Abstract
Although palatal shelf adhesion is a crucial event during palate development, little work has been carried out to determine which molecules are responsible for this process. Furthermore, whether altered palatal shelf adhesion causes the cleft palate presented by Tgf-β3 null mutant mice has not yet been clarified. Here, we study the presence/distribution of some extracellular matrix and cell adhesion molecules at the time of the contact of palatal shelves in both wild-type and Tgf-β3 null mutant palates of two strains of mice (C57/BL/6J (C57), and MF1) that develop cleft palates of different severity. We have performed immunohistochemistry with antibodies against collagens IV and IX, laminin, fibronectin, the α5- and β1-integrins, and ICAM-1; in situ hybridization with a Nectin-1 riboprobe; and palatal shelf cultures treated or untreated with TGF-β3 or neutralizing antibodies against fibronectin or the α5-integrin. Our results show the location of these molecules in the wild-type mouse medial edge epithelium (MEE) of both strains at the time of the contact of palatal shelves; the heavier (C57) and milder (MF1) alteration of their presence in the Tgf-β3 null mutants; the importance of TGF-β3 to restore their normal pattern of expression; and the crucial role of fibronectin and the α5-integrin in palatal shelf adhesion. We thus provide insight into the molecular bases of this important process and the cleft palate presented by Tgf-β3 null mutant mice.
Keywords: cleft palate, Tgf-β3, mouse, collagen, laminin, fibronectin, α5β1-integrin, ICAM-1, Nectin-1
Introduction
In mammals, the palatal region develops from the embryonic primary and secondary palates. The primary palate builds the anterior palate up to the incisal foramen, while the secondary palate forms the remaining hard and soft palates. The secondary palate starts as two downward extensions—the palatal shelves—that arise from the medial side of the left and right maxillary processes of the first branchial arch. Palatal shelves initially grow along the lateral surface of the tongue, but subsequently they rotate, become horizontally placed over the tongue and approach each other (Ferguson, 1988). Then, the epithelia covering their tips (medial edge epithelium [MEE]) stop proliferating (Hudson and Shapiro, 1973; Brinkley, 1984; Cui et al., 2003), adhere strongly, and form the midline epithelial seam (MES). MES cells rapidly intercalate (Tudela et al., 2002) and eventually disappear by means of cell death (Mori et al., 1994; Taniguchi et al., 1995; Martínez-Álvarez et al., 2000a; Cuervo et al., 2002; Cuervo and Covarrubias, 2004; Vaziri Sani et al., 2005), epithelial to mesenchymal transformation (Fitchett and Hay, 1989; Griffith and Hay, 1992; Shuler et al., 1992; Martínez-Álvarez et al., 2000a; Nawshad et al., 2004; Jin and Ding, 2006), and migration to the oral or nasal epithelium (Carette and Ferguson, 1992; Cuervo and Covarrubias, 2004; Takigawa and Shiota, 2004; Jin and Ding, 2006). Finally, mesenchyme confluence is achieved in the midline, giving rise to the intact secondary palate (Ferguson, 1988).
Several of these mechanisms fail in Tgf-β3 null mutant mice. In these mice, the palatal shelves grow, rotate, approach each other, and contact in the midline as in the wild type, but soon afterwards they separate partially or totally, resulting in cleft palate (Kaartinen et al, 1995; Proetzel et al., 1995). The main alteration in these palates resides in the MEE, whose cells do not cease DNA proliferation at the time of palatal fusion (Cui et al., 2003) and do not die (Martínez-Álvarez et al., 2000a, 2004). These cells neither migrate (unpublished) nor intercalate (Tudela et al., 2002). They express Snail abnormally, and eventually keratinize, as does the palatal oral epithelium (Martínez-Álvarez et al., 2004). In this MEE, Smad-2 is not phosphorylated, contrary to what is observed in the wild-type MEE, while Smad-3 is normal (Cui et al., 2003).
Little work has been devoted to analyzing MEE cell adhesion in Tgf-β3 null mutant mice. Although a failure of palatal shelf adhesion was initially suggested to explain the cleft palate presented by these mice (Proetzel et al., 1995), further studies in culture discarded this possibility (Taya et al., 1999). However, we found that palatal shelf adhesion is greatly reduced in Tgf-β3 null palate cultures (Gato et al., 2002) and that the presence of several cell adhesion molecules is altered in their MEE (Tudela et al., 2002). These disparities could be due to differences in the adhesion abilities between strains, because Taya's work was performed using Tgf-β3 null mutants of the MF1 strain (Manchester colony [129 × CF1]), while the mutants we used had a C57/BL/6J background. Actually, the cleft palate presented by these mutants has different severities: the great majority of the 129 × CF1 Tgf-β3 null palates show incomplete cleft palate with partial adhesion of palatal shelves while in the C57/BL/6J background about half of the palates are completely cleft (Kaartinen et al., 1995; Proetzel et al., 1995; Taya et al., 1999). In this work, we have postulated that this variation in palatal shelf adhesion between these two strains' Tgf-β3 null mutants correlates with differences in the presence of extracellular (ECM) and cell adhesion (CA) molecules in the MEE just before the contact of palatal shelves. This investigation should help to understand why in some cases the mutation of the TGF-β3 gene in humans leads to the appearance of cleft palate and in others it does not. To investigate our hypothesis, we have looked into the presence/distribution of several ECM and CA molecules in the pre-contact MEE of both C57/BL/6J (C57) and MF1 Tgf-β3 wild-type and null mutant mice. Among all ECM molecules present during palate development, collagens IV and IX, laminin, and fibronectin were selected. Laminin and collagen IV were chosen as representative of MEE basement membrane ECM molecules (Ferguson, 1988; Dixon et al., 1993; Singh et al., 1997; Montenegro et al., 1998). Collagen IX was investigated because it has been proposed as a good candidate to participate in MEE cell adhesion given the time of its appearance in the MEE (Ferguson, 1988). Fibronectin, demonstrated repeatedly in the palatal mesenchyme (Silver et al., 1981; Kurisu et al., 1987; Ohsaki et al., 1995), has also been observed on the MEE surface (Silver et al., 1981). Although it has never been reported to act in palatal shelf adhesion, fibronectin is involved in the adhesion of other epithelial anlagen (Menko et al., 1998). Our results show the location of these molecules in the wild-type mouse pre-contact MEE of both strains, the heavier (C57) and milder (MF1) alteration of their presence in the Tgf-β3 null mutants and the importance of TGF-β3 to restore their normal pattern of expression. The apical location of fibronectin that we found in the wild-type mouse pre-contact MEE prompted us to investigate the presence of its receptor, the α5β1-integrin, in the two mouse strains' MEE. Our results show a differently altered pattern of expression of the two subunits between the two strains' mutants and demonstrate the importance of fibronectin and the α5-integrin for palatal shelf adhesion. We have also analyzed in the two strains contacting MEE the presence of ICAM-1, a ligand of the LFA-1 integrin, whose abnormal appearance was previously observed in the C57 Tgf-β3 null mouse MEE surface at the time of initial contact of palatal shelves (Tudela et al., 2002). We demonstrate that ICAM-1 follows the same pattern of expression of its receptor in the two strains' Tgf-β3 null mutant MEE. Our ultimate objective was to investigate in these mice palates the expression of the Nectin-1 gene, an immunoglobulin-like cell adhesion molecule whose mutation increases the risk to develop cleft palate in humans (Suzuki et al., 2000; Sozen et al., 2001; Scapoli et al., 2006). We show a pattern of expression of Nectin-1 in the wild-type MEE that suggests its involvement in the initial adhesion of opposing MEE and the loss of this pattern in the C57 Tgf-β3 null mutants.
Material and methods
Animals
C57/BL/6J (Jackson Laboratories, Bar Harbor, ME) or MF1 (Manchester Colony (129 × CF1), previously constructed by Proetzel et al., 1995) Tgf-β3 heterozygous mice were mated, and the day of vaginal plug detection was designated as day 0. Time mated pregnant mice were killed by an overdose of chloroform. The embryos were removed by cesarean section, placed in sterile cold 1/1 Dulbecco's modified Eagle's medium/HAM's F12 growth medium (DMEM/F12) (Sigma, Aldrich Inc., St. Louis, MO) supplemented with 1% penicillin/streptomycin, and decapitated. Embryos to be used for culture experiments were removed under sterile conditions. Once heads were obtained, the jaw and tongue were removed. Genotyping was performed as described in Proetzel et al. (1995).
Immunohistochemistry
E14.5 wild-type and Tgf-β3 null mutant C57 or MF1 mouse heads were fixed overnight in either buffered formaldehyde (for anti-fibronectin, -collagen IV, -laminin, and -β1-integrin), cold acetone, following the AMeX protocol described in Sato et al., 1986 (for anti-collagen IX and -α5-integrin), or Zinc fixative (BD PharMingen, San Diego, CA) (for anti-ICAM-1). Because we aimed to study the presence of ECM molecules immediately before palatal shelf adhesion, only specimens where palatal shelves had close approach were selected, with the exception of those to be labeled with anti-ICAM-1, in which initial contact between opposing MEE was required. To avoid regional differences along the anterior–posterior axis of the palate, all sections were taken from its middle third. TGF-β3-treated or untreated Tgf-β3 null palatal shelf cultures were fixed in buffered formaldehyde for 2 hr. Standard paraffin embedding was then performed. Epitope was unmasked in 5-μm-thick sections using a 0.2% solution of pepsin (Sigma-Aldrich) in HCl 0.1 N (for anti-collagen IV) or 1 mM EDTA (Sigma-Aldrich) (for anti-fibronectin, -laminin, and -β1-integrin). Sections were then incubated for 2 hr at room temperature with either 1:75 monoclonal mouse IgG anti-human fibronectin (BD Transduction Laboratories, Franklin Lakes, NJ), 1:50 policlonal rabbit IgG anti-mouse laminin (Sigma-Aldrich), 1:50 monoclonal mouse IgG anti-sheep collagen IX (Developmental Studies Hybridoma Bank, Iowa City, IA), 1:100 policlonal rabbit IgG anti-human collagen IV (ICN Biomedicals Inc., Aurora, OH), 1:20 monoclonal mouse IgG anti-human β1-integrin (BD Transduction Laboratories), 1:50 monoclonal mouse IgG anti-hamster α5-integrin (Developmental Studies Hybridoma Bank), or 1:10 policlonal hamster IgG anti-mouse ICAM-1 (CD-54) (BD PharMingen). Negative controls were performed using mouse or rabbit IgG (controls) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), at the same concentrations and conditions used for the respective experimental studies.
Labeling was developed using the Rabbit/Mouse EnVision™ Peroxidase System, a peroxidase-conjugated dextran polymer (Dako Corp., Carpinteria, CA), and 3,3′-diaminobenzidine (DAB kit) as chromogen (Dako Corp.).
Some sections were counterstained with hematoxylin for a few seconds before mounting. No less than four different specimens per antibody and experimental condition were analyzed.
Palatal shelf organ cultures
Under sterile conditions, E13.5 C57 wild-type or E14 C57 Tgf-β3 null mutant mouse palatal shelves were extracted microsurgically, placed on a 2 × 2 mm Millipore filter (0.8 μm pore size) (Millipore Corp., Bedford, MA), and cultured in Trowell's tissue culture in DMEM/F12 supplemented with 2% penicillin/streptomycin and 1% ascorbic acid, at 37°C in a 5% CO2 incubator. Wild-type palatal shelves were cultured in close apposition and used to block the action of fibronectin or the α5-integrin. In these cultures medium, 1:50 rabbit anti-fibronectin (Novotec, Lyon, France), 40 μg/ml rat anti-α5-integrin (BD PharMingen), or similar amounts of PBS, rabbit IgG (control) (Calbiochem, Darmstadt, Germany), or rat IgG (control) (BD Pharmingen) at similar concentrations (controls) were added. Cultures were maintained for 36 hr, changing the medium after 24 hr. Tgf-β3 null palatal shelf cultures were added 10 ng/ml rhTGF-β3 (R&D Systems Inc., Minneapolis, MN) (n = 6) or a similar amount of PBS (controls, n = 5), and they were cultured for 12 hr. Specimens for each group were taken from three different experiments.
Assessment of palatal shelf adhesion in palate cultures
Wild-type paired palatal shelf cultures treated with either PBS (n = 11), rabbit IgG control (n = 9), rat IgG (control) (n = 10), anti-fibronectin (n = 8), or anti-α5-integrin (n = 10) were used (Table 1). Cultures were fixed in buffered formaldehyde, dehydrated in a graded ethanol series and embedded in paraffin. They were then sectioned along the anterior–posterior axis. 7 μm-thick sections were hematoxylin and eosin stained following standard procedures. Sections were studied using a Nikon Optiphot light microscope (Nikon Corp., Tokyo, Japan) and photographed with a Nikon Coolpix 995 camera (Nikon Corp.). To measure the length of the adhered opposing MEE in all palate cultures, a measuring grid inserted in a × 10 ocular lens was used. Because in the areas where palatal shelf adhesion fails ulterior fixation causes separation of opposing MEE, all places where opposing MEE were in full contact were considered adhered. Likewise, those areas where fusion had occurred (mesenchymal confluence) were also taken as places where opposing MEE had adhered previously, and were included for measurement. Therefore, the length of adhered MEE was the sum of the length of contacted opposing MEE and “disappeared” MEE, if applicable. Figure 1 shows an example on how measures were taken. The length of the adhered MEE was measured in one of every 10 sections taken from the middle hundred sections of each palate culture, and measures from cultures of each experimental group were added. The average length of adhered MEE for each group was then calculated. The final values are expressed as the arithmetic mean ± standard error. For comparison of the average measurements between experimental and control samples, data were entered into a computer database and statistically analyzed using SPSS for Windows (version 12.5; SPSS Inc., Chicago, IL). The Student t-test was used to determine any significant difference. Criterion for statistical significance was set at p<0.01.
Table 1.
Palatal shelf adhesion in control or treated palate cultures
| Type of palate culture | No. of cultures studied | No. of sections studied | Average length of adhered MEE |
|---|---|---|---|
| Control (PBS) | 11 | 110 | 275.03 ± 34.01 μm (100%) |
| Control (rabbit IgG) | 9 | 90 | 259.86 ± 71.06 μm (100%) |
| Control (rat IgG) | 10 | 100 | 286.75 ± 29.92 μm (100%) |
| Anti-fibronectin treated | 8 | 80 | 115.85 ± 48.16 μm1 (42%–44%)2 |
| Anti-α5-integrin treated | 10 | 100 | 194.51 ± 35.08 μm1 (67%–70%)3 |
Statistically significant with respect to the controls. Measurements are mean ± standard error. In all cases p<0.01 by two-tailed Student's test.
Percentage of adhesion respecting the controls (PBS and rabbit IgG, respectively).
Percentage of adhesion respecting the controls (rat IgG and PBS, respectively).
MEE, medial edge epithelium.
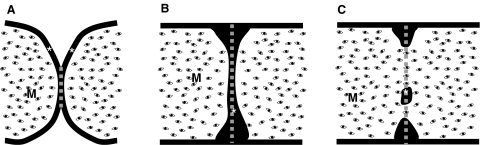
Fig. 1.
Scheme showing the way as palatal shelf adhesion was assessed. Scheme in (A) corresponds to a palate culture section with scarcely adhered opposing mouse medial edge epithelium (MEE). Schemes in (B) and (C) correspond to sections with an intact (B) or partially disrupted (C) midline epithelial seam (MES). The gray discontinuous line represents the length of adhered MEE measured in each situation. *MEE; M, mesenchyme.
In situ hybridization
The Nectin-1 probe was kindly provided by Dr. Lars Haarr, University of Bergen, Norway, and constructed as in Haarr et al., 2001. E14.5 C57 wild-type (n = 5) and Tgf-β3 null (n = 5), and MF1 wild-type (n = 4) and Tgf-β3 null (n = 4) mouse heads were extracted in ice-cold PBS/DEPC and fixed overnight in 4% paraformaldehyde in PBS/DEPC. All mandibles, cranial vaults, and encephala were then removed. In situ hybridization was performed in toto as described in Martínez-Álvarez et al. (2004). After hybridization, heads were subsequently incubated with an alkaline phosphatase-conjugated anti-digoxigenin antibody. After developing, whole-mount heads were embedded in gelatin, sectioned with a Leica VT 1000M vibratome (Leica Geosystems AG., St. Gallen, Switzerland), and stored in PBS containing 50% glycerol. Sections were studied using a Leica DMR microscope (Leica Geosystems) and photographed with a Leica DFC 320 digital camera (Leica Geosystems).
Results
Presence of extracellular matrix molecules in the pre-contact MEE of C57 and MF1 Tgf-β3 wild-type and null mutant mice
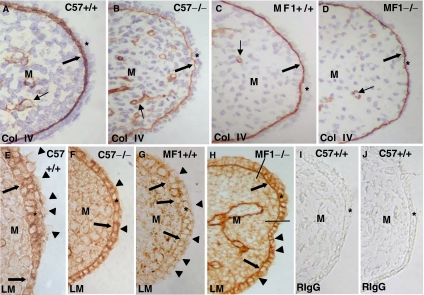
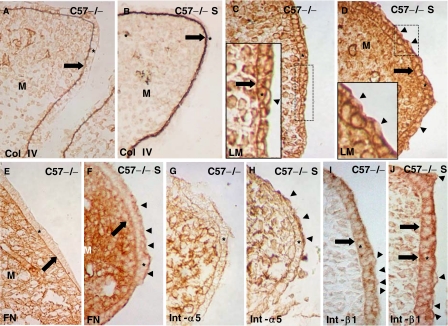
We labeled E14.5 wild-type and Tgf-β3 null mutant C57 and MF-1 mouse heads with antibodies against collagen IV, laminin, collagen IX, and fibronectin (Figs. 2 and 3). A thick layer of collagen IV was observed in the MEE basement membrane of C57 wild-type palates (Fig. 2A), while this layer was thinner in the MF1 strain (Fig. 2C). When compared with the wild type, the presence of collagen IV in the basement membrane of C57 null palates was very scarce and curled (Fig. 2B). Collagen IV immunostaining in the MF1 null mutant MEE was only slightly reduced when compared with the wild type (compare Figs. 2C and 2D). Laminin was present intercellularly in the wild-type MEE of both strains (Figs. 2E,2G). A careful observation of its distribution revealed that its presence in the pre-contact MEE basement membrane is disrupted (Figs. 2E,2G). However, laminin was continuous at this location in both strains Tgf-β3 null mouse MEE (Figs. 2F,2H). Interestingly, the apical surface of the two strains' wild-type mouse MEE superficial cells was unlabeled (Figs. 2E,2G), while it was labeled in the Tgf-β3 null palates (Figs. 2F,2H). In the Tgf-β3 null MF-1 mouse, a small part of the pre-contact MEE showed both anti-laminin labeling in the MEE surface and morphological appearance closely resembling that observed in the wild type, although the presence of laminin in the basement membrane was continuous in this small area (Fig. 2H). Labeling with a rabbit IgG (control) at the same dilutions used for experiments was negative (Figs. 2I,2J).
Fig. 2.
Changes in the presence of collagen IV and laminin in C57 and MF1 wild-type and Tgf-β3 null mutant palates. Presence of collagen IV (A–D) and laminin (E–H) in the pre-contact palatal shelves of E14.5 wild-type (A, C, E, G) and Tgf-β3 null mutant (B, D, F, H) mice. Palates in (A), (B), (E), (F), (I) and (J) correspond to the C57 strain, while those in (C), (D), (G) and (H) correspond to the MF1 strain. (I) and (J) are sections labeled with rabbit IgG (control). The layer of collagen IV labeling (thick arrow) is thicker in the C57 wild type (A) and very thin in the mutant (B). These differences are reduced in the MF1 strain (C and D). Laminin is present around mouse medial edge epithelium (MEE) cells and absent in some areas of the basement membrane in the wild type of both strains (arrows in E and G) and absent in the apical surface of the superficial cells (arrowheads in E and G). Laminin is present in the basal cell layer of the mutant of both strains (arrows in F and H) and in the apical surface of the MEE (arrowheads in F and H). Notice the existence of a small part of the MEE (between the two lines in H) with similar morphology and laminin distribution in the apical MEE surface compared with the wild type. *MEE; M, mesenchyme. Thin arrows in (A–D) indicate the presence of collagen around blood vessels.
Fig. 3.
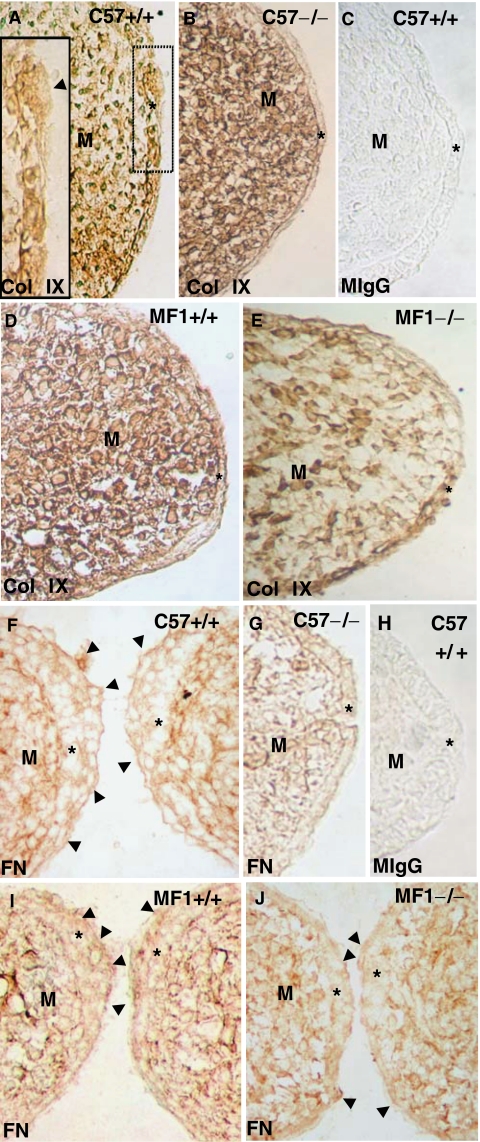
Changes in the presence of collagen IX and fibronectin in C57 and MF1 wild-type and Tgf-β3 null mutant palates. Presence of collagen IX (A, B, D, E) and fibronectin (F, G, I, J) in the pre-contact palatal shelves of E14.5 wild-type (A, D, F, I) and Tgf-β3 null mutant (B, E, G, J) mice. Palates in (A), (B), (C), (F), (G), and (H) correspond to the C57 strain, while those in (D), (E), (I) and (J) correspond to the MF1 strain. (C) and (H) are sections labeled with mouse IgG (control). The inset in (A) corresponds to a high power image of the squared area in (A). In the C57 wild-type palate, collagen IX is present around mouse medial edge epithelium (MEE) cells and absent on the apical surface of the superficial cells (arrowhead in the inset in A), but it is totally absent in the mutant MEE (B). These differences are not marked in the MF1 strain (compare D and E). Fibronectin is present around the most superficial MEE cells in the wild-type palate of both strains (arrowheads in F and I), absent in the C57 mutant MEE (G), and weak in the same location in the MF1 mutant MEE (arrowheads in J). *MEE; M, mesenchyme.
Collagen IX was intercellularly located in the wild-type pre-contact MEE of both strains and absent in the MEE surface (Figs. 3A,3D). Its presence was somewhat weaker in the MF-1 Tgf-β3 null mutant (Fig. 3E), but it was absent in the mutant of the C57 strain (Fig. 3B). Both strains wild-type mouse pre-contact MEE showed strong anti-fibronectin labeling around its cells, mostly located at the apical layers (Figs. 3F,3I). Interestingly, both bulging MEE cells and the MEE apical surface were strikingly anti-fibronectin positive (Figs. 3F,3I). Tgf-β3 null MF-1 MEE showed a weaker but still positive anti-fibronectin staining at the location observed in the wild type (Fig. 3J). However, fibronectin was totally absent around MEE cells and on the MEE apical surface in the C57 Tgf-β3 null mouse (Fig. 3G). Labeling with a mouse IgG (control) at the same dilution used for experiments was negative (Figs. 3C,3H).
Summarizing, there are little or no differences in the presence of collagen IV, IX, laminin, and fibronectin in the pre-contact MEE of both strains wild-type palates. Tgf-β3 null mice MEE show an altered presence/localization of collagen IV and IX, laminin, and fibronectin when compared with the wild type, which is much weaker in the strain with less-severe cleft palate.
Presence of the fibronectin receptor in C57 and MF1 Tgf-β3 wild-type and null mutant pre-contact MEE
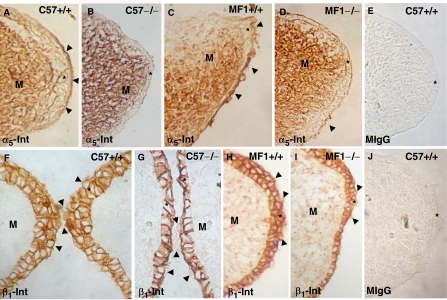
Fibronectin was the only ECM molecule studied here that was located in the most apical pre-contact MEE cells, which strongly suggested its participation in opposing MEE adhesion. We thus focused on fibronectin and studied the presence/distribution of its receptor, the α5β1-integrin, in the two mouse strains. We investigated the two subunits independently and immunolabeled E14.5 C57 and MF-1 wild-type and Tgf-β3 null mutant mouse palates with antibodies against the α5- and β1-integrins (Fig. 4). The α5-integrin was located apically in both strains' wild-type mouse MEE (Figs. 4A,4C). Its presence was strikingly reduced in the MF1 Tgf-β3 null mutant (Fig. 4D) but it was absent in the C57 mutant (Fig. 4B). The localization pattern of the β1-integrin was similar in the two strains' wild-type mice: β1-integrin was present around all MEE cell surfaces, including the apical surface of the most apical cells (Figs. 4F,4H). In the C57 Tgf-β3 null mutant, the few bulging cells present and the basal MEE surface were anti-β1-integrin negative (Fig. 4G), although the rest of the MEE was positive. However, the MF-1 Tgf-β3 null mouse MEE showed no differences with the wild type (compare Figs. 4H and 4I). Labeling with a mouse IgG (control) at the concentrations used for experiments was negative (Figs. 4E,4J).
Fig. 4.
Changes in the presence of the α5- and β1-integrins in C57 and MF1 wild-type and Tgf-β3 null mutant palates. Presence of α5- (A–D) and β1- (F–I) integrins in the pre-contact palatal shelves of E14.5 wild type (A, C, F, H) and Tgf-β3 null mutant (B, D, G, I) mice. Palates in (A), (B), (E), (F), (G) and (J) correspond to the C57 strain, while those in (C), (D), (H) and (I) correspond to the MF1 strain. (E) and (J) Sections labeled with mouse IgG (control). α5-integrin is present on the mouse medial edge epithelium (MEE) apical surface of C57 and MF1 wild-type palates (arrowheads in A and C), absent in this location in the C57 Tgf-β3 null mutant (B), and very scarce in the MF1 Tgf-β3 null mutant (arrowhead in D). β1-integrin is present in all MEE cells surfaces in the C57 wild type and in the MF1 wild type and Tgf-β3 null mutant palates (F, H, and I), but absent in the MEE basal surface and bulging cells (arrowheads in G) in the C57 Tgf-β3 null mutant. *MEE; M, mesenchyme.
Summarizing, our results reveal no changes in the localization of the α5 and β1-integrins between the pre-contact MEE of both strains' wild-type palates with differences for both subunits between the Tgf-β3 null and wild-type MEE in the C57 strain and for the α5 subunit in the MF1 strain.
Tgf-β3 rescue of extracellular matrix and cell adhesion molecules in the Tgf-β3 null mutant pre-contact MEE
To demonstrate the influence of TGF-β3 on the presence/distribution of these molecules in the MEE, we cultured palatal shelves from E14 C57 Tgf-β3 null mutant mice in the presence or absence of TGF-β3. To perform these experiments, we chose the C57 strain because it had the most marked alterations of any strain in the presence of these molecules. Cultures were maintained for 12 hr to impede the MEE disappearance that occurs in isolated cultured palatal shelves in longer period cultures (Takigawa and Shiota, 2004). We immunolabeled these palate cultures with antibodies directed against collagen IV, laminin, and fibronectin, as representatives of ECM molecules located at the MEE basal membrane, intercellularly and on the MEE surface, respectively, and the α5- and β1-integrins (Fig. 5). As expected, immunolabeling of Tgf-β3 null palate cultures with these antibodies showed a pattern of presence/distribution of the molecules studied similar to the one observed in vivo (compare Figs. 5A,5C,5E,5G,5I with 2B,2F,3G,4B,4G, respectively). The addition of TGF-β3 for 12 hr to these cultures' medium resulted in a great increment in collagen IV in the basement membrane (compare Figs. 5A with 5B) and the appearance of fibronectin and the α5-integrin on the MEE apical surface (compare Fig. 5E with 5F, 5G with 5H). These 12 hr TGF-β3-treated Tgf-β3 null palate cultures developed bulging cells on their MEE surface, whose apical surfaces were anti-laminin negative (Fig. 5D) and anti-β1-integrin positive (Fig. 5J), although the presence of laminin in the basal membrane was unchanged (compare Figs. 5D and 5C). The amount of β1-integrin greatly increased in these cultures' MEE basal surface (compare Figs. 5J and 5I).
Fig. 5.
Rescue of the normal presence of collagen IV, laminin, fibronectin, and the α5- and β1-integrins in Tgf-β3 null mutant palates by TGF-β3. C57 E14 Tgf-β3 null mutant palatal shelves were cultured for 12 hr in the absence (A, C, E, G, and I) or presence (B, D, F, H, and J) of TGF-β3. Sections were labeled with an anti-collagen IV (A, B), anti-laminin (C, D), anti-fibronectin (E, F), anti-α5-integrin (G, H), and anti-β1-integrin (I, J) antibody. Insets in (C) and (D) show high-power images of the squared areas in (C) and (D). (A, B) Anti-collagen IV labeling increased in the basement membrane (arrows) of supplemented palate cultures. (C, D) Bulging cells appeared on the mouse medial edge epithelium (MEE) surface of TGF-β3 supplemented Tgf-β3 null mutant palatal shelf cultures, whose apical surface was devoid of laminin (arrowheads in D). This was not observed in untreated cultures (arrowhead in the inset in C). Similar to what was observed in untreated cultures, laminin still persisted in the basement membrane of the supplemented cultures (arrows in the insert in C and in D). (E–H) Supplemented cultures showed the appearance of fibronectin and α5-integrin on the apical MEE surface (arrowheads in F and H, respectively), which was absent in the untreated cultures (E and G). The presence of fibronectin decreased in the basement membrane (arrows in E and F). (I, J) β1-integrin labeling was intense in the apical surface of the MEE bulging cells (arrowheads in J), and in the apical and basal (arrow in J) surfaces of TGF-β3 supplemented cultures, while it was weak or absent in these locations in untreated cultures (arrowheads and arrow in I). *MEE; M, mesenchyme.
Thus, our results demonstrate the importance of TGF-β3 for the correct amount and distribution of collagen IV, laminin, fibronectin, and the α5- and β1-integrins in the pre-contact MEE.
Role of fibronectin and its receptor in palatal shelf adhesion
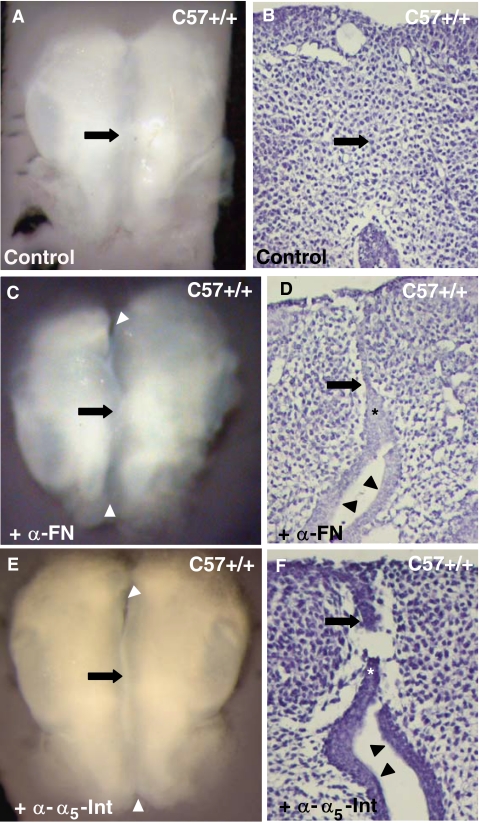
Because the superficial presence of fibronectin and its receptor, the α5-integrin, in the wild-type mouse pre-contact MEE strongly suggested its participation in their initial adhesion, we performed experiments blocking their action. We added antibodies that had proved to efficiently neutralize their activity in other organs' culture (Sakai et al., 2003) to E13.5 C57 wild-type paired palatal shelf cultures (Fig. 6). We then measured palatal shelf adhesion as described in ‘Material and methods.’ As expected, the addition of the anti-fibronectin antibody at a concentration of 1:50 resulted in a palatal shelf adhesion of 42%–44% with respect to the controls (Figs. 6A–6D and Table 1). A significant reduction of palatal shelf adhesion (67%–70%) compared with controls was appreciated when the antibody against the α5-integrin was added to the culture medium (Figs. 6E,6F and Table 1).
Fig. 6.
Palatal shelf adhesion after fibronectin and α5-integrin action blockage. Wild-type palatal shelves cultured for 36 hr in the presence of PBS (A, B), anti-fibronectin (C, D), or anti-α5-integrin neutralizing antibodies (E, F). (B, D and F) are sections of the cultures in (A, C, and E), respectively. In the untreated cultures, opposing mouse medial edge epithelium (MEE) adhesion (arrow in A and B) is complete. However, in the treated cultures, opposing MEE only adhered in a short length (arrow in C–F), while most of them remain separated (arrowheads in C–F). *MEE.
Thus, our results show the importance of fibronectin and its receptor for palatal shelf adhesion.
ICAM-1 in C57 and MF1 Tgf-β3 wild-type and null mutant MEE
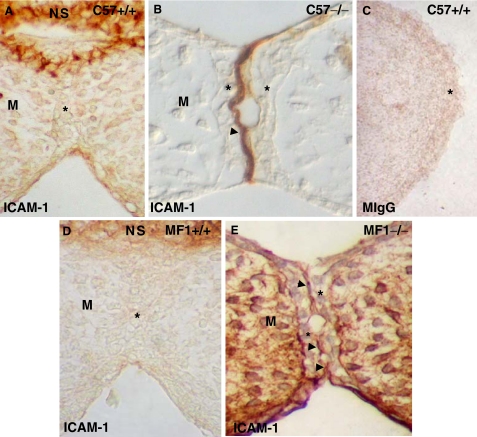
The abnormal presence of the LFA-1 integrin between contacting opposing MEE in C57 Tgf-β3 null mouse palates, which was absent in the wild type (Tudela et al., 2002), prompted us to investigate the presence of its ligand, ICAM-1, in the two mouse strains' contacting palatal shelves (Fig. 7). As expected, ICAM-1 was absent in both strains' wild-type mouse contacting MEE (Figs. 7A,7D) but was observed on the apical surface in the null mutants (Figs. 7B,7E). This suggests that the presence of ICAM-1 in the MEE is an abnormal condition associated with the appearance of cleft palate. Labeling with a mouse IgG (control) at the same concentration used for experiments was negative (Fig. 7C).
Fig. 7.
Changes in the presence of ICAM-1 in C57 and MF1 wild type and Tgf-β3 null mutant palates. Presence of ICAM-1 in the contacting palatal shelves of E14.5 wild type (A, D) and Tgf-β3 null mutant (B, E) mice. Palates in (A), (B), and (C) belong to mice of the C57 strain, while those in (D) and (E) to the MF1 strain. (C) Corresponds to a palate section labeled with mouse IgG -control-. ICAM-1 is absent between contacting opposing mouse medial edge epithelium (MEE) in wild-type palates, although present in the nasal septum (NS), and evident on the MEE apical surface in the Tgf-β3 null mutants of both strains (arrowheads in B and E). *MEE; M, mesenchyme.
Nectin-1 during palatal shelf adhesion and fusion
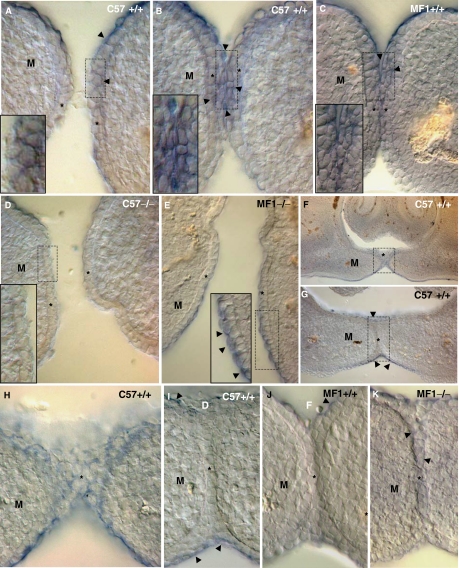
On the basis of epidemiological observation that mutations of the Nectin-1 gene increase the risk of developing cleft palate (Suzuki et al., 2000; Sozen et al., 2001; Scapoli et al., 2006), we looked into the expression of Nectin-1 in the MEE before and during palatal shelf contact in C57 and MF1 wild-type and Tgf-β3 null mouse palates (Fig. 8). In situ hybridization with a Nectin-1 probe showed that in the two strains' wild-type palate Nectin-1 was expressed in the most apical MEE cells at the time of the contact of palatal shelves (Figs. 8A–8C). Staining still persisted in the multilayered MES cells (Figs. 8F,8H), but it disappeared as the MES developed (Figs. 8G,8I,8J). Nectin-1 was absent in the Tgf-β3 null mouse MEE of the C57 strain (Fig. 8D) but present in the most apical MEE cells in the MF1 (Fig. 8E). Interestingly, there was Nectin-1 expression in these MF1 mutants' developed MES cells (Fig. 8K).
Fig. 8.
Expression of Nectin-1 in C57 and MF1 wild-type and Tgf-β3 null mutant palates. Expression of Nectin-1 in the E14.5 wild-type (A–C, F–J) and Tgf-β3 null mutant (D, E, and K) palates at the time of contact between palatal shelves. Palates in (A), (B), (D), (F), (G), (H) and (I) belong to mice of the C57 strain, while those in (C) (E), (J) and (K), to those of the MF1 strain. (F) and (G) are sections taken from the same specimen, where (G) is slightly posterior to (F). (H) and (I) are high-power images of the squared areas in (F) and (G). Insets in (A), (B), (C), (D) and (E) show high-power images of the squared areas in (A), (B), (C), (D) and (E). Observe the presence of Nectin-1 in the most apical pre-contact and contact mouse medial edge epithelium (MEE) cells of both strains wild-type mouse (arrowheads in A–C), the expression in these mice multilayered midline epithelial seam (MES) cells (F and H) and that there is no expression in the developed MES cells (G, I and J), although still persisting in the oral and nasal palatal epithelia (arrowheads in G, I, and J). Nectin-1 expression is absent in the Tgf-β3 null mutant MEE of the C57 strain (D) but it is present in the MEE apical surface of the MF1 mutant (arrowheads in the inset in E). Notice the presence of Nectin-1 in the developed MES cells of these mutants (arrowheads in K). *MEE or MES; M, mesenchyme.
Discussion
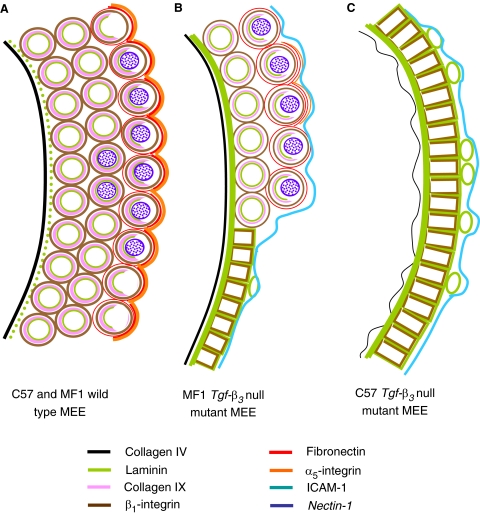
In this work we have analyzed the presence and distribution of several ECM and CA molecules in the MEE of two strains of Tgf-β3 null mutant mice presenting cleft palates of different severity and have compared their presence with that observed in wild-type palates. A scheme summarizing these results is shown in Figure 9. We have also investigated the influence of TGF-β3 in their appearance and the importance of fibronectin and its receptor in palatal shelf adhesion.
Fig. 9.
Scheme representing the localization of the different molecules studied in the mouse medial edge epithelium (MEE) of C57 and MF1 wild-type and Tgf-β3 null mutant palates. Scheme in (A) corresponds to wild-type MEE of C57 and MF1 strains, while those in (B) and (C) represent the MEE of C57 and MF1 Tgf-β3 null mutant palates, respectively. Anti-collagen IV labeling is represented in black, anti-laminin in green, anti-collagen IX in pink, anti-β1-integrin in brown, anti-fibronectin in red, anti-α5-integrin in orange, anti-ICAM1 in blue, and the expression of Nectin-1 in purple.
Palatal shelf adhesion is a crucial event during palate development in which two different and practically simultaneous processes occur. The first is the redistribution of the MEE cells that takes place immediately before the contact of palatal shelves (Brinkley, 1984) to produce the great mass of cells needed in opposing MEE adhesion. Up to this moment, the MEE is formed by an inner layer of cuboidal cells covered by an outer layer of flat cells, the superficial periderm (Fitchett and Hay, 1989), and only when palatal shelves approach each other does the MEE become multilayered (Brinkley, 1984; Tudela et al., 2002). These pre-contact MEE cells are round, develop many focal contacts, and show a patchy presence of vinculin, thus suggesting small movements among them (Tudela et al., 2002). As we demonstrate here, these cells have a basement membrane with a clear presence of collagen IV but where laminin is discontinuous. This discontinuity could be related to the occurrence of cell death in the MEE basal cell layer (Martínez-Álvarez et al., 2000a; Takigawa and Shiota, 2004), which motivates laminin disintegration in isolated palatal shelf cultures (Cuervo and Covarrubias, 2004). In this pre-contact MEE we note the presence of laminin and the β1 integrin intercellularly, which had not been documented earlier, and that of collagen IX, previously reported by Ferguson (1988). Laminin and collagen IX are ligands of β1-integrins, and because of their location among MEE cells, their role could be to ensure pre-contact MEE cells redistribution. E-cadherin, α-, and β-catenin have been identified as well among pre-contact MEE cells (Tudela et al., 2002; Martínez-Álvarez et al., 2004) and could also cooperate.
The importance of these molecules in the pre-contact MEE cells redistribution is supported by the fact that in the C57 Tgf-β3 null mutant, with severe cleft palate, the MEE is unable to become multilayered (Tudela et al., 2002) and the presence of most of these molecules is strikingly altered: the distribution pattern of E-cadherin, α-, and β-catenin (Tudela et al., 2002; Martínez-Álvarez et al., 2004), laminin and collagen IV (this work) is greatly distorted, while collagen IX is totally absent (this work). Interestingly, collagen IX was strongly suggested to play a role in palatal epithelial cell adhesion (Ferguson, 1988), and mutations of CDH1/E-cadherin, which delete the extracellular cadherin repeat domains required for cell–cell adhesion, have been recently associated with the appearance of cleft lip and palate (Frebourg et al., 2006).
The most important event in palatal shelf adhesion is the process of opposing MEE adhesion. To achieve it, the MEE surface increases its area by developing bulging cells and filopodia (Taya et al., 1999; Martínez-Álvarez et al., 2000a, 2000b), while desmosomes form on the MEE surface (Ferguson, 1988). In parallel, cell adhesion molecules are synthesized by the most apical MEE cells. This is the case with the chondroitin sulfate proteoglycan (CSPG), which appears on the MEE surface just before the contact of palatal shelves, increases as this contact becomes imminent, and disappears once palatal shelf adhesion occurs (Gato et al., 2002). As its timely presence suggests, altering the synthesis of CSPG results in a reduction of palatal shelf adhesion (Gato et al., 2002). We show here that fibronectin, its receptor (the α5β1-integrin), and Nectin-1 are all especially evident at the MEE apical surface at the time of contact of palatal shelves. The presence of small amounts of fibronectin on the MEE surface at this time point was previously reported, but it was considered amniotic fluid fibronectin without activity in this process (Silver et al., 1981). Together with a patchy distribution of fibronectin on the MEE surface, a regular distribution of fibronectin all around the superficial MEE cells clearly rules out this possibility. The importance in palatal shelf adhesion of fibronectin on the MEE surface is confirmed by the fact that fibronectin neutralization in culture and that of its receptor, the α5-integrin, results in a significant reduction of palatal shelf adhesion. In agreement with this, mutations of the MID1 gene, which encodes for a protein with a fibronectin type III domain, are associated with the Opitz phenotype, which includes cleft palate (Perry et al., 1999). The presence of the α5- and β1-integrins in the pre-contact MEE is described here for the first time. Interestingly, the α5-integrin is only present on the MEE surface, consistent with the role that we demonstrate in palatal shelf adhesion, while the β1-integrin has a broader presence in the MEE. This means that β1-integrins other than the α5β1-integrin are present in the MEE. This may play other functions in MEE cells, as suggested earlier. The expression of Nectin-1 in the MEE is compatible with a role in initial palatal shelf adhesion. Nectin-1 is expressed in MEE cells before the contact of palatal shelves (Suzuki et al., 2000), but we show here that it is especially evident in the most superficial MEE cells both immediately before and during the initial contact of palatal shelves. Once the MES becomes bi- or monolayered, Nectin-1 disappears, thus indicating that it does not participate in the developed MES intercellular adhesion. Although Nectin-1-deficient mice do not develop cleft palate (Irie et al., 2004 and references therein), the mutation of the PVRL-1 gene, codifying for the nectin-1 receptor, seems to constitute a genetic risk factor for non-syndromic cleft palate (Suzuki et al., 2000; Sozen et al., 2001; Scapoli et al., 2006), demonstrating the importance of this molecule in palate fusion.
It thus seems that opposing MEE adhesion results from the cooperation of different cell adhesion molecules (fibronectin, the α5β1-integrin, nectin-1, CSPG), all working together to guarantee the process. All of them are absent in the C57 Tgf-β3 null pre-contact MEE apical surface (this work; Gato et al., 2002). In addition, other molecules such as laminin, ICAM-1, and the LFA-1 integrin appear abnormally in this surface, while E-cadherin, β-, and α-catenin lose their apicobasal polarity (this work; Tudela et al., 2002; Martínez-Álvarez et al., 2004). This important alteration of the ECM molecules in the Tgf-β3 null cleft palate is congruent with the changes of both the presence of ECM molecules and Tgf-β3 expression observed in palate fibroblasts of cleft lip and palate patients (Baroni et al., 2006). It is clear that the C57 Tgf-β3 null pre-contact MEE is very different from its wild type-counterpart regarding its morphology (altered epithelial redistribution, absence of bulging cells) and the presence of ECM and CA molecules, which justifies the great impairment of palatal shelf adhesion occurring in these mice. All this suggests a role for TGF-β3 on the differentiation of the C57 pre-contact MEE toward a pre-fusion epithelium and, in fact, the addition of TGF-β3 to these null mice palate cultures not only normalizes the presence of most of the molecules studied (Gato et al., 2002; this work), but also the development of bulging cells (this work), palatal shelf adhesion (Gato et al., 2002), cell intercalation (Tudela et al., 2002), and epithelial to mesenchymal transformation (Kaartinen et al., 1997). Actually, the eventual fate of the C57 Tgf-β3 null mutant MEE is identical to that of the palatal oral epithelium (Martínez-Álvarez et al., 2004), which is not influenced by TGF-β3 (Fitzpatrick et al., 1990; Pelton et al., 1990). We do not know whether the altered MEE differentiation in these mice is the primary consequence of the absence of TGF-β3 or, as observed in other systems (Adams and Watt, 1993; Hay, 1993), it is due to a primary failure of cell–ECM adhesion caused by this absence. Nor do we know whether the lack of TGF-β3 causes the abnormal or null presence of only one of the studied molecules and the alteration of the others' results. It is accepted that ECM and CA molecules greatly interact among themselves (reviewed in Chen and Gumbiner, 2006). Another possibility is that the primary effects of the TGF-β3 absence was a disregulation of MEE cell matrix metalloproteinases, whose expression is altered in Tgf-β3 null palates (Blavier et al., 2001), leading to the ECM-CA alteration observed here. This needs to be clarified with further investigation.
The alteration of pre-contact MEE cell redistribution and opposing MEE adhesion is milder in the MF1 Tgf-β3 null MEE. In this strain, the pre-contact MEE achieves some degree of stratification, and opposing MEE adhesion occurs in part of the palate. However, in the adhered area the MES is formed by a reduced number of cells and persists until birth as either a bilayered or a multilayered epithelium (Kaartinen et al., 1995; Proetzel et al., 1995; Taya et al., 1999; Martínez-Álvarez et al., 2000a). Contrary to the observed in the C57 strain (Gato et al., 2002), MF1 Tgf-β3 null palatal shelves adhere properly in vitro (Taya et al., 1999). Consistent with this, the presence of the molecules studied in the pre-contact MEE is less altered than in the C57 strain. Collagens IV and IX, fibronectin, and the α5-integrin are less reduced, while the β1-integrin and the expression of Nectin-1 are normal. However, laminin and ICAM-1 are still present in the MEE basal and apical surfaces, respectively, while the expression of Nectin-1 persists in the developed MES, which may be the reason as to why MF1 Tgf-β3 null mutant MES cells never disappear (Kaartinen et al., 1995, 1997; Proetzel et al., 1995). The question raised is why the absence of TGF-β3 leads to these dissimilarities depending on the strain. Undoubtedly, TGF-β3 is important to the differentiation of the MF1 Tgf-β3 null pre-contact MEE toward the pre-fusion phenotype, because otherwise these mutants would not develop cleft palate. However, in this strain, growth factors other than TGF-β3 could be also involved in the process. Both TGF-β1 and TGF-β2 have been seen to improve palatal shelf fusion in MF1 Tgf-β3 null palate cultures (Taya et al., 1999) and are potent inductors of the synthesis of ECM molecules during palate development (Ferguson, 1988; Dixon and Ferguson, 1992). Interestingly, TGF-β1 is overexpressed in the mesenchyme of the C57 Tgf-β3 null mice immediately before the contact of palatal shelves (Martínez-Álvarez et al., 2004), probably as an attempt to compensate the absence of TGF-β3. However, Taya et al. (1999) did not find any difference in the intensity or distribution of TGF-β1 or TGF-β2 immunostaining in the heads of MF1 wild-type and Tgf-β3 null mutant mice, which may indicate a higher participation of these growth factors in palate fusion in this strain wild type and less impact caused by the absence of TGF-β3. Likewise, other growth factors could be involved in palatal shelf adhesion and participate differently in this process in both strains. In fact, although somewhat impaired, palatal shelf adhesion occurs between opposing Tgf-βr2 ablated MEE, where TGF-β signaling is blocked (Xu et al., 2006). TGF-α, EGF, or FGF, expressed during palatal fusion (Ferguson, 1988; Citterio and Gaillard, 1994; Britto et al., 2002; Rice et al., 2004), increase the presence of collagens, fibronectin, and glycosaminoglycans in palate cultures (Foreman et al., 1991; Dixon et al., 1993) and can modify the expression of matrix metalloproteinases (Miettinen et al., 1999), thus changing ECM molecules composition. Interestingly, their mutation or that of their receptors has been associated with increased risk to develop cleft palate (Miettinen et al., 1999; Jugessur et al., 2003). Investigations on the role of these growth factors in palatal shelf adhesion and on their expression in the two strains' Tgf-β3 null mutants need to be carried out to give more insight on the pathogenesis of the cleft palate produced by mutations of the Tgf-β3 gene.
Acknowledgments
The authors wish to thank Dr. Lars Haarr, University of Bergen, Norway, for kindly providing the Nectin-1 riboprobe, Mrs. Alicia Cerro and Mrs. Dolores Arroyo for help with histology, Mr. Kevin Wood (Linguistic Centre of University Complutense of Madrid [CSIM]) and the anonymous referees for revision of the manuscript. This work was supported by grants from the Fondo de Investigación Sanitaria (PI03/0185), Comunidad Autónoma de Madrid (08.6/0001/2003 and GR/SAL/0539/2004) and University Complutense-Comunidad Autónoma de Madrid to the Complutense Research Group 920202 in 2005.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Baroni T, Belluci C, Lilli C, Pezzetti F, Carinci F, Beccetti E, Carinci P, Stabellini G, Calvitti M, Lumare E, Bodo M. Retinoic acid, GABA-ergic, and TGF-beta signaling systems are involved in human cleft palate fibroblast phenotype. Mol Med. 2006;12:237–245. doi: 10.2119/2006-00026.Baroni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavier L, Lazaryev A, Groffen J, Heisterkamp N, DeClerck YA, Kaartinen V. TGF-beta3-induced palatogenesis requires matrix metalloproteinases. Mol Biol Cell. 2001;12:1457–1466. doi: 10.1091/mbc.12.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley LL. Changes in cell distribution during mouse secondary palate closure in vivo and in vitro. I. Epithelial cells. Dev Biol. 1984;102:216–227. doi: 10.1016/0012-1606(84)90186-6. [DOI] [PubMed] [Google Scholar]
- Britto JA, Evans RD, Hayward RD, Jones BM. Toward pathogenesis of Apert cleft palate: FGF, FGFR, and TGF beta genes are differentially expressed in sequential stages of human palatal shelf fusion. Cleft Palate Craniofac J. 2002;39:332–340. doi: 10.1597/1545-1569_2002_039_0332_tpoacp_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Carette MJM, Ferguson MWJ. The fate of medial edge epithelial cells during palatal fusion in vitro: an analysis by DiI labelling and confocal microscopy. Development. 1992;114:379–388. doi: 10.1242/dev.114.2.379. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Crosstalk between different adhesion molecules. Curr Opin Cell Biol. 2006;18:572–578. doi: 10.1016/j.ceb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Citterio HL, Gaillard DA. Expression of transforming growth factor alpha (TGF alpha), epidermal growth factor receptor (EGF-R) and cell proliferation during human palatogenesis: an immunohistochemical study. Int J Dev Biol. 1994;38:499–505. [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogénesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- Cuervo R, Valencia C, Chandaratna RAS. Programmed cell death is required for Palate Shelf Fusion and is regulated by retinoic acid. Dev Biol. 2002;245:145–156. doi: 10.1006/dbio.2002.0620. [DOI] [PubMed] [Google Scholar]
- Cui XM, Chai Y, Chen J, Yamamoto T, Ito Y, Bringas P, Shuler CF. TGF-β3-dependent SMAD2 phosphorylation and inhibition of MEE proliferation during palatal fusion. Dev Dyn. 2003;227:387–394. doi: 10.1002/dvdy.10326. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Carette MJ, Moser BB, Ferguson MW. Differentiation of isolated murine embryonic palatal epithelium in culture: exogenous transforming growth factor alpha modulates matrix biosynthesis in defined experimental conditions. In Vitro Cell Dev Biol. 1993;29A:51–61. doi: 10.1007/BF02634371. [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Ferguson MW. The effects of epidermal growth factor, transforming growth factors alpha and beta and platelet-derived growth factor on murine palatal shelves in organ culture. Arch Oral Biol. 1992;37:395–410. doi: 10.1016/0003-9969(92)90024-3. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ. Palate development. Development. 1988;103(Suppl.):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989;131:455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DR, Denhez F, Kondaiah P. Differential expresion of TGFβ isoforms in murine palatogenesis. Development. 1990;109:585–595. doi: 10.1242/dev.109.3.585. [DOI] [PubMed] [Google Scholar]
- Foreman DM, Sharpe PM, Ferguson MW. Comparative biochemistry of mouse and chick secondary-palate development in vivo and in vitro with particular emphasis on extracellular matrix molecules and the effects of growth factors on their synthesis. Arch Oral Biol. 1991;36:457–471. doi: 10.1016/0003-9969(91)90137-j. [DOI] [PubMed] [Google Scholar]
- Frebourg T, Oliveira C, Hochain P, Karam R, Manouvrier S, Graziadio C, Vekemans M, Hartmann A, Baert-Desurmont S, Alexandre C, Lejeune Dumoulin S, Marroni C, Martin C, Castedo S, Lovett M, Winston J, Machado JC, Attie T, Jabs EW, Cai J, Pellerin P, Triboulet JP, Scotte M, Le Pessot F, Hedouin A, Carneiro F, Blayau M, Seruca R. Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet. 2006;43:138–142. doi: 10.1136/jmg.2005.031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gato A, Martínez ML, Tudela C, Alonso I, Moro JA, Formoso MA, Ferguson MWJ, Martinez-Alvarez C. TGF-β3-induced chondroitin sulphate proteoglycan mediates palatal shelf adhesion. Dev Biol. 2002;250:393–405. [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial–mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Haarr L, Shukla D, Rodahl E, Dal Canto MC, Spear PG. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 2001;287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix alters epithelial differentiation. Curr Opin Cell Biol. 1993;5:1029–1035. doi: 10.1016/0955-0674(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Hudson CD, Shapiro BL. A radioautographic study of deoxiribonucleic acid synthesis in embryonic rat palatal shlef epithelium with reference to the concept of programmed cell death. Archs Oral Biol. 1973;18:77–84. doi: 10.1016/0003-9969(73)90022-8. [DOI] [PubMed] [Google Scholar]
- Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y. Roles and modes of action of nectins in cell–cell adhesion. Semin Cell Dev Biol. 2004;15:643–656. doi: 10.1016/j.semcdb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Jin JZ, Ding J. Analysis of cell migration, transdifferentiation and apoptosis during mouse secondary palate fusion. Development. 2006;133:3341–3347. doi: 10.1242/dev.02520. [DOI] [PubMed] [Google Scholar]
- Jugessur A, Lie RT, Wilcox AJ, Murray JC, Taylor JA, Saugstad OD, Vindenes HA, Abyholm FE. Cleft palate, transforming growth factor alpha gene variants, and maternal exposures: assessing gene–environment interactions in case-parent triads. Genet Epidemiol. 2003;25:367–374. doi: 10.1002/gepi.10268. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Cui XM, Heisterkamp N, Groffen J, Shuler CF. Transforming growth factor-β3 regulates transdifferentiation of medial edge epithelium during palatal fusion and associated degradation of the basement membrane. Dev Dyn. 1997;209:255–260. doi: 10.1002/(SICI)1097-0177(199707)209:3<255::AID-AJA1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kurisu K, Ohsaki Y, Nagata K, Kukita T, Yoshikawa H, Inai T. Immunocytochemical demonstration of simultaneous synthesis of types I, III and V collagen and fibronectin in mouse embryonic palatal mesenchymal cells in vitro. Coll Relat Res. 1987;7:333–340. doi: 10.1016/s0174-173x(87)80026-2. [DOI] [PubMed] [Google Scholar]
- Martínez-Álvarez C, Blanco MJ, Perez R, Rabadan MA, Aparicio M, Resel E, Martinez T, Nieto MA. Snail family members and cell survival in physiological and pathological cleft palates. Dev Biol. 2004;265:207–218. doi: 10.1016/j.ydbio.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Martínez-Álvarez C, Bonelli R, Tudela C, Gato A, Mena J, O'kane S, Ferguson MWJ. Bulging medial edge epithelial cells and palatal fusion. Int J Dev Biol. 2000b;44:331–335. [PubMed] [Google Scholar]
- Martínez-Álvarez C, Tudela C, Pérez-Miguelsanz J, Ókane S, Puerta J, Ferguson MWJ. Medial edge epithelial cell fate during palatal fusion. Dev Biol. 2000a;220:343–357. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann NY Acad Sci. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Chin JR, Shum L, Slavkin HC, Shuler CF, Derynck R, Werb Z. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nat Genet. 1999;22:69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- Montenegro MA, Rojas M, Domínguez S, Rosales CJ. Differences in extracellular matrix components and cell density during normal and dexamethasone-treated secondary palate development in two strains of mice with different susceptibility to glucocorticoid induced-clefting. J Craniofac Genet Dev Biol. 1998;18:100–106. [PubMed] [Google Scholar]
- Mori C, Nakamura N, Okamoto Y, Osawa M, Shiota K. Cytochemical identification of programmed cell death in the fusing fetal mouse palate by specific labelling of DNA fragmentation. Anat Embryol. 1994;190:21–28. doi: 10.1007/BF00185843. [DOI] [PubMed] [Google Scholar]
- Nawshad A, LaGamba D, Hay ED. Transforming growth factor beta (TGFbeta) signalling in palatal growth, apoptosis and epithelial mesenchymal transformation (EMT) Arch Oral Biol. 2004;49:675–689. doi: 10.1016/j.archoralbio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Nagata K, Kurisu K. Localization of types I and III collagen and fibronectin in the developing mouse palatal shelves. Acta Anat (Basel) 1995;153:161–167. doi: 10.1159/000147696. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Dickinson ME, Moses HL, Hogan BLM. In situ hybridization analysis of TGFβ3 RNA expression during mouse development: comparative studies with TGFβ1 and β2. Development. 1990;110:609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Perry J, Short KM, Romer JT, Swift S, Cox TC, Ashworth A. FXY2/MID2, a gene related to the X-linked Opitz syndrome gene FXY/MID1, maps to Xq22 and encodes a FNIII domain-containing protein that associates with microtubules. Genomics. 1999;62:385–394. doi: 10.1006/geno.1999.6043. [DOI] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Dotschman T. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DP. Disruption of Fgf10/Fgfr2b-coordinated epithelial–mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986;125:431–435. [PMC free article] [PubMed] [Google Scholar]
- Scapoli L, Palmieri A, Martinelli M, Vaccari C, Marchesini J, Pezzetti F, Baciliero U, Padula E, Carinci P, Carinci F. Study of the PVRL1 gene in Italian nonsyndromic cleft lip patients with or without cleft palate. Ann Hum Genet. 2006;70(Part 3):410–413. doi: 10.1111/j.1529-8817.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- Shuler CF, Halpern DE, Guo Y, Sank AC. Medial edge epithelium fate traced by cell lineage analysis during epithelial–mesenchymal transformation in vivo. Dev Biol. 1992;154:318–330. doi: 10.1016/0012-1606(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Silver MH, Foidart JM, Pratt RM. Distribution of fibronectin and collagen during mouse limb and palate development. Differentiation. 1981;18:141–149. doi: 10.1111/j.1432-0436.1981.tb01115.x. [DOI] [PubMed] [Google Scholar]
- Singh GD, Johnston J, Ma W, Lozanoff S. Cleft palate formation in fetal Br mice with midfacial retrusion: tenascin, fibronectin, laminin, and type IV collagen immunolocalization. Cleft Palate Craniofac J. 1997;35:65–76. doi: 10.1597/1545-1569_1998_035_0065_cpfifb_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Sozen MA, Suzuki K, Tolarova MM, Bustos T, Fernandez Iglesias JE, Spritz RA. Mutation of PVRL1 is associated with sporadic, non-syndromic cleft lip/palate in northern Venezuela. Nat Genet. 2001;29:141–142. doi: 10.1038/ng740. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell–cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Shiota K. Terminal differentiation of palatal medial edge epithelial cells in vitro is not necessarily dependent on palatal shelf contact and midline epithelial seam formation. Int J Dev Biol. 2004;48:307–317. doi: 10.1387/ijdb.041840tt. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Sato N, Uchiyama Y. Apoptosis and heterophagy of medial edge epithelial cells of the secondary palatine shelves during fusion. Arch Histol Cytol. 1995;58:191–203. doi: 10.1679/aohc.58.191. [DOI] [PubMed] [Google Scholar]
- Taya Y, O'kane S, Ferguson MWJ. Pathogenesis of cleft palate in TGF-β3 knockout mice. Development. 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Tudela C, Formoso MA, Martínez T, Pérez R, Aparicio M, Maestro C, Del Río A, Martínez E, Ferguson MWJ, Martínez-Álvarez C. TGF-β3 is required for the adhesion and intercalation of medial edge epithelial cells during palate fusion. Int J Dev Biol. 2002;46:333–336. [PubMed] [Google Scholar]
- Vaziri Sani F, Hallberg K, Harfe BD, McMahon AP, Linde A, Gritli-Linde A. Fate-mapping of the epithelial seam during palatal fusion rules out epithelial–mesenchymal transformation. Dev Biol. 2005;285:490–495. doi: 10.1016/j.ydbio.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Urata MM, Chai Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–248. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]