652(199605)167:2<290::AID-JCP13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Wakil A E, Wang Z E, Locksley R M. Exp Parasitol. 1996;84:214–222. doi: 10.1006/expr.1996.0107. [DOI] [PubMed] [Google Scholar]
- 32.Cheever A W, Williams M E, Wynn T A, Finkelman F D, Seder R A, Cox T M, Hieny S, Caspar P, Sher A. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 33.Czaja M J, Weiner F R, Eghbali M, Giambrone M A, Zern M A. J Biol Chem. 1987;262:13348–13351. [PubMed] [Google Scholar]
- 34.Granstein R D, Deak M R, Jacques S L, Margolis R J, Flotte T J, Whitaker D, Long F H, Amento E P. J Invest Dermatol. 1989;93:18–27. doi: 10.1111/1523-1747.ep12277336. [DOI] [PubMed] [Google Scholar]
- 35.Pittet B, Rubbia-Brandt L, Desmouliere A, Sappino A P, Roggero P, Guerret S, Grimaud J A, Lacher R, Montandon D, Gabbiani G. Plast Reconstr Surg. 1994;93:1224–1235. doi: 10.1097/00006534-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Armendariz-Borunda J, Seyer J M, Kang A H, Raghow R. FASEB J. 1990;4:215–221. doi: 10.1096/fasebj.4.2.2298342. [DOI] [PubMed] [Google Scholar]