Abstract
Many food products, particularly fruits and vegetables, contain natural products that affect biotransformation enzymes. These may be expected to affect the rate of biotransformation of PCBs that are metabolized by the affected enzymes. The first step in PCB metabolism is cytochrome P450-dependent monooxygenation. Natural products present in cruciferous vegetables have been shown to selectively up-regulate CYP1A1 and CYP1A2 isozymes on chronic ingestion, and may lead to increased metabolism of those PCB congeners that are substrates for the induced P450s. On the other hand, several natural products selectively inhibit monooxygenation, especially in the intestine, and may lead to increased bioavailability and reduced metabolism of dietary PCBs. Food natural products are known to affect phase II pathways important in the detoxication of hydroxylated PCBs, namely UDP-glucuronosyltransferase and PAPS-sulfotransferase. Continual dietary exposure to chrysin and quercetin, found in fruits and vegetables, induces UGT1A1 and may reduce exposure to hydroxylated PCBs through increased glucuronidation. These and other natural products are also inhibitors of glucuronidation and sulfonation, potentially leading to transient decreases in the elimination of hydroxylated PCBs. In summary, the expected effects of food natural products on PCB biotransformation are complex and may be biphasic, with initial inhibition followed by enhanced biotransformation through monooxygenation and conjugation pathways.
1. Introduction
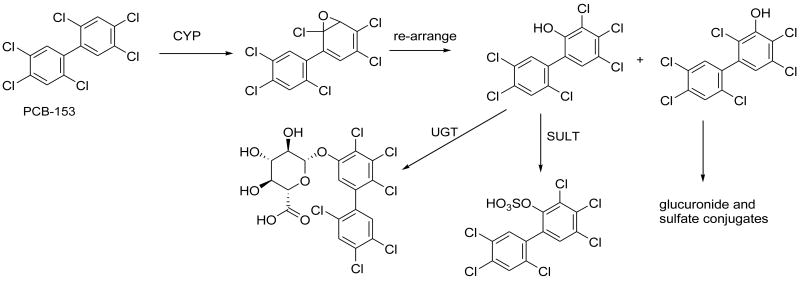
It has long been recognized that some PCB congeners are slowly biotransformed in animals, leading to their slow clearance from the body (Letcher et al., 2000; James, 2001), while other congeners are quite rapidly metabolized and excreted, especially in animals with induced biotransformation enzymes (Yoshimura et al., 1987). As well as being important for clearance of PCBs from the body (Mathews and Anderson, 1975), the biotransformation of PCBs is of interest because of its effect on toxicity. Four classes of PCB metabolites, polychlorobiphenylols (OH-PCBs), PCB-epoxides, PCB-catechols and PCB-methylsulfones are considered toxic or potentially toxic due to their effects on biological systems. The pathways leading to these metabolites may be impacted by exposure to dietary components such as phytochemicals, and well as the composition of the diet. This paper will focus on the effects of food natural products on the formation of OH-PCBs and their detoxication by glucuronidation and sulfonation. As illustrated in figure 1 with 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB153) as an example, one or more cytochrome P450 isoforms may catalyze the formation of OH-PCBs, which could then be detoxified by glucuronidation and sulfonation, catalyzed by UDP-glucuronosyltransferases (UGTs) and PAPS-sulfotransferases (SULTs).
Figure 1.
Biotransformation of PCB-153 to hydroxylated and conjugated metabolites. Other metabolites may arise from the intermediate epoxide, but these are not the focus of this paper.
2.1 Formation and properties of polychlorobiphenylols (OH-PCBs)
The only enzymes known to form OH-PCBs are cytochromes P450 (P450). The OH-PCB can be formed from re-arrangement of an epoxide (Fig 1), or direct insertion of oxygen into the C-H bond. In general more highly chlorinated PCBs are hydroxylated more slowly than PCBs with few chlorine substituents (Mathews and Anderson, 1975), although the ring positions of chlorine substituents also influence the rate of P450-dependent monooxygenation. There have been studies of the isoform-specific biotransformation of PCB congeners, particularly by the major Phenobarbital-inducible CYP2B isoforms and 3-methylcholanthrene-inducible CYP1A isoforms, e.g. (Kaminsky et al., 1981). Human CYP2B6 and rat CYP2B1 were the major isoforms for metabolism of PCB153 (Duignan et al., 1987; Ariyoshi et al., 1992), shown in figure 1, although it should be noted that PCB153 is relatively resistant to metabolism, and is therefore one of the more biologically persistent PCB congeners (Letcher et al., 2000).
Analysis of blood and tissues of PCB-exposed people and animals has revealed the presence of not only parent PCBs, but also metabolites, including OH-PCBs (Sandau et al., 2000), which are potentially toxic. OH-PCBs with a 4-hydroxy-3,5-dichloro substitution pattern in one ring, and one or more chlorine atoms in the other ring are retained in blood, bound to thyroid hormone binding protein (Bergman et al., 1994). Depending on the exact structural features, OH-PCB congeners may interact directly with estrogen receptors, as agonists or antagonists, or they may inhibit estrogen sulfotransferase and be indirect endocrine disruptors (Kester et al., 2000; Arulmozhiraja et al., 2005). Some OH-PCBs interact with thyroid hormone binding proteins and thyroxine sulfotransferase (Schuur et al., 1998), and some OH-PCBs inhibit glucuronidation and sulfonation of hydroxylated xenobiotics (van den Hurk et al., 2002; Wang et al., 2005; Wang and James, 2006; Wang et al., 2006). OH-PCBs also affect intercellular communication (Machala et al., 2004).
2.2 Effects of food natural products on cytochrome P450 concentrations
Alteration of P-450 enzyme concentrations in liver and other organs, through exposure to agents that up- or down-regulate synthesis of P450 isozymes, is likely to affect the formation of OH-PCBs. Studies in animals have shown that natural products present in food and herbal drugs will induce synthesis of P450 isoforms in the 1, 2 and 3 families (Table 1). Phytochemicals known to induce CYP1A1 and CYP1A2 synthesis include isothiothiocyanates, indole-3-carbinol and its condensation products (including di-indolyl-methane), flavonoids and tea polyphenolic compounds (Gross-Steinmeyer et al., 2004; Moon et al., 2006). The herbal drug, St. John's wort, which has the active component, hyperforin, induces hepatic CYP3A4 (Komoroski et al., 2004). Interestingly, many of the phytochemicals that upon chronic administration upregulate P450 synthesis will acutely inhibit P450, through binding of the natural product or its metabolite to P450 (Moon et al., 2006).
Table 1.
Examples of food natural products known to affect biotransformation pathways.
| Compound | Food sources | Effect | Reference: |
|---|---|---|---|
| Chrysin | Fruits, Honey | Induce CYP1A1
Induce UGT1A6 Inhibit UGT |
Moon et al., 2006
Galijatovic et al., 2000 Mizuma and Awazu, 2004 |
| Curcumin | Turmeric spice | Induce CYP1A1
Inhibit CYP1A1 Induce UGT1A1 and UGT1A6 |
Gross-Steinmeyer et al., 2004
Thapliyal and Maru, 2001 Naganuma et al., 2006 |
| 3,3′-Di-indolylmethane | Cruciferous vegetables (cabbage, broccoli, cauliflower, Brussels sprouts) | Inhibit CYP1A1
Induce CYP1A1 |
Gross-Steinmeyer et al., 2004 |
| Bergamottin, 6′,7′-Di-hydroxy-bergamottin | Grapefruit | Inhibit CYP3A4 | Dahan and Altman 2004 |
| Epigallocatechin gallate | Tea | Induce UGT
Inhibit UGT |
Xhu et al., 1998
Mizuma and Awazu, 2004 |
| Epicatechin-3-gallate | |||
| Genistein | Soybeans, peas, alfalfa | Inhibit CYP1A2,
CYP2E1, CYP3A4 Inhibit SULT1A1 and SULT1E1 |
Moon et al., 2006
Wang and James, 2006 |
| Hyperforin | St. John's Wort | Induce CYP3A4 | Komoroski et al., 2004 |
| Phenyl isothiocyanate | Cruciferous vegetables | Induce CYP1A1
Induce CYP1A2 Inhibit several CYP isoforms |
Gross-Steinmeyer et al., 2004
Nakajima et al., 2001 |
| Quercetin | Onions, apples, other fruits and vegetables | Induce CYP1A1
Inhibit CYP1A2, CYP3A4 Induce UGT Inhibit UGT1A1 Inhibit SULT1A1 and SULT1E1 |
Moon et al., 2006
Galijatovic et al., 2000 Zhu et al., 1998 Wang and James, 2006 |
With respect to PCB metabolism, the toxicological significance of induction of P450 enzymes by phytochemicals is not clear. As noted above, OH-PCBs are toxic metabolites of PCBs, so it may be expected that increased formation of these metabolites would lead to increased toxicity. However, most phytochemical inducing agents are themselves poorly bioavailable (Manach et al., 2005), and of weak potency as inducers, so that quite large quantities, taken chronically, are needed to produce effects (Walle, 2004). Exceptions are diindolyl-methane and St. John's wort, which have been demonstrated to increase expression of CYP1A1/2 and CYP3A4, respectively, at normally ingested concentrations of the food or herb (Table 1). Another important point is that while induction of P450 will lead to increased formation of OH-PCBs, the same phytochemicals that induce P450 also induce UDP-glucuronosyltransferases (UGTs), which catalyze the detoxication of OH-PCBs by glucuronidation (Zhu et al., 1998; Galijatovic et al., 2000).
2.3 Direct effects on P450 activity
Several phytochemicals have been shown through in vitro studies to inhibit or stimulate P450s in an isozyme-selective fashion. For example, alpha-naphthoflavone inhibits CYP1A1 and CYP1A2, but stimulates CYP3A4 (Slaga et al., 1977; Emoto et al., 2001). Quercetin inhibits CYP1A1 (Moon et al., 2006). Furanocoumarins present in grapefruit juice, such 6′,7′-dihydroxybergamottin, are potent inhibitors of CYP3A4 (Dahan and Altman, 2004). These effects have been demonstrated with substrates other than PCBs, but would be expected to apply to PCB metabolism.
Inhibition of P450 isozymes involved in PCB metabolism could have toxicological significance, in that reduced monooxygenation would result in reduced elimination of PCBs from the body. If this happens, effects that are attributed to the parent compounds may be more pronounced. The intestine is the most likely site in the body for effects of phytochemical inhibitors of the P450-dependent monooxygenation of PCBs to occur. If food products such as fish or shellfish taken from PCB-contaminated environments are consumed at the same time as grapefruit juice or vegetables such as onions that contain inhibitory phytochemicals, the intestinal P450s are likely to be inhibited, and intestinal first-pass metabolism reduced. However, although the intestine has been shown to metabolize PCBs (Doi et al., 2000), it is not considered a major site of PCB metabolism. It is likely that only a small amount of the ingested dose of PCBs will be subject to intestinal first-pass metabolism, and thus inhibition of this already small amount of monooxygenation should result in little effect of co-ingested phytochemicals on the bioavailability of the PCBs. Studies have shown that the effects of grapefruit juice on drug bioavailability are due to effects only in the intestine (Culm-Merdek et al., 2006), since the systemic bioavailability of bergamottin, 6′,7′-dihydroxybergamottin and other inhibitory natural products is quite low, and the concentrations achieved in liver are too low for inhibition of hepatic biotransformation. Most phytochemicals, including flavonoids, furanocoumarins and anthocyanins are poorly bioavailable, in part because of extensive glucuronidation and sulfonation of the phytochemicals (Walle, 2004). Therefore, the levels of active chemicals taken up from food into the liver, the major site of PCB metabolism, are unlikely to be high enough to inhibit metabolism. Even if there were sufficient absorption for inhibition in the liver, the effect would be transient, since most phytochemicals are rapidly excreted with elimination half-lives of 2-8 hr (Manach et al., 2005). An exception is quercetin, the most abundant flavonoid in many foods (Hertog et al., 1992): although it is incompletely absorbed, quercetin has an elimination half-life of 10 to 20 hr, suggesting the possibility of accumulation with repeated ingestion (Manach et al., 2005).
3. Metabolism of OH-PCBs
The major pathways for metabolism of OH-PCBs are further P450-dependent monooxygenation and phase II conjugation. The P450-dependent metabolism of OH-PCBs leads to the formation of potentially toxic catechols, hydroquinones and quinones, and these pathways will be susceptible to the same influences as those discussed above for primary metabolism of PCBs to OH-PCBs. The major phase II pathways for OH-PCB conjugation are glucuronidation, catalyzed by UGTs, and sulfonation, catalyzed by PAPS-sulfotransferases (SULTs). Glucuronide and sulfate conjugates of OH-PCBs are likely to be excreted in feces and urine and removed from the body, and are therefore considered to be detoxication pathways.
3.1 Glucuronidation
The rate of glucuronidation of a particular OH-PCB depends on the tissue concentrations of the OH-PCB and the co-substrate for glucuronidation, UDP-β-D-glucuronic acid (UDPGA), as well as the UGT isoforms present. Glucuronidation of several OH-PCBs has been studied in rat liver microsomes, with expressed human UGTs and in catfish intestinal and hepatic microsomes (Tampal et al., 2002; Daidoji et al., 2005; Sacco, 2006).
Substrate kinetics for the glucuronidation of selected OH-PCBs in rat liver microsomes under saturating conditions of UDPGA (3-4 mM) are shown in Table 2. One study (Tampal et al., 2002) found apparent Km values for OH-PCB between 0.1 and 0.27 mM and Vmax from 0.31 to 31.3 nmol/min/mg, while another (Daidoji et al., 2005) reported OH-PCB apparent Km values between 0.03 and 0.14 mM and Vmax from 3 to 11 nmol/min/mg. The rat studies showed a general trend towards lower Vmax values with higher numbers of Cl atoms in the OH-PCB. Another trend that emerged from comparing results in the two rat liver studies was that OH-PCBs with a 4-OH-3,5-dichloro- substitution pattern in the phenolic ring showed lower Vmax values than OH-PCBs with a 4-OH-3-chloro- substitution pattern, if the substituents in the aphenolic ring were constant (Figure 2). Other studies have shown that 4-OH-3,5,-dichloro-OH-PCBs are retained in the body longer than OH-PCBs with other substitution patterns (Bergman et al., 1994). The retention has been attributed to selective binding of 4-OH-3,5,-dichloro-OH-PCBs to transthyretin in the blood, however it is possible that slow glucuronidation contributes to the retention. It is noteworthy that UDPGA concentrations of >2mM were needed for maximal rates of glucuronidation in rat hepatic microsomes. The apparent Km and Vmax values reported in Table 2 were obtained with UDPGA concentrations of 3 mM (Daidoji et al., 2005) or 4 mM (Tampal et al., 2002). Hepatic concentrations of UDPGA in rats are 0.2–0.4 mM (Goon and Klaassen, 1992), thus, actual rates of glucuronidation of OH-PCBs in rat liver are likely to be lower than the Vmax values.
Table 2.
Glucuronidation of OH-PCBs in rat liver microsomes. Values taken from Tampal et al., 2002 and Daidoji et al., 2005.
| Compound | Name | Rat liver microsomes | ||
|---|---|---|---|---|
| Km mM |
Vmax nmol/min/mg |
Efficiency
μL/min/mg |
||
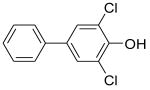
|
4-OH-CB14 | 0.27 | 31.3 | 116 |
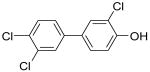
|
4′-OH-CB35 | 0.09 | 8.4 | 93 |
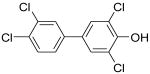
|
4′-OH-CB79 | 0.09 | 2.1 | 23 |
|
4′-OH-CB121 | 0.07 | 4.5 | 68 |
|
4-OH-CB146 | 0.2 | 0.62 | 3 |
|
4-OH-CB187 | 0.13 | 0.31 | 2 |
Values shown are means, obtained from studies with n = 3 different rat livers.
Figure 2.
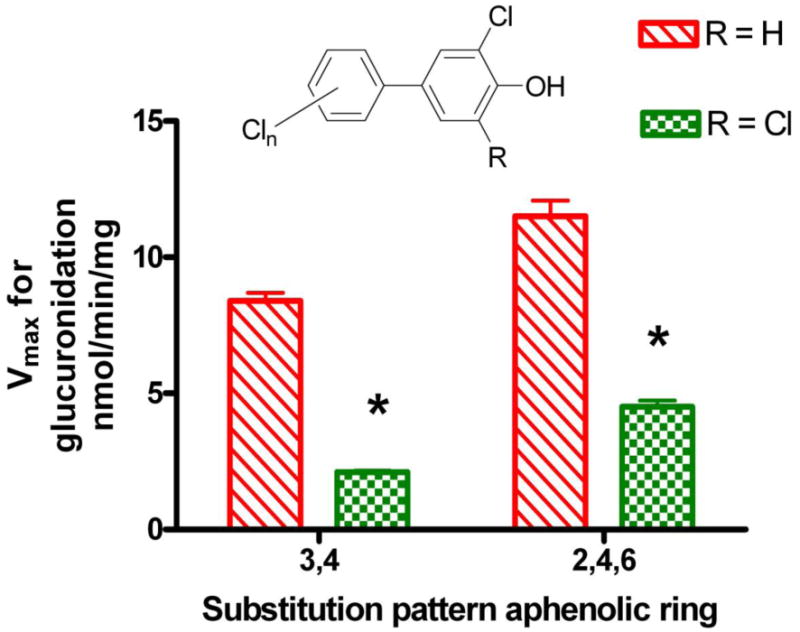
Influence of a second flanking chlorine atom on the maximal rates of glucuronidation of polychlorobiphenylols in rat liver microsomes. Values taken from (Tampal et al., 2002) and (Daidoji et al., 2005).
In the channel catfish, studies were conducted with intestinal and hepatic microsomes, under saturating conditions of UDPGA. The properties of channel catfish UGTs were less favorable than those of the rat UGTs for glucuronidation of OH-PCB. In both intestine and liver, apparent Km values for the OH-PCBs were higher than found with rat liver, and Vmax values were lower. Under saturating conditions of UDPGA, Vmax was higher in catfish hepatic than intestinal microsomes. The enzymatic efficiencies (Vmax/Km) in catfish liver or intestinal microsomes were one to two orders of magnitude lower than reported in the two rat studies (Sacco, 2006). In the catfish, the apparent Km values for UDPGA were considerably lower with intestinal microsomes (0.03 mM) than with hepatic microsomes (0.67 mM) (Sacco et al., in preparation).
The measured physiological concentrations of UDPGA in channel catfish liver, proximal and distal intestine were determined in the spring, with catfish that were fed a commercial chow diet or a nutritionally complete semi-purified diet (NRC, 1983), using a direct chromatographic method (Sacco, 2006). The results demonstrate that UDPGA concentrations in liver and distal intestine were not affected by the type of diet, but UDPGA concentrations in proximal intestine were higher (p<0.05) in catfish fed the chow diet (Figure 3). Average hepatic UDPGA concentrations were 133 ± 33 nmol/g liver (mean ± S.D., n=8 ), or roughly 0.15 mM, a concentration that was well below the UDPGA apparent Km value. We may expect that in the catfish liver, glucuronidation of OH-PCBs will be relatively inefficient, both because the Km values for the OH-PCBs are much higher than expected environmental concentrations of these metabolites, and because the physiological concentration of UDPGA, the required co-substrate, is considerably lower than the concentration needed for optimal activity.
Figure 3.
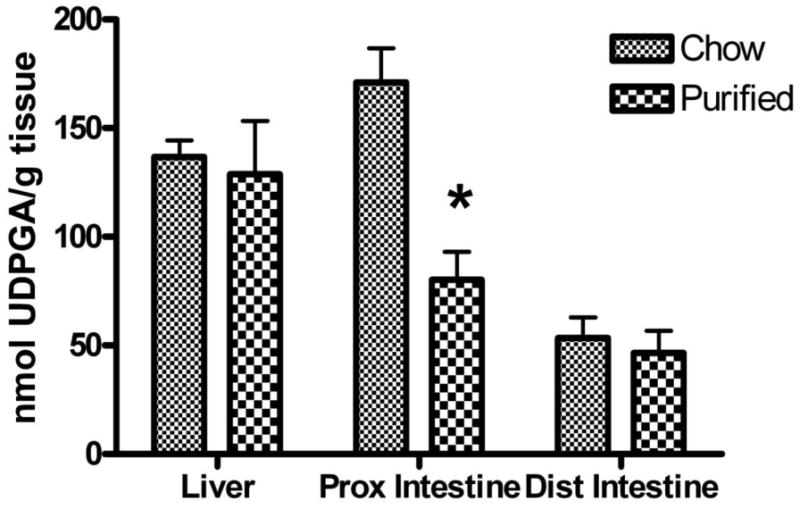
Concentrations of UDP-glucuronic acid (UDPGA) in channel catfish liver, proximal intestine and distal intestine. The catfish were fed a commercial chow diet (Silvercup trout chow, Utah), or a nutritionally complete semi-purified diet (NRC, 1983) for three weeks before sacrifice. Values shown are mean ± S.D., n=4 and * indicates a significant effect of diet, p<0.01 on UDPGA concentrations in proximal intestine.
In the proximal intestine, however, the measured concentrations of UDPGA on either of the studied diets were above the apparent Km for UDPGA. The chow diet, shown previously to contain inducers of CYP1A (James et al., 1997), was associated with two-fold higher concentrations of UDPGA than the reference diet, for reasons that are as yet unclear.
3.1.1 Induction of UGT by food natural products
Several studies have reported upregulation of UGTs following ingestion or exposure to food natural products. In one study mice and rats were given a solution of lyophilized green tea in water, containing 5 or 10 mg tea solids per ml, as the sole source of drinking fluids for 18 days (Zhu et al., 1998). The concentration used is two to three times that in brewed green tea, which contains approximately 3 mg tea solids per ml. This sub-chronic ingestion of tea polyphenols was found to induce hepatic UGTs, and the glucuronidation of p-nitrophenol and estrone substrates (Zhu et al., 1998). Analysis showed that the major components of the tea solids were epicatechin, epigallocatechin and their gallate esters. The bioavailability of the tea polyphenols was not studied, but the results showed that enough was absorbed to have an effect on glucuronidation. Exposure of CACO-2 cells to the flavonoids chrysin and quercetin (10 μM) resulted in upregulation of UGT1A1 and perhaps other UGTs, as well as increased glucuronidation of chrysin (Galijatovic et al., 2000; Walle, 2004). Foods such as onions, kale, broccoli, beans, apples and cherries contain quercetin in concentrations ranging from 0.03 to 0.35 mg per g fresh vegetable or fruit (Hertog et al., 1992), concentrations that are sufficient to cause the effects observed in cell culture. The effects of food natural products on OH-PCB glucuronidation have not been studied, but OH-PCBs that are substrates for UGT1A1, or other isoforms induced by these natural products, should be more rapidly glucuronidated following exposure to flavonoids. To date, only one study has published information on the specific UGT isoforms that metabolize OH-PCBs (Daidoji et al., 2005). Expressed human UGT2B1 metabolized eight of the eleven OH-PCBs studied, UGT1A6 metabolized three of the eleven and UGT1A1 catalyzed glucuronidation of ten of the eleven OH-PCBs. UGT1A5 and UGT1A7 had very low or no activity with these eleven OH-PCBs. Since UGT1A1 could metabolize most of the OH-PCBs examined, it is possible that chronic exposure to flavonoids or tea polyphenols could result in more rapid glucuronidation of OH-PCBs, and reduce exposure to these potentially toxic PCB metabolites.
3.1.2 Inhibition of glucuronidation by natural products
As is the case with cytochrome P450, acute exposure to flavonoids and polyphenols results in direct inhibition of glucuronidation, while chronic exposure results in up-regulation of UGTs, provided a sufficient dose is absorbed. The synthetic compound, beta-naphthoflavone, inhibited glucuronidation of 3-OH-BaP (James et al., 1997), and naturally occurring tea polyphenols and flavonoids were inhibitors of the glucuronidation of p-nitrophenol and estrone with IC50 values in the low μM range (Zhu et al., 1998; Mizuma and Awazu, 2004). Flavonoids and related natural products may acutely inhibit OH-PCB glucuronidation, particularly in the intestine, where concentrations will be highest after food consumption, and may therefore increase bioavailability and exposure to OH-PCBs present in foodstuffs.
3.2 Sulfonation
An alternate pathway for elimination of the potentially toxic OH-PCBs is sulfate conjugation. Five OH-PCBs were examined for their properties as substrates of human hepatic cytosolic sulfotransferases, and only three of these, 3′OH-CB3, 4′-OH-CB3 and 4′OH-CB112 had measurable rates of sulfate conjugation at low concentrations of the OH-PCB substrate (Wang et al., 2006). Recent studies showed that 4-OH-CB34 and 4′OH-CB68 but not 4′OH-CB9 were substrates for expressed human SULT2A1 (Liu et al., 2006). In rat hepatocytes (Daidoji et al., 2005), there was less sulfonation than glucuronidation of 4′-OH-2,4,6-trichlorobiphenyl (4′OH-CB30) and 4′OH-2,3,4,5-tetrachlorobiphenyl (4′OH-CB61). While further study is needed, particularly with human liver, it seems likely that sulfonation is a minor pathway of elimination of OH-PCBs, as compared with glucuronidation.
3.2.1 Inhibition of sulfonation
Several sulfotransferase enzymes are quite susceptible to inhibition by natural products. As recently reviewed (Wang and James, 2006), studies have shown that SULT1A1 and SULT1A3 are potently inhibited by natural products present in juices (apple, grape, grapefruit), teas (green and black) and coffee. As discussed above, it is likely that the effects of ingestion of beverages that contain inhibitors of sulfotransferase will be more pronounced in the intestine than the liver, due to the relatively poor bioavailability of polyhydroxylated natural products, however as yet, the effect of sulfotransferase inhibition on the bioavailability of phenolic compounds, such as OH-PCBs, has not been studied. Quercetin, found in many fruits, vegetables and wine, was a potent inhibitor of the sulfation of p-nitrophenol, estradiol, and several drug substrates in human liver cytosol and with expressed human SULT1A1, with IC50 values below 1 μM. Quercetin was a less potent inhibitor of SULT1E1 (IC50 about 1 μM) and only a weak inhibitor (IC50 64 μM) of SULT2A1 activity (Wang and James, 2006). If further studies show that SULT2A1 is the major enzyme that catalyzes sulfonation of OH-PCBs, as suggested by the studies of Liu et al (2006), it is possible that foods containing quercetin will have little effect on their sulfonation.
4. Summary
In summary, food natural products are known to affect at least three enzyme families involved in the biotransformation of PCBs and their phase I metabolites, namely P450, UGT and SULT. Induction of P450-dependent monooxygenation of PCBs by food components may lead to more rapid formation of potentially toxic metabolites such as OH-PCBs, however the same food components often induce UGTs, which detoxify the OH-PCBs. Inhibition of biotransformation enzymes by polyhydroxylated food natural products is most likely to occur in the intestine, and may increase bioavailability of PCBs and OH-PCBs present in the diet. Further studies are warranted to determine if upregulation of biotransformation enzymes by daily exposure to food natural products will increase the elimination of PCBs and OH-PCBs.
Acknowledgments
The authors' work discussed in this paper was supported in part by 1-P42-ES-07375 and 1-P42-ES-13661.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariyoshi N, Koga N, Oguri K, Yoshimura H. Metabolism of 2,4,5,2′,4′,5′-hexachlorobiphenyl with liver microsomes of phenobarbital-treated dog; the possible formation of PCB 2,3-arene oxide intermediate. Xenobiotica. 1992;22:1275–1290. doi: 10.3109/00498259209053156. [DOI] [PubMed] [Google Scholar]
- Arulmozhiraja S, Shiraishi F, Okumura T, Iida M, Takigami H, Edmonds JS, Morita M. Structural requirements for the interaction of 91 hydroxylated polychlorinated biphenyls with estrogen and thyroid hormone receptors. Toxicol Sci. 2005;84:49–62. doi: 10.1093/toxsci/kfi063. [DOI] [PubMed] [Google Scholar]
- Bergman A, Klasson-Wehler E, Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culm-Merdek KE, von Moltke LL, Gan L, Horan KA, Reynolds R, Harmatz JS, Court MH, Greenblatt DJ. Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther. 2006;79:243–254. doi: 10.1016/j.clpt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability--mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1–9. doi: 10.1038/sj.ejcn.1601736. [DOI] [PubMed] [Google Scholar]
- Daidoji T, Gozu K, Iwano H, Inoue H, Yokota H. UDP-glucuronosyltransferase isoforms catalyzing glucuronidation of hydroxy-polychlorinated biphenyls in rat. Drug Metab Dispos. 2005;33:1466–1476. doi: 10.1124/dmd.105.004416. [DOI] [PubMed] [Google Scholar]
- Doi AM, Lou Z, Holmes E, Li C, Venugopal CS, James MO, Kleinow KM. Effect of micelle fatty acid composition and 3,4,3′, 4′-tetrachlorobiphenyl (TCB) exposure on intestinal [(14)C]-TCB bioavailability and biotransformation in channel catfish in situ preparations. Toxicol Sci. 2000;55:85–96. doi: 10.1093/toxsci/55.1.85. [DOI] [PubMed] [Google Scholar]
- Duignan DB, Sipes IG, Leonard TB, Halpert JR. Purification and characterization of the dog hepatic cytochrome P-450 isozyme responsible for the metabolism of 2,2′,4,4′,5,5′-hexachlorobiphenyl. Arch Biochem Biophys. 1987;255:290–303. doi: 10.1016/0003-9861(87)90396-1. [DOI] [PubMed] [Google Scholar]
- Emoto C, Yamazaki H, Iketaki H, Yamasaki S, Satoh T, Shimizu R, Suzuki S, Shimada N, Nakajima M, Yokoi T. Cooperativity of alpha-naphthoflavone in cytochrome P450 3A-dependent drug oxidation activities in hepatic and intestinal microsomes from mouse and human. Xenobiotica. 2001;31:265–275. doi: 10.1080/00498250110052120. [DOI] [PubMed] [Google Scholar]
- Galijatovic A, Walle UK, Walle T. Induction of UDP-glucuronosyltransferase by the flavonoids chrysin and quercetin in Caco-2 cells. Pharm Res. 2000;17:21–26. doi: 10.1023/a:1007506222436. [DOI] [PubMed] [Google Scholar]
- Goon D, Klaassen CD. Effects of microsomal enzyme inducers upon UDP-glucuronic acid concentration and UDP-glucuronosyltransferase activity in the rat intestine and liver. Toxicol Appl Pharmacol. 1992;115:253–260. doi: 10.1016/0041-008x(92)90330-u. [DOI] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Stapleton PL, Liu F, Tracy JH, Bammler TK, Quigley SD, Farin FM, Buhler DR, Safe SH, Strom SC, Eaton DL. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004;34:619–632. doi: 10.1080/00498250412331285481. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. [Google Scholar]
- James MO. Polychlorinated biphenyls: metabolism and metabolites. In: Robertson LW, Hansen LG, editors. PCBs- Recent Advances in Environmental Toxicology and Health Effects. University of Kentucky Press; Lexington: 2001. pp. 35–46. [Google Scholar]
- James MO, Altman AH, Morris K, Kleinow KM, Tong Z. Dietary modulation of phase 1 and phase 2 activities with benzo(a)pyrene and related compounds in the intestine but not the liver of the channel catfish, Ictalurus punctatus. Drug Metab Dispos. 1997;25:346–354. [PubMed] [Google Scholar]
- Kaminsky LS, Kennedy MW, Adams SM, Guengerich FP. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry. 1981;20:7379–7384. doi: 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141:1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Komoroski BJ, Zhang S, Cai H, Hutzler JM, Frye R, Tracy TS, Strom SC, Lehmann T, Ang CY, Cui YY, Venkataramanan R. Induction and inhibition of cytochromes P450 by the St. John's wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos. 2004;32:512–518. doi: 10.1124/dmd.32.5.512. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Wehler EK, Bergman A. Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasiverta J, editor. New types of persistent halogenated compounds. Springer-Verlag; Berlin: 2000. pp. 317–157. [Google Scholar]
- Liu Y, Apak TI, Lehmler HJ, Robertson LW, Duffel MW. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol. 2006;19:1420–1425. doi: 10.1021/tx060160+. [DOI] [PubMed] [Google Scholar]
- Machala M, Blaha L, Lehmler HJ, Pliskova M, Majkova Z, Kapplova P, Sovadinova I, Vondracek J, Malmberg T, Robertson LW. Toxicity of hydroxylated and quinoid PCB metabolites: inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem Res Toxicol. 2004;17:340–347. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Mathews HB, Anderson MW. Effect of chlorination on the distribution and excretion of polychlorinated biphenyls. Drug Metab Dispos. 1975;3:371–380. [PubMed] [Google Scholar]
- Mizuma T, Awazu S. Dietary polyphenols (-)-epicatechin and chrysin inhibit intestinal glucuronidation metabolism to increase drug absorption. J Pharm Sci. 2004;93:2407–2410. doi: 10.1002/jps.20146. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Naganuma M, Saruwatari A, Okamura S, Tamura H. Turmeric and curcumin modulate the conjugation of 1-naphthol in Caco-2 cells. Biol Pharm Bull. 2006;29:1476–1479. doi: 10.1248/bpb.29.1476. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–1113. [PubMed] [Google Scholar]
- NRC. Nutrient Requirements of Warm-water Fishes and Shellfishes, National Research Council. National Academy Press; Washington, D.C.: 1983. [Google Scholar]
- Sacco JC. Phase II biotransformation of xenobiotics in polar bear (Ursus maritimus) and channel catfish (Ictalurus punctatus) University of Florida; United States -- Florida: 2006. [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, Norstrom RJ. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian inuit. Environ Health Perspect. 2000;108:611–616. doi: 10.1289/ehp.00108611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuur AG, Brouwer A, Bergman A, Coughtrie MW, Visser TJ. Inhibition of thyroid hormone sulfation by hydroxylated metabolites of polychlorinated biphenyls. Chem Biol Interact. 1998;109:293–297. doi: 10.1016/s0009-2797(97)00140-3. [DOI] [PubMed] [Google Scholar]
- Slaga TJ, Thompson S, Berry DL, Digiovanni J, Juchau MR, Viaje A. The effects of benzoflavones on polycyclic hydrocarbon metabolism and skin tumor initiation. Chem Biol Interact. 1977;17:297–312. doi: 10.1016/0009-2797(77)90093-x. [DOI] [PubMed] [Google Scholar]
- Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) Chem Res Toxicol. 2002;15:1259–1266. doi: 10.1021/tx0200212. [DOI] [PubMed] [Google Scholar]
- Thapliyal R, Maru GB. Inhibition of cytochrome P450 isozymes by curcumins in vitro and in vivo. Food Chem Toxicol. 2001;39:541–547. doi: 10.1016/s0278-6915(00)00165-4. [DOI] [PubMed] [Google Scholar]
- van den Hurk P, Kubiczak GA, Lehmler HJ, James MO. Hydroxylated polychlorinated biphenyls as inhibitors of the sulfation and glucuronidation of 3-hydroxy-benzo[a]pyrene. Environ Health Perspect. 2002;110:343–348. doi: 10.1289/ehp.02110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;36:829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Wang LQ, James MO. Inhibition of sulfotransferases by xenobiotics. Curr Drug Metab. 2006;7:83–104. doi: 10.2174/138920006774832596. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, Falany CN, James MO. In vitro inhibition of human hepatic and cDNA-expressed sulfotransferase activity with 3-hydroxybenzo[a]pyrene by polychlorobiphenylols. Environ Health Perspect. 2005;113:680–687. doi: 10.1289/ehp.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LQ, Lehmler HJ, Robertson LW, James MO. Polychlorobiphenylols are selective inhibitors of human phenol sulfotransferase 1A1 with 4-nitrophenol as a substrate. Chem Biol Interact. 2006;159:235–246. doi: 10.1016/j.cbi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Yonemoto Y, Yamada H, Koga N, Oguri K, Saeki S. Metabolism in vivo of 3,4,3′,4′-tetrachlorobiphenyl and toxicological assessment of the metabolites in rats. Xenobiotica. 1987;17:897–910. doi: 10.3109/00498258709044189. [DOI] [PubMed] [Google Scholar]
- Zhu BT, Taneja N, Loder DP, Balentine DA, Conney AH. Effects of tea polyphenols and flavonoids on liver microsomal glucuronidation of estradiol and estrone. J Steroid Biochem Mol Biol. 1998;64:207–215. doi: 10.1016/s0960-0760(97)00163-5. [DOI] [PubMed] [Google Scholar]