Abstract
Interleukin 3 (IL-3)-dependent survival of hematopoietic cells is known to rely on the activity of multiple signaling pathways, including a pathway leading to activation of phosphoinositide 3-kinase (PI 3-kinase), and protein kinase Akt is a direct target of PI 3-kinase. We find that Akt kinase activity is rapidly induced by the cytokine IL-3, suggesting a role for Akt in PI 3-kinase-dependent signaling in hematopoetic cells. Dominant-negative mutants of Akt specifically block Akt activation by IL-3 and interfere with IL-3-dependent proliferation. Overexpression of Akt or oncogenic v-akt protects 32D cells from apoptosis induced by IL-3 withdrawal. Apoptosis after IL-3 withdrawal is accelerated by expression of dominant-negative mutants of Akt, indicating that a functional Akt signaling pathway is necessary for cell survival mediated by the cytokine IL-3. Thus Akt appears to be an important mediator of anti-apoptotic signaling in this system.
The development of immature bone marrow progenitor cells is governed by a delicate balance between proliferation and apoptosis and is controlled by the availability of cytokines in vivo (for review see ref. 1). Many cytokines not only stimulate cell growth, but also are necessary for cell survival. Defining the precise mechanism of cytokine function in cells is therefore critical for understanding hematopoiesis (for review see ref. 1).
The cytokines interleukin 3 (IL-3) and granulocyte/macrophage colony-stimulating factor (GM-CSF) exert their survival and proliferative functions through a common signaling subunit, the βc receptor (2). Signal transduction events that regulate survival by IL-3 and GM-CSF are in part mediated by cytosolic tyrosine kinases (i.e., Lyn) and subsequent mitogen-activated protein kinase (MAPK) activation (2–6). A region of the βc receptor that is sufficient for IL-3 and GM-CSF-dependent survival induces MAPK activation (2, 3). In the absence of IL-3, IL-3-dependent progenitor cells quickly undergo apoptosis, but expression of Bcl-2 can support survival upon IL-3 withdrawal (7, 8).
Another signaling molecule that is activated by IL-3 and GM-CSF is phosphoinositide 3-kinase (PI 3-kinase) (4, 9, 10). PI 3-kinase has been implicated in the regulation of survival in several cell types (for review see ref. 11). PI 3-kinase also is implicated in IL-3-dependent survival, and a region on the βc receptor important for IL-3/GM-CSF-dependent survival is necessary for PI 3-kinase activation (2). Furthermore, an activated mutant of Ras that stimulates PI 3-kinase activity (12) can complement mutant βc receptors that are defective in survival in response to GM-CSF (2). Several downstream targets of PI 3-kinase recently have been identified, including p70 ribosomal protein S6 kinase (13), several protein kinase C isoforms (for review see ref. 14), and the serine/threonine kinase Akt (15–17).
Akt (also referred to as Racα or PKBα), which also was identified by its homology to protein kinases A and C, is the cellular homolog of the v-akt oncogene (18–20). The protein kinase activity of Akt is directly stimulated by products of PI 3-kinase in vitro (15, 21, 22). In cells, Akt is activated by factors that stimulate PI 3-kinase activity in cells, such as thrombin, platelet-derived growth factor, and insulin (for review see ref. 11). Akt also is activated under conditions of cellular stress by a pathway not involving PI 3-kinase (23). The N-terminal regulatory domain of Akt contains a Pleckstrin homology domain (PH) that is important for Akt activation (for review see ref. 11). Phosphatidylinositol-3,4-bisphosphate directly binds to the Akt PH domain to stimulate Akt protein kinase activity (15, 22, 24). Full activation of Akt also requires phosphorylation on serine and threonine residues (25).
Overexpression experiments using Akt and oncogenic v-akt, and the use of Akt mutants that interfere with its function in cells have underscored the importance of Akt in several cell systems (for review see ref. 11). Akt is able to support the survival of primary cerebellar granule cells after survival factor deprivation (26). Activated Akt also prevents apoptosis that is induced after exposure of fibroblast and epithelial cells to ultraviolet radiation (27), after conditional expression of c-myc in fibroblast cell lines (28, 29), and after detachment of MDCK epithelial cells from their extracellular matrix (anoikis) (30). Little knowledge, however, exists about the function of Akt in hematopoietic cells, even though c-akt originally was identified as a transduced oncogene in a T cell lymphoma-inducing retrovirus in AKR mice (31). One study recently has examined the ability of activated forms of Akt to prevent cell death induced by withdrawal of IL-2 from BAF/3 cells expressing the IL-2 receptor (32).
Here we show that Akt activity is induced rapidly by the cytokine IL-3 and that the activation of Akt by IL-3 is dependent upon the activity of PI 3-kinase. Our results suggest a central role for Akt in PI-3 kinase signaling in IL-3-dependent cells. Akt and v-akt expression significantly prevented apoptosis of IL-3-dependent cells in the absence of IL-3, suggesting an important role for the Akt kinase in cell survival. In addition, the expression of dominant-negative mutants of Akt interfered with Akt activation by IL-3 and promoted apoptosis. We failed to observe the induction of Bcl-2 expression in cells overexpressing Akt. The role for Akt in IL-3-dependent cell survival could explain the transforming potential of v-akt in hematopoietic cells.
MATERIALS AND METHODS
Cell Lines, Expression Constructs, and Transfections.
32D cells were maintained in RPMI medium 1640 with 10% fetal calf serum (FCS) and 10% WEHI-3 conditioned medium (33). Human Bcl-2 in pSV is described in ref. 7. The retroviral vector pLXSN and v-akt in pLXSN were previously described (34). Hemagglutinin (HA) epitope-tagged expression constructs of HA-Akt, HA-Akt(K179M), and HA-Akt(PH) were generated by transferring pre-existing constructs from pJ3Ω (15, 35) into the retroviral vector pBabe (36). Inserts of HA-Akt, HA-Akt(K179M), and HA-Akt(PH) were excised by digestion with HindIII and BamHI, and blunted and inserted into the SnaBI site of pBabe by blunt-end ligation. All constructs were verified by sequence analysis.
Cells expressing the various Akt constructs were generated by transfecting 32D cells. Mass cultures of 32D cells were selected in 2 μg/ml puromycin (Sigma). To generate v-akt expressing cells, 32D cells were either electroporated with v-akt in pLXSN or infected with retroviruses that were generated by transient expression of v-akt in pLXSN in the retroviral packaging line BOSC-23 (37). Mass cultures of v-akt expressing cells were selected in 400 μg/ml G418 (GIBCO/BRL).
Cell Proliferation and Survival Assays.
Cell proliferation was monitored by [3H]thymidine uptake as previously described (33). Cell survival was determined by counting viable cells using the trypan blue exclusion method (38). Briefly, 32D cells carrying control vectors and cells expressing v-akt, HA-Akt, HA-Akt(K179M), HA-Akt(PH), and/or Bcl-2 were washed with RPMI containing 10% FCS and antibiotics to deplete of IL-3. For [3H]thymidine uptake experiments, cultures of 1 × 105 cells were grown in 24-well plates at different concentrations (0.01% to 10%) of WEHI-3 conditioned medium. After 48 hr, 1 μCi of [3H]thymidine was added to each well for 6 hr. Incorporated radioactivity was estimated by liquid scintillation counting. For survival assays, IL-3-depleted cells were seeded at a density of 2 × 105 per ml in RPMI containing 10% FCS. To determine the effect of DNA damage reagents on cell survival, etoposide (Sigma) was added at different concentrations to RPMI medium containing 10% FCS and WEHI-3 conditioned medium. Subsequently, the ratio of viable to dead cells was determined by trypan blue exclusion.
Immunoblotting, Immunoprecipitation, and Immunocomplex Kinase Assays.
Cultures of 32D cells transfected with control vectors and various Akt constructs were depleted of IL-3 by brief washing in RPMI containing 10% FCS. Cells were starved for 30 min in the same media, and then transferred to RPMI without serum. Wortmannin (Sigma) was added at 100 nM where indicated. Cells (5 × 106) were stimulated 30 min later with 1–2 ng/ml of recombinant IL-3 (R & D Systems or PeproTech, Boston) in RPMI 1640 medium and lysed by the addition of an equal volume of 2× Nonidet P-40 lysis buffer (40 mM Tris⋅HCl, pH 8.0/274 mM NaCl/20% glycerol/2% Nonidet P-40/2 mM phenylmethylsufonyl fluoride/4 μg/ml aprotinin/4 μg/ml leupeptin). Endogenous Akt was immunoprecipitated from cell lysates using Akt-CT antibody (15). HA-epitope tagged Akt constructs were immunoprecipitated using monoclonal anti-HA antibody (Boehringer). Akt activity was determined by in vitro kinase assays using histone H2B as a substrate (15). Immunocomplex kinase assays were displayed by 12.5% crosslinked SDS/PAGE and blotted onto nitrocellulose. After exposure to PhosphoImager (Bio-Rad) and x-ray film, blots were probed with anti-Akt-CT to ensure equal protein loading.
To visualize the Akt N terminus in cells transfected with HA-Akt[PH], immunoblots were performed using the Akt-NT antibody (21). To examine the phosphorylation state of MAPK, whole-cell lysates were resolved by crosslinked 7.5% SDS/PAGE and transferred onto nitrocellulose membranes. Immunoblots were performed using anti-ERK (Santa Cruz Biotechnology).
RESULTS
To determine whether Akt is involved in the IL-3-dependent survival pathway, we tested whether Akt is activated by IL-3 in the myeloid progenitor cell line 32D. Addition of IL-3 to 32D cells increased the activity of immunoprecipitated Akt toward histone H2B >3-fold within 1 min (Fig. 1A). Akt activation by IL-3 was PI 3-kinase-dependent based on inhibition by the PI 3-kinase inhibitor wortmannin (Fig. 1A). To further investigate the activation of Akt, we generated stable 32D cell lines expressing HA-tagged wild-type Akt (HA-Akt) and kinase-inactive Akt with a point mutation in the ATP binding site [HA-Akt(K179M)] (15). Both proteins were expressed (Fig. 1B). As judged by histone H2B kinase activity in anti-HA immunoprecipitates, the protein kinase activity of HA-Akt, but not HA-Akt(K179M), was stimulated about 3–4 fold by IL-3 in 32D cells (Fig. 1B). Thus, the observed histone H2B kinase activity after IL-3 stimulation can be demonstrated with HA-tagged proteins and requires the active site, implying that direct activation of Akt is responsible for the activity in anti-Akt immunoprecipitates rather than a coprecipitating kinase being responsible.
Figure 1.
Akt is activated by IL-3 depending upon the activity of PI-3 kinase. (A) 32D cells were starved of IL-3 as described above and stimulated with 1 ng/ml IL-3 (Materials and Methods). Subsequently, the kinase activity of Akt was determined by histone H2B phosphorylation in immunoprecipitates using anti-Akt-CT. Akt kinase activity also was determined in immunoprecipitates from cells that were pretreated with 100 nM wortmannin before IL-3 stimulation. Subsequently, the immunoprecipitates were probed with anti-Akt-CT to ensure equal protein loading. (B) 32D cells expressing HA-Akt or HA-Akt(K179M) were starved of IL-3 and stimulated for various times with 2 ng/ml IL-3. HA-Akt and HA-Akt(K179M) proteins were immunoprecipitated with monoclonal anti-HA before and after stimulation, and their kinase activity was monitored by in vitro kinase. The lower panel shows an anti-Akt-CT immunoblot of the immunoprecipitates.
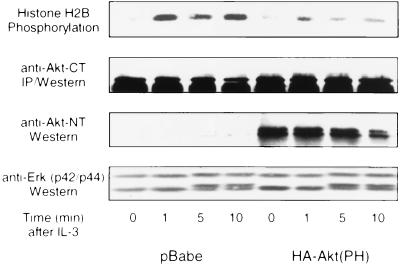
Kinase-inactive Akt can block okadaic acid-induced inhibition of glycogen synthase kinase-3 (39). Furthermore, recent findings demonstrated that kinase-inactive Akt and a mutant of Akt that lacks the kinase domain block the survival of cerebellar granule cells, indicating the dominant-negative effect of these mutants in the Akt-dependent survival pathway (26). To test whether overexpression of the Akt regulatory domain also could interfere with IL-3-dependent activation of endogenous Akt, we measured the activity of endogenous Akt in cells expressing the N terminus of Akt, which includes the PH domain but lacks the kinase domain [HA-Akt(PH)]. After IL-3 stimulation, control cells and cells expressing HA-Akt(PH) were lysed and endogenous Akt was immunoprecipitated (15). The expression of HA-Akt(PH) reduced the activity of endogenous Akt in starved cells and cells that were stimulated with IL-3 to less than 20% of the activity observed in the control cells (Fig. 2). Even though the inhibition of Akt activity was not complete, our results indicated that HA-Akt(PH) acts as a dominant-negative mutant for the Akt survival pathway by reducing its activity and blocking its ability to be activated by IL-3. To test if the signaling block by HA-Akt(PH) was specific for Akt, we also tested mobility shift (Fig. 2) and activity (data not shown) of MAPK that is activated by IL-3 in 32D cells. Consistent with previous studies, addition of IL-3 to starved 32D cells led to a mobility shift of MAPK within 5 min, indicative of the activation of these kinases (Fig. 2). MAPK activation by IL-3 was not affected by expression of HA-Akt(PH), indicating that the inhibitory effect of HA-Akt(PH) on Akt signaling is specific for the Akt pathway. This result also suggests that Akt is not required for IL-3-dependent activation of MAPK.
Figure 2.
Activation of endogenous Akt by IL-3 is inhibited by expression of the Akt N terminus. The kinase activity of endogenous Akt was determined by histone H2B phosphorylation in anti-Akt-CT immunoprecipitates from 32D cells stimulated with IL-3 (1 ng/ml). Akt activity was measured in immunoprecipitates from cells transfected with pBabe vector alone as well as from cells transfected with HA-Akt(PH) in pBabe. Whole-cell lysates were blotted with anti-Akt-CT and anti-Akt-NT to determine expression of Akt and the Akt N terminus, respectively. In parallel, MAPK mobility shifts were determined by anti-Erk immunoblotting of whole-cell lysates after electrophoresis in 7.5% SDS/PAGE.
The role of Akt in IL-3-dependent biological responses was studied in 32D cells expressing different forms of Akt or oncogenic v-akt. A consistent promotion of IL-3-dependent DNA synthesis coincident with expression of v-akt or HA-Akt was observed at low, but not high, doses of IL-3 (Fig. 3 A and B). Furthermore, a dominant-negative mutant of Akt inhibited DNA synthesis at both high and low IL-3 (Fig. 3 A and B). These results imply that Akt may be involved in proliferative responses although it is difficult to separate proliferation and survival.
Figure 3.
Proliferation of cells expressing Akt constructs. The proliferation of 32D cells expressing various Akt constructs was measured at various levels of IL-3 using the [3H]thymidine uptake assay (see Materials and Methods). The error bars indicate the standard error of four experiments. (Left) [3H]thymidine uptake was determined in cells transfected with vector alone or v-akt in pLXSN. (Right) [3H]thymidine uptake also was measured in cells transfected with pBabe alone and HA-Akt or HA-Akt(K179M) in pBabe.
Previous studies have shown that withdrawal of IL-3 induces apoptosis in IL-3-dependent 32D cells (7). DNA laddering that is characteristic of apoptosis occurred after switching 32D cells to IL-3-free growth medium (data not shown). Treatment of cells with the PI 3-kinase inhibitor LY294002 in the presence of IL-3 induced apoptosis in IL-3-dependent cells (10), suggesting that PI 3-kinase may play an important role in IL-3-dependent cell survival. To test whether Akt can mediate survival, we investigated cells expressing various Akt constructs. Mass cultures of cells expressing either vector alone or v-akt were starved of IL-3, and cell survival was monitored over 5 days using the trypan blue dye exclusion assay (38). For the cells expressing the vector control, within 16 hr substantial apoptosis occurred (approximately 50% dead cells) and by 72 hr virtually all cells were dead (Fig. 4A). Expression of human Bcl-2 partially prevented apoptosis of 32D cells, in agreement with previous studies (7). Expression of oncogenic v-akt, a constitutively activated Akt kinase (15), also partially blocked cell death, though the effect was not as potent as that of Bcl-2. At 24 hr after IL-3 withdrawal, >50% of the v-akt-expressing cells were viable compared with 35% of control cells (Fig. 4A). At 72 hr, 99% of control cells had died; in contrast, approximately 20% of v-akt-expressing cells still remained viable after 5 days, suggesting a significant protective function of v-akt (data not shown). However, this protocol may underestimate the protection by v-akt, because pools of G418-resistant cells were used and the protein expression level of v-akt was almost 10-fold lower than that of endogenous Akt (data not shown).
Figure 4.
Overexpressed Akt and oncogenic v-akt prevent apoptosis that is induced by IL-3 withdrawal. The viability of cells was measured by the trypan blue exclusion assays. The percentages of surviving cells at different times after IL-3 withdrawal are presented. (A) Control cells transfected with vector alone and cells expressing Bcl-2 or v-akt were starved of IL-3 for 24 hr and then assayed for trypan blue exclusion. The error bars indicate the standard error of four experiments. (B) Cells transfected with pBabe alone and cells expressing HA-Akt, HA-Akt(PH), HA-Akt (K179M), or Bcl-2 were starved of IL-3 for 24 hr, and subsequently assayed by trypan blue dye exclusion assays. The survival of cells that expressed Bcl-2 and wild-type HA-Akt or kinase-deficient HA-Akt(K179M) also was estimated 24 hr after IL-3 depletion. Error bars indicate the standard error of three experiments.
To further examine the ability of Akt to increase cell survival, pools of cells were transfected with pBabe vector alone or with constructs expressing HA-Akt, HA-Akt(K179M), or HA-Akt(PH). Overexpression of HA-Akt significantly attenuated apoptosis that was triggered by depletion of IL-3. At 24 hr of IL-3 starvation, more than 60% of HA-Akt-expressing cells were still alive, but less than 20% of the control cells survived (Fig. 4B). The level of protection by Akt at this time was nearly that reached by Bcl-2 expression (Fig. 4B). Dominant-negative mutants of Akt accelerated IL-3 depletion-induced cell death: less than 8% of the cells expressing HA-Akt(PH) and only 1% of the cells expressing HA-Akt(K179M) survived after 24 hr. Therefore, Akt both can mediate cell survival in the absence of IL-3 and is needed for IL-3-mediated survival. The survival function of Akt was transient when compared with that of constitutively activated v-akt: by 48 hr of IL-3 depletion, 95% of cells expressing HA-Akt also had died (data not shown).
Because both Akt and Bcl-2 can protect 32D cells from apoptosis, we tested whether the Akt- and Bcl-2-mediated survival pathways overlapped. First, we studied the effects of Akt expression on Bcl-2-dependent cell survival. 32D cells expressing human Bcl-2 and murine Akt were generated by cotransfecting constructs directing the expression of Bcl-2 and HA-Akt or its mutants. Bcl-2 was expressed at similar amount in all cells (data not shown). Expression of Akt and its mutants and the activation of Akt and HA-Akt by IL-3 were not affected in cells that were expressing Bcl-2 (data not shown). Expression of HA-Akt and Bcl-2, however, impaired survival of 32D cells and reduced it to the level of protection that was observed after expression of HA-Akt alone (Fig. 4B). Dominant-negative HA-Akt(K179M) had no significant effect on Bcl-2-mediated survival (Fig. 4B).
Akt also may act by reducing apoptosis induced through DNA repair. To test if v-akt was able to protect 32D cells from apoptosis that is induced by DNA damaging reagents, we exposed control cells and cells expressing Bcl-2 or v-akt for 16 hr to the DNA damaging reagent etoposide in growth medium. Etoposide has been shown to induce apoptosis in other IL-3-dependent cell lines, including BAF/3 cells, by inhibiting topoisomerase II (40). Cell death, as measured by the dye exclusion method, was induced by increasing concentrations of etoposide, and the expression of v-akt efficiently blocked etoposide-induced apoptosis (Fig. 5). Bcl-2 was somewhat more efficient at blocking etoposide-induced apoptosis (Fig. 5).
Figure 5.
v-akt protects cells from apoptosis induced by the DNA damaging reagent etoposide. Percentages of surviving cells were estimated at 16 hr post-etoposide addition to IL-3-containing medium. Error bars indicate the difference between two experiments.
DISCUSSION
Akt acts in multiple signaling systems as a downstream target of PI 3-kinase: this includes activation of Akt in signaling pathways that originate from activated receptor tyrosine kinases (15, 16, 41) and G protein-coupled receptor systems like the thrombin receptor (21). Here, we report that Akt is activated as a consequence of cell stimulation by a cytokine in agreement with a recent study (32). IL-3-dependent activation of Akt probably is mediated by activation of PI 3-kinase because an inhibitor of PI 3-kinase, wortmannin, blocks Akt activation in response to IL-3. Previous studies have shown that the activity of Akt is regulated directly by products of PI 3-kinase that bind to the Akt PH domain (21, 22, 24). Phosphorylation of Akt is important for its activation and the major phosphorylation sites, threonine 308 and serine 473, have been identified (25). Hence, IL-3-dependent activation of Akt is likely to be governed by lipid binding and phosphorylation events. Furthermore, the Akt signaling pathway in 32D cells is probably distinct from pathways leading to MAPK activation, given that dominant-negative mutants of Akt inhibited endogenous Akt activation without blocking MAPK activation.
Consistent with PI-3 kinase being a survival factor in several different tissue culture systems and Akt being a direct target of PI-3 kinase (for review see ref. 11), we demonstrate here that Akt also promotes survival after IL-3 withdrawal. Expression of Akt and its oncogenic form, v-akt, prevented apoptosis that is initiated by IL-3 withdrawal (Fig. 4). Although Akt and v-akt were equally potent in protecting cells from apoptosis immediately after IL-3 starvation, only v-akt showed a long-term anti-apoptotic effect (Fig. 4A). This difference is consistent with differences in the activity of the two molecules: cytoplasmic Akt is transiently activated upon stimulation of PI 3-kinase and phosphorylation, whereas v-akt is constitutively activated by being localized to the plasma membrane (15). Our studies using dominant-negative forms of Akt underscored the importance of Akt for cell survival. Expression of dominant-negative HA-Akt(PH) and HA-Akt(K179M) accelerated apoptosis in 32D cells (Fig. 4). In the presence of IL-3, dominant-negative Akt also partially inhibited IL-3-dependent proliferation (Fig. 3). Recently, by using our set of dominant-negative mutants, Akt was shown to be important for IGF-1-dependent survival of primary cerebellar neurons (26). These data are in good agreement with our present findings. Taken together with other recent work (27–29, 32), they suggest a general anti-apoptotic function for Akt. Thus, among the various targets of PI-3 kinase, Akt is probably the major mediator of the survival function of PI-3 kinase.
Our results suggest that Akt is using a mechanism for cell survival that seems not to involve Bcl-2. Akt and Bcl-2 failed to synergize in protection of cells from apoptosis. Furthermore, the overexpression of HA-Akt reduced the level of Bcl-2-mediated protection to the level that was observed after expressing HA-Akt alone. More importantly, expression of dominant-negative Akt with Bcl-2 did not compromise the Bcl-2-dependent survival. Even though these results do not exclude Bcl-2 or another family member being downstream of Akt, they suggest that Akt does not play a role in Bcl-2-dependent survival. In addition, we failed to observe a change in the migration pattern of Bcl-2 in HA-Akt overexpressing cells that is indicative of Bcl-2 phosphorylation (data not shown), which is important for its anti-apoptotic function (42). A recent paper has implicated Akt in prevention of apoptosis after withdrawal of IL-2 by a mechanism that is claimed to lead to an increase in the expression levels of c-myc and Bcl-2 (32). Bad, a member of the Bcl-2 family that is rapidly phosphorylated upon IL-3 stimulation (43), can attenuate Bcl-2 activity by heterodimerization with Bcl-2 (for review see refs. 44 and 45). It will be interesting to study whether IL-3-dependent Bad phosphorylation is mediated by Akt. To date, only glycogen synthase kinase-3 has been identified as a direct downstream target of Akt (39), and it remains to be determined if the role of glycogen synthase kinase-3 in the regulation of cell proliferation by phosphorylating the adenomatous polyposis coli gene product APC (46) is important for Akt-mediated survival.
Our data indicate that Akt, presumably acting as a downstream effector of PI 3-kinase, has an important role in the survival of IL-3-dependent cells. Thus, Akt differs from other signaling molecules such as MAPK because of its rapid activation (this report) and from p70 ribosomal protein S6 kinase because of its role in cell survival (26, 29). Expression of oncogenic v-akt also prevented apoptosis induced by the DNA damaging agent etoposide (Fig. 5). This may be relevant to the therapy of those human cancer forms that are characterized by enhanced expression of the human protooncogene AKT2 that is closely related to Akt and v-akt (90% similarity on the protein level) (47, 48).
The discovery of phosphatases that regulate the ratio of D3-phosphorylated phosphoinositides (49–54) adds further complexity to the understanding of signaling cascades downstream of PI 3-kinases. Recent studies have demonstrated that the phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase SH2-containing inositol phosphatase (SHIP) associates with the activated IL-3 receptor (55, 56). Interestingly, SHIP appears to counteract the protective function of PI 3-kinase (2) and Akt (this report) in IL-3-dependent survival by inducing apoptosis in an IL-3-dependent cell system (57). This could be due to the ability of SHIP to recruit negative regulatory molecules to the IL-3-dependent signaling complex.
Acknowledgments
We thank M. Boudreau, H. Chang, R. Friedrich, A. Kazlauskas, R. Khosravi-Far, G. Thomas, and X. Yang for critical reading of the manuscript. This work was supported by Public Health Service Grants CA51962 (D.B.) and GM41890 (L.C.C.) and by the National Cancer Institute of Canada (D.R.K.). Z.S. is a recipient of the Irvington Institute Fellowship. T.F.F. is a recipient of the K.M. Hunter Fellowship in Cancer Research from the National Cancer Institute of Canada supported with funds provided by the Terry Fox Run.
ABBREVIATIONS
- IL
interleukin
- PI 3-kinase
phosphoinositide 3-kinase
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- MAPK
mitogen-activated protein kinase
- PH
Pleckstrin homology domain
- FCS
fetal calf serum
- HA
hemagglutinin
References
- 1.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita T, Yokota T, Arai K-i, Miyajima A. EMBO J. 1995;14:266–275. doi: 10.1002/j.1460-2075.1995.tb07000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato N, Sakamaki K, Terada N, Arai K-i, Miyajima A. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corey S, Eguinoa A, Puyana-Theall K, Bolen J B, Cantley L, Mollinedo F, Jackson T R, Hawkins P T, Stephens L R. EMBO J. 1993;12:2681–2690. doi: 10.1002/j.1460-2075.1993.tb05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson S M, Jorgensen B. J Immunol. 1995;155:1660–1670. [PubMed] [Google Scholar]
- 6.Torigoe T, O’Connor R, Santoli D, Reed J C. Blood. 1992;80:617–624. [PubMed] [Google Scholar]
- 7.Nunez G, London L, Hockenbery D, Alexander M, McKearn J P, Korsmeyer S J. J Immunol. 1990;144:3602–3610. [PubMed] [Google Scholar]
- 8.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 9.Gold M R, Duronio V, Saxena S P, Schrader J W, Aebersold R. J Biol Chem. 1994;269:5403–5412. [PubMed] [Google Scholar]
- 10.Scheid M P, Lauener R W, Duronio V. Biochem J. 1995;312:159–162. doi: 10.1042/bj3120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Viciana P, Warne P H, Dhand R, Vanhaesebroeck B, Gout I, Fry M J, Waterfield M D, Downward J. Nature (London) 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 13.Chung J, Grammer T C, Lemon K P, Kazlauskas A, Blenis J. Nature (London) 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter C L, Cantley L C. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 15.Franke T F, Yang S-I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 16.Burgering B M T, Coffer P J. Nature (London) 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 17.Kohn A D, Takeuchi F, Roth R A. J Biol Chem. 1996;271:21920–21926. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 18.Bellacosa A, Franke T F, Gonzales-Portal M E, Datta K, Taguchi T, Gardner J, Cheng J Q, Testa J R, Tsichlis P N. Oncogene. 1993;8:745–754. [PubMed] [Google Scholar]
- 19.Coffer P J, Woodgett J R. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- 20.Jones P F, Jakubowicz T, Pitossi F J, Maurer F, Hemmings B A. Proc Natl Acad Sci USA. 1991;88:4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke T F, Kaplan D R, Cantley L C, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 22.Klippel A, Kavanaugh W M, Pot D, Williams L T. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S I, Kikkawa U. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frech M, Andjelkovic M, Ingley E, Reddy K K, Falck J R, Hemmings B A. J Biol Chem. 1997;272:8474–8481. doi: 10.1074/jbc.272.13.8474. [DOI] [PubMed] [Google Scholar]
- 25.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 26.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 27.Kulik G, Klippel A, Weber M J. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 29.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Nature (London) 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja A, Rodriguez-Viciana P, Wennström S, Warne P H, Downward J. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staal S P, Hartley J W, Rowe W P. Proc Natl Acad Sci USA. 1977;74:3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koyasu S, Nakauchi H, Kitamura K, Yonehara S, Okumura K, Tada T, Yahara I. J Immunol. 1985;134:3130–3136. [PubMed] [Google Scholar]
- 34.Ahmed N N, Franke T F, Bellacosa A, Datta K, Gonzales-Portal M-E, Taguchi T, Testa J R, Tsichlis P N. Oncogene. 1993;8:1957–1963. [PubMed] [Google Scholar]
- 35.Datta K, Franke T F, Chan T O, Makris A, Tang S-I, Kaplan D R, Morrison D K, Golemis E A, Tsichlis P N. Mol Cell Biol. 1995;15:2304–2310. doi: 10.1128/mcb.15.4.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesner P W, Winters T R, Green S H. J Cell Biol. 1992;119:1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cross D A E, Alessi D R, Cohen P, Andjelkovic M, Hemmings B A. Nature (London) 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 40.Collins M K, Marvel J, Malde P, Lopez-Rivas A. J Exp Med. 1992;176:1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohn A D, Kovacina K S, Roth R A. EMBO J. 1995;14:4288–4295. doi: 10.1002/j.1460-2075.1995.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito T, Deng X, Carr B, May W S. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 43.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 44.Golstein P. Science. 1997;275:1081–1082. doi: 10.1126/science.275.5303.1081. [DOI] [PubMed] [Google Scholar]
- 45.Gajewski T F, Thompson C B. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- 46.Peifer M. Science. 1997;272:974–975. doi: 10.1126/science.272.5264.974. [DOI] [PubMed] [Google Scholar]
- 47.Bellacosa A, de Feo D, Godwin A K, Bell D W, Cheng J Q, Altomare D A, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa J R. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 48.Cheng J Q, Godwin A K, Bellacosa A, Taguchi T, Franke T F, Hamilton T C, Tsichlis P N, Testa J R. Proc Natl Acad Sci USA. 1992;89:9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damen J E, Liu L, Rosten P, Humphries R K, Jefferson A B, Majerus P W, Krystal G. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guilherme A, Klarlund J K, Krystal G, Czech M P. J Biol Chem. 1996;271:29533–29536. doi: 10.1074/jbc.271.47.29533. [DOI] [PubMed] [Google Scholar]
- 51.Jackson S P, Schoenwaelder S M, Matzaris M, Brown S, Mitchell C A. EMBO J. 1995;14:4490–4500. doi: 10.1002/j.1460-2075.1995.tb00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kavanaugh W M, Pot D A, Chin S M, Deuter-Reinhard M, Jefferson A B, Norris F A, Masiarz F R, Cousens L S, Majerus P W, Williams L T. Curr Biol. 1996;6:438–445. doi: 10.1016/s0960-9822(02)00511-0. [DOI] [PubMed] [Google Scholar]
- 53.McPherson P S, Garcia E P, Slepnev V I, David C, Zhang X, Grabs D, Sossin W S, Bauerfeind R, Nemoto Y, De Camilli P. Nature (London) 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 54.Woscholski R, Waterfield M D, Parker P J. J Biol Chem. 1995;270:31001–31007. doi: 10.1074/jbc.270.52.31001. [DOI] [PubMed] [Google Scholar]
- 55.Liu L, Damen J E, Ware M D, Krystal G. J Biol Chem. 1997;272:10998–11001. doi: 10.1074/jbc.272.17.10998. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Jefferson A B, Zhang X, Norris F A, Majerus P W, Krystal G. J Biol Chem. 1996;271:29729–29733. doi: 10.1074/jbc.271.47.29729. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Damen J E, Hughes M R, Babic I, Jirik F R, Krystal G. J Biol Chem. 1997;272:8983–8988. doi: 10.1074/jbc.272.14.8983. [DOI] [PubMed] [Google Scholar]