Abstract
Current guidelines for the prevention of coronary heart disease emphasize the importance of global cardiovascular risk, which requires the evaluation and treatment of multiple risk factors. Cardiovascular risk can be stratified with the Framingham algorithm, which produces a numerical score related to the presence of risk factors, such as hypertension, dyslipidemia, and smoking. However, this algorithm is not generally applicable to European countries, particularly for those countries where the risk for cardiovascular disease is low. The SCORE (Systematic COronary Risk Evaluation) project has produced risk charts that are based on cholesterol, blood pressure, and age for low-risk European countries (Belgium, France, Greece, Italy, Luxembourg, Spain, and Switzerland) and high-risk countries. Assessments of end-organ damage can provide further prognostic information, particularly in intermediate-risk patients, but the value of including additional biomarkers in risk stratification remains to be confirmed. Risk for coronary heart disease is high or very high in more than 50% of hypertensive patients. Risk appears to be underestimated in clinical practice, particularly in those patients at highest risk. Major intervention trials with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have shown that these agents reduce the risk for cardiovascular events in patients at all levels of risk, with the greatest benefits seen in those at highest risk.
Introduction
Cardiovascular disease, particularly coronary heart disease (CHD), remains a major cause of mortality and morbidity in industrialized countries, despite advances in prevention and treatment. The problem is also spreading to developing countries and is thus becoming a worldwide threat.[1] Although the impact of individual risk factors, such as hypertension or dyslipidemia, is well established, the past decade has seen a growing emphasis on the management of global cardiovascular risk, which requires evaluation and treatment of multiple risk factors. This trend has been driven by the finding in large epidemiologic studies that cardiovascular risk factors have synergistic, rather than additive, effects on total risk. Data from the Framingham Heart Study, for example, show that hypertension (defined as a systolic blood pressure [SBP] of ≥ 150 mm Hg) increases the 8-year risk for cardiovascular disease 1.5-fold, and dyslipidemia (total cholesterol ≥ 6.5 mmol/L [≥ 260 mg/dL]) increases the risk 2.3-fold, compared with that in a 40-year-old man with normal blood pressure (SBP ≤ 120 mm Hg systolic) and cholesterol (total cholesterol ≤ 4.6 mmol/L [≤ 185 mg/dL]). However, the presence of these 2 risk factors together increases the risk 3.5-fold. Furthermore, the additional presence of glucose intolerance results in a 6.2-fold increase in risk.[2–5] A further analysis from the same study showed that, for any given level of total cholesterol, the risk for CHD increases exponentially with the number of additional risk factors (Figure 1).[6,7]
Figure 1.

Risk for coronary heart disease according to total cholesterol level and number of additional risk factors (ECG = electrocardiography; LVH = left ventricular hypertrophy; SBP = systolic blood pressure). Reproduced with permission from Kannel.[7]
Such findings highlight the importance of effective interventions to reduce global cardiovascular risk in patients with multiple risk factors. This article discusses the question of how such patients can be identified in clinical practice and reviews insight from major outcome trials in patients at different levels of cardiovascular risk.
Identification of High-Risk Patients by Algorithms and Risk Assessment Charts
According to the hypertension management guidelines published by the European Society of Hypertension-European Society of Cardiology (ESH/ESC), patients with elevated blood pressure (SBP ≥ 130 mm Hg, diastolic blood pressure [DBP] ≥ 85 mm Hg) and associated clinical conditions, such as proteinuria or a history of myocardial infarction, or target-organ damage, such as atherosclerotic plaques, are considered to be at “very high” risk for cardiovascular disease.[8] In addition, cigarette smoking is a well-documented and potent risk factor for cardiovascular disease.[9] For instance, a meta-analysis of 32 studies estimated the relative risk for ischemic stroke to be 1.9 (95% confidence interval [CI] 1.7, 2.2) in smokers vs nonsmokers.[10] In the United States, an estimated 21,400 (without adjustment for potential confounding factors) and 17,800 (with adjustments) stroke deaths annually can be attributed to smoking, suggesting that smoking contributes to 12% to 14% of all stroke deaths.[11] A history of smoking also predicted an increased risk for acute myocardial infarction (adjusted odds ratio, 1.81; 95% CI 1.75, 1.87).[12] Smoking cessation is associated with a substantial decrease in the risk for clinical cardiovascular events, such as all-cause mortality (relative risk reduction, 36%; 95% CI 29, 42) and nonfatal myocardial infarction (relative risk reduction, 32%; 95% CI 18, 43) compared with those who continue to smoke.[13] One year after quitting smoking, the risk for CHD has been shown to decrease by 50%.[14]
Whereas the patients described above are easily recognized in clinical practice, the identification of patients at lower levels of risk is more problematic.
The European guidelines define patients as being at high multifactorial risk if the 10-year absolute risk for cardiovascular death is ≥ 5%, or if the risk will exceed 5% if projected to the age of 60 years.[8] By contrast, the US National Cholesterol Education Program (NCEP) guidelines define high-risk patients as having a 10-year absolute risk for CHD events of ≥ 20%, on the basis of the presence of various risk factors.[15] In the latter guidelines, risk is calculated with the Framingham algorithm, in which points are assigned according to age, smoking status, SBP, and total and high-density lipoprotein cholesterol to give a total score that is related to the 10-year risk for CHD.[15] This algorithm stratifies individuals with multiple risk factors according to their 10-year risk for CHD (< 10%, 10% to 20%, > 20%). It is derived from a large and robust database, and has been shown to be widely applicable to US populations.[16] However, population risks for CHD differ widely between European countries; thus, recalibration of the Framingham algorithm is necessary in low-risk European populations. The impact of such differences was highlighted by an analysis from the Prospective Epidemiological Study of Myocardial Infarction study (PRIME), which compared the 5-year CHD risk predicted from the Framingham algorithm with that observed in men aged 50–59 years in Belfast, United Kingdom (a high-risk population) and France (a low-risk population) free of CHD at baseline.[17] Among the Belfast subjects (n = 2399), there was generally good agreement between the predicted risk and observed CHD events (coronary death, myocardial infarction, and angina pectoris) among men at all deciles of estimated risk; the estimated ratio of predicted-to-observed CHD events was 1.34 (95% CI 1.12, 1.60). On the other hand, in the French population (n = 7359), the Framingham algorithm tended to overestimate CHD risk by approximately 2-fold (estimated ratio, 2.35; 95% CI 2.05, 2.71). Such findings highlight the need for specific risk evaluations in populations at different levels of risk.
Recognition of these differences in risk between US and some other populations led to the initiation of the Systematic COronary Risk Evaluation (SCORE) project, in order to develop a risk scoring system that is broadly applicable within Europe.[18] This system was developed from 12 European cohort studies, most of which were population-based, involving a total of 205,178 subjects (117,098 men, 88,080 women); the total duration of follow-up amounted to 2.7 million person-years. Among this population, there were 7934 deaths from cardiovascular causes, 5652 of which were attributable to CHD. The SCORE system predicts the 10-year risk for fatal cardiovascular events according to either total cholesterol (Figure 2) or the total cholesterol:high-density lipoprotein cholesterol ratio and is sex-specific. An individual is considered to be at high risk if their risk for fatal cardiovascular disease is ≥ 5%. Separate risk scoring charts are available for countries with low cardiovascular risk (Belgium, France, Greece, Italy, Luxembourg, Spain, and Switzerland) and high risk (all other European countries). No separate charts were produced for persons with diabetes, because in most cohorts the diagnosis of diabetes was based on self-report.[18] However, in view of the strong association between diabetes and increased cardiovascular risk,[19] and the higher risk for fatal CHD among diabetic women compared with diabetic men,[20] the instructions for the use of the SCORE charts state that in diabetic patients the estimated risk should be doubled in men and quadrupled in women.[18]
Figure 2.
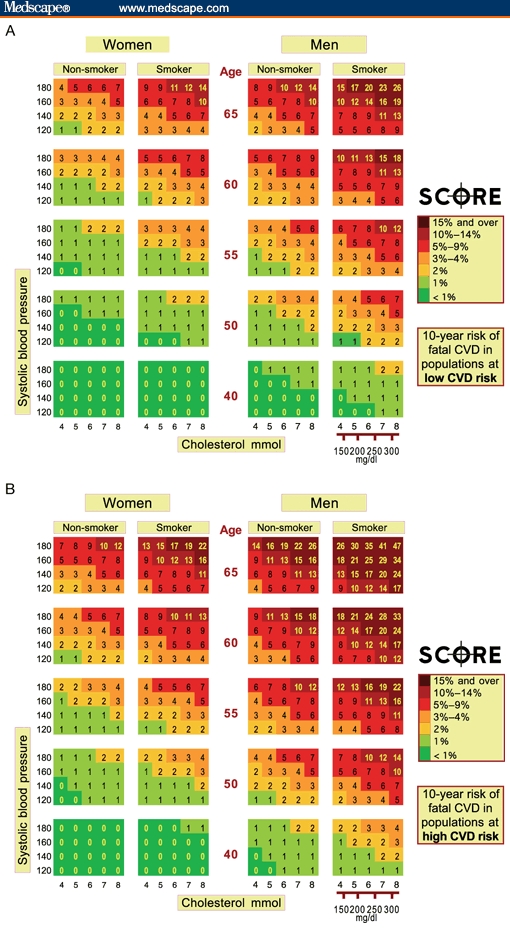
SCORE (Systematic COronary Risk Evaluation) risk charts based on total cholesterol, showing 10-year risk for fatal cardiovascular disease in countries with (A) low risk (Belgium, France, Greece, Italy, Luxembourg, Spain, and Switzerland) and (B) high risk for cardiovascular disease. Reproduced with permission from Conroy and coworkers.[18]
It should be noted that death rates from acute myocardial infarction have declined markedly since the late 1970s in some, but not all, European countries. The most dramatic decrease has occurred in Norway, where death rates have decreased from approximately 108 per 100,000 in 1980 to approximately 35 per 100,000 in 2000. Indeed, annual mortality from acute myocardial infarction in Norway is now lower than in Greece (a country considered low-risk in the SCORE charts[18]), where mortality has remained almost unchanged during the same period. Hence, it can be anticipated that it will become necessary to adapt the SCORE system according to these historical trends.
How Effective Is Risk Assessment in Clinical Practice?
The current guidelines for the prevention of CHD, emphasizing the importance of global risk assessment, are widely accepted by physicians. In the Reassessing European Attitudes about Cardiovascular Treatment (REACT) survey of 754 primary care physicians from 5 European countries (France, Germany, Italy, Sweden, and the United Kingdom) conducted in 2002, 89% of the respondents agreed with the content of the then current guidelines and 81% reported using these guidelines.[21] However, only 18% of respondents believed that the guidelines were being widely implemented: Potential barriers to greater implementation included lack of time (reported by 38% of physicians), prescription costs, and patient compliance.
Furthermore, there is growing evidence that, even when risk management guidelines are followed, the risk is often stratified inaccurately. The recent CONTROLRISK study conducted in Spain investigated the accuracy of risk stratification in 8920 hypertensive patients recruited from primary care (n = 4485) or specialist (n = 4435) practices.[22] Overall, 75.1% of patients treated by specialists and 60.3% of those treated in primary care were at high or very high risk for cardiovascular disease: Target organ damage, such as left ventricular hypertrophy, was present in 57.6% and 34.3%, respectively, and associated clinical conditions, such as CHD, were present in 21.5% and 13.1%, respectively. The level of cardiovascular risk, assessed according to the ESH/ESC guidelines, was stratified correctly in 54.6% of patients treated by specialists and in only 48% of patients treated in primary care. The level of risk was underestimated by 1 level in approximately 30% of patients in both settings, and by 2 levels in approximately 10% of patients in both settings (Figure 3). Most worryingly, patients with a very high level of risk were only identified as such in 44.9% of cases by specialists and in 25.3% by primary care physicians (P < .001).
Figure 3.

Accuracy of risk stratification (according to European Society of Hypertension-European Society of Cardiology [ESH/ESC] guidelines) in hypertensive patients treated in primary care or specialist practice in the CONTROLRISK study. Reproduced with permission from Barrios and colleagues.[22]
Similar results were obtained in a UK study in which global cardiovascular risk was assessed in 397 hypertensive patients aged between 60 and 79 years by general practitioners and practice nurses using Framingham-based tables.[23] The level of risk was estimated correctly in only 21% of patients, and was underestimated in 63%, there being no systematic difference between estimates made by general practitioners and practice nurses.
Assessment of Target Organ Damage in Risk Stratification
Target organ damage is a common finding in hypertensive patients,[22] and offers a potential means of improving risk stratification in patients at intermediate risk for CHD.[24] Such approaches include measurement of arterial intima-medial thickness and flow-mediated vasodilation by arterial ultrasonography, and tonometric assessments of arterial function (eg, the ankle-brachial index, augmentation index, and pulse wave velocity). These techniques can provide additional prognostic information to that derived from conventional risk factors.[25] As a result, a recent review has proposed that they should be used in patients assessed as being at low risk in whom suspicions are raised by factors, such as a family history of early CHD, the metabolic syndrome, or substantial elevation of a single risk factor.[24]
An alternative approach is based on the detection of subclinical atherosclerosis by measurement of high-sensitivity C-reactive protein (hs-CRP) and computed tomographic evaluation of coronary artery calcification (CAC). A recent study has shown that CAC is a stronger predictor of cardiovascular risk than classical risk factors, such as hypertension, smoking, or diabetes.[26] The relative risk for CHD in men with high CAC, compared with those with low CAC, was 18.2 (95% CI 10.6, 31.3). By comparison, the relative risks (95% CI) associated with a history of hypertension, diabetes, or dyslipidemia were 1.7 (1.3, 2.2), 1.9 (1.4, 2.5), or 2.5 (1.9, 3.3), respectively. Other potential biomarkers include hs-CRP,[27] microalbuminuria,[28] and homocysteine.[29] Although such markers may provide useful additional information, particularly in low- or intermediate-risk patients, prospective trials combining clinical assessment and diagnostic imaging are needed to establish their precise role in risk stratification.
Insights From Clinical Trials
As noted above, the ESH/ESC guidelines define a patient as being at high risk if the absolute risk for cardiovascular death is ≥ 5% over 10 years, or will exceed 5% if projected to 60 years of age.[8] Several, recent, large-scale outcome trials with angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme (ACE) inhibitors have focused on patients in this category. The cardiovascular mortality rates observed in these trials emphasize the impact of multiple risk factors, target organ damage, and associated clinical conditions.
Primary Prevention Studies
The Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial involved 15,245 hypertensive patients (SBP 160–210 mm Hg, DBP 95–105 mm Hg), aged 50 years and over, who were considered to be at high risk for cardiovascular disease due to the presence of at least 1 risk factor or associated disease.[30] The projected 10-year cardiovascular mortality in these patients was 9.2%, confirming that this was indeed a high-risk patient population.
Similarly, in the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study, the 10-year projected cardiovascular mortality was 9.2% in patients with ARBs and 10.6% in those treated with atenolol.[31] In LIFE, patients were between the ages of 55 and 80 years at baseline and had a history of treated or untreated hypertension (SBP 160–200 mm Hg, DBP 95–115 mm Hg). In addition, all patients had electrocardiographic evidence of left ventricular hypertrophy (Cornell voltage product > 2440 mm × ms or Sokolow-Lyon voltage > 38 mm). Thus, this trial illustrates how markers of end-organ damage can help to identify patients at high risk for cardiovascular death.
More recently, the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) has compared the effects of various antihypertensive drug regimens on cardiovascular events in hypertensive patients aged 40–79 years with no history of myocardial infarction or current CHD.[32] Patients in this study were considered to be at high cardiovascular risk due to the presence of at least 3 other cardiovascular risk factors. The projected 10-year cardiovascular mortality rates were 4.9% in patients receiving amlodipine alone or with perindopril, and 6.5% in those receiving atenolol alone or with bendroflumethiazide.
Secondary Prevention Studies
The Clinical Outcomes Utilizing Revascularization and AGgressive drug Evaluation (COURAGE) study was a secondary prevention trial that compared the efficacy of intensive pharmacologic treatment and percutaneous coronary intervention in 2287 patients with objective evidence of myocardial ischemia and significant CHD.[33] Approximately two thirds of the enrolled patients were hypertensive; one third had diabetes; and there was a history of myocardial infarction in 38%. Patients assigned to pharmacologic therapy received antiplatelet therapy with aspirin or clopidogrel in addition to treatment for hypertension and/or dyslipidemia: In total, 77% were treated with ARBs or ACE inhibitors, and 93% were receiving statins. After a median of 4.6 years' follow-up, there was no significant difference in the incidence of all-cause mortality or nonfatal myocardial infarction between patients receiving pharmacologic therapy and those undergoing percutaneous coronary intervention (hazard ratio, 1.05; 95% CI 0.87, 1.27; P = .62). Thus, in these patients, the high cardiovascular risk appeared to be related more to the presence of multiple risk factors or associated diseases than to narrowing of the coronary arteries.
The effect of ACE inhibitors and ARBs on the secondary prevention of cardiovascular events in high-risk patients with CHD with or without hypertension has been assessed in numerous outcome studies.[31,33,35–42] However, to date, the evidence for whether more aggressive management is necessary by dual blockade of the renin-angiotensin system is limited to Candesartan in Heart Failure-Assessment of Reduction in Mortality and morbidity (CHARM) in patients already receiving ACE inhibitor therapy (CHARM-Added).[43] The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET), the results of which will be available in early 2008, is the first study to evaluate the efficacy of a combination of an ARB and an ACE inhibitor in a broad range of high-risk patients.[44] The 25,620 patients with CHD, cerebrovascular disease, peripheral vascular disease, or high-risk diabetes with evidence of end-organ damage enrolled are being treated with telmisartan and ramipril or either monotherapy. At the completion of the trial, patients will have received treatment for 3.5–5.5 years. The primary endpoint is a composite of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for congestive heart failure. Secondary endpoints are newly diagnosed congestive heart failure, revascularization procedures, newly diagnosed diabetes mellitus, the development of dementia or cognitive decline, nephropathy, and newly diagnosed atrial fibrillation. A number of substudies will examine the effect of telmisartan on cardiac structure and function, arterial stiffness, and potential biomarkers of cardiovascular disease.
Benefits of Renin-Angiotensin System Blockade in Relation to Level of Risk
The evidence from secondary prevention trials shows that the clinical benefits achieved with ACE inhibitors or ARBs are proportional to the patients' level of risk (Figure 4). The highest risk for cardiovascular mortality is seen in patients with heart failure after acute myocardial infarction, such as those enrolled in the Acute Infarction Ramipril Efficacy (AIRE)[33] and TRAndolapril Cardiac Evaluation (TRACE)[35] studies, and the relative reductions in mortality achieved with ACE inhibitors are also highest in these patients. The Heart Outcomes Prevention Evaluation (HOPE) study showed that significant reductions in mortality can also be achieved in high-risk patients with vascular disease without heart failure.[39] By contrast, in the Prevention of Events with Angiotensin Converting Enzyme inhibitors (PEACE) study, which enrolled patients with stable coronary artery disease and preserved left ventricular function, the relative risk for cardiovascular death was low, and the addition of an ACE inhibitor to standard therapy produced a relatively small reduction in cardiovascular mortality.[41] Nevertheless, given the large numbers of such patients encountered in routine clinical practice, even a small reduction in cardiovascular mortality translates into a substantial benefit.
Figure 4.

Relative risk for cardiovascular mortality, compared with a healthy 60-year-old individual, and relative reductions in mortality (orange bars) achieved with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in major outcome trials (AIRE = Acute Infarction Ramipril Efficacy trial;[34] TRACE = TRAndolapril Cardiac Evaluation;[35] SOLVD = Studies Of Left Ventricular Dysfunction;[36,37] SAVE = Survival And Ventricular Enlargement study;[38] HOPE = Heart Outcomes Prevention Evaluation;[39] EUROPA = EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease;[40] PEACE = Prevention of Events with Angiotensin Converting Enzyme inhibition).[41]
Conclusions
Accurate assessment of global cardiovascular risk is essential for the optimal prevention of cardiovascular events in at-risk patients. This will require careful attention to multiple risk factors, including hypertension, dyslipidemia, and cigarette smoking. In addition, it is important that age should be taken into account in view of the fact that the population of most industrialized countries is aging: It is anticipated that by 2025, approximately 30% of the population in many European countries will be aged 60 years and older.[45]
The proportion of hypertensive patients who are at high or very high risk for cardiovascular disease exceeds 50%. However, there is increasing evidence that both specialists and primary care physicians tend to underestimate cardiovascular risk, particularly in patients at the highest risk. Although both the SCORE charts and the Framingham risk algorithm provide useful assessments of global cardiovascular risk, they still need to be implemented more widely. Furthermore, it is essential that, in European countries, risk assessment be adapted to the level of risk prevailing in a given country, and that temporal changes in cardiovascular risk are taken into account. Numerous intervention studies have shown that renin-angiotensin system blockade with ACE inhibitors or ARBs reduces the risk for cardiovascular events in patients at all levels of risk, with the greatest benefits being seen in patients at highest risk. Future studies should investigate whether routine risk calculation in all patients will improve understanding and optimal treatment of cardiovascular risk among physicians.
Acknowledgments
Writers employed by PAREXEL MedCom drafted the manuscripts, on the basis of the authors' slide presentations and the audio from the symposium. The authors reviewed and amended, and approved the content and the final drafts. No authors received any payment, either directly or indirectly, for their work on the manuscripts. PAREXEL MedCom received payment from Boehringer Ingelheim (BI), the sponsor of the symposium. The writers who contributed to this supplement are as follows: Anne Jakobsen, Jose Heroys, Ann Ralph, Tomas Rees, and Michael Shaw.
Footnotes
Reader Comments on: Cardiovascular High-Risk Patients – Treat to Protect, But Whom? See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at f.zannad@chu-nancy.fr or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
References
- 1.Mitka M. Heart disease a global health threat. JAMA. 2004;291:2533. doi: 10.1001/jama.291.21.2533. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Importance of hypertension as a major risk factor in cardiovascular disease. In: Genest J, Koiw E, Kuchel O, editors. Hypertension: Physiopathology and Treatment. New York: McGraw-Hill; 1977. pp. 888–910. [Google Scholar]
- 3.Wilson PW, Kannel WB, Silbershatz H, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 4.Poulter N. Coronary heart disease is a multifactorial disease. Am J Hypertens. 1999;12:92S–95S. doi: 10.1016/s0895-7061(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 5.Fagot-Campagna A, Rolka DB, Beckles GLA, et al. Prevalence of lipid abnormalities, awareness, and treatment in U.S. adults with diabetes. Diabetes. 2000;49(suppl):A78–A79. [Google Scholar]
- 6.Kannel WB. Bishop Lecture. Contribution of the Framingham Study to preventive cardiology. J Am Coll Cardiol. 1990;15:206–211. doi: 10.1016/0735-1097(90)90203-2. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB. The lipid-atherogenesis connection. 12 questions frequently asked by physicians. Consultant. 1988;28:25–38. [Google Scholar]
- 8.Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Doll R, Peto R, Wheatley K, et al. Mortality in relation to smoking: 40 years' observation on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298:789–794. doi: 10.1136/bmj.298.6676.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA. 2000;284:706–712. doi: 10.1001/jama.284.6.706. [DOI] [PubMed] [Google Scholar]
- 12.Delaney JA, Daskalopoulou SS, Brophy JM, et al. Lifestyle variables and the risk of myocardial infarction in the General Practice Research Database. BMC Cardiovasc Disord. 2007;7:38. doi: 10.1186/1471-2261-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD003041.pub2. CD003041. [DOI] [PubMed] [Google Scholar]
- 14.American Heart Association. Heart Disease and Stroke Statistics: 2005 Update. Dallas: American Heart Association; 2004. p. 33. [Google Scholar]
- 15.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Bethesda, Md: National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health; 2002. NIH Publication No. 02-521. [Google Scholar]
- 17.Empana JP, Ducimetiere P, Arveiler D, et al. Are the Framingham and PROCAM coronary heart disease risk functions applicable to different European populations? The PRIME Study. Eur Heart J. 2003;24:1903–1911. doi: 10.1016/j.ehj.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 19.Wood DA, De Backer G, Faergeman O, et al. Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Eur Heart J. 1998;19:1434–1503. doi: 10.1053/euhj.1998.1243. [DOI] [PubMed] [Google Scholar]
- 20.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs FDR, Erhardt L. Acceptance of guideline recommendations and perceived implementation of coronary heart disease prevention among primary care physicians in five European countries: the Reassessing European Attitudes about Cardiovascular Treatment (REACT) survey. Fam Pract. 2002;19:596–604. doi: 10.1093/fampra/19.6.596. [DOI] [PubMed] [Google Scholar]
- 22.Barrios V, Escobar C, Calderon A, et al. Cardiovascular risk profile and risk stratification of the hypertensive population attended by general practitioners and specialists in Spain. The CONTROLRISK study. J Hum Hypertens. 2007;21:479–485. doi: 10.1038/sj.jhh.1002167. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery AA, Fahey T, Mackintosh C, et al. Estimation of cardiovascular risk in hypertensive patients in primary care. Br J Gen Pract. 2000;50:127–128. [PMC free article] [PubMed] [Google Scholar]
- 24.Kullo IJ, Malik AR. Arterial ultrasonography and tonometry as adjuncts to cardiovascular risk stratification. J Am Coll Cardiol. 2007;49:1413–1426. doi: 10.1016/j.jacc.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Almahameed A. Peripheral arterial disease: recognition and medical management. Cleve Clin J Med. 2006;73:621–626. 628, 632–634. doi: 10.3949/ccjm.73.7.621. passim. [DOI] [PubMed] [Google Scholar]
- 26.Erbel R, Mohlenkamp S, Lehmann N, et al. Sex related cardiovascular risk stratification based on quantification of atherosclerosis and inflammation. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2007.02.031. 2007 March 25; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 28.Klausen KP, Scharling H, Jensen JS. Very low level of microalbuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med. 2006;260:231–237. doi: 10.1111/j.1365-2796.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 29.Boushey CJ, Beresford SA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 30.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 31.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 32.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 33.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 34.The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet. 1993;342:821–828. [PubMed] [Google Scholar]
- 35.Kober L, Torp-Pedersen C, Carlsen JR, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 36.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 37.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 38.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 40.Fox KM, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- 41.Braunwald E, Domanski MJ, Fowler SE, et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMurray J, Solomon S, Pieper K, et al. The effect of valsartan, captopril, or both on atherosclerotic events after acute myocardial infarction: an analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT) J Am Coll Cardiol. 2006;47:726–733. doi: 10.1016/j.jacc.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 43.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 44.Teo K, Yusuf S, Sleight P, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. A Policy Framework. Geneva, Switzerland: World Health Organization; 2002. Active Ageing. [PubMed] [Google Scholar]


