Abstract
Polychlorinated biphenyl (PCB) congeners exhibit a broad range of adverse biological effects including neurotoxicity. The mechanisms by which PCBs cause neurotoxic effects are still not completely understood. The blood-brain barrier (BBB) is a physical and metabolic barrier separating brain microenvironment from the peripheral circulation and is mainly composed of endothelial cells connected by tight junctions. We examined the effects of several highly-chlorinated PCB congeners on expression of tight junction proteins in human brain endothelial cells. Treatment for 24 h with selective PCB congeners disrupted expression of the cytosolic scaffold proteins of tight junctions, such as zonula occludens (ZO)-1, ZO-2, and AF6. In contrast, PCB exposure did not alter expression of integral membrane proteins, junctional adhesion molecule-A (JAM-A), and claudin-1. Based on these data, we suggest that PCB-mediated selective alterations of tight junction protein expression may contribute to their neurotoxic effects in the central nervous system.
Keywords: Polychlorinated biphenyls, blood-brain barrier, tight junctions, zonula occludens
1. Introduction
Homeostasis of the central nervous system (CNS) microenvironment is essential for normal functions of the brain and is maintained by the blood-brain barrier (BBB). The BBB is mainly formed by highly specialized endothelial cells and it protects the brain from blood-borne substances. At the same time, the BBB ensures the supply of nutrients to the brain by specific transport systems (Abbott, 2005; Neuwelt, 2004). The BBB acts as a physical and metabolic barrier because a complex tight junction system between adjacent endothelial cells restricts most paracellular movement of ions and solutes across the brain endothelium (Pardridge, 2002). Tight junctions (TJ) of the BBB are composed of an intricate combination of at least three integral membrane proteins (claudins, junctional adhesion molecules [JAMs], and occludin) and cytoplasmic accessory proteins, such as zonula occludens (ZO)-1, ZO-2, and AF6.
ZO-1 was the first tight junction-associated protein identified and characterized among cytosolic accessory proteins of TJ (Stevenson et al., 1986). The ZO proteins belong to the family of membrane-associated guanylate kinases (MAGUK), which possess a distinct modular organization and associate peripherally with the cellular membranes. ZO-1 is essential for TJ assembly and it forms a scaffold complex with other cytosolic accessory proteins, such as ZO-2 and AF6, to anchor integral membrane proteins to actin cytoskeleton (Denker and Nigam, 1998; Wolburg and Lippoldt, 2002). Disruption of ZO-1 at TJ has been observed in response to bacterial toxins (Chen et al., 2002), drugs (Liu et al., 1999), growth factors (Hollande et al., 2001), cytokines (Blum et al., 1997), and hypoxia (Fischer et al., 2004) and correlated with increased permeability and/or decreased electrical resistance. In addition, ZO proteins are believed to form a complex for transcription factors and signaling proteins involved in regulation of cell proliferation and differentiation (Balda and Matter, 2000; Ryeom et al., 2000).
Relatively less information is available on the role of ZO-2 and AF6 in the regulation of integrity of the brain endothelium. ZO-2 shares with ZO-1 three defined core regions of MAGUK family proteins: a SH3 domain, a guanylate cyclase and a PDZ domain. SH3-domains bind signaling proteins and cytoskeletal elements. Guanylate cyclase domains are involved in the ATP-dependent transformation of GMP to GDP, and PDZ-domains mediate specific binding to carboxy-terminal cytoplasmic ends of transmembrane proteins (Wolburg and Lippoldt, 2002). ZO-2 acts as a signaling molecule to communicate the state of cell-cell contact of the TJ (Hawkins and Davis, 2005.). It appears that ZO-2 can modulate these interactions by its influence on the transcription factors c-Jun, c-Fos, and C/EBP (Betanzos et al., 2004). ZO-2 expression decreases in response to several pathological conditions, including amyloid beta treatment (Marco and Skaper, 2006), hypoxia (Fischer et al., 2004), cerebral infarction in spontaneously hypertensive rats (Hom et al., 2007), and exposure to HIV proteins, such as gp120 (Kanmogne et al., 2005) or Tat (Andras et al., 2003).
AF6 is a multidomain actin-binding protein that serves as a scaffold protein between transmembrane proteins and the actin cytoskeleton (Boettner et al., 2000). It interacts with ZO-1, nectin, Eph receptors, and the actin-regulatory protein profilin (Boettner et al., 2000; Yamamoto et al., 1997). It binds to Ras-like small GTPases and is suggested to be an effector of both Ras and Rap. It has been shown that activation of Ras can result from interaction of AF6 with ZO-1 (Yamamoto et al., 1997). AF6 may regulate adhesion of leukocytes to extracellular matrix. Indeed, overexpression of AF6 in Jurkat cells increased its adhesion to fibronectin. In contrast, knockdown of AF6 in T cells results in an enhancement of cell adhesion (Zhang et al., 2005).
Exposure to polychlorinated biphenyls (PCBs) can contribute to induction of neurological disorders including morphological changes of neurons (Carpenter, 2006), brain tumor-promoting effects (Knerr and Schrenk, 2006), and the developmental defects of the nervous system (Lee and Opanashuk, 2004; Winneke et al., 2002). However, the mechanisms by which PCBs cause these neurotoxic effects are not fully understood. Because of the role of the BBB in protecting brain environment, we have explored the hypothesis that exposure of human cerebrovascular endothelial cells to PCBs can alter expression of TJ proteins and thus impair BBB integrity.
2. Materials and Methods
2.1 Materials
PCB congeners were purchased from AccuStandard (New Haven, CT) and dissolved in dimethyl sulfoxide (DMSO). Collagen type I was obtained from BD Biosciences (Bedford, MA). Antibodies against ZO-1, claudin-1, and JAM-A were obtained from Invitrogen Zymed (San Francisco, CA). Anti-ZO-2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-AF6 antibody was obtained from BD Transduction (Lexington, KY). All other chemicals and reagents were purchased from Sigma (St. Louis, MO).
2.2 Cell cultures and PCBs treatment
Human brain endothelial cells (hCMEC/D3) are an immortalized cell line derived from a primary cell culture through co-expression of hTERT and the SV40 large T antigen. This cell line retains most of the morphological and functional characteristics of brain endothelial cells (Weksler et al., 2005). hCMEC/D3 were cultured using EBM-2 medium (Cambrex BioScience, Wokingham, UK) supplemented with growth factors and fetal bovine serum. Prior to each experiment, the cells were serum-starved in experimental medium containing 0.1% FBS without supplements for at least 12 h.
In the present study, highly-chlorinated PCBs, including both coplanar and non-coplanar congeners, were evaluated. As coplanar PCBs, we used PCB 77 (3,3’4,4’-tetrachlorobiphenyl) and PCB 126 (3,3’,4,4’,5-pentachlorobiphenyl). In addition, PCB104 (2,2’,4,5,5’-pentachlorobiphenyl) and PCB153 (2,2’,4,4’,5,5’-hexachlorobiphenyl) were employed as non-coplanar PCBs. PCB118 (2,3’,4,4’,5-pentachlorobiphenyl), which was also included in the present study, is a major mono-ortho-substituted PCB (mixed-type inducer of cytochrome P450). Due to its binding affinity to the arylhydrocarbon receptor (AhR), TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) was used as a positive control for coplanar PCBs effects. Stock solution of PCBs was prepared in DMSO and the same amounts of DMSO as in PCBs-treated cells were added to control cultures. Levels of DMSO in experimental media were less than 0.05%.
Serum concentration of PCBs can reach approximately 3 µM in people exposed to these toxicants (Jensen, 1989; Wassermann et al., 1979). However, local micro-environmental levels of PCBs in the extracellular space are not known. Therefore, in the present study, cells were treated with specific PCBs at the concentration of 5 µM. Treatment at this concentration did not show cytotoxic effects over the time course of the experiments as determined by the MTT conversion assay (data not shown).
2.3 Immunoblotting
To prepare plasma membrane fraction, treated hCMEC/D3 were harvested and were disrupted by sonication. Cell debris and nuclei were removed by centrifugation at 600 g for 10 min. The supernatant was centrifuged again at 100,000 g for 1 h. The pellet was resuspended in lysis buffer (40 mM Tris-HCl, pH 7.4, 1.0% SDS, 1 mM Na3VO4, 1 mM EDTA, 0.5 mM EGTA, 1mM PMSF, and 1 × protease inhibitor cocktail) and used for experiments. For total cell extracts, treated hCMEC/D3 cells were lysed with same lysis buffer as that used for preparation of plasma membrane fraction. Protein samples were electrophoresed in SDS-PAGE and transferred onto a Hybond-ECL membrane (Amersham Biosciences, Piscataway, NJ). Immunoreactive protein bands were visualized with the enhanced chemiluminescence system (Amersham).
2.4 Immunofluorescence
Brain endothelial cells cultured on Type I collagen-coated chamber slide were fixed with ethanol for 30 min at 4°C and non specific binding was blocked with 3% bovine serum albumin (BSA) for 30min. Samples were incubated overnight at 4°C with primary antibody and then incubated with FITC-conjugated secondary antibody. The cells were observed and photographed using a confocal fluorescence microscope (Olympus FluoView 300; Olympus America Inc., Center Valley, PA).
2.5 Real-Time RT-PCR
Total RNA was isolated and purified using RNeasy Mini Kit (Qiagen) according to the protocol of the manufacturer. Then, 1 µg of total RNA was reverse transcribed at 25 °C for 15 min, 42 °C for 45 min and 99 °C for 5 min in 20 µl of 5 mM MgCl2, 10 mM Tris-HCl, pH 9.0, 50 mM KCl, 0.1% Triton X-100, 1 mM dNTP, 1 unit/µl of recombinant RNasin ribonuclease inhibitor, 15 units/µg of AMV reverse transcriptase, and 0.5 µg of random hexamers. For quantitative PCR, amplifications of individual genes were performed on ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) using TaqMan® Universal PCR Master Mix, gene-specific TaqMan PCR probes and primers, and a standard thermal cycler protocol (50 °C for 2 min before the first cycle, 95 °C for 15 sec and 60 °C for 1 min, repeated 45 times). The primers and probes were obtained from Applied Biosystems. The threshold cycle (CT) from each well was determined using ABI Prism 7000 SDS software. Relative quantification, which represents the change in gene expression from real-time quantitative PCR experiments between PCB-treated group and untreated control group, was calculated by the comparative CT method as described by Livak and Schmittgen (2001). The data were analyzed using equation 2−ΔΔCT, where ΔΔCT = [CT of target gene - CT of housekeeping gene] treated group – [CT of target gene - CT of housekeeping gene] untreated control group. For the treated samples, evaluation of 2−ΔΔCT represents the fold change in gene expression, normalized to a housekeeping gene (□-actin) and relative to the untreated control.
2.6 Statistical Analysis
Results are expressed as means ± S.D. Data were statistically analyzed using one-way ANOVA, followed by Student’s t test. Statistical probability of p<0.05 was considered significant.
3. Results
3.1 PCBs decrease expression of selective tight junction proteins
Tight junctions of the BBB are composed of transmembrane proteins, such as claudins, JAMs, and occludin, which are linked to the cytoskeleton by the accessory proteins, such as ZO-1, ZO-2, and AF6. As indicated in Fig. 1A, treatment with both coplanar and non-coplanar PCBs for 24 h markedly reduced expression of ZO-1, ZO-2 and AF6 in the membrane fractions of endothelial cells. To exclude the possibility that these effects were caused by translocation of tight junction proteins, expression of ZO-1, ZO-2, and AF6 was also measured in total extracts of hCMEC/D3 treated with PCBs. Fig. 1B shows that decreased levels of ZO-1, ZO-2 and AF6 in total cell extracts are similar to those in plasma membrane fraction. In contrast, tight junction transmembrane proteins, such as claudin-1 and JAM-A, were not affected by PCB treatment in both fraction of plasma membrane and total cell lysate (Figs. 1A and 1B).
Fig. 1. PCB congeners decrease expression of tight junction proteins in human brain endothelial cells.
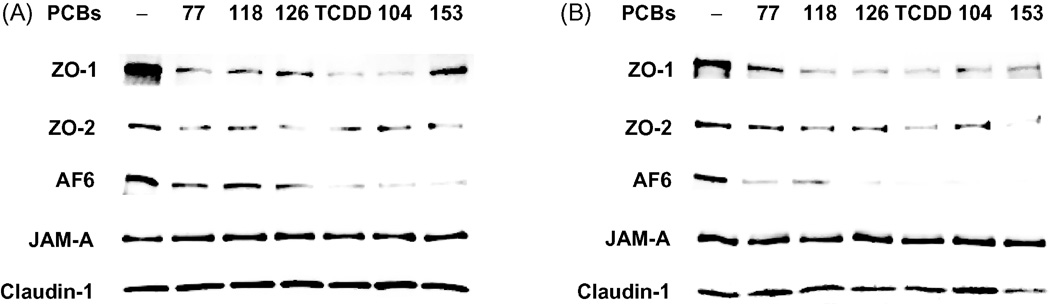
Confluent hCMEC/D3 were treated for 24 h with 5 µM of individual PCB congeners or 10 nM TCDD. Expression of tight junction proteins was determined by Western blot in plasma membrane fractions (A) and in total cell extracts (B) using antibodies against ZO-1, ZO-2, AF6, claudin-1 or JAM-A. The blots are representative images from three independent experiments.
In order to further confirm PCB-induced alterations of tight junction accessory protein levels, ZO-1 expression was evaluated by immunochemistry in hCMEC/ D3 treated with specific PCB congeners. In agreement with Western blot results, treatment with both coplanar and non-coplanar PCBs markedly decreased ZO-1 immunoreactivity (Fig. 2).
Fig. 2. PCB congeners decrease ZO-1 immunoreactivity.
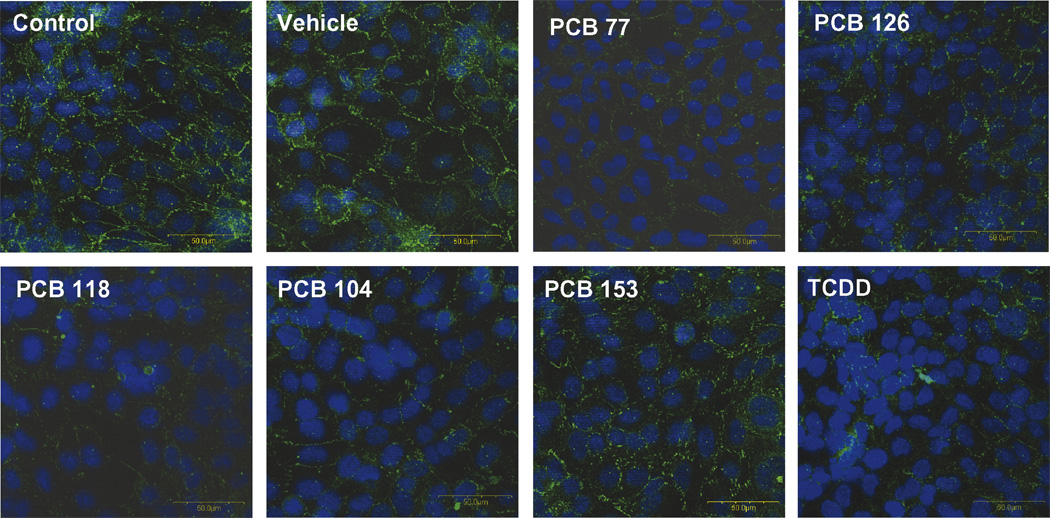
Cultures of hCMEC/D3 were grown to confluency on collagen type I-coated chamber slide and exposed for 24 h to 5 µM of individual PCB congeners or 10 nM TCDD (positive control). ZO-1 immunoreactivity was analyzed using polyclonal anti-ZO-1 antibody. PCB exposure resulted in a weaker or absent ZO-1 immunoreactivity at the cell–cell borders. The photographs are representative images from three independent experiments. Original magnification × 400.
3.2 PCB exposure does not alter steady mRNA level of ZO-1, ZO-2 and AF6
We next evaluated the possibility that PCB-mediated breakdown of tight junction proteins results from changes at the mRNA levels. hCMEC/D3 were exposed to individual PCB congeners at the concentration of 5 µM for 6, 12, or 18h and mRNA levels of ZO-1, ZO-2, and AF6 were analyzed by real-time RT-PCR. As shown in Fig. 3, steady mRNA levels encoding for these tight junction proteins were not changed in PCB-treated brain endothelial cells. Thus, PCB-induced alterations of tight junction protein expression are not mediated by changes in gene expression. Instead, our results suggest that the breakdown of tight junction proteins may result from the changes at the translational or posttranslational levels.
Fig. 3. PCB-induced disturbances in tight junction protein expression are not associated with alternation of steady mRNA levels.
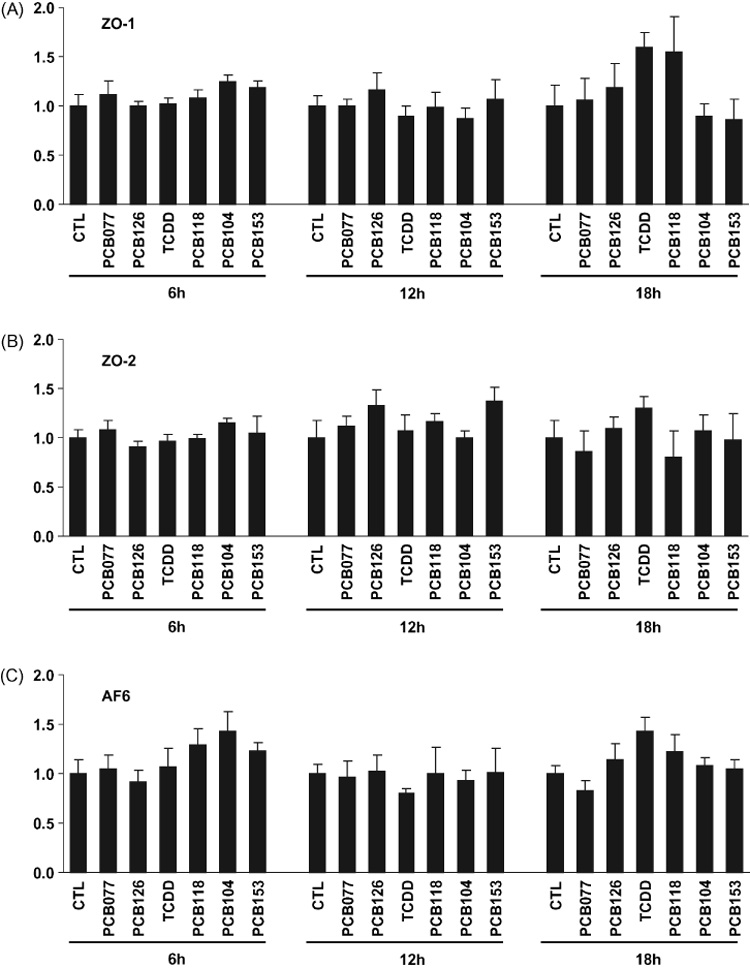
Confluent hCMEC/D3 cultures were exposed to 5 µM of individual PCB congeners or 10 nM TCDD (positive control) for 6, 12, or 18 h. Then mRNA levels of ZO-1, ZO-2, and AF6 were determined by real-time RT-PCR. Data are mean ± SD.
4. Discussion
There is considerable evidence that exposure to PCBs can induce neurotoxic effects including morphological changes of neurons and the developmental defects of the nervous system, which may lead to neurobehavioral and intellectual disorder as well as brain tumor-promoting effects (Seegal, 2000; Winneke et al., 2002). In several cohort studies, a positive correlation was found between prenatal or early postnatal PCB-exposure at environmental levels and adverse effects on neurodevelopmental changes (Carpenter, 2006). However, the mechanisms by which PCBs may influence the nervous system are largely unknown. In this study, we showed first evidence which PCBs exposure induces TJ disruption of human brain microvascular endothelial cells, which may lead to perturbation of the BBB integrity. Since the role of the BBB is to maintain an intact brain environment, the changes in the BBB may affect neuronal homeostasis and induce neurotoxic effects.
Loss of TJ proteins is commonly observed in neuroinflammatory and neurodegenerative disorders that are frequently associated with stroke (Brown and Davis, 2002), Alzheimer’s disease (Fiala et al., 2002), HIV-1 encephalitis (Persidsky et al., 2006), and traumatic brain injury (Morganti-Kossmann et al., 2002). Disruption of TJ in brain endothelial cells can increase serum protein extravasation through impaired BBB and lead to the development of vasogenic brain edema (Davies, 2002). For example, the CNS accumulation of serum proteins may increase osmotic load, impair neuronal function, and cause white matter pallor (Kustova et al., 1999). Perivascular edema, which occurs as the effects of the BBB dysfunction, is likely to impair the movement of oxygen, nutrients and metabolites across the microvessel wall as well as induce swelling of astrocyte end-feet (Norenberg, 1994).
The microvasculature of brain tumors characteristically lose their BBB properties and leak fluid into the brain through opening of microvascular endothelial cell TJ (Groothuis et al., 1991; Shibata, 1989). Thus, the disruption of TJ between microvascular endothelial cells in astrocytomas and metastatic cells were proposed to be associated with edema formation (Davies, 2002). In addition, the formation of brain metastases may be accelerated by disruption of TJ structures (Jiang et al., 2000).
Several highly-chlorinated PCBs that have both coplanar and non-coplanar structures were employed in the present study. Coplanar PCBs have dioxin-like activity as arylhydrocarbon receptor (AhR) agonists. Considerable evidence demonstrates that exposure to this class of compounds can induce vascular effects. Using an AhR-deficient mouse model, we have previously indicated that a functional AhR is critical for the proinflammatory events mediated by coplanar PCBs (Hennig et al., 2002). Other in vivo studies with AhR knockout mice also demonstrated that AhR activation is involved in the inflammatory response induced by the AhR ligands (Thatcher et al., 2007). Toxicity of coplanar PCBs has been linked to activation of the cytochrome P450 1A (CYP1A) subfamily via the interaction with the AhR (Alsharif et al., 1994; Safe and Krishnan, 1995). It was demonstrated that PCB77 can uncouple the catalytic cycle of CYP1A1, allowing heme iron within the active site of this enzyme complex to act as a Fenton catalyst and generating hydroxyl radicals from hydrogen peroxide (Schlezinger et al., 2006; Schlezinger et al., 1999). However, non-coplanar PCBs can also exert vascular effects. We have previously reported that exposure of endothelial cells to highly chlorinated ortho-substituted PCBs, such as PCB104, increased cellular oxidative stress, the release of VEGF, and MMP expression in microvascular endothelial cells (Eum et al., 2004; 2006; Toborek et al., 1995).
Breakdown of endothelial tight junctions and the disturbances in the BBB integrity are hallmarks of pathological alterations of the CNS, including brain tumors or neuroinflammatory diseases when homeostasis within the CNS is disturbed (Tsukita et al., 1999). In recent years, several proteins associated with epithelial and endothelial TJ have been identified. They include the MAGUK family, such as ZO-1, and ZO-2, as well as other non-MAGUK cytosolic proteins, such as AF6 that are less characterized (Neuwelt, 2004; Wolburg and Lippoldt, 2002). Expression of these proteins was selectively diminished by treatment with PCBs. Specifically, PCB exposure disrupted expression and pericellular distribution of ZO-1 (Fig. 1 and Fig. 2) and reduced protein expressions of ZO-2 and AF6 (Fig. 1), which bind ZO-1 to complete a scaffold complex of TJ plaque. In contrast, integral membrane proteins, JAM-A and claudin-1, were not changed in response to PCB treatment.
To evaluate possible mechanisms by which PCBs can mediate the breakdown of tight junction proteins, we examined the steady mRNA levels of TJ cytosolic accessory proteins, ZO-1, ZO-2 and AF6 in hCMEC/D3 exposed to specific PCB congeners. Surprisingly, mRNA levels encoding for these proteins were not altered as a result of PCB treatment (Fig. 3). Thus, it is plausible to suggest that PCB-induced disruption of ZO-1, ZO-2 and AF6 expression might result from the translational or post-translational regulations. One of the possible mechanisms may be related to proteolysis of tight junction proteins by matrix metalloproteinases (MMPs). Emerging data implicated MMPs in the regulation of tight junction expression (Hawkins et al., 2007; Lohmann et al., 2004) and we demonstrated that PCBs can induce expression of MMPs (Eum et al., 2006). Increased MMP expression may lead to degradation of tight junction proteins and opening of the BBB as demonstrated in a hypoxia/reperfusion model of stroke in spontaneously hypertensive rats (Yang et al., 2007).
Another potential mechanism that may play a role in PCB-mediated tight junction degradation may involve ubiquitination-proteasome systems. It has been demonstrated that the cytoplasmic NH2 terminus of occludin can bind Itch, an E3 ubiquitin-protein ligase, and UBC4, an ubiquitin-conjugating enzyme (Lui and Lee, 2005; Traweger et al., 2002). To support these mechanisms, dibutyl-cAMP-induced degradation of occludin was prevented by MG-132, a proteasome inhibitor. These effects were associated with accumulation of ubiquitin-conjugated and Itch-conjugated occludin (Traweger et al., 2002).
Alterations of tight junction protein expression may significantly contribute to PCB pathology. For example, alterations of ZO-1 expression can adversely affect permeability across monolayers of brain capillary endothelial cells (Bolton et al., 1998; Fischer et al., 2004; Youakim and Ahdieh, 1999). In addition, loss of ZO-1 from cerebral vascular endothelium was observed during CNS inflammation caused by injection of LPS in juvenile rats (Bolton et al., 1998). Other studies have also demonstrated that a decrease of the ZO-1 level was induced by pro-inflammatory molecules, such as tumor necrosis factor (TNF) and interferon (Youakim and Ahdieh, 1999). Thus, these data are in agreement with our reports that treatment with PCBs can induce endothelial hyperpermeability (Eum et al., 2004; Toborek et al., 1995).
In conclusion, the present study is the first research report indicating that exposure to highly chlorinated PCBs can diminish expression of tight junction proteins, such as ZO-1, ZO-2 and AF6 in brain endothelial cells. These effects are specific because the transmembrane tight junction proteins, such as JAM-A or claudin-1, were not affected by PCB treatment. Due to their role in maintaining brain homeostasis, disruption of tight junctions may directly contribute to the neurotoxic effects of PCBs.
ACKNOWLEDGMENTS
This study was supported by NIH (P42 ES 07380, MH63022, MH072567, and NS39254).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol. Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif NZ, Lawson T, Stohs SJ. Oxidative stress induced by 2,3,7,8-Tetrachlorodibenzo-p-dioxin Is mediated by the aryl hydrocarbon (Ah) receptor complex. Toxicology. 1994;92:39–51. doi: 10.1016/0300-483x(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 Expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp. Cell Res. 2004;292:51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O'Donnell L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am. J. Physiol. 1997;273:H286–H294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Davis TP. Calcium modulation of adherens and tight junction function: A potential mechanism for blood-brain barrier disruption after stroke. Stroke. 2002;33:1706–1711. doi: 10.1161/01.str.0000016405.06729.83. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): Routes of exposure and effects on human health. Rev. Environ. Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Chen ML, Pothoulakis C, LaMont JT. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to clostridium difficile toxin A. J. Biol. Chem. 2002;277:4247–4254. doi: 10.1074/jbc.M109254200. [DOI] [PubMed] [Google Scholar]
- Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J. Anat. 2002;200:639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am. J. Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. VEGF Regulates PCB104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp. Cell Res. 2004;296:231–244. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Eum SY, Lee YW, Hennig B, Toborek M. Interplay between epidermal growth factor receptor and janus kinase 3 regulates polychlorinated biphenyl-induced matrix metalloproteinase-3 expression and transendothelial migration of tumor cells. Mol. Cancer Res. 2006;4:361–370. doi: 10.1158/1541-7786.MCR-05-0119. [DOI] [PubMed] [Google Scholar]
- Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur. J. Clin. Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J. Cell Physiol. 2004;198:359–369. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- Groothuis DR, Vriesendorp FJ, Kupfer B, Warnke PC, Lapin GD, Kuruvilla A, Vick NA, Mikhael MA, Patlak CS. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann. Neurol. 1991;30:581–588. doi: 10.1002/ana.410300411. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased Blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Hennig B, Meerarani P, Slim R, Toborek M, Daugherty A, Silverstone AE, Robertson LW. Proinflammatory properties of coplanar PCBs: In vitro and in vivo evidence. Toxicol. Appl. Pharmacol. 2002;181:174–183. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- Hollande F, Blanc EM, Bali JP, Whitehead RH, Pelegrin A, Baldwin GS, Choquet A. HGF regulates tight junctions in new nontumorigenic gastric epithelial cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G910–G921. doi: 10.1152/ajpgi.2001.280.5.G910. [DOI] [PubMed] [Google Scholar]
- Hom S, Fleegal MA, Egleton RD, Campos CR, Hawkins BT, Davis TP. Comparative changes in the blood-brain barrier and cerebral infarction of SHR and WKY rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1881–R1892. doi: 10.1152/ajpregu.00761.2005. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jensen AA. Background levels in humans, in halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxines and related products. Elsevier Science Publishers; 1989. [Google Scholar]
- Jiang WG, Martin TA, Llaffafian I, Mansel RE. Tight junctions, a critical structure in the control of cancer invasion and metastasis. In: Jiang WG, Manse RE, editors. Cancer metastasis: molecule and cellular mechanisms and clinical intervention. Amsterdam: Kluwer Academic; 2000. [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Knerr S, Schrenk D. Carcinogenicity Of "non-dioxinlike" polychlorinated biphenyls. Crit. Rev. Toxicol. 2006;36:663–694. doi: 10.1080/10408440600845304. [DOI] [PubMed] [Google Scholar]
- Kustova Y, Grinberg A, Basile AS. Increased blood-brain barrier permeability in LP-BM5 infected mice is mediated by neuroexcitatory mechanisms. Brain Res. 1999;839:153–163. doi: 10.1016/s0006-8993(99)01734-5. [DOI] [PubMed] [Google Scholar]
- Lee DW, Opanashuk LA. Polychlorinated biphenyl mixture Aroclor 1254-induced oxidative stress plays a role in dopaminergic cell injury. Neurotoxicology. 2004;25:925–939. doi: 10.1016/j.neuro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Liu DZ, LeCluyse EL, Thakker DR. Dodecylphosphocholine-mediated enhancement of paracellular permeability and cytotoxicity in Caco-2 cell monolayers. J. Pharm. Sci. 1999;88:1161–1168. doi: 10.1021/js990094e. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–196. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM. Camp perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J. Cell. Physiol. 2005;203:564–572. doi: 10.1002/jcp.20254. [DOI] [PubMed] [Google Scholar]
- Marco S, Skaper SD. Amyloid beta-peptide1-42 alters tight junction protein distribution and expression in brain microvessel endothelial cells. Neurosci Lett. 2006;401:219–224. doi: 10.1016/j.neulet.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA. Mechanisms of disease: the blood-brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. discussion 141-132. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Astrocyte responses to CNS injury. J. Neuropathol. Exp. Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: The vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Paul D, Goodenough DA. Truncation mutants of the tight junction protein ZO-1 disrupt corneal epithelial cell morphology. Mol. Biol. Cell. 2000;11:1687–1696. doi: 10.1091/mbc.11.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Krishnan V. Cellular and molecular biology of aryl hydrocarbon (Ah) receptor-mediated gene expression. Arch. Toxicol. Suppl. 1995;17:99–115. doi: 10.1007/978-3-642-79451-3_8. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by coplanar polychlorinated biphenyl congeners. Aquat. Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, White RD, Stegeman JJ. Oxidative inactivation of Cytochrome P-450 1A (CYP1A) stimulated by 3,3′,4,4′-Tetrachlorobiphenyl: production of reactive oxygen by vertebrate CYP1As. Mol. Pharmacol. 1999;56:588–597. doi: 10.1124/mol.56.3.588. [DOI] [PubMed] [Google Scholar]
- Seegal RF. The Neurotoxicological consequences of developmental exposure to PCBs. Toxicol. Sci. 2000;57:1–3. doi: 10.1093/toxsci/57.1.1. [DOI] [PubMed] [Google Scholar]
- Shibata S. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. (Berl) 1989;78:561–571. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (Zonula Occludens) in a variety of epithelia. J. Cell. Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher TH, Maggirwar SB, Baglole CJ, Lakatos HF, Gasiewicz TA, Phipps RP, Sime PJ. Aryl hydrocarbon receptor-deficient mice develop heightened inflammatory responses to cigarette smoke and endotoxin associated with rapid loss of the nuclear factor-kappab component RelB. Am. J. Pathol. 2007;170:855–864. doi: 10.2353/ajpath.2007.060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Espandiari P, Robertson LW, Hennig B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J. Biochem. Toxicol. 1995;10:219–226. doi: 10.1002/jbt.2570100406. [DOI] [PubMed] [Google Scholar]
- Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase Itch. J. Biol. Chem. 2002;277:10201–10208. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Wassermann M, Wassermann D, Cucos S, Miller HJ. World PCBs map: Storage and effects in man and his biologic environment in the 1970s. Ann. N. Y. Acad. Sci. 1979;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Winneke G, Walkowiak J, Lilienthal H. PCB-nduced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology. 2002:181–182. 161–165. doi: 10.1016/s0300-483x(02)00274-3. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight unctions of the blood-brain barrier: Development, composition and regulation. Vascul. Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J. Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Rehmann H, Price LS, Riedl J, Bos JL. AF6 negatively regulates Rap1-induced cell adhesion. J. Biol. Chem. 2005;280:33200–33205. doi: 10.1074/jbc.M505057200. [DOI] [PubMed] [Google Scholar]


