Abstract
A model [6 + 5] segment coupling process involving a C-terminal valine hexapeptide acid and a resin-attached pentapeptide amide which N-terminated in a hindered Aib unit was examined using a variety of HOAt-derived coupling reagents. Best results were observed with HAPyU in DCM solvent in which loss of configuration amounted to 5.8%.
It is known that as the amino acid residue to which a chiral amino acid or peptide residue is coupled becomes more-and-more sterically hindered, the rate of coupling decreases and correspondingly the risk of loss of configuration at the reacting chiral carboxylic acid residue increases1. The activated intermediate presumably has more chance for undergoing loss of configuration, possibly via an oxazolone intermediate, during the slow coupling process. Examples include the coupling of the oxazolone derived from Z-Aib-Phe-OH to the series of amino acid esters H-Gly-OEt, H-Ala-OMe and H-Aib-OMe1.
Because newer coupling reagents based on 1-hydroxy-7-azabenzotriazole (HOAt)2 have made it possible to decrease the loss of configuration for coupling at ordinary proteinogenic amino acids we have now examined some of these newer reagents in the case of couplings to segments N-terminating in an Aib unit. A common system which includes a number of Aib units at various positions and thus represents a convenient model for such systems is the alamethicin class of peptaibols3.
Prior to the development of a convenient stepwise solid-phase route to the alamethicins via Fmoc amino acid fluorides4 these naturally occurring materials had routinely been approached via segment coupling techniques5. For success in such cases the requisite segment couplings were always designed to occur, for the nucleophilic component, at an ordinary proteinogenic amino acid with the carboxylic acid component terminating in either a non-chiral or “safe” chiral amino acid such as Gly, Aib or Pro6. Indeed the fact that the achiral Aib unit can be used at the C-terminal position illustrates the fact that coupling at carboxylic acid Aib units are much more easily achieved than coupling to the amino group of an Aib residue. During an early study of a segment-based route to alamethicin recorded by Schmitt and Jung5b the coupling of Boc-Pro-Val-OH to H-Aib-Aib-OMe was shown to be compromised by the formation of 20–30% of the D-Val epimer of the desired tetrapeptide. Therefore the scheme adopted by these workers reversed the identities of the nucleophilic and electrophilic residues involved in the coupling process. Recently Peggion, Coin and Toniolo7 promoted a related segment coupling technique as a convenient approach for the synthesis of a wide variety of alamethicin analogs.
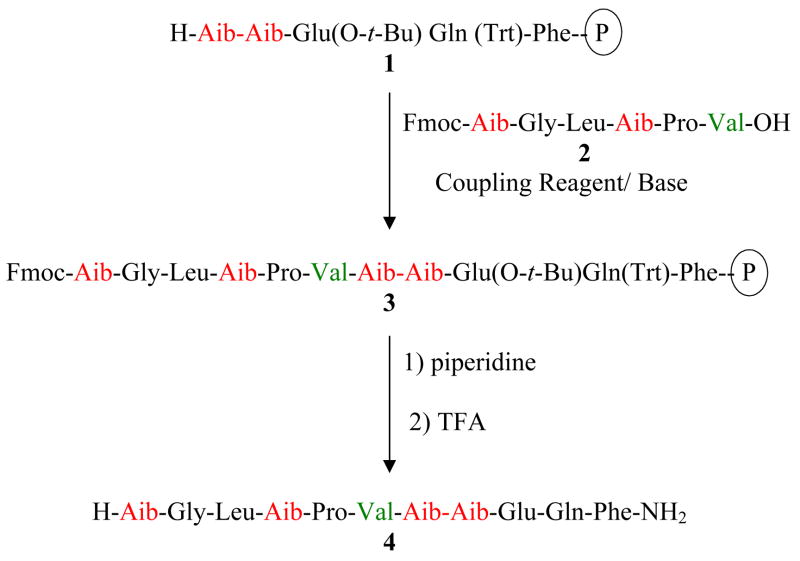
In the present work we have reversed the positions of the key amino acid units, deliberately placing the nucleophilic Aib unit, as in the Schmitt/Jung study at the N-terminal position of the nucleophilic segment in order to determine if this “illogical” design could succeed in the case of the newer coupling reagents. In order to effect a solid-phase segment coupling process the desired pentapeptide unit was built onto a solid support to give 1. The carboxylic acid segment 2 to be coupled to 1 terminated in valine. Coupling was expected to provide the undecapeptide attached to the support 3. Upon Fmoc deblocking and removal from the support by consecutive treatment with piperidine and TFA the extent of absorption in the HPLC trace near 9.36 min due to residual unreacted pentapeptide amide 5 can be used as a measure of the completeness of the coupling process.
Upon comparing N-TBTU/DIEA and N-HATU/DIEA as coupling reagents for this system in DMF over a period of 35 min. only the latter gave evidence of the desired undecapeptide amide and if the latter reaction were allowed to proceed for 16 h the reaction was nearly complete. However, loss of configuration was massive (65.4% of the D-epimer) as seen in Table 1. Formation of over 50% of the D-epimer suggests the incursion of asymmetric induction8, presumably via the intermediacy of the peptide oxazolone derived from 2. Substitution of the weaker base TMP for DIEA gave slightly less D-epimer (52.7%) although the reaction appeared to be less complete.
Table 1.
[6+5] Coupling of 2 with 1 to Give Resin 3a
| Run | Coupling Reagent | Base | Coupling Time (h) | Solvent | % D-Val Epimer |
|---|---|---|---|---|---|
| 1 | N-HATU (3 eq) | DIEA, (6 eq) | 16 | DMF | 65.4 |
| 2 | N-HATU (3 eq) | TMP, (6 eq) | 24 | DMF | 52.7 |
| 3 | N-HATU (3 eq) | DB(DMAP) (6 eq) | 16 | DMF | 39.2 |
| 4 | N-HATU (3 eq) | DB(DMAP) (6 eq) | 5 | DMF | 28.6 |
| 5 | N-HATU (3 eq). | PS, (6 eq) | 5 | DMF | 33.9 |
| 6 | N-HATU (3 eq) | DB(DMAP) (3 eq) PS, (3 eq) | 5 | DMF | 26.0 |
| 7 | N-HATU (3 eq). | DB(DMAP) (3 eq) PS, (3 eq) | 14 | DCM | 18.7 |
| 8 | N-HAPyU (3 eq) | DB(DMAP) (3 eq) PS, (3 eq) | 14 | DCM | 5.84 |
Under the conditions given only runs 7 and 8 were judged to be nearly complete based on the absence of significant absorption in the HPLC trace due to recovered pentapeptide amide 5. Yields for 4 cannot be given since the crude reaction mixtures were only examined by HPLC analysis for evidence of completion of the coupling process and the relative extent of formation of the D-Val epimer of 4.
Previously it had been shown that DB(DMAP)9 is a tertiary base which is comparable to TMP in avoiding loss of configuration during peptide coupling although as a stronger base (pKa about 9 vs. 7.43 for TMP) it should allow for a greater extent of coupling. This was confirmed by comparison of runs involving coupling over 16 h which with N-HATU/DIEA and N-HATU/DB(DMAP) led to 65.4% and 39.2% of the D-Val epimer, respectively. With a 1:1 mixture of the still stronger base proton sponge along with DB(DMAP) coupling over a period of 5 h led to a reduction in the extent of D-Val epimer formation to 26.0%.
Further improvements in DMF solvent were not achieved and it was only by switching to DCM that significantly better results were observed. Thus with DB(DMAP)/PS/N-HATU in DCM coupling was nearly complete after 14 h and loss of configuration was reduced to 18.7% (run 7, Table 1). Finally when the more efficient coupling reagent N-HAPyU9 was substituted for N-HATU these conditions gave nearly complete coupling and only 5.84% of the D-Val epimer (run 8, Table 1). The only system which achieved better results involved the HOAt/DIC system10 in DCM (4.5% D-Val epimer) and while the coupling yield was low after 14 h, presumably extending the reaction time might allow for completion of the reaction.
These results appear to set the limits to what we could achieve with these newer reagents in this highly hindered system. With two strong, highly hindered bases DB-(DMAP) and PS, coupling is more effective than with either of these bases alone or with either the strong base DIEA alone or the much weaker base TMP alone11,13.
In summary, it is shown that for the solid phase segment coupling of 2 to 1, for avoiding loss of configuration at the D-Val carboxylic acid unit, the guanidinium-type coupling reagent N-HAPyU is superior to N-HATU, DCM is preferable as solvent to the more polar DMF and that careful selection of the base is important. Thus a mixture of DB(DMAP) and PS is more effective than either one alone and much more effective than DIEA or TMP. While the use of a strong base is important in boosting the coupling process toward completion these bases must be sterically hindered in order to avoid extensive loss of configuration. As yet no explanation can be offered as to why the mixture of DB(DMAP) and PS is more effective than either base alone.
Supplementary Material
Supplementary Data
Experimental details for carrying out stepwise solid phase syntheses for authentic samples of all peptides used and solid phase segment coupling reactions. In addition high- and low-resolution MS spectra and HPLC traces for all model peptides are presented as well as HPLC traces for the undecapeptide amide obtained by segment condensation under various conditions.
Scheme 1.
Acknowledgments
We are indebted to the National Institutes of Health (GM-09706) for support of this work. The National Science Foundation is also thanked for grants used to purchase the high field NMR spectrometers used in this work.
Footnotes
Abbreviations used: Aib = α-aminoisobutyric acid residue; Boc = tert-butyloxycarbonyl; DCC = dicyclohexylcarbodiimide; DB(DMAP) = 2,6-di-t-butyl-4-(dimethylamino)pyridine; DCM = dichloromethane; DIC = N,N′-diisopropylcarbodiimide; DIEA= N,N diisopropylethylamine; DMF,= N,N-dimethylformamide; Fmoc = 9-fluorenemethyloxycarbonyl,; N-HAPyU = 1-(dipyrrolidinylmethylene)-1H-1,2,3-triazolo [4,5-b] pyridinium hexafluorophosphate 3-oxide; N-HATU = 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b] pyridinium, hexafluorophosphate 3–oxide; PS = proton sponge = 1,8-bis(dimethylamino)naphthalene; N-TBTU = 1-[bis (dimethylamino)methylene]-1H-benzotriazolium tetrafluoroborate 3-oxide; TMP = 2,4,6-trimethylpyridine; TFA = trifluoroacetic acid.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Goodman M, McGahren WJ. Tetrahedron. 1967;23:2031. doi: 10.1016/0040-4020(67)80037-1. [DOI] [PubMed] [Google Scholar]
- 2.(a) Carpino LA. J Amer Chem Soc. 1993;115:4397. [Google Scholar]; (b) Carpino LA, El-Faham A. J Org Chem. 1994;59:695. [Google Scholar]; (c) Carpino LA, El-Faham A, Albericio F. J Org Chem. 1995;60:3561. [Google Scholar]
- 3.For a recent review on the synthesis of peptides containing α,α-dialkyl amino acid including those incorporating these amino acids in consecutive positions see: Formaggio F, Broxterman QB, Toniolo C. In: Houben-Weyl, Methods in Organic Chemistry. Goodman M, Felix A, Moroder L, Toniolo C, editors. E22c. Thieme; Stuttgart, Germany: 2003. pp. 292–310.
- 4.(a) Wenschuh H, Beyermann M, Krause E, Brudel M, Winter R, Schümann M, Carpino LA, Bienert M. J Org Chem. 1994;59:3275. [Google Scholar]; (b) Wenschuh H, Beyermann M, Haber H, Seydel JK, Krause E, Bienert M, Carpino LA, El-Faham A, Albericio F. J Org Chem. 1995;60:405. [Google Scholar]
- 5.(a) Balasubramanian TM, Kendrick NCE, Taylor M, Marshall GR, Hall JE, Vodyanoy F, Reusser F. J Amer Chem Soc. 1981;103:6127. [Google Scholar]; (b) Schmitt H, Jung G. Liebigs Ann Chem. 1985:321. [Google Scholar]; (c) Gisin BF, Davis DG, Borowska ZK, Hall JE, Kobayashi S. J Amer Chem Soc. 1981;103:6373. [Google Scholar]
- 6.Although couplings of peptide segments C-terminating in proline have long been considered safe, this is not the case for coupling to an Aib residue, especially if the proline residue is attached to an Aib unit or some other sterically hindered residue, e.g. the pivaloyl group. Loss of configuration in such systems is believed to proceed via a positively charged oxazolonium intermediate. See: Nagaraj R, Balaram P. Tetrahedron. 1981;37:2001.
- 7.Peggion C, Coin I, Toniolo C. Pept Sci. 2004;76:485. doi: 10.1002/bip.20161. [DOI] [PubMed] [Google Scholar]
- 8.Davies JS, Thomas RJ. J Chem Soc Perkin. 1981;1:1639. [Google Scholar]
- Benoiton NL, Young CL, Chen FMF. Int J Pept Protein Research Chem. 1991;38:574. doi: 10.1111/j.1399-3011.1991.tb01542.x. [DOI] [PubMed] [Google Scholar]
- 9.Carpino LA, Ionescu D, El-Faham A. J Org Chem. 1996;61:2460. [Google Scholar]
- 10.Carpino LA, El-Faham A. Tetrahedron. 1999;55:6813. [Google Scholar]
- 11.General Procedure for Solid-Phase Synthesis: Syntheses were carried out manually using as reaction vessels, plastic syringes which could be attached to a water aspirator line12. The starting resin (500 mg) of Fmoc-PAL-PEG-PS resin (0.2 mmol/g resin) was washed with DMF (3 × 10 ml), DCM (3 × 10 ml) and DMF (3 × 10 ml). The Fmoc group was deblocked by means of 20% piperidine in DMF for 10 min. After complete deprotection, solvent was removed via the aspirator. The deblocked resin was washed with DMF (3 × 10 ml) and the first coupling was carried out using 3 equiv. of Fmoc AA-OH, 3 equiv. of N-HATU and 6 equiv. of DIEA with a preactivation time of 2–3 min., a coupling time of 35 min. and a deblocking time of 10 min.(20% piperidine in DMF). A Kaiser test was carried out after each coupling. After assembling the complete sequence of the desired peptide, the Fmoc-group was deblocked and the resin washed with DMF (3 × 10 ml), DCM (3 × 10 ml), ethanol (2 × 10 ml) and ether (1 × 10 ml). The resin was dried under vacuum for 15 min. and then treated with a mixture of 50% TFA/DCM, 5% H2O, 5% phenol and 2% triisopropylsilane for 2 h. The filtrate was collected and the resin was further washed with DCM (3 × 5 ml). The combined filtrates were evaporated in vacuo at room temperature and the residue was precipitated by adding cold ether, the solvent was decanted, the residue dried under vacuum and the crude peptide was examined by MS (MALDI) and HPLC analysis carried out on a C18 5μm Waters Novapak column, 150 × 3.9 mm, flow rate 1 ml/min., detection at 220 nm with a Waters 996 PDA detector using the following system: 5–60% ACN/H2O 0.1% TFA in 25 min. (linear gradient) followed by holding at 60% ACN/H2O 0.1%TFA for 15 min.
- 12.Carpino LA, Ismail M, Truran GA, Mansour EME, Iguchi S, Ionescu D, El-Faham A, Riemer C, Warrass R. JOrg Chem. 1999;64:4324. [Google Scholar]
- 13.Solid-Phase Segment Synthesis of H-Aib-Gly-Leu-Aib-Pro-Val-Aib-Aib-Glu-Gln-Phe-NH2: The undecapeptide amide was assembled on 50 mg of pentapeptide resin 1 using 3 equiv. of acid 2 (23 mg), 3 equiv. of the coupling reagent and either 6 equiv. of a single base or 3 equiv. of each of two bases. Coupling times were varied and final cleavage from the resin was carried out using a mixture of 50% TFA in DCM, 5% H2O, 5% phenol and 2% triisopropylsilane for 2 h. All reactions involving DMF alone were carried out in plastic syringes as described in the general procedure whereas those involving mixtures of DMF/DCM or DCM alone were carried out in capped vials with stirring only in the case of DCM. HPLC analyses were carried out as described above. Data from the various runs are collected in Table 1. The species formed during the coupling processes were identified in each case by the retention times of the L- or D-epimers of the undecapeptide amide 4 or the residual unreacted pentapeptide amide 5 as determined by analysis of the authentically synthesized model compounds. The completeness of the coupling process could be judged qualitatively by the absence of significant absorption in the HPLC trace near the retention time (9.36 min) of the recovered amide 5. Integration of the peaks at 15.19 and 16.14 min. due to the D-Val-epimer and the all–L epimer, respectively, of the undecapeptide amide 4 gave the extent of loss of configuration as noted in Table 1. Details for representative runs are given in the Supporting Information Section.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Experimental details for carrying out stepwise solid phase syntheses for authentic samples of all peptides used and solid phase segment coupling reactions. In addition high- and low-resolution MS spectra and HPLC traces for all model peptides are presented as well as HPLC traces for the undecapeptide amide obtained by segment condensation under various conditions.