Abstract
Spectrin (βIΣ∗) and ankyrin (AnkG119) associate with Golgi membranes and the dynactin complex, but their role in vesicle trafficking remains uncertain. We find that the actin-binding domain and membrane-association domain 1 (MAD1) of βI spectrin together form a constitutive Golgi targeting signal in transfected MDCK cells. Expression of this signal in transfected cells disrupts the endogenous Golgi spectrin skeleton and blocks transport of α- and β-Na,K-ATPase and vesicular stomatitis virus-G protein from the endoplasmic reticulum (ER) but does not disrupt the formation of Golgi stacks, the distribution of β-COP, or the transport and surface display of E-cadherin. The Golgi spectrin skeleton is thus required for the transport of a subset of membrane proteins from the ER to the Golgi. We postulate that together with polyfunctional adapter proteins such as AnkG119, Golgi spectrin forms a docking complex that acts prior to the cis-Golgi, presumably with vesicular–tubular clusters (VTCs or ERGIC), to sequester specific membrane proteins into vesicles transiting between the ER and Golgi, and subsequently (probably involving other isoforms of spectrin and ankyrin) to mediate cargo transport within the Golgi and to other membrane compartments. We hypothesize that this vesicular spectrin–ankyrin adapter-protein trafficking (or tethering) system (SAATS) mediates the capture and transport of many membrane proteins and acts in conjunction with vesicle-targeting molecules to effect the efficient transport of cargo proteins.
Complex pathways of coated vesicle budding, transport, and fusion link the synthesis of membrane proteins in the endoplasmic reticulum (ER) to Golgi transport and membrane assembly (1–5). Three types of vesicle coats are recognized in this process: Clathrin/AP, COPI, and COPII. All form perivesicular, electron-dense layers of uniform composition, and all assemble under ADP-ribosylation factor (ARF) control. Novel isoforms of spectrin (βIΣ∗) and ankyrin (ANKG119) may form a fourth coat (6, 7) possibly in association with the dynactin complex required for microtubule-based vesicular transport (8). The Golgi-associated ankyrins include a fully characterized, novel, small ankyrin (AnkG119) (7) and larger isoforms identified immunologically (9). Although spectrin differs from other coat proteins in that it does not form geometrically precise or easily visualized coats, its association with Golgi is stimulated by ARF (Godi, A., Santone, I., Pertile, P., P.D., P.R.S., J.S.M., Di Tullio, G., Polishuck, R., Petrucci, T. C., Luini, A., and M.A.D., unpublished work). Yet, direct evidence that Golgi spectrin participates in vesicle or membrane protein trafficking is lacking.
We have used Madin Darby canine kidney (MDCK) cells transfected with human βIΣ1 and βIΣ2 spectrin to determine the regions of spectrin necessary for its association with the Golgi, and evaluated the impact of these constructs on the native Golgi spectrin skeleton, Golgi integrity, and the transport of Na,K-ATPase, VSV-G protein, and E-cadherin from the ER to the plasma membrane. Our results demonstrate a requirement for Golgi spectrin in membrane protein transport and suggest that the vesicular spectrin skeleton acts to select cargo molecules destined for anterograde vesicular transport.
Portions of this work have been presented in abstract form (10).
METHODS
Preparation of Spectrin Constructs and Transfected Cell Lines.
The various βI spectrin peptides were expressed from existing human βI spectrin constructs (11–13) usually using the pcDNA3 vector (Invitrogen); stable MDCK cell lines were established by selection with G418 after lipofectamine transfection (13). All constructs were verified by DNA sequencing. Newly confluent cells were infected for 60 min with vesicular stomatitis virus in Costar Transwell filters (100 μl, 108 titer virus) (14). Infected cells were incubated for 3 hr and fixed.
Antibodies and Immunodetection Procedures.
Constructs epitope-labeled by the 8-aa FLAG marker (IBI) were detected with Mab M5 (IBI). βI spectrin antibodies included Mab VIIIC7, Mab VD4, Mab IVC9, and Mab IVF8 (15); Pab C19 against 19 COOH-terminal residues of βIΣ1 spectrin (gift from V. Marchesi, Yale University); and Pab MUS1 against region III of βIΣ2 spectrin (16). Pab 10D detected βII spectrin (7). Other antibodies were Mab β-COP (I. Mellman, Yale University); Pab E-cadherin (Transduction Laboratories, Lexington, KY); Mab α-Na,K-ATPase (Upstate Biotechnology); Mab β-Na,K-ATPase (M. Caplan, Yale University); Mab VSV-G (J. Rose, Yale University); and Pab centractin (E. Holzbaur, University of Pennsylvania, Philadelphia). Cells were grown on glass coverslips or culture dishes, washed three times with PBS, fixed 15 min in acetone, blocked 30 min in goat serum, and incubated with 1° antibody in 2% BSA in PBS + 10% goat serum at room temperature for 60 min. All spectrin Mabs were used at 1:100; E-cadherin, at 1:5,000; and β-COP, 10D, C19, at 1:200. Detection used 2° antibodies conjugated to CY3 or CY2 (Vector Laboratories).
Immunoprecipitations.
Confluent MDCK cells were PBS-washed, lysed 20 min at 4°C by gentle rocking in IP buffer (10 mM Tris⋅HCl, pH 7/150 mM NaCl/5 mM EDTA/1 mM EGTA/2 mg/ml BSA/0.5% deoxycholate/1% Nonidet P-40/0.5 mM pefablock/1 mM PMSF/1 mM leupeptin). The lysate (2 ml) was decanted and centrifuged 1 min at 10,000 × g and precleared by 60-min incubation with 25 μl of nonimmune rabbit serum with 200 μl of a 50% Protein A Sepharose (Pharmacia). The remaining supernatant was treated 4 hr at 4° with 200 μl of 1° antibody, pelleted by Protein A Sepharose, and analyzed.
Electron Microscopy.
Cell monolayers fixed in situ for 1 hr in Karnovsky’s fixative were washed in 0.1 M sodium cacodylate, pH 7.4, and postfixed in 1% OsO4 in 0.1 M S-collidine. After dehydrating in graded ethanol and washing with propylene oxide, samples were embedded in Epox-812 (Ernest F. Fullam, Schenectady, NY). Ultrathin sections stained with aqueous uranyl acetate and lead citrate were viewed on a Zeiss EM-910 electron microscope at 80 kV.
RESULTS
Region I + MAD1 of βI Spectrin is a Golgi Targeting Signal.
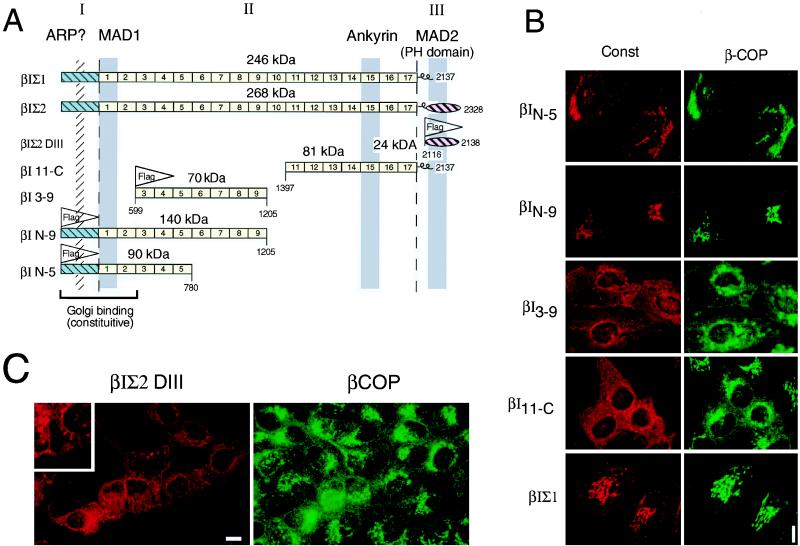
MDCK cells were transfected with a series of βIΣ1 and βIΣ2 spectrin constructs that collectively represented all known, functional domains of spectrin (reviewed in ref. 17). These were presented to the cell either individually or together as a single construct and, when necessary, were epitope-tagged with FLAG (Fig. 1A). Quantitative Western blot analysis of equivalent numbers of cells from the various transfected lines revealed approximately equivalent (within 2-fold) molar expression levels of βI11−C(1.31 ± .08), βI3−9(1.63 ± .08), βIN−9(0.85 ± .06), and βIN−5(1.69 ± .03) relative to βIΣ1 (unity), indicating that differences in expression levels could not account for the differences between constructs. Despite the presence of an ankyrin binding site in βI spectrin repeat 15 (18) and a strong phosphatidylinositol 4,5-bisphosphate (PtdInsP2)-dependent membrane-association domain in MAD2 (10, 19, 20), these functional domains were not sufficient per se to target spectrin efficiently to the Golgi, as evidenced by their lack of coincident staining with β-COP (Fig. 1 B and C). Instead, polypeptides containing these domains dispersed into a finely punctate cytoplasmic pattern, presumably due to their association with membranous organelles outside the Golgi or with other structures. This was most apparent with the MAD2/PH domain containing construct (βIΣ2-DIII). The nature of the cellular complexes formed with these peptides remains undetermined, although in the case of those encompassing the MAD2/PH domain (βIΣ2-DIII), their pattern of perinuclear tubulovesicular and punctate staining (Fig. 1C Inset) is reminiscent of the pattern observed for the ER–Golgi intermediate compartment 53-kDa protein (ERGIC-53) after microtubule disruption (21) or the pattern of sec13p (COPII) vesicles budding from the yeast nuclear envelope (reviewed in ref. 3). Conversely, region I + MAD1 (19) of βI spectrin together formed a necessary and sufficient Golgi targeting signal, as seen with the βIN−5 construct (Fig. 1B). All polypeptides encompassing these two regions of spectrin sorted to the Golgi with high fidelity, whereas peptides that lacked these domains sorted to the Golgi much less efficiently or not at all. In additional experiments (data not shown), we have explored the limits of this activity by expressing just MAD1 alone, region I alone, or constructs containing just region I + MAD1. These experiments are complicated by poor cell survival and instability of expression, but they do reveal that both region I and MAD1 appear to be essential for the sorting of spectrin to the Golgi.
Figure 1.
Stable transfection of βI spectrin constructs in MDCK cells. (A) Diagram representing the βI constructs used and the relationship of these to the known functional domains of βI spectrin. Classically, based on internal homologies, spectrin has been considered to be composed of three “domains” or regions, labeled I, II, and III in this figure (17). To lessen confusion, these are referred to as regions I, II, and III in this paper. Three direct membrane-association domains (MAD1 to MAD3) have been determined by in vitro assay (reviewed in refs. 17, 19, and 36). The locations of MAD1 and MAD2 are shown. MAD3 has been identified within the broad region of repeat 3–9 (36). MAD2 contains βIΣ2 spectrin’s pleckstrin homology (PH) domain, which binds directly to isolated Golgi membranes in an ARF- and PtdInsP2-dependent manner (Godi, A., et al., unpublished work). The locus of the ankyrin binding domain in βI spectrin is as indicated (18), and ARP? marks the location of the actin (and putative centractin) binding domain in βI spectrin (8, 37). FLAG represents an eight-residue epitope tag that was incorporated into some constructs to allow their identification by indirect immunofluorescent microscopy. (B) The distribution of the transfected spectrin vs. the distribution of β-COP, as revealed by double immunofluorescence. Note that only constructs containing NH2-terminal βI spectrin sequences assume a Golgi distribution, and that with all constructs the distribution of β-COP is largely preserved. (C) The MAD2 domain of βIΣ2 spectrin (with an intact PH domain) remains in a punctate cytoplasmic and perinuclear distribution, neither associating with the Golgi nor disrupting β-COP distribution. The punctate and tubulovesicular pattern of staining with this construct is better appreciated at the ×2 magnified Inset. Because the binding of this construct alone to Golgi membranes in vitro is PtdInsP2-dependent (Godi, A., et al., unpublished work), and G418 is an agent that can potentially sequester PtdInsP2 (38), in separate experiments cells were grown in the absence of G418 and the distribution of βI-DIII again was examined; no differences were detected (data not shown). Note that even in these stable transfected lines, not all cells express the recombinant spectrin construct. (Bar = 10 μ.)
The βIN-5 Construct Disrupts the Endogenous Golgi Spectrin Skeleton and Na,K-ATPase Transport.
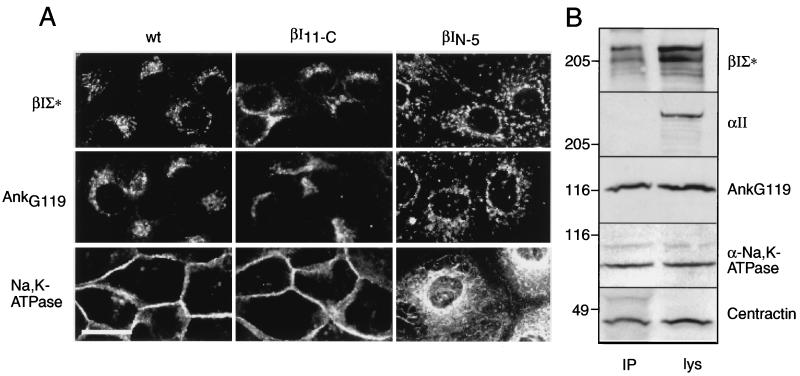
In wild-type (wt) MDCK cells, Golgi spectrin and AnkG119 are localized with the Golgi, whereas α-Na,K-ATPase is predominately distributed at the plasma membrane, where it is tethered via the plasma membrane form of ankyrin to αIIβII spectrin (22, 23) (Fig. 2). Immunoprecipitation with Mab VIIIC7 reveals that a fraction of α-Na,K-ATPase also exists in a complex with βIΣ∗spectrin, AnkG119, and centractin (Fig. 2B). Centractin (ARP-1) is an actin-related protein that is part of the dynactin complex. This pool of α-Na,K-ATPase associated with βIΣ∗ spectrin, AnkG119, and centractin appears to be distinct from the pool of plasma membrane α-Na,K-ATPase, because αII spectrin was not present in these complexes (Fig. 2B) and presumably represents Na,K-ATPase not yet assembled at the plasma membrane. The distribution of the endogenous Golgi spectrin–ankyrin skeleton, as well as membrane assembly of Na,K-ATPase, was disrupted by βI spectrin constructs that contained the region I/MAD1 Golgi targeting signal. (Fig. 2A). In the presence of such peptides, endogenous Golgi βIΣ∗ spectrin and AnkG119 were dispersed into coarse punctate cytosolic patches. (Figs. 2A and 3A). Even more dramatic was the unexpected effect of these peptides on the distribution of Na,K-ATPase, which became widely dispersed throughout the cytoplasm in a pattern resembling the endoplasmic reticulum, with very little protein identifiable at the cell surface (Figs. 2A and 3A). The βIN−5 expressing cells were also swollen compared with control cells (mean diameter 29 ± 6.7 μ vs. 17 ± 3.7 μ) and grew more slowly presumably due to insufficient plasma membrane Na,K-ATPase (or other membrane proteins; see below). This effect on Na,K-ATPase distribution was not evident with constructs lacking region I/MAD1 (e.g., βI11-C, Fig. 2A), nor was it apparent in cells expressing full-length βIΣ1 spectrin (that contains the ankyrin–Na,K-ATPase binding domain but lacks MAD2- and PtdInsP2-dependent Golgi binding).
Figure 2.
The expression of amino-terminal βI spectrin constructs in MDCK cells disrupts the native Golgi spectrin–ankyrin skeleton and Na,K-ATPase assembly. (A) The intracellular distribution of wt Golgi spectrin was monitored using Mab VIIIC7, which reacts with full-length Golgi spectrin but not the shorter FLAG-tagged βIN−5 construct (refs. 7 and 39; Godi, A., et al., unpublished work). The distribution of βIΣ∗ spectrin in cells transfected with βI11−C was monitored by Mab VD4, which does not react with the βI11−C peptide. AnkG119 was monitored by the antibody “Jasmin” (7); α-Na,K-ATPase was monitored by a Mab from Upstate Biotechnology. Note the dispersal of the endogenous Golgi spectrin, AnkG119, and α-Na,K-ATPase by βIN−5 (but not by βI11−C). All shortened spectrin constructs containing region I and MAD1 displayed a similar effect, whereas constructs lacking these regions of βI spectrin did not significantly alter the native distributions of βIΣ∗ spectrin, AnkG119, or Na,K-ATPase. (Bar = 10 μ.) (B) In wt cells, βIΣ∗ spectrin, AnkG119, centractin, and Na,K-ATPase are coprecipitated from detergent lysates by Mab VIIIC7 (lane IP). Soluble αIIβII spectrin (as detected here by immunoblotting for αII spectrin), the form found predominately at the plasma membrane, is also present in these detergent lysates (lane lys) but is not part of the precipitable Golgi spectrin complex. Control experiments with preimmune or irrelevant antibody did not precipitate any of these components (data not shown). Similarly, immunoprecipitates with Mab VIIIC7 of the βIN−5-transfected cells also demonstrated coprecipitation of AnkG119, and Na,K-ATPase with βIΣ∗ spectrin, indicating that despite the dispersal of these elements induced by the βIN−5 construct, these elements remained associated in the detergent lysates (data not shown). Western blots after SDS/PAGE were visualized by enhanced chemiluminescence.
Na,K-ATPase Is Blocked at the ER-to-Medial Golgi Transition by βIN−5 Spectrin.
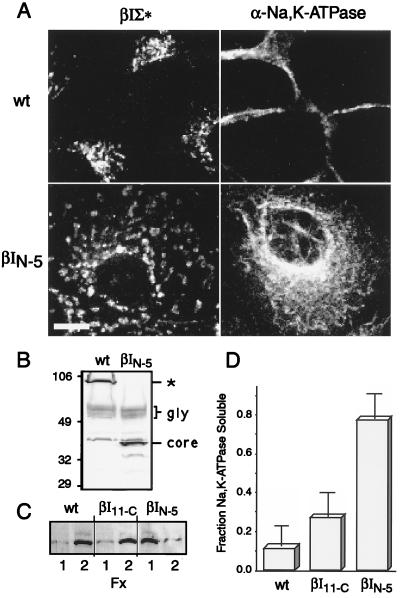
Normally, after synthesis and core glycosylation in the ER, β-Na,K-ATPase undergoes further glycosylation in the medial Golgi and then is vectorally incorporated as an α,β-Na,K-ATPase heterodimer into a detergent-insoluble αIIβII spectrin and ankyrin cortical skeletal lattice (22–25). The distribution of Na,K-ATPase in the βIN−5 expressing cells suggested that the exit of Na,K-ATPase from the ER might be blocked (Fig. 3A). Western blot analysis confirmed that the spectrin βIN−5 polypeptide blocked Golgi-mediated glycosylation of β-Na,K-ATPase (Fig. 3B). Although these experiments did not seek to quantitatively measure the degree of transport inhibition, the results of several separate determinations suggested that the efficiency of β-Na,K-ATPase transport inhibition by the βIN−5 peptide is quite high, because the level of processed β-Na,K-ATPase that could be detected correlated inversely with the fraction of cells in these cultures that were actually expressing the recombinant peptide. Also blocked in these cells was the transition of α-Na,K-ATPase to detergent insolubility (Fig. 3C), indicating a failure of Na,K-ATPase to join a stable plasma membrane-associated spectrin lattice. The presence of the βI11−C polypeptide, with its competent ankyrin binding domain but lack of region I/MAD1 sequences (cf. Fig. 2C), only marginally impacted the assembly of Na,K-ATPase into a detergent-insoluble skeleton. Collectively, these data indicate that truncated βI spectrin peptides containing the Golgi targeting signal but lacking downstream sequences including the ankyrin/Na,K-ATPase binding domain, block the ER-to-medial Golgi transport of both subunits of Na,K-ATPase.
Figure 3.
Dispersal of Golgi spectrin by βIN−5 blocks Na,K-ATPase transport to the medial Golgi and its incorporation into a detergent stable skeletal complex. (A) The intracellular distribution of the endogenous βIΣ∗ spectrin (determined by Mab VIIIC7, which does not react with the expressed βIN−5 peptide) and Na,K-ATPase in MDCK cells transfected with βIN−5 spectrin. (B) Golgi-dependent glycosylation of β-Na,K-ATPase is blocked by the βIN−5 spectrin peptide. Control MDCK cells (wt) or cells transfected with the βIN−5 construct (βIN−5) were analyzed by Western blotting using a β-Na,K-ATPase-specific antibody (Mab B1–13). The apparent molecular mass of the various products are depicted. Note the broad band above 50 kDa (gly) representing mature glycosylated β-Na,K-ATPase, and the intense band at 44 kDa in the βIN−5 cells (core) representing the ER-dependent core β-Na,K-ATPase glycosylation product. It thus appears that the βIN−5 spectrin peptide inhibits mature glycosylation of β-Na,K-ATPase, a process characteristic of medial Golgi processing, but does not interfere with the formation of the core glycosylation product, which occurs in the ER. In this experiment, approximately half of the βIN−5 cells are expressing the βIN−5 construct, as judged by immunofluorescence (data not shown), which appears to account for most of the mature glycosylated product observed in the βIN−5 cell lines. The band ≈106 kDa in the wt cells (∗) is inconsistent in its appearance and may represent a cross-linked adduct of β-Na,K-ATPase with α-Na,K-ATPase or other protein, as is commonly observed after reduced SDS/PAGE in MDCK cells (22). (C) Fraction of Na,K-ATPase present in the soluble and detergent-insoluble pools of MDCK cells transfected with different βI spectrin constructs. Fx1 (soluble fraction), material solubilized by 0.5% Triton X-100 in 100 mM NaCl; Fx2 (cytoskeletal fraction), material solubilized by 0.5% Triton X-100 and 250 mM NH4SO4 (40). Shown are Western blots for α-Na,K-ATPase after SDS/PAGE. (D) The results of three separate determinations of α-Na,K-ATPase extractability of wt and two transfected MDCK cell lines were quantified in duplicate by densitometry, and all analyses were averaged. (Error bars represent ± 1 SD.) Note the substantial loss of fully assembled and detergent-insoluble Na,K-ATPase in the βIN−5-expressing cells.
The Transport of only a Subset of Membrane Proteins Is Blocked by βIN−5. Spectrin.
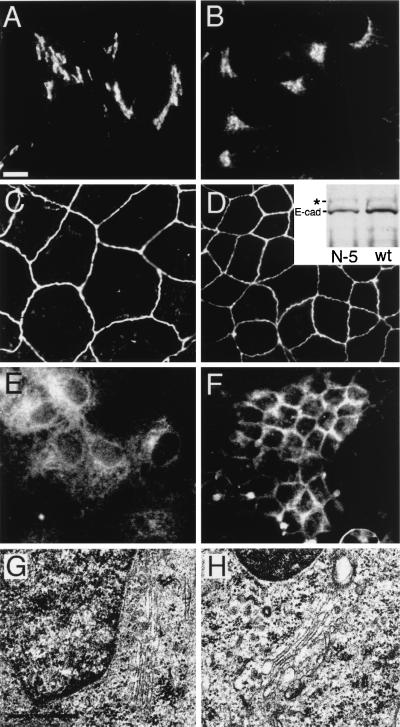
Finally, it was of interest to evaluate the transport of other membrane proteins, as well as the overall integrity of the Golgi, in the βIN−5 transfected cells. Infection with vesicular stomatitis virus demonstrated that VSV-G transport to the plasma membrane, which is packaged exclusively by the COPII coat (review ref. 3), like Na,K-ATPase, was blocked by the βIN−5 spectrin construct (Fig. 4E and F). The pattern of VSV-G staining suggests a block prior to the intermediate compartment, but additional experiments will be required to resolve this issue. Conversely, E-cadherin, another type I basolaterally targeted membrane protein, was fully expressed at the plasma membrane (Fig. 4 C and D). Western blot analysis of E-cadherin processing, which involves a protease cleavage in the trans-Golgi compartment that reduces the precursor protein from 135 to 120 kDa (26), could detect no increase in uncleaved E-cadherin pools (Fig. 4D Inset). Thus, unlike Na,K-ATPase and VSV-G, the transport of E-cadherin was not blocked at the ER-to-Golgi transition.
Figure 4.
Comparison of the effect of βIN−5 spectrin on Golgi and the assembly of different proteins in MDCK cells. Wild-type (B, D, F, H) and βIN−5-transfected cells (A, C, E, and G) are shown. The localization of VSV-G protein (E and F) was measured after transient infection. E-cadherin (C and D) was monitored with a Mab from Transduction Laboratories and also by the extent to which the precursor peptide was proteolyzed (Inset, Western blot) from 135 kDa (∗) to 120 kDa (E-cad) (a process that occurs in the trans-Golgi 26). There was no significant difference in the extent of E-cadherin processing or its level of assembly at the plasma membrane in the βIN−5 line vs. wt cells. Despite the disruption of Na,K-ATPase and VSV-G transport (and the wt Golgi spectrin skeleton; Fig. 3), the Golgi appears to remain largely intact as measured by the distribution of β-COP (A and B) and by the presence of normal-appearing juxtanuclear Golgi structures in uranyl acetate and lead-stained electron microscopy (G and H). [Bar = 10 μ (A–F) and 0.5 μ (G and H)]. [Original magnification = ×63,000 (G and H)].
The Overall Golgi Architecture Is Preserved in Cells Expressing βIN−5 Spectrin.
The dispersed pattern of the endogenous βIΣ∗ spectrin and AnkG119 observed in cells expressing βIN−5 was reminiscent of the pattern observed for many Golgi markers after treatment with Brefeldin A (ref. 6; Godi, A., et al., unpublished work) or after the dissolution of microtubules with nocodazole (6, 21). Both of these treatments disperse the Golgi membrane complex. With microtubule disruption, Golgi components (including markers such as ERGIC-53) redistribute to peripheral sites of protein exit from the ER (21, 27). Although the βIN−5 spectrin polypeptide dispersed the endogenous spectrin skeleton and disrupted in a specific way the transport of at least some integral membrane proteins, it did not disturb the distribution of β-COP (Fig. 4 A and B). Preservation of a juxtanuclear Golgi complex in the βIN−5 expressing cells was also evident by electron microscopy (Fig. 4), because in both wt and βIN−5 cells rough ER and normal Golgi stacks were apparent in an approximately normal distribution. Thus, it appears that the disruption of ER-to-Golgi transport of Na,K-ATPase and VSV-G caused by the βIN−5 spectrin polypeptide is a selective blockage of the transport of these proteins and is not attributable to global disruption of the transport of other membrane proteins or of the secretory apparatus itself.
DISCUSSION
Collectively, these findings point to a specific role for the Golgi-associated spectrin skeleton. Previously it has been demonstrated that Golgi spectrin associates with the dynactin complex, a motor responsible for microtubule-mediated transport of intracellular organelles (8). This association was hypothesized to link the dynactin complex to the organelle membranes, based on the well known membrane-association properties of spectrin. We have also shown previously that spectrin’s MAD1 is a direct membrane-binding domain, and that a second direct membrane-association domain (MAD2) also exists (19). Of these two, only MAD1 in association with region I represents a strong and sufficient constitutive Golgi assembly signal. The role of MAD2 in vesicular transport (exemplified by the behavior of βIΣ2-DIII construct) is more speculative. Although MAD2 does not appear to be required for the transport of either α-Na,K-ATPase, VSV-G, or E-cadherin, this domain does target punctate tubulovesicular structures that may represent specialized regions at which protein is exported from the ER (the so-called VTCs or ERGIC 5). G protein-regulated COPII-to-COP I exchange is also believed to occur in these regions (28, 29). Because in other work we have demonstrated that Golgi spectrin can bind via its PH domain within MAD2 to PtdInsP2 phospholipid, and that this binding is stimulated by the G protein ARF (Godi, A., et al., unpublished work), it is interesting to speculate that the MAD2/PH region of spectrin is independently seeking VTCs, perhaps in an ARF-dependent fashion (Godi, A., et al., unpublished work). It thus appears that in addition to a strong binding signal in region I/MAD1, mechanisms exist to fine-tune the affinity of Golgi spectrin with specific internal membrane compartments.
The data reported here also demonstrate that spectrin with MADI and the actin-binding region I (which presumably binds centractin in the dynactin complex) constitute a molecule capable not only of associating with Golgi structures but also mediating the trafficking of many integral membrane proteins. We hypothesize that even a truncated spectrin (as exemplified by βIN−5) is sufficient to link transport vesicles to microtubule-based or other motors and thereby mediate the transport of vesicles containing many integral and secretory (cargo) proteins. However, the failure of truncated spectrins that lack spectrin’s ankyrin-binding domain (and therefore cannot bind ankyrin and indirectly Na,K-ATPase) to mediate Na,K-ATPase transport suggests that at least some integral membrane proteins are not efficiently sorted into vesicles exiting the ER or otherwise transported unless they are able to bind to the ER/Golgi spectrin skeleton. We believe that this binding initially occurs at regions in which cargo proteins are being packaged for export from the ER, most likely in association with COPII-coated vesicles. Presumably, the failure of VSV-G to transport also reflects its failure to bind to ankyrin or components of the βIΣ∗ spectrin vesicular skeleton that are deleted in the βIN−5 construct, although such direct binding has not been formally demonstrated in these experiments. These findings are also of considerable interest because in recent work a di-acidic signal (Asp-X-Glu) has been postulated to be necessary in the cytoplasmic domain of integral membrane proteins to ensure their export from the ER (30). However, the di-acidic signal is relatively nonspecific and found in a variety of type I membrane proteins. We do not know whether the di-acidic signal binds to component(s) of the Golgi spectrin–ankyrin skeleton but note the even greater selectivity imparted by the Golgi spectrin skeleton, which transports only one of two di-acidic motif-containing proteins (E-cadherin vs. VSV-G). Thus, more than a simple membrane tether, Golgi spectrin, together with various adapter proteins such as AnkG119, appears to form a cryptic protein-sorting apparatus that is required to capture specific cargo proteins into vesicles destined for transport from the ER to the Golgi and possibly through the Golgi. Although demonstrated here for Na,K-ATPase and VSV-G, this phenomenon, we believe, is general, and we hypothesize that Golgi spectrin, together with ankyrin and other adapter proteins, forms a complex multifunctional coat system mediating the sequestration of a diverse array of integral membrane proteins into transport vesicles. For purposes of euphonic simplicity, we term this system SAATS for spectrin-ankyrin-adapter protein trafficking (or tethering) system. Based on comparisons with the plasma membrane spectrin skeleton, other adapter proteins besides ankyrin likely to be involved in SAATS include members of the protein 4.1, adducin, α-catenin, and actin-related protein gene families (17, 31). Like other coatomer complexes, SAATS assembly to nascent vesicles or at the exit portals of the ER may be regulated by ARF (Godi, A., et al., unpublished work), and presumably SAATS recognizes complex targeting signals resident in the cytoplasmic domains of integral membrane proteins. Based on earlier studies of the packing of targeting molecules such as v-SNARE [soluble N-ethylmaleimide-sensitive fusion attachment protein (SNAP) receptor] into vesicles, which occurs independently of the packaging of cargo molecules (32), and the fact that cargo molecule sorting depends on apparently complex determinants (30, 33), we propose that SAATS functions primarily as a system that manages the trafficking of specific cargo molecules destined for the plasma membrane or other intracellular compartments.
An important question is why SAATS or an equivalent system has not emerged in studies of the secretory pathway in yeast. We do not understand this but note that yeast do not express spectrin nor do they form an organized juxtanuclear Golgi complex. We hypothesize that this simple organism evolved alternative solutions to the problem of cargo protein sequestration and transport. Unicellular organisms may also simply not require the efficiency and specificity of cargo loading provided by SAATS. In future studies, it will be important to determine in mammalian cells the precise membrane compartment to which βIΣ∗ spectrin initially binds; which proteins utilize SAATS; the nature of the adapter proteins involved; and the relationship of SAATS to the targeting pathways involving known targeting signals, docking proteins, SNAPS, and SNAP receptors (1). It also seems likely, given the presence of novel spectrins and ankyrins associated with the trans Golgi compartment (6, 9), endolysosomes (34), and axonally transported vesicles (16, 35), that SAATS will also function in protein trafficking pathways beyond the ER-to-Golgi transition.
Acknowledgments
We thank Drs. S. Kennedy, S. Weed, and B. Forget for early assistance, and Drs. J. Rose, I. Mellman, E. Holzbaur, and M. Caplan for antibodies and reagents. Mr. T. Ardito’s assistance with the electron microscopy studies is acknowledged. This work was supported by grants from the National Institutes of Health to J.S.M. and P.D. and from the Consiglio Nazionale delle Ricerche to A.D.M.
ABBREVIATIONS
- ARF
ADP ribosylation factor
- ERGIC
endoplasmic reticulum-Golgi intermediate compartment
- Mab
monoclonal antibody
- MAD
membrane association domain
- Pab
polyclonal antibody
- PH
pleckstrin homology domain
- PtdInsP2
phosphatidylinositol 4,5-bisphosphate
- VTC
vesicular tubular compartment
- wt
wild type
- MDCK cells
Madin Darby canine kidney cells
References
- 1.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 2.Schmid S L, Damke H. FASEB J. 1995;9:1445–1453. doi: 10.1096/fasebj.9.14.7589986. [DOI] [PubMed] [Google Scholar]
- 3.Schekman R, Orci L. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 4.Kreis T E, Pepperkok R. Curr Opin Cell Biol. 1994;6:533–537. doi: 10.1016/0955-0674(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 5.Bannykh S I, Balch W E. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck K A, Buchanan J A, Malhotra V, Nelson W J. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarajan P, Stabach P R, Mann A S, Ardito T, Kashgarian M, Morrow J S. J Cell Biol. 1996;133:819–830. doi: 10.1083/jcb.133.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holleran E A, Tokito M K, Karki S, Holzbaur E L F. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck K, Buchanan J A, Nelson W J. J Cell Sci. 1997;110:1239–1249. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]
- 10.Devarajan P, Stabach P, Morrow J S. Mol Biol Cell. 1996;7:324a. doi: 10.1083/jcb.133.4.819. (abstr.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkelmann J C, Chang J G, Tse W T, Scarpa A L, Marchesi V T, Forget B G. J Biol Chem. 1990;265:11827–11832. [PubMed] [Google Scholar]
- 12.Winkelmann J C, Costa F F, Linzie B L, Forget B G. J Biol Chem. 1990;265:20449–20454. [PubMed] [Google Scholar]
- 13.Weed S A, Stabach P R, Oyer C E, Gallagher P G, Morrow J S. Lab Invest. 1996;74:1117–1129. [PubMed] [Google Scholar]
- 14.Pimplikar S W, Ikonen E, Simons K. J Cell Biol. 1994;125:1025–1035. doi: 10.1083/jcb.125.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris A S, Anderson J P, Yurchenco P D, Green L A D, Ainger K J, Morrow J S. J Cell Biochem. 1986;30:51–70. doi: 10.1002/jcb.240300107. [DOI] [PubMed] [Google Scholar]
- 16.Malchiodi-Albedi F, Ceccarini M, Winkelmann J C, Morrow J S, Petrucci T C. J Cell Sci. 1993;106:67–78. doi: 10.1242/jcs.106.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Morrow J S, Rimm D L, Kennedy S P, Cianci C D, Sinard J H, Weed S A. In: Handbook of Physiology. Hoffman J, Jamieson J, editors. London: Oxford Univ. Press; 1997. pp. 485–540. [Google Scholar]
- 18.Kennedy S P, Warren S L, Forget B G, Morrow J S. J Cell Biol. 1991;115:267–277. doi: 10.1083/jcb.115.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lombardo C R, Weed S A, Kennedy S P, Forget B G, Morrow J S. J Biol Chem. 1994;269:29212–29219. [PubMed] [Google Scholar]
- 20.Wang D S, Shaw G. Biochem Biophys Res Commun. 1995;217:608–615. doi: 10.1006/bbrc.1995.2818. [DOI] [PubMed] [Google Scholar]
- 21.Cole N B, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow J S, Cianci C, Ardito T, Mann A, Kashgarian M T. J Cell Biol. 1989;108:455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson W J, Hammerton R W. J Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mays R W, Siemers K A, Fritz B A, Lowe A W, van Meer G, Nelson W J. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson W J, Veshnock P J. J Cell Biol. 1986;103:1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shore E M, Nelson W J. J Biol Chem. 1991;266:19672–19680. [PubMed] [Google Scholar]
- 27.Bannykh S I, Rowe T, Balch W E. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aridor M, Bannykh S I, Rowe T, Balch W E. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe T, Aridor M, McCaffery J M, Plutner H, Nuoffer C, Balch W E. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura N, Balch W E. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 31.Bennett V. J Biol Chem. 1992;267:8703–8706. [PubMed] [Google Scholar]
- 32.Yeung T, Barlowe C, Schekman R. J Biol Chem. 1995;270:30567–30570. doi: 10.1074/jbc.270.51.30567. [DOI] [PubMed] [Google Scholar]
- 33.Campbell J L, Schekman R. Proc Natl Acad Sci USA. 1997;94:837–842. doi: 10.1073/pnas.94.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoock T C, Peters L L, Lux S E. J Cell Biol. 1997;136:1059–1070. doi: 10.1083/jcb.136.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman S R, Lopresti L L, Riederer B M, Sikorski A, Zagon I S. Brain Res Bull. 1989;23:311–316. doi: 10.1016/0361-9230(89)90214-1. [DOI] [PubMed] [Google Scholar]
- 36.Davis L H, Bennett V. J Biol Chem. 1994;269:4409–4416. [PubMed] [Google Scholar]
- 37.Karinch A M, Zimmer W E, Goodman S R. J Biol Chem. 1990;265:11833–11840. [PubMed] [Google Scholar]
- 38.Gabev E, Kasianowicz J, Abbott T, McLaughlin S. Biochim Biophys Acta. 1989;979:105–112. doi: 10.1016/0005-2736(89)90529-4. [DOI] [PubMed] [Google Scholar]
- 39.Harris A S, Green L A D, Ainger K J, Morrow J S. Biochim Biophys Acta. 1985;830:147–158. doi: 10.1016/0167-4838(85)90022-6. [DOI] [PubMed] [Google Scholar]
- 40.Devarajan P, Scaramuzzino D A, Morrow J S. Proc Natl Acad Sci USA. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]