Abstract
A novel bifunctional maleimido CHX-A”-DTPA chelator 5 was developed and conjugated to the monoclonal antibody trastuzumab (Herceptin) and subsequently radiolabeled with 111In. The resulting 111In labeled immunoconjugate 2 was demonstrated to bind to SKOV3 ovarian cancer cells comparably to an isothiocyanato CHX-A” DTPA modified native trastuzumab, 1. Through efficient thiol-maleimide chemistry, antibodies, peptides or other targeting vectors can now be modified with an established radioactive metal chelating agent CHX-A” DTPA for imaging and/or therapies of cancer.
The use of monoclonal antibodies (mAbs) as targeting vectors to selectively deliver a lethal dose of radiation to cancer cells through either a β- or α-emitting radionuclide, radioimmunotherapy (RIT), has been extensively investigated for cancer therapies.1-3 The targeted nature of such therapies as well as imaging with γ- or β+-emitters (SPECT / PET), radioimmunoimaging (RII), offers the promise of greater efficacy and less toxicity.4 This field has attracted greater attention particularly after the recent Food and Drug Administration approval of two radionuclide-bearing monoclonal antibody therapies (90Y-ibritumomab and 131I-tositumomab) for the treatment of lymphohematopoietic malignancies.5, 6
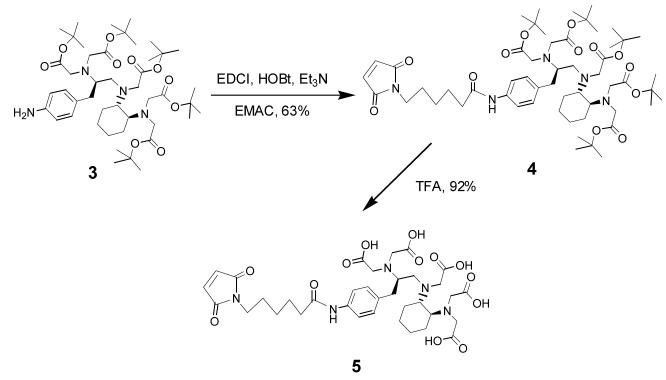
The development of suitable bifunctional chelating agents for modification of proteins for RII and RIT continues to receive great attention. To achieve useful radiolabeled mAbs in the clinical setting, chelating agents must form thermodynamically and kinetically stable complexes to prevent loss of radionuclides in vivo, while retaining mAb immunoreactivity.7, 8 A variety of DTPA and DOTA derivatives bearing isothiocyanate reactive groups are well documented for this purpose and some have been carried forward into antibody-targeted radiation therapy clinical trials (Figure 1).4 The acyclic bifunctional ligand, CHX-A” DTPA (N-[(R)-2-amino-3-(p-aminophenyl)propyl]-trans-(S,S)-cyclohexane-1,2-diamine-N,N,N’,N”,N”-pentaacetic acid) reported in 1997, has been found to be excellent at sequestering radionuclides such as 86/90Y, 212/213Bi, 111In, and 177Lu with great in vivo stability. 9-12 In particular, radiolabeling of CHX-A” DTPA modified mAb with the therapeutic α-emitter 212Bi was found to be statistically comparable to protein conjugates formed with a bifunctional DOTA derivative (2-(p-isothiocyanatobenzyl)-l,4,7,10-tetraazacyclododecane tetraacetic acid) in stability, without the detraction of the slow formation kinetics inherent to the DOTA macrocycle.11
Figure 1.
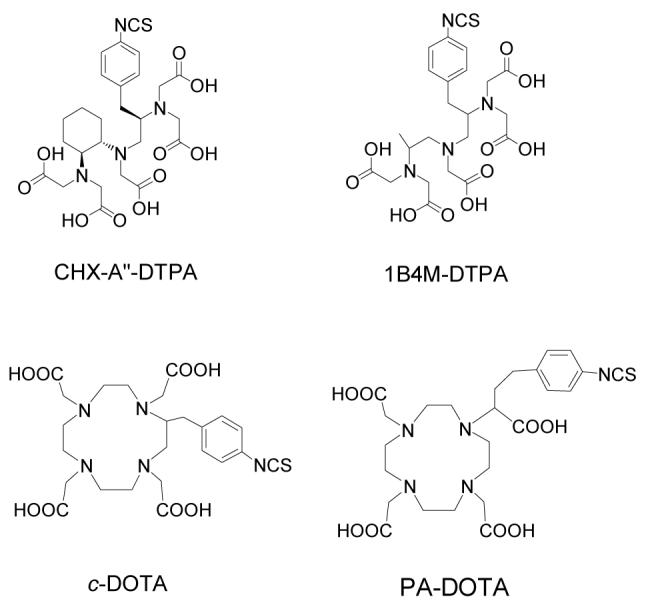
Structures of CHX-A” DTPA, 1B4M-DTPA, c-DOTA and PA-DOTA.
In this laboratory, the isothiocyanate form of CHX-A” DTPA reacts with primary ε-amine groups of lysine or terminal α-amine residues of proteins and has routinely been used for conjugation to mAb (Figure 2). This conjugation method can be problematic in controlling the precise number and exact position of chelates that have been attached. This can lead to concerns regarding potential interference with antigen binding, particularly so if there are lysine residues in the antigen binding region.
Figure 2.
A schematic presentation of CHX-A” DTPA conjugated trastuzumab 1, conjugation through an isothiocyanato CHX-A” DTPA chelator, and 2 conjugation through the maleimido CHX-A” DTPA chelator, 5, to prepare 2.
An alternate conjugation strategy is available via the selectivity of the Michael addition between a thiol and a maleimide.13 The maleimide group reacts efficiently and specifically with thiol-terminal biomolecules and has been widely used to form stable protein conjugates through thioether linkages. This conjugation method is widely considered to give greater control over the position of the chelating agent on a protein when the thiol group(s), e.g., engineered cysteine residues, can be introduced at the genetic level. One the other hand, thiol groups are less likely than charged groups (e.g. amino groups) currently used for conjugation to be found in at mAb’s binding sites, thereby offering better chemistry to retain immunoreactivity of mAbs. A small number of chelating agents that exploit this strategy have been reported,14-17 but few have seen extensive use for in vivo studies of radiolabeled antibodies or peptides for either imaging or therapy. In part, this may be due to a lack of familiarity with this chemistry and/or more problematically, the lack of appropriate derivatives of previously established chelating agents.
Herein, the synthesis of a novel maleimido CHX-A” DTPA chelator, 5, is described as well as its successful conjugation to the monoclonal antibody trastuzumab. This novel malemide CHX-A” DTPA derivative thus offers an alternative route to modify antibodies, peptides, and other targeting vectors with an established radiometal chelate. This conjugation strategy might be particularly useful considering the availability of engineered Fab or Fab’ antibody fragments containing free sulfhydryl groups.
For the synthesis of 5, we used the tert-butyl ester protected p-amino functionalized CHX-A” DTPA, 3, which was recently developed in this laboratory (Figure 3).18, 19 This aniline derivative was reacted with N-ε-maleimidocaproic acid (EMCA) using standard peptide coupling conditions to provide maleimide 4 (63%).20 The tert-butyl esters in 4 were quantitatively cleaved by TFA as monitored by TLC to generate 5.21 Thereafter, the liberated five carboxyl groups, along with the three tertiary amines, became available for sequestering radioactive metals, such as 111In, 86/90Y, 212/213Bi or 177Lu. The maleimide moiety in 5 provides a highly reactive group towards thiol groups either extant or introduced into proteins or peptides. To conjugate 5 to trastuzumab, the mAb was first thiolated with 15 equivalents of Traut’s agent using standard procedures with minor modifications.22 Routinely, ∼ 2 - 4 thiol groups per trastuzumab were introduced as quantified by Ellman’s reagent. Thiolated trastuzumab was then reacted with 10 equivalents of 5 to produce conjugate 2.23 Unreacted thiolated conjugate was capped with iodoacetamide to minimize cross-linking of the mAb conjugate product and to promote a longer shelf life of the immunoconjugate. Finally, the reaction mixture was dialyzed into PBS buffer at 4 °C to remove all small molecules from the protein solution. The final average ratio of CHX-A” DTPA moieties per trastuzumab achieved by this process was calculated to be ∼ 2 based on an Arsenazo (III) assay.24 The immunoconjugate 2 was radiolabeled efficiently (> 95%) with the SPECT radionuclide 111In within 30 min at room temperature.25 Figure 4 shows the size-exclusion HPLC profile of the isothiocyanate and maleimide-functionalized trastuzumab as compared to their respective 111In labeled analogs as well as to the unmodified mAb. The profiles of the maleimide-modified analogs were similar to those of the previously reported isothiocyanate-modified analogs, both radiolabeled and unlabeled, as well as to unmodified trastuzumab. Immunoreactivity of the resulting 111In labeled immunoconjugate 2 was demonstrated by binding to SKOV-3, a human ovarian carcinoma cell line. There was comparable binding between 111In labeled immunoconjugate 2 (44.9%) and the 111In-labeled isothiocyanato CHX-A” modified trastuzumab, 1 (38.6%).19,26
Figure 3.
Synthesis of the bifunctional maleimido CHX-A” DTPA chelator, 5.
Figure 4.
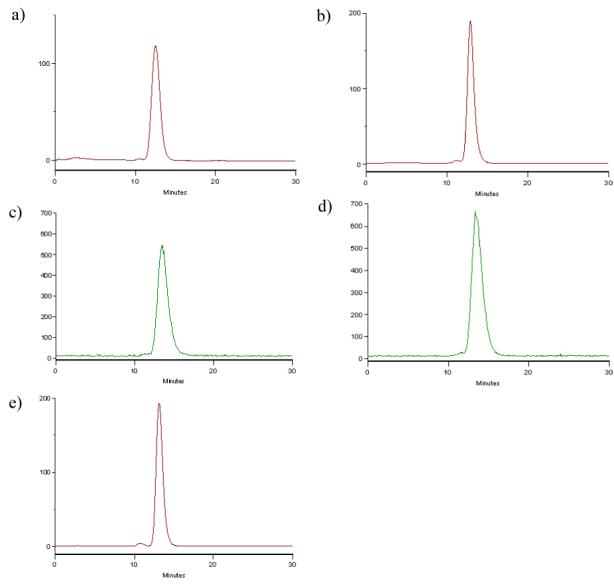
SE-HPLC chromatograph of (a) 1; (b) 2; (c) 111In-1; (d) 111In-2; (e) trastuzumab. (a), (b), and (e) were monitored by UV at 280 nm, while (c) and (d) were recorded by an in-line HPLC radiodetector.
In conclusion, a novel bifunctional maleimido DTPA derivative 5 was designed, synthesized and characterized for conjugation to thiol containing biomolecules such as antibodies, peptides or other targeting vectors, which was based on a previously established chelating agent, CHX-A”. Successful conjugation of 5 to the monoclonal antibody trastuzumab (Herceptin) was achieved by efficient thiol-maleimide chemistry. One should note that the direct use of 4 would also be possible for solution or solid phase conjugation to peptides and other vectors. Subsequent cleavage of the esters would provide a conjugate product suitable for radiolabeling. Therefore, this newly developed maleimido CHX-A” DTPA allows a new route to providing an established radioactive metal chelating agent on biomolecules for imaging and/or therapies of cancers.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References and notes
- 1.Goldenberg DM. Cancer Immunol. Immunother. 2003;52:281. doi: 10.1007/s00262-002-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milenic DE, Brechbiel MW. Cancer Biol. Therapy. 2004;3:361. doi: 10.4161/cbt.3.4.790. [DOI] [PubMed] [Google Scholar]
- 3.Wu AM, Senter PD. Nat. Biotech. 2005:1137. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 4.Milenic DE, Brady ED, Brechbiel MW. Nat. Rev. Drug Discov. 2004;3:488. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 5.Wahl RL. J. Nucl. Med. 2005;46(suppl 1):128s. [PubMed] [Google Scholar]
- 6.Borghaei H, Schilder RJ. Semin. Nucl. Med. 2004;34:4. doi: 10.1053/j.semnuclmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Clin. Cancer Res. 2004;10:7834. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 8.Milenic DE, Garmestani K, Brady ED, Albert P, Abdulla A, Flynn J, Brechbiel MW. Clin. Cancer Res. 2007;13:1926. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Kobayashi H, Sun B, Yoo M, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Bioorg. Med. Chem. 1997;5:1925. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, Wu C, Yoo TM, Sun B, Pastan I, Paik CH, Gansow OA, Carrasquillo JA, Brechbiel MW. J. Nucl. Med. 1998;39:829. [PubMed] [Google Scholar]
- 11.Nikula TK, McDevitt MR, Finn RD, Wu C, Kozak RW, Garmestani K, Brechbiel MW, Curcio MJ, Pippin CG, Tiffany-Jones L, Geerlings MW, Sr., Apostolidos C, Molinet R, Geerlings MW, Jr., Gansow OA, Scheinberg DA. J. Nucl. Med. 1999;40:166. [PubMed] [Google Scholar]
- 12.Milenic DE, Garmestani K, Chappell LL, Dadachova E, Yordanov A, Ma D, Schlom J, Brechbiel MW. Nucl. Med. Biol. 2002;29:431. doi: 10.1016/s0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 13.Hermanson GT. Bioconjugate Techniques. Academic Press; London: 1996. pp. 419–455. [Google Scholar]
- 14.Arano Y, Uezono T, Akizawa H, Ono M, Wakisaka K, Nakayama M, Sakahara H, Konishi J, Yokoyama A. J. Med. Chem. 1996;39:3451. doi: 10.1021/jm950949+. [DOI] [PubMed] [Google Scholar]
- 15.Ali MS, Quadri SM. Bioconjugate Chem. 1996;7:576. doi: 10.1021/bc960051e. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MR, Shively JE. Bioconjugate Chem. 1998;9:72. doi: 10.1021/bc970136v. [DOI] [PubMed] [Google Scholar]
- 17.Lattuada L, Gabellini M. Synth. Commun. 2005;35:2409. [Google Scholar]
- 18.Clifford T;, Boswell AC, Biddlecombe GB, Lewis JS, Brechbiel MW. J. Med. Chem. 2006;49:4297. doi: 10.1021/jm060317v. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Baidoo K, Gunn AJ, Boswell CA, Milenic DE, Choyke PL, Brechbiel MW. J. Med. Chem. 2007;50:4759. doi: 10.1021/jm070657w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Synthesis of maleimido CHX-A” penta tert-butyl ester 4: N-ε-Maleimidocaproic acid (0.28 g, 1.32 mmol), 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDCI) (0.50 g, 2.62 mmol), 1-hydroxybenzotriazole (HOBt) (0.35 g, 2.62 mmol), and Et3N (0.18 mL, 1.32 mmol) were added to a stirred solution of 3 (1.00 g, 1.20 mmol) in DMF (50 mL). The mixture was stirred for 24 hr at room temperature (RT) and then concentrated under reduced pressure, diluted with CH2Cl2 (100 mL), and washed successively with water (2 × 100 mL), 5% NaHCO3 (2 × 100 mL), and water (1 × 100 mL). The organic layer was concentrated and the residue was chromatographed on silica gel eluted with EtOAc:MeOH (9:1) to afford 4 (0.78g, 63%). 1H NMR (DMSO-d6) δ 9.70(s, 1H), 7.45(d, J = 8.1 Hz, 2H), 7.15(d, J = 8.1Hz, 2H), 7.00(s, 2H), 3.5-3.20 (m, 12H), 3.15(br d, J = 15.6Hz, 1H), 2.8-3.0(m, 2H), 2.75(m, 1H), 2.55(m, 3H), 2.39(m, 1H), 2.24(t, J = 7.2Hz, 2H), 1.90(br s, 2H), 1.70-1.10(m, 10H), 1.37(s, 45H), 1.03(m, 4H); 13C NMR (CDCl3) δ 171.7, 171.3, 170.8, 136.5, 134.1, 129.6, 119.5, 80.4, 63.9, 63.2, 62.6, 53.5, 53.0, 52.5; 39.0, 37.2, 36.1, 28.1, 27.2, 26.2, 25.8, 25.0; ES-MS: calcd for C55H88N5O13 [M + H+]: 1026.63786, found 1026.63822.
- 21. Synthesis of maleimido CHX-A” DTPA 5: CHX-A” derivative 4 (0.30 g, 0.29 mmol) was stirred with 15 mL of TFA for 4 hr. The reaction mixture was concentrated under reduced pressure, treated with Et2O (50 mL), and filtered to afford 5 (0.20 g, 92%). 1H NMR (DMSO-d6) δ 9.78 (s, 1H), 7.45(d, J = 8.1Hz, 2H), 7.18(d, J = 8.1Hz, 2H), 7.00(s, 2H), 3.60-2.40(m, 17H), 2.24(t, J = 7.2Hz, 2H), 2.00(m, 2H), 1.50(m, 8H), 1.20(m, 6H); 13C NMR (DMSO-d6) δ 173.1, 172.7, 171.3, 171.2, 168.8, 137.8, 134.6, 133.4, 129.6, 119.3, 61.5, 53.2, 52.4, 37.2, 36.4, 28.0, 26.1, 24.8, 24.5, 24.5, 24.1; ES-MS: calcd for C35H48N5O13 [M + H+]: 744.30922, found 744.30692.
- 22.Jue R, Lambert JM, Pierce LR, Traut RR. Biochemistry. 1978;17:5399. doi: 10.1021/bi00618a013. [DOI] [PubMed] [Google Scholar]
- 23. Thiolation of and conjugation with Trastuzumab: Trastuzumab (∼ 7.7 mg) was reacted with Traut’s reagent at a 1:15 molar ratio for 1 hr at RT in 1 mL of PBS containing 5mM EDTA buffer. Excess Traut’s reagent was removed by passage of the reaction solution through a PD-10 column eluted with PBS containing 5mM EDTA buffer. Just prior to protein conjugation, 5 was dissolved in the same buffer and then added drop wise to the mAb solution to achieve a molar reaction ratio of 10:1 (5: Trastuzumab) and gently vortexed. The solution was then gently agitated in the dark at 25 °C for 1 hr. Excess free unreacted SH groups were capped by the addition of iodoacetamide solution (2.0 mM). Finally, the reaction mixture was dialyzed into PBS buffer at 4 °C with 4 buffer changes (4 × 1 L) over 48 hr to afford immunoconjugate 2. Protein concentration and the number of the CHX-A” chelate per protein were determined by Lowry assay and Arsenazo(III) assay respectively. The purity of immunoconjugate 2 was evaluated by both SE-HPLC and SDS-PAGE (data not shown), and was found to be comparable to native trastuzumab.
- 24.Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Bioconjugate Chem. 1992;3:342. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- 25. 111In Radiolabeling of immunoconjugate 2: Caution: 111In (t1/2 = 2.8 d) is a γ-emitting radionuclide. Appropriate shielding and handling protocols should be in place when using this radionuclide. NH4OAc buffer (0.15 M, pH 7, 100 μL) was added to a solution of the maleimido CHX-A” DTPA chelator, 5, conjugated to trastuzumab, 2, in PBS (1 mg/mL, 50 μL). 111InCl3 in 0.05 M HCl (1 mC, 3 μL) (PerkinElmer) was then added to the mixture and vortexed. The reaction mixture was incubated at room temperature for 30 min. The reaction was quenched by the addition of a solution of EDTA (0.1M EDTA, 4 μL). The 111In labeled product was purified on a PD-10 column using PBS as the eluent. The radiolabeling efficiency was 95.2%.
- 26. Radioimmunoassay: Immunoreactivities of the radiolabeled preparations were assessed in a radioimmunoassay using methanol-fixed cells. Briefly, SKOV-3 cells were trypsinized, pelleted, re-suspended in PBS (pH 7.2) and fixed with cold methanol at a final concentration of 80% methanol. After 1 hr at 4°C, the cells were washed with PBS (3 times) and re-suspended in PBS containing 1% BSA (PBS/BSA). Serial dilutions of radiolabeled trastuzumab (∼140 to 8 nCi) were added in duplicate to cells (1×106/50 μL) in 50 μL of PBS/BSA. The cells were washed with 3 mL of 1% BSA in PBS following an 18 hr incubation at room temperature, pelleted at 1,000 × g for 5 min and the supernatant decanted. The radioactivity was measured in a γ-counter (WizardOne, PerkinElmer, Shelton, CT) and the percent bound calculated for each dilution. The value presented is the average percent bound.