Summary
GCC185 is a large coiled coil protein at the trans Golgi network that is required for receipt of transport vesicles inbound from late endosomes, and for anchoring non-centrosomal microtubules that emanate from the Golgi. Here we demonstrate that recruitment of GCC185 to the Golgi is mediated by two Golgi-localized small GTPases of the Rab and Arl families. GCC185 binds Rab6 and mutation of residues needed for Rab binding abolishes Golgi localization. The crystal structure of Rab6 bound to the GCC185 Rab binding domain reveals that Rab6 recognizes a two-fold symmetric surface on a coiled coil immediately adjacent to a C-terminal GRIP domain. Unexpectedly, Rab6 binding promotes association of Arl1 with the GRIP domain. We present a structure-derived model for dual GTPase membrane attachment that highlights the potential ability of Rab GTPases to reach binding partners at a significant distance from the membrane via their unstructured and membrane-anchored, hypervariable domains.
Introduction
Vesicle-mediated transport between membrane bound compartments is essential for protein secretion, cell signaling and cell growth. Transport involves the collection of specific cargo molecules into vesicles, movement of vesicles by motors along cytoskeletal tracks, and tethering, docking and fusion of vesicles at the target membrane (Pfeffer, 1999). GTPases of the Rab and Arf families play central roles in the regulation of these processes by recruiting specific partner ("effector") proteins to the membranes upon which they are bound (Grosshans et al., 2006; Gillingham and Munro, 2007). The GTPases interconvert between active, GTP bound forms that are capable of effector interactions, and inactive, GDP-bound forms; interconversion is catalyzed by GTPase-specific enzymes.
Among GTPase effectors are proteins that have been demonstrated to function as transport vesicle tethers. Tethers function to bring partner membranes into close proximity before membrane fusion (Stzul and Lupashin, 2006). One class is comprised of macromolecular assemblies such as the Exocyst and TRAPP complexes; another class is comprised of large coiled coil proteins such as p115 and EEA1 (Stzul and Lupashin, 2006). GCC185 is a putative coiled coil tether of 185 kDa that belongs to the Golgi-localized Golgin family of proteins. GCC185 is required for Rab9-dependent transport of mannose 6-phosphate receptors from late endosomes to the trans-Golgi network (TGN; Reddy et al., 2006; Derby et al., 2007). In support of the notion that this protein functions as a tether, cells depleted of GCC185 accumulate their cargo in peripheral, Rab9-positive vesicles (Reddy et al., 2006). A recent study demonstrated that GCC185 is also required for the attachment of non-centrosomal microtubules to the TGN via CLASPs, proteins that stabilize microtubule plus ends and prolong their elongation from the TGN (Efimov et al., 2007). Since GCC185 is a key player in both membrane traffic and cytoskeletal organization, it is important to determine how GCC185 is localized to the TGN.
GCC185 is one of four human Golgins (Golgin-245, Golgin-97, GCC88, GCC185) that contain a GRIP domain. The ∼45 residue GRIP domain represents an Arl1 GTPase binding unit and is sufficient to specify Golgi-targeting of Golgin-97 and Golgin-245 via Arl1 binding (Gillingham and Munro, 2007). The crystal structure of Arl1 bound to the Golgin-245 GRIP domain has been determined (Panic et al., 2003; Wu et al., 2004) and confirmed the importance of an invariant GRIP domain tyrosine residue for Arl1 binding and Golgi localization of Golgin-245 and Golgin-97. Surprisingly, despite its relatively well conserved GRIP domain, we and others found that GCC185 binds very poorly to Arl1, and mutation of the invariant GRIP domain tyrosine residue interfered only weakly with GCC185's Golgi localization (Lu and Hong 2003, Derby et al., 2004, Reddy et al., 2006). GCC185 differs from other GRIP domain proteins in its TGN sub-localization (Luke et al., 2003; Derby et al., 2004). In addition, attempts to re-localize GCC185 in cells expressing an early endosome-targeted Arl1 were not successful, despite efficient re-localization of Golgin-97 and Golgin-245 (Derby et al., 2004). Together, these data suggested that GCC185 is localized to the TGN by a mechanism that is distinct from that used by Golgin-245 and Golgin-97.
In this study we define an independent Rab GTPase binding site near the C-terminus of GCC185. We show that Rab6 binds to this site and we present the three dimensional crystal structure of the Rab6:Rab binding domain complex. Analysis of mutant proteins revealed that the Rab binding domain regulates both Golgi association and Arl1 binding to the GCC185 GRIP domain. Thus, two members of distinct GTPase families cooperate to localize GCC185 to the Golgi.
Results
We tested the Golgi associated, Rab6 GTPase for its possible interaction with GCC185. Rab6 was an obvious candidate, as it localizes to the Golgi and both yeast (Ypt6p) and human proteins interact with several putative tethering proteins, including the yeast VFT/GARP complex (Siniossoglou and Pelham, 2001), and the yeast protein Sgm1 and its mammalian homolog, TMF (Fridmann-Sirkis et al., 2004). In addition, there is strong genetic interaction between the genes encoding Ypt6p and the sole, yeast GRIP domain protein, Imh1p (Li and Warner, 1996, Tsukada and Gallwitz, 1996).
For these experiments, a construct of GST fused to the GCC185 C-terminus was employed that includes a ∼45 residue GRIP domain, plus ∼40 amino acids upstream of this domain and ∼20 amino acids that extend to the C-terminus (C-110, Figure 1A). As shown in Figure 1B (left panel), C-110 bound Rab6-GTP with a 5-fold preference over Rab6-GDP, which defines GCC185 as a Rab6 effector. This interaction was specific in that the C-110 fragment did not bind Rab1 (Figure 1C), Rab5 (Reddy et al., 2006) or Arl1-GTP (Figure 1C) at comparable concentrations. However Arl1 binding is 2006). observed when a higher (approximately 3 fold) C-110 concentration is used (see below). A somewhat larger, 158residue- residue-construct did not yield a higher bound fraction of Rab6 protein (not shown), demonstrating that an entire Rab binding site lies within the 110 C-terminal residues of GCC185.
Figure 1.
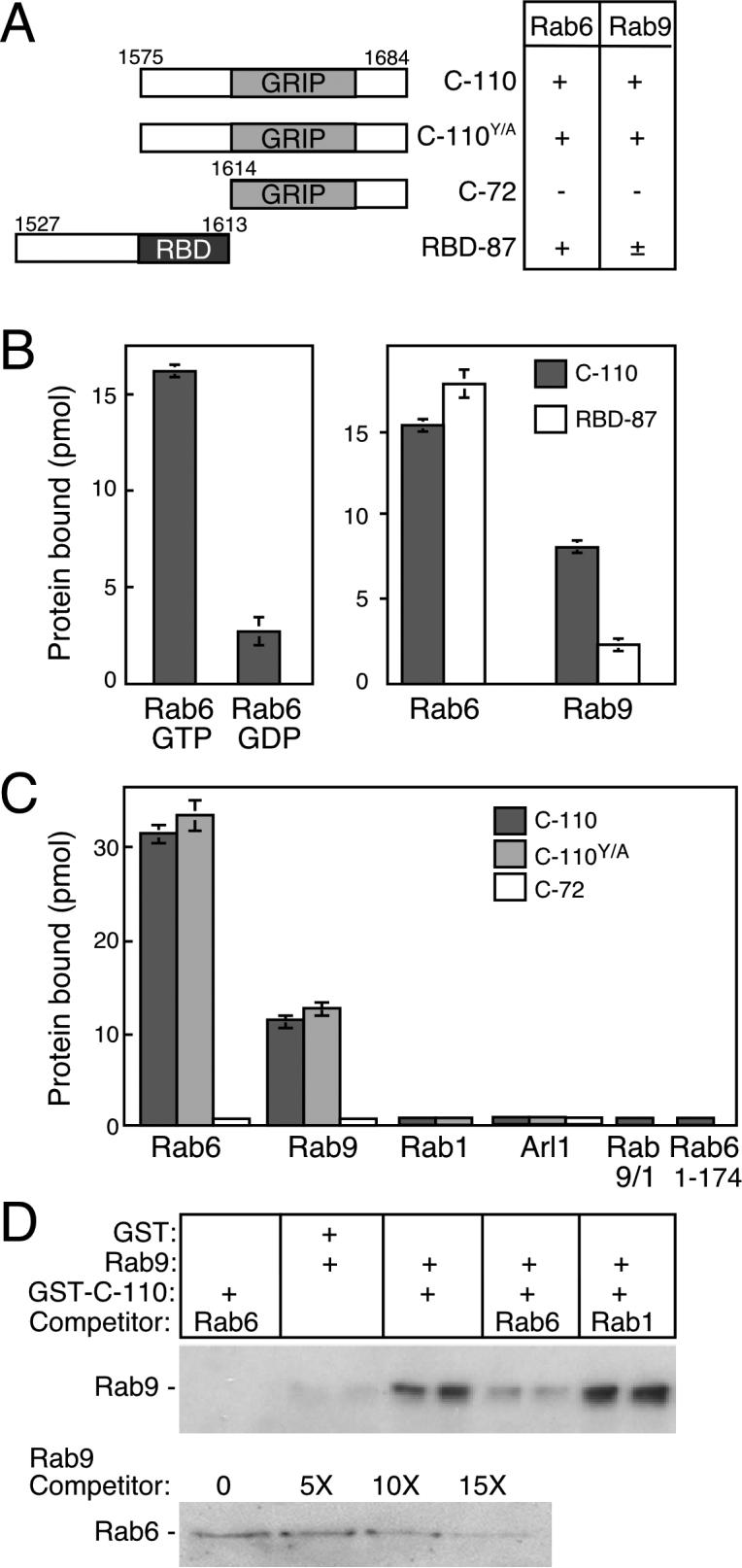
Identification of a GCC185 Rab-binding domain. (A) Constructs used to map Rab-GCC185 interactions; numbers represent amino acid residues. The GRIP domain and a Rab binding domain (RBD) are shown. At right: summary of binding to Rab6 or Rab9. (B) GCC185 preferentially binds Rab6-GTP via residues upstream of the GRIP domain. Reactions contained 50 pmol His-Rab6 (left) or 1.2 nmol His-Rab6, or Rab9-His (right) using 35S-GTPγS or 3H-GDP-preloaded GTPase and either GST-C110 or GST-RBD-87 (2.8 μM). (C) The GRIP domain is not sufficient for Rab binding. GST-C-110, C-110Y/A, or C-72 (2 μM) was incubated with 35S-GTPγS-preloaded GTPases (∼500 pmol) (as in B. except Rab1 and Rab9 were untagged and or ArlQ71L-His was used). Untagged Rab9/1 and His-Rab6 1−174 were employed. (D) Rab6 specifically competes with Rab9 for GCC185 binding. GST-C-110:Rab9 complexes (pair of lanes in the center) were incubated for 3 min with ten fold excess competitor. Rab9-His was detected by immunoblot using a monoclonal anti-Rab9 antibody that did not cross react with Rab6 (see pair of lanes at far left). Lower panel, same as upper panel using indicated amounts of competitor Rab9. Data are mean ± SD.
A specific, conserved GRIP domain tyrosine residue was previously shown to be critical for Arl1-binding and Golgi recruitment of Golgin-245 and Golgin-97 (Barr, 1999; Kjer-Nielsen et al., 1999). Mutation of the corresponding tyrosine residue in GCC185 (Y1618A) had no influence on Rab6 (Figure 1C) or Rab9 binding (Reddy et al., 2006; Figure 1C), consistent with a different mode of interaction for Rabs with GCC185. As a construct comprised of the 72 C-terminal residues of GCC185 (C-72; Figure 1A) failed to bind Rab6 or Rab9 (Figure 1C), we conclude that the GRIP domain is dispensible for Rab binding.
Residues just upstream of the GRIP domain (1575−1613; RBD in Figure 1A) comprise a Rab binding domain. As shown in Figure 1B (right panel), the so-called RBD-87 polypeptide that includes this region but lacks the GRIP domain was sufficient for full Rab6 binding. In contrast, the RBD-87 construct bound only about 25% of Rab9 as compared to C-110 (Figure 1B, right panel), suggesting that Rab9 interacts at least in part with the GRIP domain. Interestingly, Rab6 and Rab9 C-terminal hypervariable domains contributed to GCC185 binding, as a chimeric Rab9 protein containing the hypervariable domain of Rab1 (Rab9/1; Aivazian et al., 2006) or a Rab6 construct lacking the hypervariable domain (Rab6 1−174) failed to bind C-110 (Figure 1C). Thus, residues 1575−1613 comprise a region in GCC185 that is necessary for Rab binding, and additional downstream sequences are needed for Rab9 but not Rab6.
An anti-Rab9 immunoblot-based binding assay was used to test whether Rab6 and Rab9 compete for interaction with GCC185. Briefly, the C-110 fragment was incubated with Rab9, either alone or with excess Rab6 or Rab1. When a ten-fold excess Rab6 competitor was added to the preformed Rab9-GCC185 complex, Rab9 binding was reduced to background levels (Figure 1D, upper panel; compare lanes 7 & 8 with 5 & 6). An equal amount of Rab6 competitor decreased the Rab9-bound fraction to 50% (not shown). In contrast, a ten-fold excess of Rab1 did not compete with Rab9 for GCC185 binding (Figure 1D, lanes 9 & 10). In addition, Rab9 could compete for Rab6 binding, although it was a less potent competitor (Figure 1D, lower panel). Thus, Rab6 Rab9and Rab9 compete for GCC185 binding both specifically and efficiently. Together, these data confirm that Rab6 and Rab9 bind to at least partially overlapping sites on GCC185.
The binding affinities for GCC185 with Rab6 and Rab9 were determined by isothermal titration calorimetry. Supplemental Figure 1A and B show representative, calorimetric raw data for nucleotide-preloaded, Rab6-GTP and Rab9-GTP upon injection into a cell containing GST-C-110 (upper panels). Peak size was diminished with each injection as the binding reaction reached saturation. As expected, titrations of Rab6-GTP and Rab9-GTP into buffer (upper panel insets) or into GST (lower panel insets) resulted in heats comparable to baseline values in the GCC185 binding reaction. The affinities of C-110 for Rab6-GTP and Rab9-GTP are 2.3 ± 0.3 μM S.D. and 4.5 ± 1.2 μM S.D., respectively, with equimolar stoichiometry. Since GST-C-110 is dimeric as determined by gel filtration in conjunction with multiple angle light scattering (not shown), we conclude that the Rab6/Rab9:C-110 complexes have 2:2 stoichiometry in solution.
Characterization of the Rab binding domain of GCC185
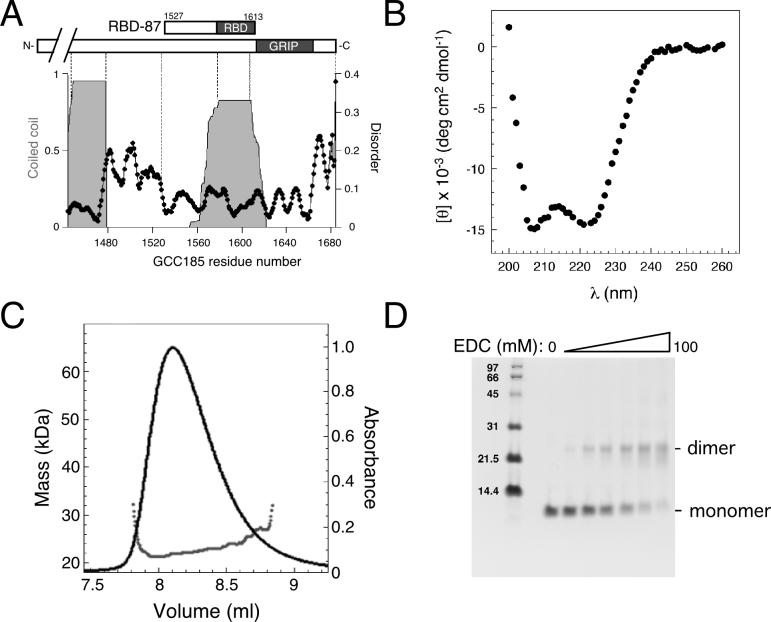
Structure prediction of the GCC185 C-terminus using the Paircoil algorithm (Berger et al., 1995) revealed a short dimeric, coiled coil that precisely spans the Rab binding domain (shaded area, Figure 2A, top). This region and the GRIP domain were free of predicted unstructured loops (Linding et al., 2003) (Figure 2A). In addition, flexible regions were detected downstream of the GRIP domain (∼20 residues), and spanning ∼40 residues upstream of residue 1527 (Figure 2A).
Figure 2.
The GCC185 Rab binding domain is a helical dimer. (A) Structure prediction of the GCC185 C-terminus. Coiled coil (shaded gray; cutoff=0.8) and disordered regions (black line and dots; cutoff=0.12) in GCC185 residues 1444−1684 were predicted with Paircoil and the DisEMBL™ programs, respectively. At top: bar diagram of the GCC185 C-terminus. (B) Circular dichroism spectrum of untagged RBD-87 (mean residue ellipticity). (C) Untagged RBD-87 forms a dimer in solution. Black line, A280. The mass at different elution volumes was calculated from multiple angle static light scattering data (dots). The polydispersity of the peak was 1.024. The mass of RBD-87 (92aa) monomer is 10.3 kDa. (D) Analysis of RBD-87 by crosslinking. Untagged protein (5 μM) was reacted at 20°C for 2 hr with 0−100 mM EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride).
The RBD-87 Rab binding domain-containing polypeptide (Figure 1A and top Figure 2A) was predicted to be ∼33% helical by the coiled coil prediction algorithm. The α-helix content of the RBD-87 polypeptide was determined by circular dichroism to be 40% (Figure 2B). Gel filtration coupled to direct mass measurement by multiple angle static light scattering showed that untagged RBD-87 eluted in a single peak, with a mass of 21.9 kDa, and is therefore a dimer (Figure 2C). Consistent with this finding, chemical crosslinking of RBD-87 yielded efficient conversion to a dimer in solution (Figure 2D). Full length GCC185 is also dimeric (Luke et al., 2005), consistent with the RBD-87 polypeptide forming a helical dimer.
A helical wheel projection of the predicted coiled coil spanning the Rab binding domain identified the hydrophobic face of an amphipathic helix, with residues at heptad positions 'a' and 'd' expected to reside at the interface of a dimeric coiled coil (Harbury et al., 1993; Figure 3A). Close inspection of the hydrophilic surface revealed two hydrophobic residues (I1588 and L1595), both at heptad position ('c'), that would be solvent-exposed if this model is correct (residues boxed in Figure 3A). Such residues were outstanding candidates to mediate hydrophobic protein-protein interactions.
Figure 3.
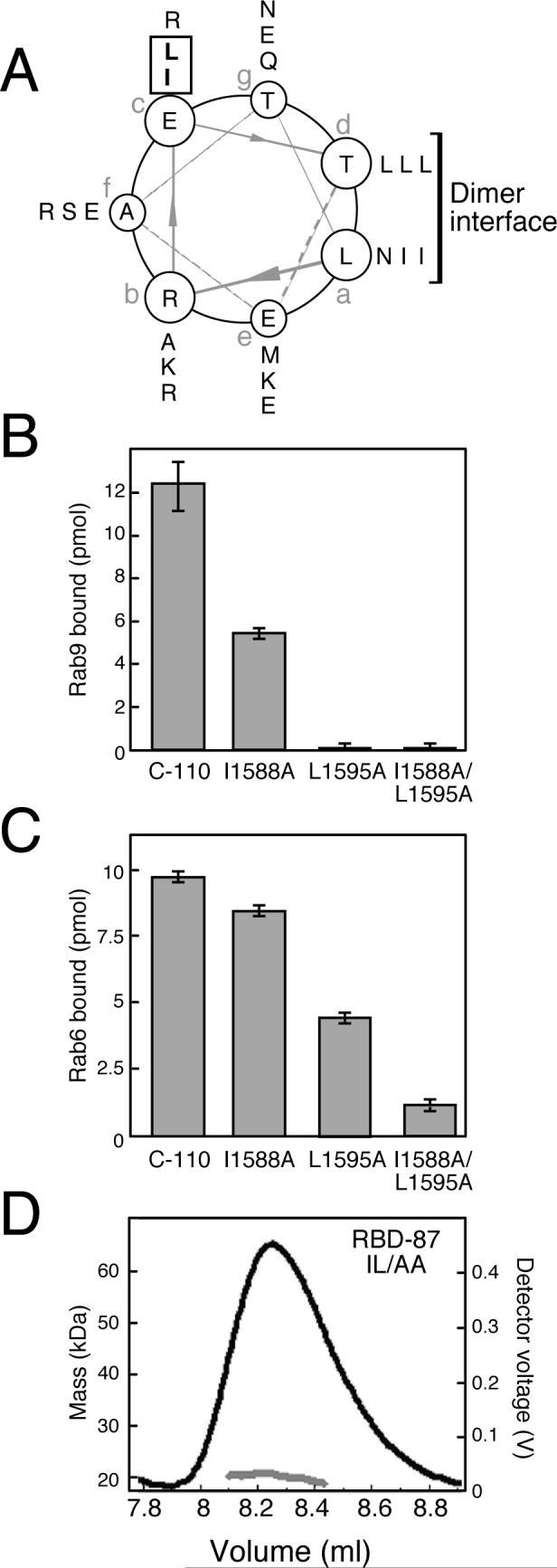
Accessible hydrophobic residues in the predicted coiled coil are critical for Rab binding. (A) Helical wheel projection of a coiled coil predicted for GCC185 residues 1579−1606. Residues in registers “a-f” were predicted by the Paircoil program. Residues at positions “a” and “d” lie in the dimer interface. Boxed residues are candidates for binding interactions with Rab GTPases. (B, C) Effect of alanine substitutions on Rab binding. Reactions contained wild type or mutant GST-C-110 (B; 3 μM, C; 2 μM) and 35S-GTPγS-preloaded GTPases (B; 170 pmol Rab9-His, C; 190 pmol His-Rab6). Data are mean ± SD. (D) Mass determination of untagged RBD-87 I1588A/L1595A by multiple angle static light scattering. The gel filtration elution profile of the protein (black line) and molecular mass (grey line) are shown. Polydispersity of the peak was 1.001.
I1588 and L1595 were therefore mutated to alanine within the C-110 polypeptide; binding of these mutant proteins to Rab6 and Rab9 was then tested. The I1588A mutant bound ∼50% less to Rab9 than its wild type counterpart (Figure 3B). The L1595A mutation abolished Rab9 binding completely; a double mutant was also binding incompetent, as expected. I1588 and L1595 were also critical for Rab6 binding (Figure 3C). The I1588A mutant showed slightly less (14%) binding than the wild type polypeptide; L1595A was decreased about 50%, and the double mutant was the most severely impaired (88%). Because the double mutant was more impaired than either single mutant, we conclude that both residues contribute to a Rab binding interface. The lower affinity of Rab9 for the C-110 fragment (Figure S1) may explain why Rab9 is more sensitive to mutations at these positions.
Dimer formation was not disrupted in the RBD-87 I1588A/L1595A protein, as it behaved as a 20.4 kDA, mono-disperse dimer upon gel filtration and multiple angle static light scattering (Figure 3D), nearly identical to wild type RBD-87 (Figure 2C). In addition, untagged C-110 I1588A/L1595A yielded a circular dichroism spectrum that was indistinguishable from its wild type counterpart (not shown), thus the mutant protein did not appear to be misfolded. Together, these data confirm that Rab9 and Rab6 overlap in their interaction with a coiled coil Rab binding domain in GCC185.
Structure of Rab6 bound to the GCC185 Rab binding domain
The complex of human GCC185 (residues 1547−1612) and the constitutively active mutant of Rab6 (Q72L) was crystallized in the presence of GTP and Mg2+ (Table I). The structure was solved by molecular replacement using the crystal structure of GTP-bound Rab6 (Bergbrede et al., 2005, PDB ID 2gil) as the search model. A large portion of the Rab binding domain of GCC185 was clearly visible in difference electron density maps, allowing unambiguous placement of sidechains at the binding interface (Figure S2). The sidechain register was verified with an anomalous difference Fourier map of selenomethionine substituted GCC185 in complex with Rab6 (Figure 4C). The final model refined to Rfree = 26.8 and R = 22.8 at 3 Å resolution (Table I); it includes Rab6 residues 14−174, GCC185 residues 1570−1607, bound GTP and Mg2+.
Table 1.
Data Collection and Refinement Statistics.
| Native (PDB ID) | Seleno-methionine | |
|---|---|---|
| Data Collection | ||
| Space Group | P6422 | P6422 |
| Unit Cell | ||
| a (Å) | 168.6 | 167.4 |
| c (Å) | 169.0 | 167.6 |
| Stoichiometry in asymmetric unit | 3 Rab6:3 GCC185 | 3 Rab6:3 GCC185 |
| X-ray source | SSRL beamline 7−1 | SSRL beamline 11−1 |
| Detector | ADSC Q315 | Marmosaic 325 |
| Wavelength (Å) | 0.9785 | 0.9791 |
| Resolution range (Å) | 50−3.0 | 50−3.4 |
| Effective resolution (I/σ>= 2(Å)) | 3.2 | 3.6 |
| Completeness (%) | 99.7 | 99.9 |
| Rmerge (%) | 25.0 | 22.6 |
| I/σ (highest resolution shell) | 10.1 (0.5) | 10.3 (0.8) |
| Refinement statistics | ||
| Rwork/Rfree (%) | 22.8/26.8 | |
| R.m.s.d bond length (Å) | 0.003 | |
| R.m.s.d bond angles (o) | 0.46 | |
| NCS r.m.s.d. (Å, Rab6) | 0.36 | |
| NCS r.m.s.d. (Å, GCC185) | 0.5 | |
| % residues in Ramachandran: | ||
| Most favored region | 90.7 | |
| Additionally allowed | 8.7 | |
| Generously allowed | 0.7 | |
| Disallowed | 0 | |
| Crystallization | ||
| Method | Vapour diffusion at 20°C. Protein and reservoir solution (1.5 μl each) were mixed and seeded in hanging drops with 0.5 ml reservoir solution. | |
| Condition | 20% (w/v) PEG 3350, 200 mM CNNaS | |
| Cryoprotection | 20% (w/v) PEG 3350, 200 mM CNNaS, 20% (v/v) glycerol, 5 mM Hepes (pH 7.5) | |
Figure 4.
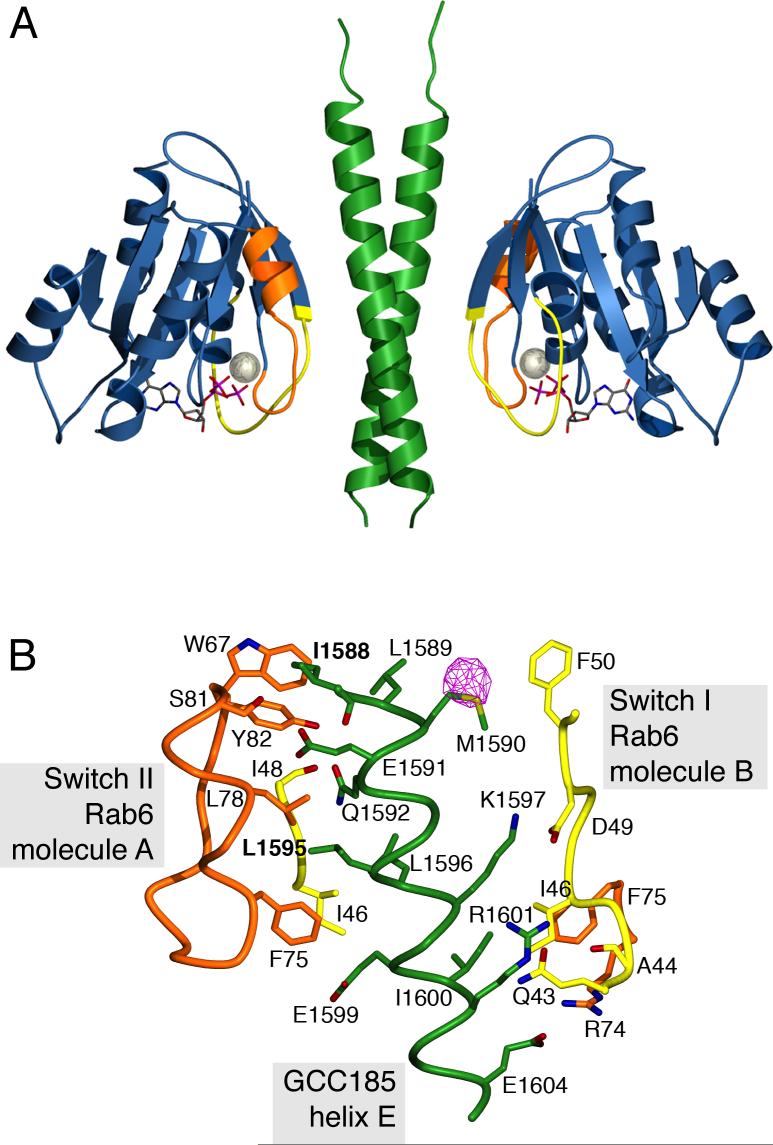
Structure of the Rab6-GCC185 complex. A. Ribbon representation of the GCC185 Rab binding domain dimer (green) and Rab6 (blue) bound to GTP (stick model) and magnesium (sphere). Switch I and II regions of Rab6 (Chattopadhyay et al., 2000) are colored yellow and orange respectively. B. View of the Rab6-GCC185 binding interface. A single GCC185 helix (E) out of the two-fold symmetric coiled coil is shown for clarity. Each helix contacts switch regions from two opposed Rab6 molecules A and B. Rab6 switch I and II (including W67), are colored yellow and orange, respectively. Protein backbone (α-carbon trace) and side chains involved in polar and hydrophobic interactions are shown. Carbonyl oxygens are shown for A44, I48 and I1588 and C-Cα bonds have been added to simplify the figure. An anomalous difference Fourier density map of the selenomethionine substituted crystal (pink, contoured at 6σ) is shown for GCC185.
Two Rab6-GTP molecules contact the GCC185 coiled coil dimer with two-fold symmetry (Figure 4A and B). Each GCC185 helix bridges two opposing Rab6 molecules (Figure 4C), and conversely, each Rab6 molecule interacts with both helices that comprise the coiled coil. This strongly suggests that Rab binding will stabilize a dimeric GCC185 structure. Extensive contacts comprise a buried interface of 1250 Å2 for a single Rab6 with the GCC185 dimer (Figure S3). For comparison, a similar area is buried in the single Arl1:GRIP dimer interface (PDB ID 1UPT; 1310 Å2; our calculations).
As expected from the nucleotide dependence of Rab interaction (Figure 1), the Rab binding interface is contributed by switch I and II regions (Figure 4) whose different conformations in the GDP and GTP bound forms primarily reflect the state of the GTPase on its surface (Stroupe and Brunger, 2000). Gratifyingly, the two GCC185 hydrophobic residues required for Rab binding (I1588 and L1595, Figure 3) are part of the interface between Rab6 and the GCC185 Rab binding domain (Figure 4C, bold; Figure S3). Moreover, two of the three invariant, hydrophobic triad residues of Rabs (F50, W67 and Y82) proposed to be important for Rab:effector interactions (Merithew et al., 2001) interact directly with I1588 (Figure S3), and the third residue also contacts GCC185.
No major conformational changes of Rab6 are observed upon binding to GCC185; Rab6-GTP (Bergbrede et al., 2005, PDB ID 2gil) superimposes with Rab6 in our complex with a root-mean-square deviation (r.m.s.d.) of 0.55 Å. This is in contrast to the complexes of Rab11:FIP3 (Eathiraj et al., 2006) and Sec4p:Sec2p (Dong, et al., 2007) where Rab binding to the coiled coil domains induces major conformational changes in the Rabs.
Residues important for Rab binding regulate Golgi localization via Rab6
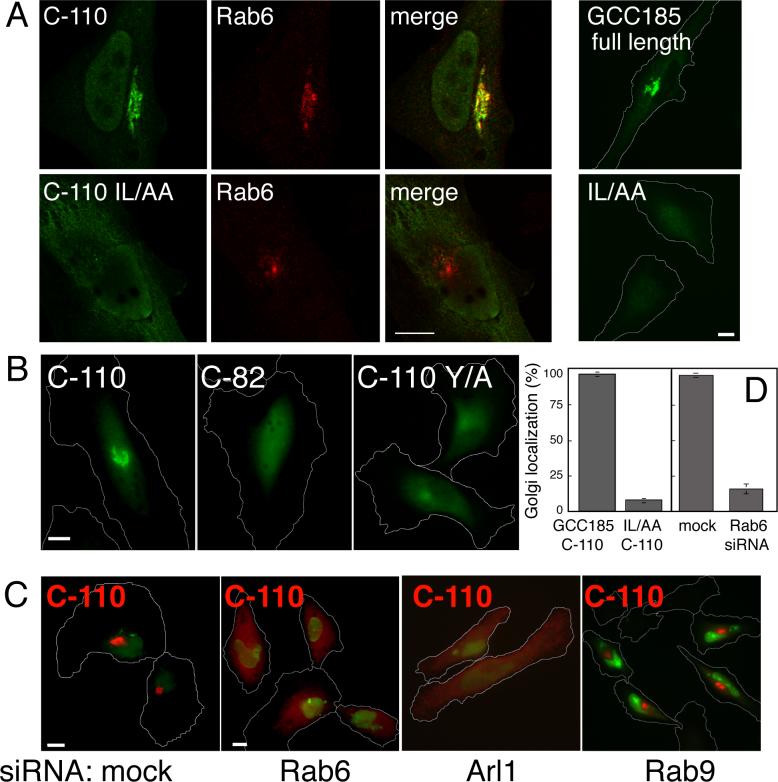
To explore the importance of Rab binding determinants in GCC185 localization, we expressed wild type or I1588A/L1595A, C-110 proteins in cultured cells and monitored their localization. Myc-tagged, wild type C-110 was localized to the Golgi complex as determined by its co-localization with Rab6 protein by confocal microscopy (Figure 5A, D). In contrast, the C-110 I1588A/L1595A protein that did not bind Rab6 or Rab9 in vitro (Figure 3B,C) failed to localize to the Golgi complex (Figure 5A, D). As expected, exogenously expressed, full length GCC185 was Golgi localized (Figure 5A top far-right); in contrast, full length GCC185 containing the I1588A/L1595A substitutions was not (Figure 5A, bottom far-right).
Figure 5.
GCC185 Golgi targeting requires the Rab binding domain and Rab6 and Arl1 GTPases. (A) Confocal micrograph of HeLa cells expressing myc-tagged wild type or I1588A/L1595A C-110, 19 h post transfection. c-myc epitope (green) and Rab6 (red) are shown. A confocal section is shown; image step size was 15.2 nm. Far right panels were analyzed as in B. (B) Conventional fluorescence micrograph of HeLa cells transfected with myc-C-110, myc-C-82 (20 h post transfection) or myc-C-110 Y1618A (29 h post transfection). Cells were stained as in A. (C) Conventional immunofluorescence micrograph of HeLa cells transfected with 6-FAM-conjugated RNA oligonucleotides (green, mock) or these plus siRNAs targeting Rab6, Arl1 or Rab9 as indicated. Post depletion, and 17 h prior to fixation, cells were transfected with myc-GCC185 C-110 (red). siRNA-siRNA-transfected cells are indicated by nuclear green fluorescence from 6-FAM conjugated RNA oligonucleotides.myc-C- (D). Quantification of panels A and part of C. Perinuclear myc-fluorescence above background that overlapped with Rab6 staining was scored as Golgi localization. Standard deviations are from two experiments, 100 cells counted per bar shown. For quantitation of C, perinuclear fluorescence above background was scored in mock (n=159 and 113) and Rab6 siRNA (n=314 and 210) treated cells in duplicate experiments. Bars, 10 μm.
The requirement for residues I1588 and L1595 in Golgi localization showed that Rab binding domain-determinants dominate the Golgi association process. Consistent with this, a myc-tagged fragment ("C-82") comprised of the 82 C-terminal most residues of GCC185 (and lacking I1588 and L1595) also failed to associate with the Golgi complex (Figure 5B middle panel), in contrast to C-110 under identical conditions (Figure 5B, left). All transfected cells counted (n=100) displayed these phenotypes. This differs from a report of Luke et al. (2003) who showed Golgi localization of a similar, GFP-tagged construct. The two studies likely differ because of expression levels (see below).
As shown in Figure 5C, loss of Rab6 (but not Rab9) using siRNA abolished the Golgi localization of the wild type C-110 fragment. Quantitation showed that >80% of depleted cells displayed this phenotype (Figure 5D). These data demonstrate that Rab6 is important for Golgi association of GCC185.
Although not sufficient, the GRIP domain in C-82 could still participate in Golgi association. Thus, we tested localization of C-110 Y1618A, which is predicted to not bind Arl1. C-110 Y1618A was less concentrated on the Golgi than wild type C110 (Figure 5B right), suggesting that the GRIP domain also contributes to Golgi localization. Indeed, siRNA depletion of Arl1 confirmed a requirement for this GTPase in C-110 localization in living cells (Figure 5C). As expected, Golgin-97 and Golgin-245 also lost Golgi association in Arl1 depleted cells (Supp. Figure 4) however global TGN organization Sinceremained intact as determined by TGN46 localization (Supp. Figure 4). Yet the Y1618A mutation did not completely abolish Golgi localization, from which we conclude that C-110 associates predominantly via Rab binding domain interactions.
Rab6 facilitates Arl1 binding to GCC185
The localization studies strongly suggested that both Rab binding and GRIP domains contribute to GCC185 localization. Yet initial binding experiments failed to detect Arl1 binding to the GCC185 GRIP domain (Reddy et al., 2006; Figure 1C). We therefore explored the possibility that Rab association with GCC185 may promote Arl1 binding. Untagged Arl1 GTPase was preloaded with radioactive GTP and desalted to remove unbound nucleotide. Arl1 was then incubated with increasing concentrations of the GST-C-110 fragment, in the presence or absence of Rab6 or Rab9-GTPγS; GST-C-110-bound Arl1 was measured directly in a scintillation counter.
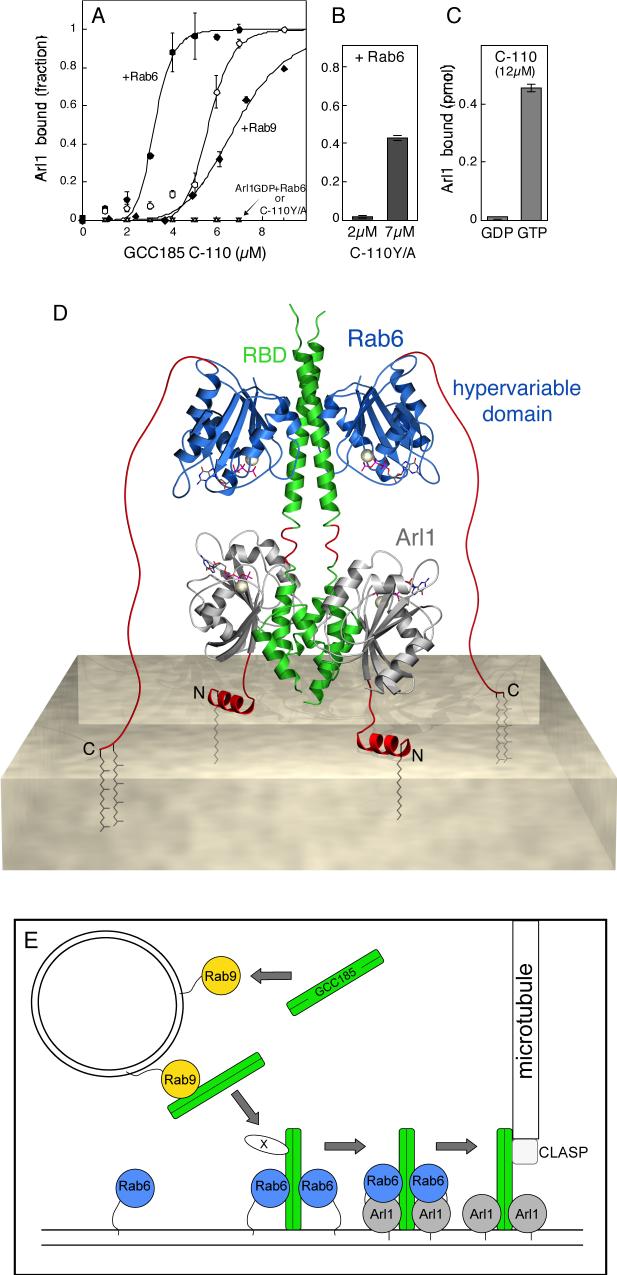
Addition of Rab6 enhanced the ability of GCC185 to bind Arl1 (Figure 6A). In reactions containing <5μM C-110 polypeptide, very low levels of Arl1 binding were detected, as shown earlier (Figure 1C; Reddy et al., 2006). For comparison, the GRIP domain of Golgin 245 binds efficiently at 2μM C-C-110 (cf. Reddy et al., 2006). When reactions were supplemented with Rab6, Arl1 binding increased up to seven-fold and a sigmoidal, saturable binding profile was observed. This binding profile is suggestive of cooperative binding, consistent with a model in which the binding of the first Arl1 monomer facilitates binding of a second Arl1 monomer to a C-110 dimer. In contrast, addition of Rab9 had a slight inhibitory effect on Arl1 binding (Figure 6A). Rab6-independent binding of Arl1 was only seen at 7μM GCC185, consistent with the dissociation constant for the Arl1:GCC185 interaction (Kd=7μM, Supplemental Figure 1C).
Figure 6.
Dual GTPase binding to GCC185. A. Rab6 stimulates Arl1 GTPase binding to GCC185. His-Rab6 or untagged Rab9 were preloaded with cold GTPγS; Arl1Δ17 was loaded with [35S] GTPγS or 3H-GDP. GST-C-110 (wild type or Y/A) was then incubated with Rab6- or Rab9-GTPγS (3.5μM) for 30 min at room temperature. Arl1Δ17 (1.2μM) bearing radioactive nucleotide was added to preformed complexes and samples were rotated for 1 h at room temperature in a total volume of 250μl. Data in (A) are fitted to an equation reflecting cooperative binding. Maximal binding was 2.2% of input Arl1 protein. Wild type C-110 binding to: Open circles, Arl1 alone; filled circles, Arl1 + Rab6; closed diamonds, Arl1 + Rab9; small triangles, Arl1GDP + Rab6; C-110 Y/A binding to: inverted triangles, Arl1 alone. B. Rab6 (3.5μM) was incubated with GST-C-110 Y/A at the indicated concentrations; Arl1 was then added as in A. Shown is the fraction Arl1 protein bound. C. Nucleotide dependence of Arl1 binding to GST-C-110. Reactions contained 22.8 pmol Arl1 protein. A-C, data are mean ± SD. D. Model of Rab6 and Arl1 bound to the GCC185 Rab binding domain (RBD) and GRIP domains, respectively. GCC185 (green) bound to Rab6 (blue) and Arl1 (grey). Modeled regions (red) include the Rab6 hypervariable domain (extended), the Arl1 N-terminus at the membrane and the junction between the GRIP and Rab binding domains of GCC185. Lipid anchors (brown) and the cytosolic leaflet of the membrane bilayer are shown. See text for details. E. Model for tether transfer from a vesicle to the Golgi membrane. Dimeric, cytosolic GCC185 is first recruited onto a Rab9-bearing vesicle. GCC185 on the vesicle is proposed to interact with a Golgi-bound GCC185 via a hypothetical linking protein (X) that may represent CLASP. Initial docking would permit SNARE pairing (not shown); independently, Rab6 displaces Rab9 from GCC185 and then promotes Arl1 binding. Soluble GCC185 may also be recruited to the Golgi by a Rab9 independent process. Proteins in E are not drawn to scale. At far right: GCC185-bound CLASP that nucleates microtubule polymerization at the Golgi.
Specificity of Arl1 binding was confirmed by its nucleotide dependence in the presence (Figure 6A) or absence (Figure 6C) of Rab6-GTPγS. It also required the conserved tyrosine residue documented to be important for GRIP domain-Arl1 interactions (Figure 6A, B). Arl1 did not bind C-110 Y1618A at concentrations as high as 7μM mutant protein (Figure 6A bottom). At this concentration, addition of Rab6 restored ∼40% binding of Arl1 protein to C-110 Y1618A, despite the loss of the key tyrosine residue (Figure 6B). This provides independent confirmation that Rab6 can indeed enhance the interaction between Arl1 and GCC185. In summary, our study has revealed a novel and unexpected mechanism by which Rab6 regulates GCC185's ability to bind to Arl1 via its C-terminal GRIP domain.
A model for dual GTPase regulation of GCC185
We used the crystal structure of the dimeric Golgin-245 GRIP domain bound to two Arl1 molecules (Panic et al., 2003) to generate a structure model of the GCC185 GRIP domain bound to Arl1 (see Methods). Simultaneous binding of Rab6 (blue) and Arl1 (gray) to GCC185 was then simulated by combining the Arl:GRIP modeled complex with our Rab6:GCC185 Rab binding domain structure (Figure 6C). The modeled GCC185 GRIP domain was linked to the Rab binding domain by addition of the only four residues that were not present in either of the two combined structures (red; Figure 6C) to form a continuous helical junction between the GRIP N-termini and the Rab binding domain coiled coil. Analysis of this junction shows that the two domains cannot be linked by a continuous coiled coil, which implies that this junction may be flexible or even disordered.
Several features of this model are especially noteworthy. The C-termini of the Rab6 core domains (blue) face away from the membrane surface and are connected to the 34 amino acid long, Rab6 hypervariable domains (red) which are not visible in our crystal structure. Our model positions Rab6 at the farthest possible distance (up to ∼105 Å) from the membrane surface. Nevertheless, the Rab6 hypervariable domain is long enough to accommodate membrane anchoring by two C-terminal prenyl groups. Moreover, this conformation allows insertion of both Arl1 N-terminal helices and myristoyl groups into the membrane (as proposed by Panic et al., 2003; Wu et al., 2004) along an axis that is perpendicular to that of the Rab6 prenyl insertions. Together, the hetero-hexameric complex would form a stable platform to support GCC185 on the membrane with six lipid anchors and two protein helices distributed over four radial attachment points. The GCC185 C-terminal tail may also be inserted in the membrane, further stabilizing the lipid association (Panic et al., 2003). The upright orientation of GCC185 could be important for its role in connecting the TGN to non-centrosomal microtubule tracks (Efimov et al., 2007) and also for its putative function as a tether for transport vesicles.
In summary, the presence of two adjacent GTPase binding sites suggests an unexpected interplay between these proteins in mediating GCC185 localization and function. A striking aspect of our model is the potential ability of extended Rab hypervariable domains to reach a considerable distance away from the membrane surface where the GTPase catalytic domain contacts and regulates important binding partners.
Discussion
We have shown here that the GCC185 C-terminus contains a Rab GTPase binding site located just upstream of its GRIP domain. Binding of Rab6 to this site promotes association of Arl1 with the GRIP domain, and both GTPases act in concert to localize GCC185 to the TGN. Our structure-derived model of simultaneous GTPase binding offers two possibilities for how these proteins may cooperate. It is possible that the Rab binding and GRIP domains are conformationally flexible, such that the GRIP domain interacts better with Arl1 upon Rab6 binding. Alternatively, the GTPase pairs may be aligned to allow direct contacts between Rab6 and Arl1. In both cases, stabilization of the coiled coil dimer by Rab6 may facilitate the dimerization of the GRIP domain, a requirement for Arl1 binding.
A number of proteins rely on bipartite interactions to achieve their specific cellular localizations. For example, EEA1 is targeted to early endosomes by interaction with Rab5 and phosphatidylinositol 3-phosphate, and TIP47 binds late endosomes by interaction with Rab9 and mannose 6-phosphate receptor cytoplasmic domains (see Grosshans et al., 2006 for review). However, rather than just localizing GCC185 to the Golgi, Rab6 may regulate how and when GCC185 binds Arl1. Our experiments show that residues I1588 and L1595 that are important for Rab6 interaction are essential for correct localization of full length GCC185. We favor a model whereby Rab6 binding initiates stable association with the TGN, but could be dispensible after subsequent GCC185 engagement by two Golgi localized, Arl1 molecules.
Cooperation of G proteins has significant precedent in biology. For example, a Rab GTPase cascade acts in Golgi-to-plasma membrane vesicle transport: one Rab (YPT32) recruits an effector (Sec2) that acts as a guanine nucleotide exchange factor (GEF) to activate the subsequently acting, Sec4 Rab protein (Ortiz et al., 2002). In protein translocation, the signal recognition particle (SRP) receptor GTPases, SRα and SRβ cooperate in targeting the signal recognition particle:ribosome nacent chain complex to the endoplasmic reticulum (Halic and Beckmann, 2005). In other cases, an effector may bind two GTPases without obvious influence of one on another: for example, Rab11 and Arf5 or Arf6 bind independently to distinct sites on FIP proteins, without enhancing the other GTPase's binding affinity (Hickson et al., 2003). In our case, binding of Rab6 at one site enhances the binding of a second class of small GTPase to a second site.
Cooperation between Rab and Arl1 may be conserved across kingdoms. The single GRIP domain containing protein in Arabidopsis thaliana (Gilson et al., 2004) contains a conserved putative Rab binding domain just upstream of its C-terminal GRIP domain. Residues that comprise the GCC185:Rab6 binding interface are conserved in this protein and assume identical, coiled coil register positions. In analogy to our study, inclusion of the coiled coil region conferred efficient Golgi localization (Latijnhouwers et al., 2005; Gilson et al., 2004). Like GCC185, the plant GRIP domain protein also requires Arl1-binding for its localization (Latijnhouwers et al., 2005).
An important aspect of our model is the possibility that Rab proteins may reach some distance from the membrane (up to ∼10nm) to bind to an effector protein, due to the unstructured nature of a Rab protein's ∼30 residue, membrane anchored, C-terminal hypervariable domain (Ostermeier and Brunger, 1999; Neu et al., 1997). Thus, unlike Arf-family GTPases that are anchored closely to the membrane by N-terminal sequences and often, myristoylation, Rab proteins show a distinct mode of interaction. Gillingham and Munro (2007) have noted that membrane proximity makes Arf GTPases best suited for vesicle coat recruitment and lipid binding and deformation. The difference in the mode by which these two types of GTPases bind effectors enables Rabs and Arf-family members to cooperate in their interactions.
Our crystal structure of the Rab6:GCC185 Rab binding domain revealed two Rab molecules binding a two-fold symmetric coiled coil. This is in contrast to the asymmetric coiled coil observed in the GEF Sec2p that results in only one Sec4p molecule binding to it (Dong et al., 2007; Sato et al., 2007). Two-fold symmetric coiled coils in the Rabaptin5, RILP and FIP3 effectors offer a pair of binding sites for Rab5, Rab7 or Rab11, respectively (Zhu et al., 2004; Wu et al., 2005; Eathiraj et al., 2006; Shiba et al., 2006). Analysis of the sequence immediately upstream of the GCC88 GRIP domain suggests it will assume a coiled coil that may also be a Rab binding site. The corresponding regions in Golgin-245 and Golgin-97 are predicted to be disordered and include numerous proline residues, making it impossible for them to assume a Rab binding domain structurally similar to that in GCC185. Indeed, neither Rab6 (not shown) nor Rab9 (Reddy et al., 2006) bind the C-terminus of Golgin-245. However, these large coiled coil proteins may contain additional Rab binding sites elsewhere in their sequences.
A model for GCC185 in tethering at the Golgi complex
GCC185 is likely to play multiple roles at the Golgi complex. The discovery that it is linked via CLASP proteins to the microtubule-based cytoskeleton may enable Golgi-localized GCC185 to provide a direct track to facilitate the delivery of incoming transport vesicles to the TGN. Live cell video microscopy of Rab9-containing transport vesicles confirmed that they arrive at the TGN along cytoskeletal (and likely microtubule-based) tracks (Barbero et al., 2002).
We propose that GCC185 first associates with transport vesicles via Rab9 (Fig. 6E; Reddy et al., 2006). Once these vesicles have arrived at the Golgi (and microtubule minus ends), binding of vesicle-bound GCC185 to CLASP proteins might bring the vesicle close to the TGN to allow docking. After subsequent SNARE interaction (not shown), Rab6 on the Golgi could displace Rab9 and trigger Arl1 binding to GCC185, thus transferring GCC185 onto the TGN. In this model, tethering facilitates vesicle access to TGN-localized t-SNAREs that mediate fusion, a process that may be independent of the Rab/Arl1 transfers shown. After fusion, Rab9 is retrieved from the Golgi by GDI, a protein that extracts Rab-GDP from membranes, for return to late endosomes.
Rab9 seems to interfere with GCC185's ability to bind Arl1, thus Rab6 would need to displace Rab9 from GCC185 before Arl1 could bind. Considering these data altogether, GCC185 could be transferred in a directional manner from one membrane compartment to another, through an ordered set of binding interactions. Future experiments will provide additional mechanistic insight into when, where and how GCC185 engages distinct Rab and Arl GTPases to potentially mediate transport vesicle tethering, motility, and microtubule attachment to the TGN.
Experimental Procedures
Plasmids for protein expression
Inserts for pGST-linked to GCC185 residues1527−1684 (C-158), 1527−1613 (RBD-87) and 1614−1684-His (C-72) were generated by PCR using the parental templates pGST-GCC1851575−1684 (C-110) and pHis-GCC1851343−1684 (Reddy et al., 2006). PCR products were cloned into pGEX-6T-1. QuickChange mutagenesis was used to generate I1588A and L1595A mutations and His-Rab6 1−174 in pET15B (Stratagene, La Jolla, CA). Rab6Q72L/S180L/C181L and His-Arl1Q71L 14−181 were generated by PCR and cloned into pET47b. Wild type or mutant C-110 was amplified by PCR and cloned into pcDNA3 containing anN- N-terminal triple c-myc epitope tag to generate myc-C-82, C-110 and C-110 I1588A/L1595A. pGST-GCC185Y1618A was from Reddy et al. (2006).
Protein expression, purification and crystallization
Recombinant Rab1, Rab5, His-Rab6, His-Rab6 1−174, Rab9, Rab9-His, Rab9/1, Arl1Δ17 and ArlQ71L-His proteins were purified (Aivazian et al., 2006). Rab9/1 contains Rab9 residues 1−169 followed by Rab1 residues 173−205. pGST-GCC1851527−1684, GCC1851527−1684, pGST-GCC1851575−1684, pGST-GCC1851614−1684-His, Rab6 Q72L/S180L/C181L and ArlQ71L-His were transformed into E. coli BL21(DE3) cells and induced at an OD600 of 0.6 with 1 mM IPTG over night at 23°C. pGST-GCC1851527−1613 was induced for 3 h at 30°C. Protein purification was as described (Reddy et al., 2006). Protein was concentrated in Amicon Ultra filter units (5,000 MWCO, Millipore). For crystallization, Rab6Q72Lsc180−181LL and GCC1851547−1612 were lysed by sonication; the clarified supernatant bound to glutathione-Sepharose 4B (Amersham). GST or His tags were removed with PreScission protease (Amersham) on resin and gel filtered on Superdex 75 16/60 in buffer A (200 mM NaCl, 20 mM Hepes (pH 7.4), 1 mM MgCl2, 0.5 mM DTT). Rab6Q72Lsc180−181LL was loaded with 1 mM GTP overnight at 4°C in buffer A. Equimolar Rab6Q72Lsc180−181LL (13 mg/ml) and GCC1851547−1612 (7.5 mg/ml) (1 ml) were incubated overnight at 4°C in buffer A, 1 mM GTP. The complex was purified on Superdex 75 16/60 equilibrated with buffer A plus 100 mM NaCl and concentrated to 10 mg/ml. Selenomethionyl GCC1851547−1612 was prepared (Doublie, 1997), purified as above with 5 mM β-mercaptoethanol; complex with Rab6Q72Lsc180−181LL was formed. Initial crystals formed in sitting drops (200nl) containing a 1:1 (v/v) ratio of protein to reservoir solution (condition 61, PEGs I Suite, Qiagen). Diffraction quality crystals obtained by microseeding in hanging drops were cryoprotected prior to freezing in liquid nitrogen (Table S2). Circular dichroism, multiple angle static light scattering methods, and microscopy and antibodies are in supplemental material.
Diffraction data collection and structure determination
Diffraction data of GCC185:Rab6 complex crystals were collected at 100K and integrated and scaled using DENZO and SCALEPACK, respectively (Otwinowski and Minor, 1997). Data from a crystal containing selenomethionyl substituted GCC185 in complex with Rab6 were collected at the peak wavelength of 0.9791 Å as determined by a fluorescence scan and processed as above. The structure (PDB ID 3BBP) was solved by molecular replacement (Phaser, McCoy et al., 2005) using Rab6 (PDB ID 2gil) as a search model, stripped of solvent, nucleotide and metal ions. Using the molecular replacement phases, a σA-weighted mFo – DFc electron density map clearly indicated the presence of the Rab binding domains of GCC185 and bound nucleotide and magnesium in Rab6. The nucleotide was modeled as GTP based on a triphosphate moiety with a bridging magnesium ion. There are three Rab6 molecules and three GCC185 monomers in the asymmetric unit. Two of these Rab6 molecules interact with a GCC185 dimer forming a 2:2 complex, the third Rab6 along with a GCC185 monomer interact with crystallographic-symmetry related molecules to also form a 2:2 complex. Using a generic poly-alanine coiled coil as a starting point (derived from the crystal structure of GCN4 (Gonzalez et al., 1996, PDB ID 1ZIK), the backbone of the GCC185 Rab binding domain coiled coil, residues 1570−1607, was built. Electron density maps allowed unambiguous placement of sidechains (Figure S2). The structures of the two 2:2 complexes are nearly identical (r.m.s.d. 0.56 Å). Five percent of the observed data were set aside for cross validation, and an initial simulated annealing refinement was carried out using a maximum likelihood amplitude-based target function (CNS, Brunger et al., 1998), resulting in an R value of 30.0%. Further refinement was carried out in Phenix.refine, interspersed with manual model adjustments in Coot (Emsley and Cowtan, 2004). The final stage of refinement treated each Rab monomer as an independent TLS group and the 3 helical GCC185 segments in the asymmetric unit as an independent TLS group, resulting in a substantial decrease (5−6%) in both R and Rfree (Table I).
Molecular modeling
GCC185 (residues 1612−1665) and Golgin245 (residues 2171−2221) were manually aligned. The GCC185 GRIP domain structure was modeled in SWISS-MODEL and combined with Arl1 using PDB ID 1UPT (Panic et al, 2003). Target and template structures were identical with the only exception of an insertion of three residues in a loop between GCC185 GRIP domain helix 1 and 2. The Rab6 hypervariable domain (residues 175−208) and the Arl1 N-terminal helix (residues 2−16) were built in Chimera (Pettersen et al., 2004) and Coot using PDB files 2D4Z and 1UPT respectively. The inter-domain junction of GCC185 (residues 1608−1611) was modeled in Coot by aligning alpha carbons in a helical arrangement at ∼3.8 Å from each other.
Nucleotide loading and detection of GTPase binding to GCC185
GTPases (13−26 μM) were loaded with [35S]GTPγS (MP Biomedicals, Irvine, CA) or [3H]GDP (GE Healthcare) and equimolar cold nucleotide in 20 mM Hepes/KOH, pH7.4, 150 mM KCl, 2 mM EDTA, 1 mM MgCl2 and 0.1 mg/ml BSA 37°C(Rab9: 30 min, 37°C; Rab6 and Rab1: 3h, 37°C; Arl1, 3h, 30°C) (Reddy et al., 2006). Arl1 was loaded for 3 h at 30 °C as above but in 4 mM EDTA. Free nucleotide was removed in a NAP-5 column (GE Healthcare) pre-equilibrated in binding buffer (50 mM Hepes/KOH, pH 7.4, 150 mM KCl, 5 mM MgCl2 and 0.1 mg/ml BSA). GST-GCC185 was incubated with preloaded GTPases. Complexes were collected with glutathione resin and washed extensively on a solid support. The specific activity of 35S-GTPγS was determined by scintillation counting and bound Rab protein calculated after determining the input in a filter-binding assay. A low GST background was subtracted.
Competition assay
Rab proteins were pre-loaded with cold GTPγS as above and nucleotide exchange was stopped with 5 mM MgCl2. Rabs (0.5 μM) were then incubated with GST-GCC1851575−1684 (5 μM) for 30 min at 22° in 200 μl binding buffer (50 mM Hepes, 150 mM KCl, 5 mM MgCl2, 10 μg/ml BSA) and then bound to 25 μl of a 50% slurry of glutathione beads for 30 min at 22°C. Excess Rab-GTP competitor was added for 3 min and complexes were pelleted by centrifugation at 3500 rpm for 10 sec. The supernatant was removed by aspiration with a needle. Complexes were washed 4 × 400 μl in binding buffer and eluted with 40 mM glutathione.
RNA interference
siGLO Green fluorescent oligonucleotides (Dharmacon Research Inc.) were pooled with Rab6 (Smartpool), Rab9 (Reddy et al., 2006), or Arl1 oligos (50 nM each, Dharmacon) and transfected into HeLa cells at 30% confluency using Oligofectamine (Invitrogen) according to the manufacturer. The Arl1 siRNA target sequence was: AAGAAGAGCUGAGAAAAGCCA. Cells were fixed (Warren et al., 1984) and analyzed 72 h post transfection.
Acknowledgements
This research was funded by grants from the NIH (DK37332) to S.R.P. and the HHMI to A.T.B. We thank Drs. D. Aivazian for Rab9/1 binding data, L. Serrano for Rab purification, R. Kahn for Arl1 plasmids, and W. Weis and H. Choi for expertise. A.S.B. thanks Drs. A. Ghabrial and S. Sambashivan for critical comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplemental Figure 1. Affinity determination of GCC185:GTPase interactions by isothermal titration calorimetry. (Upper panels) Raw data of 8-μl injections of Rab6-GTPγS (247 μM) (A), (B) Rab9-GTPγS (200 μM), or (C) His-Arl1 Q71L 14−181-GTPγS (250μM) into GST-C-110 (A: 11.9 μM; B: 17 μM; C: 4μM) or buffer (inset). (Lower panels) Nonlinear least-squares fit of the integrated heat released at each injection as a function of added ligand. The total heat released in each injection was plotted against the molar ratio of GTPase protein to GST-C-110; the binding isotherm was determined using the identical-site model. Inset presents titrations into GST as a control. kcal; kilocalories. Values (kcal/mol) for ΔH, TΔS, ΔG, and n were: −4.8, 2.9,−7.7 and 0.91 for Rab6-GTP; −2.7, 4.6, −7.3 and 0.78 for Rab9-GTP. Method–GTPase (0.5 ml ∼500 μM) and GST-C-110 (1ml ∼40 μM) were applied to NAP-5 and NAP-10 columns (Amersham Biosciences, Piscataway, NJ) respectively, that were pre-equilibrated in 50 mM Hepes, pH 7.4, 150 mM KCl, 1 mM MgCl2. Proteins were eluted (GTPase in 1 ml and GST-C-110 in 1.5 ml). GTPases (0.5 ml) were then loaded with GTPγS (300 μM) after addition of 2 mM EDTA. Nucleotide exchange was stopped with 7 mM MgCl2. Equal concentrations of EDTA, MgCl2 and GTPγS were added to the GST-C-110 sample in a total volume of 2 ml in the exact same buffer. Samples were passed through a 0.4 μm Amicon Ultrafree®-MC filter (Millipore) and degassed. Protein concentrations were determined in a BioRad assay and by absorbance at 280 nm (GST-GCC185 only). GTPases were loaded in the syringe and GST-GCC185 into the cell (1.426 ml volume). Data was collected at 24°C in a VP-ITC calorimeter (Microcal LLC, Northampton, MA) according to the manufacturer, using a stirring speed of 270 rpm. Injections (8 μl) were interspersed by an equilibration time of 290 sec. An initial injection of 2 μl was performed to remove trapped air bubbles. Data was fitted to a one-set-of-sites model after baseline subtraction. The binding constant (K) was determined using the Origin software (Microcal LLC, Northampton, MA). Control experiments included GTPase injection into buffer or GST.
Supplemental Figure 2. View of side chain interactions between the GCC185 Rab binding domain and Rab6. Shown is a representative portion of a σA-weighted 2 mFo – DFc electron density map (blue, contoured at 1 σ). GCC185 helices D and E are yellow and pink respectively, and Rab6 molecules A and B are green and cyan, respectively.
Supplemental Figure 3. Schematic of Rab6-GCC185 interactions. Rab6 residues are generally divided between those in chain A (top blue box) and those in chain B (bottom blue box). GCC185 residues (green box) and coiled coil register positions (subscript) are indicated. Rab6 switch I and II amino acids are colored yellow and orange respectively. Atom distances were <3.5 Å for hydrogen bonds and salt bridges (black dashed lines) and <4.6 Å for Van der Waal interactions (grey dashed lines). Boxed residues disrupted Rab binding when mutated to alanine.
Supplemental Figure 4. Control reactions show that Arl1 siRNA mislocalizes GRIP domain proteins that require Arl1 for localization. Top panels, Golgin-97 and Golgin-245 in control cells; Lower panels, Golgin-97, Golgin-245, and TGN46 after Arl1 siRNA treatment (72 hours). Proteins are shown in red; siGlo transfected cells are green nuclei-stained.
Supplemental Methods
Preparation and circular dichroism of RBD-87–RBD-87 or RBD-87 I1599A/L1595A was bound to glutathione resin and digested with Prescission Protease according to the manufacturer (Amersham Biosciences, Piscataway,NJ), overnight, rotating at 4°C. RBD-87 was then separated from GST, GST-Prescission Protease and glutathione beads over a solid support and concentrated (Amicon Ultra, 5,000 MWCO, Millipore). The cleaved fragment includes 92 amino acids; 5 residues remain from the Prescission Protease cleavage site. CD spectra of RBD-87 (3.2 μM) were measured in 10 mM sodium phosphate using an Aviv Model 62 ADS spectropolarimeter and a 1 cm path length quartz cuvette. Data were collected at 22°C, with 1 nm resolution and at a scan rate of 6 nm min−1. The blank buffer-value at each wavelength was subtracted from each point.
Mass determination by multiple angle static light scattering–RBD-87 (300 μM) or RBD-87 I1588A/L1595A (150 μM) were injected on a Shodex kw-802.5 column equilibrated in 50 mM Hepes and 150 mM KCl, and coupled to a UV-monitor, a DAWN EOS detector and an Optilab rEX refractive index instrument (Wyatt technology Corporation). The laser wavelength was 690 nm and the flow rate 0.5 ml min−1. The native mass of the proteins was determined by software analysis of the scattered light intensity detected at 18 different angles (Wyatt technology Corporation). For refractive index, a dn/dc value of 0.185 ml/g was assumed in all calculations.
Microscopy and antibodies–HeLa cells were transfected using Fugene reagent (Roche Diagnostics, Indianapolis) according to the manufacturer. Cells were fixed as above. Images were collected with a Zeiss Axioplan2, an inverted Olympus IX70 epifluorescence microscope or a confocal Leica AOBS system. Images were processed using DeltaVision (Applied Precision), AxioVision4 (Zeiss) or Leica confocal software version 2.61 and Adobe Photoshop (Adobe Systems, Mountain View, CA) software. Mouse anti-Rab9 (Aivazian et al., 2006), and primary monoclonal antibody (9E10) against the c-myc epitope were described previously. Rabbit anti-Rab6 (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-mouse Alexa fluor 488, goat anti-rabbit and goat anti-mouse Alexa fluor 594 and 647 (Invitrogen, Carlsbad, CA) and goat anti-rabbit Cy3 (Jackson Immunoresearch) antibodies were purchased. Other antibodies were described (Reddy et al., 2006).
References
- Aivazian D, Serrano RL, Pfeffer S. TIP47 is a key effector for Rab9 localization. J. Cell Biol. 2006;173:917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J. Cell Biol. 2002;156:511–8. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr. Biol. 1999;9:381–384. doi: 10.1016/s0960-9822(99)80167-5. [DOI] [PubMed] [Google Scholar]
- Bergbrede T, Pylypenko O, Rak A, Alexandrov A. Structure of the extremely slow GTPase Rab6A in the GTP bound form at 1.8 resolution. J. Struct. Biol. 2005;152:235–238. doi: 10.1016/j.jsb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Berger B, et al. Predicting coiled coils by use of pairwise residue correlations. Proc. Natl. Acad. Sci. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D, Langsley G, Carson M, Recacha R, DeLucas L, Smith C. Structure of the nucleotide-binding domain of Plasmodium falciparum rab6 in the GDP-bound form. Acta Crystallogr. D. 2000;56:937–944. doi: 10.1107/s0907444900007575. [DOI] [PubMed] [Google Scholar]
- Derby MC, et al. Mammalian GRIP domain proteins differ in their membrane binding properties and are recruited to distinct domains of the TGN. J. Cell Sci. 2004;117:5865–5874. doi: 10.1242/jcs.01497. [DOI] [PubMed] [Google Scholar]
- Derby MC, Lieu ZZ, Brown D, Stow JL, Goud B, Gleeson PA. The trans-Golgi network Golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic. 2007;8:758–73. doi: 10.1111/j.1600-0854.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- Dong G, Medkova M, Novick P, Reinisch KM. A catalytic coiled-coil: structural insights into the activation of the Rab GTPase Sec4 by Sec2p. Mol. Cell. 2007;25:455–462. doi: 10.1016/j.molcel.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublie S. Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 1997;276:523–30. [PubMed] [Google Scholar]
- Eathiraj S, Mishra A, Prekeris R, Lambright DG. Structural basis for Rab11-medated recruitment of FIP3 to recycling endosomes. J. Mol. Biol. 2006;364:121–135. doi: 10.1016/j.jmb.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Efimov A, et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell. 2007;12:917–30. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fridmann-Sirkis Y, Siniossoglou S, Pelham HR. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 2004;5:18. doi: 10.1186/1471-2121-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingham AK, Munro S. The Small G Proteins of the Arf Family and Their Regulators. Annu. Rev. Cell Dev. Biol. 2007 Jul 10; doi: 10.1146/annurev.cellbio.23.090506.123209. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gilson PR, Vergara CE, Kjer-Nielsen L, Teasdale RD, Bacic A, Gleeson PA. Identification of a Golgi-localised GRIP domain protein from Arabidopsis thaliana. Planta. 2004;219:1050–1056. doi: 10.1007/s00425-004-1311-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Jr., Woolfson DN, Alber T. Buried polar residues and structural specificity in the GCN4 leucine zipper. Nat. Struct. Biol. 1996;3:1011–1018. doi: 10.1038/nsb1296-1011. [DOI] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic M, Beckmann R. The signal recognition particle and its interactions during protein targeting. Curr. Opin. Struct .Biol. 2005;15:116–125. doi: 10.1016/j.sbi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Harbury PB, Zhang T, Kim PS, Alber T. A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science. 1993;262:1401–1407. doi: 10.1126/science.8248779. [DOI] [PubMed] [Google Scholar]
- Hickson GR, Matheson J, Riggs B, Maier VH, Fielding AB, Prekeris R, Sullivan W, Barr FA, Gould GW. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell. 2003;14:2908–2920. doi: 10.1091/mbc.E03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Teasdale RD, van Vliet C, Gleeson PA. A novel Golgi-localisation domain shared by a class of coiled-coil peripheral membrane proteins. Curr. Biol. 1999;9:385–388. doi: 10.1016/s0960-9822(99)80168-7. [DOI] [PubMed] [Google Scholar]
- Latijnhouwers M, Hawes C, Carvalho C, Oparka K, Gillingham AK, Boevink P. An Arabidopsis GRIP domain protein locates to the trans-Golgi and binds the small GTPase ARL1. Plant J. 2005;44:459–70. doi: 10.1111/j.1365-313X.2005.02542.x. [DOI] [PubMed] [Google Scholar]
- Li B, Warner JR. Mutation of the Rab6 homologue of Saccharomyces cerevisiae, YPT6, inhibits both early Golgi function and ribosome biosynthesis. J. Biol. Chem. 1996;271:16813–16819. doi: 10.1074/jbc.271.28.16813. [DOI] [PubMed] [Google Scholar]
- Linding R, et al. Protein disorder prediction: implications for structural proteomics. Structure. 2003;11(11):1453–1459. doi: 10.1016/j.str.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Lu L, Hong W. Interaction of Arl1-GTP with GRIP Domains Recruits Autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol. Biol. Cell. 2003;14:3767–3781. doi: 10.1091/mbc.E03-01-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke MR, Houghton F, Perugini MA, Gleeson PA. The trans-Golgi network GRIP-domain proteins form alpha-helical homodimers. Biochem. J. 2005;388:835–841. doi: 10.1042/BJ20041810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke MR, Kjer-Nielsen L, Brown DL, Stow JL, Gleeson PA. GRIP domain-mediated targeting of two new coiled-coil proteins, GCC88 and GCC185, to subcompartments of the trans-Golgi network. J. Biol. Chem. 2003;278:4216–4226. doi: 10.1074/jbc.M210387200. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr. D. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- Merithew E, Hatherly S, Dumas JJ, Lawe DC, Heller-Harrison R, Lambright DG. Structural plasticity of an invariant hydrophobic triad in the switch regions of Rab GTPases is a determinant of effector recognition. J. Biol. Chem. 2001;276:13982–13988. doi: 10.1074/jbc.M009771200. [DOI] [PubMed] [Google Scholar]
- Neu M, Brachvogel V, Oschkinat H, Zerial M, Metcalf P. Rab7: NMR and kinetics analysis of intact and C-terminal truncated constructs. Proteins. 1997;27:204–209. doi: 10.1002/(sici)1097-0134(199702)27:2<204::aid-prot6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 2002;157:1005–15. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermeier C, Brunger AT. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin–3A. Cell. 1999;96:363–374. doi: 10.1016/s0092-8674(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Panic B, Perisic O, Veprintsev DB, Williams RL, Munro S. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol. Cell. 2003;12:863–874. doi: 10.1016/s1097-2765(03)00356-3. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. “UCSF Chimera - A Visualization System for Exploratory Research and Analysis.”. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1999;1:E17–22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- Reddy JV, et al. A functional role for the GCC185 golgin in mannose 6-phosphate receptor trafficking. Mol. Biol. Cell. 2006;17:4353–4363. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Shirakawa R, Horiuchi H, Dohmae N, Fukai S, Nureki O. Asymmetric coiled-coil structure with Guanine nucleotide exchange activity. Structure. 2007;15:245–252. doi: 10.1016/j.str.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Shiba T, Koga H, Shin HW, Kawasaki M, Kato R, Nakayama K, Wakatsuki S. Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc. Natl. Acad. Sci. U S A. 2006;103:15416–15421. doi: 10.1073/pnas.0605357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou S, Pelham HR. An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 2001;20:5991–5998. doi: 10.1093/emboj/20.21.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Brunger AT. Crystal structures of a Rab protein in its inactive and active conformations. J. Mol. Biol. 2000;304:585–98. doi: 10.1006/jmbi.2000.4236. [DOI] [PubMed] [Google Scholar]
- Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am. J. Physiol. Cell Physiol. 2006;290:C11–26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- Tsukada M, Gallwitz D. Isolation and characterization of SYS genes from yeast, multicopy suppressors of the functional loss of the transport GTPase Ypt6p. J. Cell Sci. 1996;109:2471–2481. doi: 10.1242/jcs.109.10.2471. [DOI] [PubMed] [Google Scholar]
- Warren G, Davoust J, Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984;3:2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Lu L, Hong W, Song H. Structural basis for recruitment of GRIP domain golgin-245 by small GTPase Arl1. Nat. Struct. Mol. Biol. 2004;11:86–94. doi: 10.1038/nsmb714. [DOI] [PubMed] [Google Scholar]
- Wu M, Wang T, Loh E, Hong W, Song H. Structural basis for recruitment of RILP by small GTPase Rab7. EMBO J. 2005;24:1491–1501. doi: 10.1038/sj.emboj.7600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, et al. Structural basis of Rab5-Rabaptin5 interaction in endocytosis. Nat. Struct. Mol. Biol. 2004;11:975–983. doi: 10.1038/nsmb832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Affinity determination of GCC185:GTPase interactions by isothermal titration calorimetry. (Upper panels) Raw data of 8-μl injections of Rab6-GTPγS (247 μM) (A), (B) Rab9-GTPγS (200 μM), or (C) His-Arl1 Q71L 14−181-GTPγS (250μM) into GST-C-110 (A: 11.9 μM; B: 17 μM; C: 4μM) or buffer (inset). (Lower panels) Nonlinear least-squares fit of the integrated heat released at each injection as a function of added ligand. The total heat released in each injection was plotted against the molar ratio of GTPase protein to GST-C-110; the binding isotherm was determined using the identical-site model. Inset presents titrations into GST as a control. kcal; kilocalories. Values (kcal/mol) for ΔH, TΔS, ΔG, and n were: −4.8, 2.9,−7.7 and 0.91 for Rab6-GTP; −2.7, 4.6, −7.3 and 0.78 for Rab9-GTP. Method–GTPase (0.5 ml ∼500 μM) and GST-C-110 (1ml ∼40 μM) were applied to NAP-5 and NAP-10 columns (Amersham Biosciences, Piscataway, NJ) respectively, that were pre-equilibrated in 50 mM Hepes, pH 7.4, 150 mM KCl, 1 mM MgCl2. Proteins were eluted (GTPase in 1 ml and GST-C-110 in 1.5 ml). GTPases (0.5 ml) were then loaded with GTPγS (300 μM) after addition of 2 mM EDTA. Nucleotide exchange was stopped with 7 mM MgCl2. Equal concentrations of EDTA, MgCl2 and GTPγS were added to the GST-C-110 sample in a total volume of 2 ml in the exact same buffer. Samples were passed through a 0.4 μm Amicon Ultrafree®-MC filter (Millipore) and degassed. Protein concentrations were determined in a BioRad assay and by absorbance at 280 nm (GST-GCC185 only). GTPases were loaded in the syringe and GST-GCC185 into the cell (1.426 ml volume). Data was collected at 24°C in a VP-ITC calorimeter (Microcal LLC, Northampton, MA) according to the manufacturer, using a stirring speed of 270 rpm. Injections (8 μl) were interspersed by an equilibration time of 290 sec. An initial injection of 2 μl was performed to remove trapped air bubbles. Data was fitted to a one-set-of-sites model after baseline subtraction. The binding constant (K) was determined using the Origin software (Microcal LLC, Northampton, MA). Control experiments included GTPase injection into buffer or GST.
Supplemental Figure 2. View of side chain interactions between the GCC185 Rab binding domain and Rab6. Shown is a representative portion of a σA-weighted 2 mFo – DFc electron density map (blue, contoured at 1 σ). GCC185 helices D and E are yellow and pink respectively, and Rab6 molecules A and B are green and cyan, respectively.
Supplemental Figure 3. Schematic of Rab6-GCC185 interactions. Rab6 residues are generally divided between those in chain A (top blue box) and those in chain B (bottom blue box). GCC185 residues (green box) and coiled coil register positions (subscript) are indicated. Rab6 switch I and II amino acids are colored yellow and orange respectively. Atom distances were <3.5 Å for hydrogen bonds and salt bridges (black dashed lines) and <4.6 Å for Van der Waal interactions (grey dashed lines). Boxed residues disrupted Rab binding when mutated to alanine.
Supplemental Figure 4. Control reactions show that Arl1 siRNA mislocalizes GRIP domain proteins that require Arl1 for localization. Top panels, Golgin-97 and Golgin-245 in control cells; Lower panels, Golgin-97, Golgin-245, and TGN46 after Arl1 siRNA treatment (72 hours). Proteins are shown in red; siGlo transfected cells are green nuclei-stained.
Supplemental Methods
Preparation and circular dichroism of RBD-87–RBD-87 or RBD-87 I1599A/L1595A was bound to glutathione resin and digested with Prescission Protease according to the manufacturer (Amersham Biosciences, Piscataway,NJ), overnight, rotating at 4°C. RBD-87 was then separated from GST, GST-Prescission Protease and glutathione beads over a solid support and concentrated (Amicon Ultra, 5,000 MWCO, Millipore). The cleaved fragment includes 92 amino acids; 5 residues remain from the Prescission Protease cleavage site. CD spectra of RBD-87 (3.2 μM) were measured in 10 mM sodium phosphate using an Aviv Model 62 ADS spectropolarimeter and a 1 cm path length quartz cuvette. Data were collected at 22°C, with 1 nm resolution and at a scan rate of 6 nm min−1. The blank buffer-value at each wavelength was subtracted from each point.
Mass determination by multiple angle static light scattering–RBD-87 (300 μM) or RBD-87 I1588A/L1595A (150 μM) were injected on a Shodex kw-802.5 column equilibrated in 50 mM Hepes and 150 mM KCl, and coupled to a UV-monitor, a DAWN EOS detector and an Optilab rEX refractive index instrument (Wyatt technology Corporation). The laser wavelength was 690 nm and the flow rate 0.5 ml min−1. The native mass of the proteins was determined by software analysis of the scattered light intensity detected at 18 different angles (Wyatt technology Corporation). For refractive index, a dn/dc value of 0.185 ml/g was assumed in all calculations.
Microscopy and antibodies–HeLa cells were transfected using Fugene reagent (Roche Diagnostics, Indianapolis) according to the manufacturer. Cells were fixed as above. Images were collected with a Zeiss Axioplan2, an inverted Olympus IX70 epifluorescence microscope or a confocal Leica AOBS system. Images were processed using DeltaVision (Applied Precision), AxioVision4 (Zeiss) or Leica confocal software version 2.61 and Adobe Photoshop (Adobe Systems, Mountain View, CA) software. Mouse anti-Rab9 (Aivazian et al., 2006), and primary monoclonal antibody (9E10) against the c-myc epitope were described previously. Rabbit anti-Rab6 (Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-mouse Alexa fluor 488, goat anti-rabbit and goat anti-mouse Alexa fluor 594 and 647 (Invitrogen, Carlsbad, CA) and goat anti-rabbit Cy3 (Jackson Immunoresearch) antibodies were purchased. Other antibodies were described (Reddy et al., 2006).