Abstract
The renin-angiotensin system plays a key role in the regulation of blood pressure, and blockade of this system now forms a central part of strategies to reduce the risk for cardiovascular events in high-risk patients. Both angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have been shown to be effective in lowering blood pressure and reducing the risk for cardiovascular events, but both classes of drug have some limitations. Plasma concentrations of angiotensin II increase during ACE-inhibitor therapy in some patients, partly as a result of the production of angiotensin II via non-ACE pathways; furthermore, elevated aldosterone concentrations can occur in a significant proportion of patients. ARBs block the deleterious effects of angiotensin II at angiotensin type 1 receptors irrespective of the origin of the peptide, but the beneficial effects of kinins may be diminished. ARB therapy results in activation of angiotensin type 2 receptors, resulting in potentially beneficial anti-inflammatory, antithrombotic, and antiproliferative effects, but the clinical significance of these effects remains controversial. Some ARBs, particularly telmisartan, have been shown to act as partial agonists of peroxisome proliferator-activated receptor gamma, thereby increasing insulin sensitivity. Combination therapy with ACE inhibitors and ARBs offers the potential for effective blood pressure control, decreased aldosterone production, enhanced kinin activity, and increased insulin sensitivity. The potential clinical benefits of this approach in high-risk patients are currently being investigated in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET), which is comparing therapy using a combination of telmisartan plus ramipril with the use of each drug in monotherapy.
Introduction
The renin-angiotensin system (RAS) plays an important role in the regulation of blood pressure. It evolved as a mechanism to preserve fluid volume in ancient times of limited salt supply and to maintain blood pressure and prevent ischemia under conditions of acute volume loss.[1] However, excessive activation of the RAS can have deleterious effects on cardiovascular and renal function. As a result, blockade of the RAS with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) is a key element of strategies to reduce cardiovascular risk.[2]
The first step in the RAS cascade (Figure 1) is the formation of angiotensin I from the precursor angiotensinogen under the action of renin; indeed, early evidence for the importance for the RAS in cardiovascular disease came from the consistent finding that renin activity is predictive of the risk for cardiovascular events.[2–4] Angiotensin I is then converted to angiotensin II, the principal effector peptide of the RAS, by ACE. In addition, angiotensin II can be produced in tissues by enzymes such as chymase. This locally produced angiotensin II is believed to mediate paracrine and autocrine functions.[5] Angiotensin II acts via 2 receptor subtypes: angiotensin type 1 (AT1) and AT2. Activation of AT1 receptors results in vasoconstriction, aldosterone and vasopressin secretion, sodium retention, and decreased renal perfusion. Hence, these receptors mediate the deleterious effects of angiotensin II, including elevated blood pressure and cardiac and vascular remodeling. The effects of the AT2 receptors have been less clearly defined because of the limited expression of these receptors in adults, because of their unconventional signaling pathways, and because many AT2-mediated actions are masked by opposing AT1-mediated effects.[6] However, it is now recognized that AT2 receptors generally oppose the actions of AT1 receptors, mediating various antiproliferative and anti-inflammatory effects and promoting tissue differentiation and regeneration and apoptosis.[6]
Figure 1.
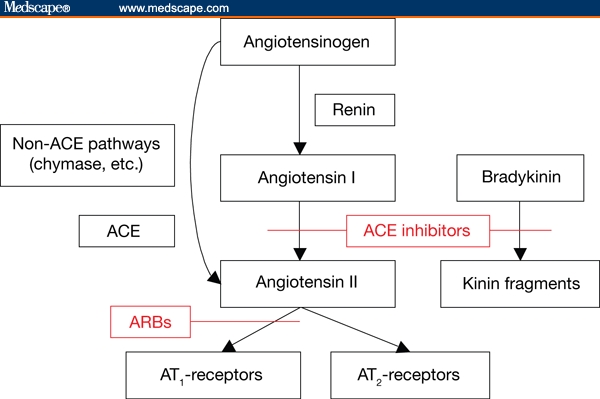
Principal elements of the renin-angiotensin system and sites of action of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs). AT1 = angiotensin type 1; AT2 = angiotensin type 2
The principal drugs available for blockade of the RAS in clinical practice are the ACE inhibitors and ARBs (Figure 1). Both classes of drugs have been shown to be effective in lowering blood pressure and reducing the risk for cardiovascular events in at-risk patients. However, they both have potential limitations and, hence, their use in combination is attracting increasing attention. For example, in the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),[7] which is reviewing the actions of ACE inhibitors and ARBs and the rationale for combination therapy with these agents in patients at high risk for cardiovascular events.
RAS Blockade With ACE Inhibitors
ACE inhibitors block the ACE-mediated production of angiotensin II from angiotensin I. However, as noted above, angiotensin II can also be produced via non-ACE pathways that are unaffected by ACE inhibitors.[5] Hence, ACE inhibitors reduce, but do not abolish, angiotensin II effects mediated via both AT1 and AT2 receptors. In addition, ACE, which is also called kininase II, catalyzes not only the conversion of angiotensin I but also the degradation of bradykinin.[8] This peptide produces vasodilation by stimulating the production of nitric oxide (NO) and prostaglandin (PG)I2 in the endothelium. Accumulation of bradykinin during ACE-inhibitor therapy, therefore, makes an important contribution to the antihypertensive efficacy of these agents.[9] However, accumulation of kinins has been implicated in the cough that is a side effect of ACE inhibitors.[10]
There is evidence that ACE itself can act as a cell-surface receptor and that binding of an ACE inhibitor to the enzyme triggers a signaling cascade that leads ultimately to PGI2 generation.[11,12] This may represent a further beneficial effect of ACE inhibitors.
Several landmark studies have shown that ACE inhibitors reduce the risk for cardiovascular events in at-risk patients. Notable among these is the HOPE (Heart Outcomes Prevention Evaluation) study,[13] which involved 9297 patients aged 55 years or older with evidence of vascular disease and at least 1 other cardiovascular risk factor. The patients were treated with ramipril or placebo for a mean of 5 years. Compared with placebo, ramipril produced a significant reduction in the risk for a composite of cardiovascular death, nonfatal myocardial infarction, or stroke (relative risk [RR] = 0.78, 95% confidence interval [CI] = 0.70, 0.86; P < .001). There were also significant reductions in each of the components of the composite endpoint, and in heart failure, revascularization procedures, cardiac arrest, and complications related to diabetes. However, certain questions concerning the potential benefits of ACE-inhibitor therapy remained unresolved. For example, a high dose of ramipril (10 mg once daily) was used in this study, and it is questionable whether a lower dose would have been equally effective. Furthermore, at the end of the study, the mean systolic/diastolic blood pressure in the ramipril group was 3/1 mmHg lower than in the placebo group, and the question of whether this difference could account for the observed reductions in clinical events remains controversial.[14–16]
During long-term treatment with ACE inhibitors, a proportion of patients exhibit a return to normal or even elevated angiotensin II concentrations (a phenomenon known as angiotensin II reactivation), and this has been shown to be associated with a poor prognosis.[17] Furthermore, aldosterone concentrations increase in some patients during chronic therapy (aldosterone escape). In a study in 91 unselected patients with heart failure, 34% of patients showed increased ACE activity while receiving ACE inhibitor treatment, 15% showed evidence of angiotensin II reactivation (angiotensin II concentrations > 10 pg/mL), and 38% showed aldosterone escape (aldosterone concentrations > 144 pg/mL).[18] Thus, in this study, the RAS was not suppressed in approximately one third of patients. Furthermore, these findings suggest that the problem of aldosterone escape may be more important than previously thought.
Angiotensin Receptor Blockade
The ARBs selectively block the AT1 receptor and have little or no affinity for the AT2 receptor. These agents offer the advantage of a highly favorable tolerability profile, which is superior to that of other classes of antihypertensive agents.[19] Moreover, based on the results of recent major outcome trials, the evidence for the benefits of ARBs in reducing cardiovascular events can be considered to be at least as strong as for ACE inhibitors.
Targeting the AT1 receptor offers the potential for more complete inhibition of the RAS than can be achieved with ACE inhibitors, because these agents block the effects of angiotensin II irrespective of the pathway by which the peptide is generated. In addition, AT1-receptor blockade allows unopposed activation of AT2 receptors, resulting in potentially beneficial antiproliferative and anti-inflammatory effects; there is also evidence for a direct stimulatory effect of AT2 receptors on the generation of NO and PGI2.[6] However, it should be noted that the clinical significance of AT2 receptor-mediated actions remains controversial.[6]
As ARBs do not result in kinin accumulation, they are associated with less kinin-induced catecholamine release compared with ACE inhibitors,[20] although the potential contribution of this to the clinical benefits of ARBs remains to be established. On the other hand, the beneficial effects of kinins such as NO and PGI2 release may be diminished during ARB therapy.
In addition to effective control of blood pressure, ARBs have been shown to offer a number of benefits in clinical practice, including anti-inflammatory and antithrombogenic effects, renoprotection, and reduced risks for stroke and new-onset diabetes.
Anti-inflammatory Effects of ARBs
Atherosclerotic disease is characterized by inflammation and increased thrombogenesis.[21] The development and stability of atherosclerotic lesions depend largely on the balance between pro-inflammatory mediators such as interleukin (IL)-6 and C-reactive protein (CRP) and anti-inflammatory agents such as IL-10.[22–24] Several studies have shown that ARBs increase the expression of anti-inflammatory cytokines and decrease that of pro-inflammatory mediators to a greater extent than ACE inhibitors. For example, Schieffer et al.[25] compared the effects of the ARB irbesartan and the ACE inhibitor enalapril on pro- and anti-inflammatory cytokines in patients with hypertension and coronary heart disease (CHD). Both agents produced a significant increase in serum IL-10 levels and a decrease in metalloprotease (MMP)-9 activity. By contrast, only the ARB was effective in reducing serum IL-6 and high sensitivity CRP (hs-CRP). Similarly, ARB treatment resulted in a significant reduction in thromboxane A2-induced platelet aggregation, whereas the ACE inhibitor had no significant effect. Thus, in this study, the ARB produced a more complete blockade of angiotensin II-mediated pro-inflammatory and procoagulatory effects than did the ACE inhibitor. Such findings suggest that ARBs may contribute to the stabilization of atherosclerotic plaques.
Renoprotective Effects of ARBs
Several major trials have shown that ARBs exert renoprotective effects that are independent of blood pressure lowering. In the IRbesartan in patients with type 2 diabetes and MicroAlbuminuria (IRMA 2) study, the addition of irbesartan 300 mg to the existing antihypertensive therapy resulted in a 70% (P < .001) reduction in the emergence of overt diabetic nephropathy compared with placebo in patients with hypertension and type 2 diabetes.[26] Blood pressure was reduced to comparable extents in irbesartan- and placebo-treated patients. In IRMA 2, it was necessary to treat just 10 patients to prevent 1 case of diabetic nephropathy, a finding which highlights the potential benefits of ARB therapy in managing patients with type 2 diabetes. Similarly, in patients with hypertension and established diabetic nephropathy, the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study[27] and the Irbesartan in Diabetic Nephropathy Trial (IDNT)[28] showed that ARBs can significantly inhibit the progression of diabetic nephropathy independently of reductions in blood pressure. A trial to compare telMisartan 40 mg titrated to 80 mg vs losArtan 100 mg in hypertensive type 2 DiabEtic with Overt nephropathy (AMADEO) found that telmisartan was superior to losartan in reducing proteinuria in patients with hypertension and diabetic nephropathy despite similar blood pressure control.[29]
ARBs and Stroke
Although reduction of blood pressure is clearly important for the prevention of stroke, evidence from clinical trials suggests that the protective effects of ARBs are not related to blood pressure alone. In a meta-analysis of 4 comparative trials with ARBs involving a total of 16,791 patients, ARBs reduced the risk for stroke by 21% (95% CI 10%, 31%), compared with other classes of antihypertensive agents (Figure 2).[30] This reduction was associated with a mean difference in blood pressure of only 2/1 mm Hg between ARB-treated patients and those receiving other agents.
Figure 2.
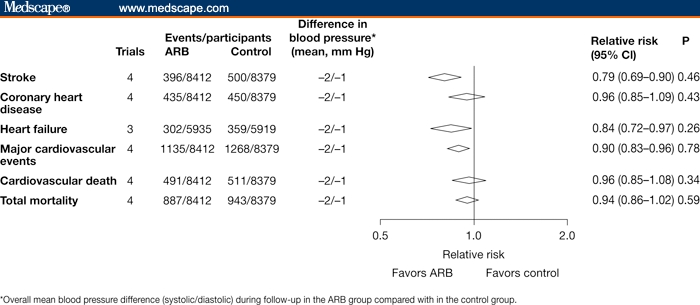
Relative risks for stroke and other cardiovascular events in 4 comparative trials with angiotensin receptor blockers (ARBs) and other antihypertensive agents. Reproduced with permission from Turnbull et al.[30]
ARBs and Diabetes Mellitus
A consistent finding in recent studies has been that ARBs significantly reduce the incidence of new-onset diabetes by approximately 20% to 25%.[31–34] In the LIFE (Losartan Intervention for Endpoint reduction in hypertension) study, for example, the RR of new-onset diabetes in patients receiving ARB was 0.75 (95% CI = 0.63, 0.88; P < .001), compared with those receiving atenolol,[30] whereas in the Candesartan in Heart failure – Assessment of Reduction in Mortality and morbidity (CHARM) studies, the corresponding RR was 0.78 (95% CI = 0.64, 0.96; P = .02) vs placebo,[31] and in the VALUE study it was 0.77 (95% CI = 0.69, 0.86; P < .0001) vs amlodipine.[33]
Several mechanisms contribute to this protective effect of ARBs against the onset of diabetes. All ARBs have been shown to improve insulin sensitivity through multiple mechanisms, including increases in muscle blood flow, decreased sympathetic nervous activity, enhanced insulin signaling, and adipose-tissue remodeling (Table).[35] For example, angiotensin II impairs insulin sensitivity by reducing activation of the phosphatidylinositol-3 signaling pathway and also decreases expression of glucose transporter 4, effects that can be reversed by ARBs.[35] In addition, ARBs can promote adipose-tissue remodeling by decreasing serum concentrations of free fatty acids and increasing the expression of adiponectin, which is known to enhance insulin sensitivity.[35]
Table.
Effects of ARBs on Insulin Sensitivity.
| Improved muscle blood flow |
| Decreased sympathetic activity |
| Favorable ionic changes (K+/Mg2+) |
| Enhanced insulin signaling (tyrosine kinase, IRS-1, PI3-kinase, GLUT4) |
| Adipose tissue remodeling (free fatty acids, adiponectin) |
| Partial PPARgamma activity (some lipophilic ARBs only) |
ARBs = angiotensin receptor blockers; IRS-1 = insulin receptor substrate 1; PI3 = phosphatidylinositol 3; GLUT4 = glucose transporter 4; PPARgamma = peroxisome proliferator-activated receptor gamma
Reproduced with permission of Scheen AJ.[35]
There is evidence that certain ARBs have a further effect on insulin sensitivity, which is mediated via peroxisome proliferator-activated receptor gamma (PPARgamma). This receptor is a nuclear transcription factor that exists as a heterodimer with the retinoid X receptor-alpha (RXR-alpha).[36] Following activation of PPARgamma by an agonist, the heterodimer binds to specific DNA response elements, triggering the expression of multiple genes affecting glucose and lipid metabolism. PPARgamma is the therapeutic target of the thiazolidinediones, which have been shown to increase insulin sensitivity and decrease serum fatty acid and triglyceride concentrations in patients with type 2 diabetes.[37] Not all ARBs, however, have been shown to bind to PPARgamma and act as partial agonists or rather as PPARgamma modulators (Figure 3). The strongest activity is seen with telmisartan.[38] In one study, the concentration producing 50% activation of PPARgamma (EC50) was 5.0 micro(mc)mol/L with telmisartan, compared with 27.0 mcmol/L with irbesartan and > 50 mcmol/L with losartan; by comparison, the EC50 for the thiazolidinedione pioglitazone was 0.2 mcmol/L.[38] Importantly, activation of PPARgamma by telmisartan occurred at drug concentrations comparable with those achieved during clinical use.[39]
Figure 3.
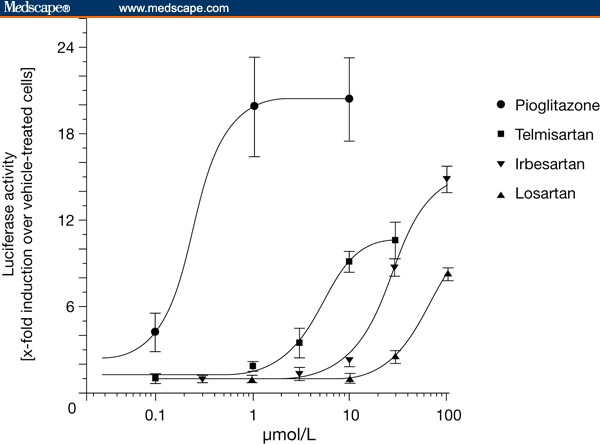
In vitro activation of peroxisome proliferator-activated receptor gamma by angiotensin receptor blockers. Reproduced with permission from Schupp et al.[38]
Combination Therapy With ACE Inhibitors and ARBs
The complementary mechanisms of action of ACE inhibitors and ARBs create a clear rationale for the use of the 2 agents in combination in patients at high risk for cardiovascular disease. Both classes of agent provide effective control of blood pressure, and there is evidence that the combination therapy may produce a larger decrease in blood pressure than either agent alone.[40,41] Plasma angiotensin II concentrations may increase during combination therapy, both as a result of the reactivation phenomenon described above that is sometimes seen with ACE inhibitors,[18] and because of the reflex increase in angiotensin II seen during treatment with ARBs.[42] However, the effect of such increases is negated by the AT1 receptor-blocking activity of ARBs. Furthermore, combination therapy may reduce the incidence of aldosterone escape associated with ACE inhibition. Evidence for this comes from the Valsartan-Heart Failure Trial (Val-HeFT) study, in which valsartan was added to standard heart-failure medication, including ACE inhibitors.[43] In this study, plasma aldosterone concentrations were significantly (P < .0001) lower in ARB-treated patients than in those receiving standard medication. A further potential advantage of combination therapy is enhancement of the beneficial effects of kinins, including NO generation, as a result of both inhibition of kinin breakdown by ACE inhibitors and a possible effect of AT2-receptor stimulation during ARB therapy.[6]
The rationale for combination therapy is further strengthened by the differential effects of ACE inhibitors and ARBs on insulin sensitivity. Both agents improve insulin sensitivity by increasing NO generation, which results in increased skeletal muscle blood flow and enhanced insulin signaling.[35] In addition, as described above, certain ARBs can act as partial agonists of PPARgamma, thereby further increasing insulin sensitivity.[38,39,44]
Several major trials have shown that both ACE inhibitors and ARBs have beneficial effects on renal function in patients with diabetic nephropathy. These benefits are even observed at the start of the renal continuum on endothelial dysfunction.[45] Currently, no data are available on the use of combination therapy in patients with established diabetic nephropathy. The results of the ONTARGET Program[7] should provide important insights in this respect.
Conclusions
Both ACE inhibitors and ARBs have been shown to have important clinical benefits in patients at risk for cardiovascular disease, although both are subject to certain limitations. There is a clear rationale for the use of combination therapy with these agents based on the potential for decreased aldosterone escape and enhanced kinin activity and NO generation. ONTARGET, which is comparing combination therapy with telmisartan and ramipril with the 2 agents given as monotherapy, should provide valuable data on the potential benefits of combination therapy in terms of protection against cardiovascular events in high-risk patients. The results of this study are awaited with anticipation.
Acknowledgments
Writers employed by PAREXEL MedCom drafted the manuscripts based on the authors' slide presentations and the audio from the symposium. The authors reviewed, amended, and approved the content and the final drafts. No author received any payment, either directly or indirectly, for his or her work on the manuscripts. PAREXEL MedCom received payment from Boehringer Ingelheim, the sponsor of the symposium. The writers who contributed to this supplement are as follows: Anne Jakobsen, Jose Heroys, Ann Ralph, Tomas Rees, and Michael Shaw.
Footnotes
Reader Comments on: Targeting Cardiovascular Protection: The Concept of Dual Renin-Angiotensin System Control See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at thomas.unger@charite.de or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
References
- 1.Ruilope LM, Rosei EA, Bakris GL, et al. Angiotensin receptor blockers: therapeutic targets and cardiovascular protection. Blood Press. 2005;14:196–209. doi: 10.1080/08037050500230227. [DOI] [PubMed] [Google Scholar]
- 2.Schmieder RE, Hilgers KF, Schlaich MP, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 3.Alderman MH, Madhavan S, Ooi WI, et al. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med. 1991;324:1098–1104. doi: 10.1056/NEJM199104183241605. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ, Woodward M, Chalmers JP, et al. Prediction of myocardial infarction by N-terminal-pro-B-type natriuretic peptide, C-reactive protein, and renin in subjects with cerebrovascular disease. Circulation. 2005;112:110–116. doi: 10.1161/CIRCULATIONAHA.104.525527. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ, Bernstein K, Celermajer D, et al. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc Drugs Ther. 2002;16:149–160. doi: 10.1023/a:1015709617405. [DOI] [PubMed] [Google Scholar]
- 6.Steckelings UM, Kaschina E, Unger T. The AT2receptor – a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Teo K, Yusuf S, Sleight P, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Linz W, Wohlfart P, Scholkens BA, et al. Interactions among ACE, kinins and NO. Cardiovasc Res. 1999;43:549–561. doi: 10.1016/s0008-6363(99)00091-7. [DOI] [PubMed] [Google Scholar]
- 9.Tschope C, Gohlke P, Zhu YZ, et al. Antihypertensive and cardioprotective effects after angiotensin-converting enzyme inhibition: role of kinins. J Card Fail. 1997;3:133–148. doi: 10.1016/s1071-9164(97)90047-6. [DOI] [PubMed] [Google Scholar]
- 10.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 11.Kohlstedt K, Brandes RP, Muller-Esterl W, et al. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004;94:60–67. doi: 10.1161/01.RES.0000107195.13573.E4. [DOI] [PubMed] [Google Scholar]
- 12.Kohlstedt K, Busse R, Fleming I. Signaling via the angiotensin-converting enzyme enhances the expression of cyclooxygenase-2 in endothelial cells. Hypertension. 2005;45:126–132. doi: 10.1161/01.HYP.0000150159.48992.11. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 14.Leenen FH. Blood pressure lowering, not vascular mechanism of action, is the primary determinant of clinical outcome. Can J Cardiol. 2004;20(Suppl B):77B–82B. [PubMed] [Google Scholar]
- 15.Sleight P, Yusuf S, Pogue J, et al. Blood-pressure reduction and cardiovascular risk in HOPE study. Lancet. 2001;358:2130–2131. doi: 10.1016/S0140-6736(01)07186-0. [DOI] [PubMed] [Google Scholar]
- 16.Staessen JA, Wang J. Do ancillary properties of antihypertensive drugs explain the outcome results of recent trials? J Nephrol. 2002;15:422–427. [PubMed] [Google Scholar]
- 17.Swedberg K, Eneroth P, Kjekshus J, et al. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation. 1990;82:1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 18.MacFadyen RJ, Lee AFC, Morton JJ, et al. How often are angiotensin II concentrations and aldosterone concentrations raised during chronic ACE inhibitor treatment in cardiac failure? Heart. 1999;82:57–61. doi: 10.1136/hrt.82.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Sierra A. Angiotensin receptor blockers in hypertension and cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2006;4:67–73. doi: 10.2174/187152506775268839. [DOI] [PubMed] [Google Scholar]
- 20.Rump LC. Advantages of Ang II receptor blockade over ACE inhibition with respect to suppression of sympathetic activity: heartening news for the kidney? Nephrol Dial Transplant. 1999;14:556–559. doi: 10.1093/ndt/14.3.556. [DOI] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis is an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 22.Pinderski Oslund LJ, Hedrick CC, Olvera T, et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–2853. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 23.Smith DA, Irving SA, Sheldon J, et al. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–749. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 24.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 25.Schieffer B, Bunte C, Witte J, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–368. doi: 10.1016/j.jacc.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 26.Parving H-H, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 27.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 28.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 29.Burgess E, Bakris G, Weir M, Koval S. Comparative long term effects of two AT1receptor blockers on proteinuria in patients with type-2 diabetes and overt nephropathy and hypertension: results of the AMADEO trial. J Hypertens. 2007;25(suppl 2):S276. [Google Scholar]
- 30.Turnbull F, Blood Pressure Lowering Treatment Trialists' Collaboration Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 31.Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 33.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 34.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 35.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 2. Overview of physiological and biochemical mechanisms. Diabetes Metab. 2004;30:498–505. doi: 10.1016/s1262-3636(07)70147-7. [DOI] [PubMed] [Google Scholar]
- 36.Pershadsingh HA. Peroxisome proliferators-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis? Expert Opin Investig Drugs. 2004;13:215–228. doi: 10.1517/13543784.13.3.215. [DOI] [PubMed] [Google Scholar]
- 37.Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs. 2003;63:1373–1405. doi: 10.2165/00003495-200363130-00004. [DOI] [PubMed] [Google Scholar]
- 38.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 39.Benson SC, Pershadsingh HA, Ho CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARf×-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 40.Ruilope LM, Aldigier JC, Ponticelli C, et al. Safety of the combination of valsartan and benazepril in patients with chronic renal disease. European Group for the Investigation of Valsartan in Chronic Renal Disease. J Hypertens. 2000;18:89–95. [PubMed] [Google Scholar]
- 41.Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ. 2000;321:1440–1444. doi: 10.1136/bmj.321.7274.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb SS, Dickstein K, Fleck E, et al. Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation. 1993;88:1602–1609. doi: 10.1161/01.cir.88.4.1602. [DOI] [PubMed] [Google Scholar]
- 43.Krum H, Carson P, Farsang C, et al. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail. 2004;6:937–945. doi: 10.1016/j.ejheart.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Clasen R, Schupp M, Foryst-Ludwig A, et al. PPARf×-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 45.Schmieder RE, Delles C, Mimran A, et al. Impact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetes. Diabetes Care. 2007;30:1351–1356. doi: 10.2337/dc06-1551. [DOI] [PubMed] [Google Scholar]


