Abstract
The ‘social intelligence hypothesis’ was originally conceived to explain how primates may have evolved their superior intellect and large brains when compared with other animals. Although some birds such as corvids may be intellectually comparable to apes, the same relationship between sociality and brain size seen in primates has not been found for birds, possibly suggesting a role for other non-social factors. But bird sociality is different from primate sociality. Most monkeys and apes form stable groups, whereas most birds are monogamous, and only form large flocks outside of the breeding season. Some birds form lifelong pair bonds and these species tend to have the largest brains relative to body size. Some of these species are known for their intellectual abilities (e.g. corvids and parrots), while others are not (e.g. geese and albatrosses). Although socio-ecological factors may explain some of the differences in brain size and intelligence between corvids/parrots and geese/albatrosses, we predict that the type and quality of the bonded relationship is also critical. Indeed, we present empirical evidence that rook and jackdaw partnerships resemble primate and dolphin alliances. Although social interactions within a pair may seem simple on the surface, we argue that cognition may play an important role in the maintenance of long-term relationships, something we name as ‘relationship intelligence’.
Keywords: avian brain, jackdaw, monogamy, pair bonding, rook, social intelligence
1. Introduction
Thirty years ago, Nicholas Humphrey (1976) suggested a radical proposal for how primates may have acquired their apparent superior intelligence when compared with other animals (see also Jolly 1966). From his observations of rhesus monkeys housed at the Sub-department of Animal Behaviour, Madingley, he was puzzled as to why these monkeys performed cognitive feats in the laboratory and yet seemed to have few, if any, problems to solve during their day-to-day existence. These laboratory-housed monkeys did not have to search for food, they had no predators to evade and the enclosure in which they lived was relatively sparse. The monkeys in this environment, however, were housed in social groups. Humphrey therefore suggested that the physical environment did not present the kind of challenges which would lead to the evolution of a flexible, intelligent mind, but that the social environment did. This proposal has been named the ‘Social Intelligence Hypothesis’ (SIH). The social environment is ever changing and largely unpredictable, particularly in those societies in which individuals have a history of interactions with other individuals (e.g. comparing monkeys with social insects). This proposal has blossomed with the support of positive data, and mutated into the ‘Machiavellian Intelligence Hypothesis’ (Byrne & Whiten 1988; Whiten & Byrne 1997) or ‘Social Brain Hypothesis’ (SBH, Dunbar 1998), which focus on the manipulative and deceptive aspects of social life and the relationship between sociality and brain power respectively. However, from its inception, the SIH was discussed primarily with respect to the evolution of primate intelligence, and not other animals. It rapidly became clear that other social animals, such as dolphins, hyenas and elephants, also demonstrated many of the biological, ecological and behavioural preconditions for intelligence; sophisticated cognitive skills in the lab and field; and many of the complex social skills found in monkeys and apes (McComb 2001; de Waal & Tyack 2003; Connor 2007; Holekamp et al. 2007).
In 1996, Peter Marler attempted to widen the comparative nature of the SIH by asking whether birds also demonstrate similar aspects of social intelligence to primates, and if so, is their social intelligence of the same kind as primates? (Marler 1996). In reviewing data on cooperation, social learning and group defence in birds, Marler concluded that the social skills of primates did not appear to be more sophisticated than birds. Indeed, he suggested that most ornithologists were not as interested in the same questions of cognitive evolution as primatologists; however, if they started looking they would find many examples of social knowledge in a wide variety of birds.
When Marler wrote his paper over 10 years ago, there was little data available on avian social intelligence. Indeed, Marler based his review primarily on data collected by field ornithologists with no interest of the underlying cognitive processes involved in social interaction. Therefore, his analysis was necessarily, in general, including data from all available species. However, based on brain size and cognitive tests in the laboratory, it is not clear why there should be a simple relationship between sociality, intelligence and brain for the Class Aves, as this is not the case for the Class Mammalia. Although there appears to be a clear relationship between social group size and brain (i.e. neocortex) size within primates (Dunbar 1992), carnivores, insectivores (Dunbar & Bever 1998), bats (Barton & Dunbar 1997) and cetaceans (Marino 2002; Connor 2007), no such relationship exists within another mammalian order, ungulates (Shultz & Dunbar 2006). Although the brain size analyses presented later in the paper were performed at the class rather than family level, it should be kept in mind that such analyses have yet to be performed in mammals.
It is the aim of the current paper to evaluate Marler's claim that avian social intelligence is the same as primate social intelligence. First, we will review whether there is a relationship between flock (group) size and brain size in birds, as there is in primates, bats, cetaceans and carnivores. Unfortunately, these analyses are not directly comparable, as the majority of comparative brain size analyses in mammals have been performed on the neocortex, whereas data on the equivalent areas of the avian brain such as the nidopallium and mesopallium is not yet available for large numbers of birds. This is likely to be important, as other ‘non-thinking’ parts of the brain are relatively unspecialized, playing a role in many aspects of behaviour and cognition. Certainly, brain size is related to other biological and ecological variables, such as body size, long developmental period, diet and habitat. With this caveat in mind, we will assess the evidence for a relationship between sociality and brain size in birds. We report data from new analyses that those species which form lifelong pair bonds, including many corvids and parrots, tend to have the largest relative sized brains. However, there are also lifelong monogamous species, such as geese, with relatively small brains. We will make a suggestion as to why these differences may exist.
In the second part of this paper, we will consider whether the SIH still applies to birds, in accordance with the differences in sociality between the majority of birds and mammals. In particular, we will assess how lifelong monogamy, in combination with other socio-ecological factors, may have led to increased brain size. We will briefly review the socio-ecology of three lifelong monogamous species; greylag geese, jackdaws and rooks, to determine whether life-history traits and/or ecology may have influenced sociality in these species and subsequently the evolution of social intelligence. We will then present some data from behavioural observations of juvenile rooks suggesting that they form alliances, support one another in fights, exchange different behavioural commodities (e.g. food, social support and preening), recognize relationships between third parties and demonstrate third-party post-conflict affiliation. These behaviours have so far only been described for primates and dolphins. We conclude that rooks (and probably other large-brained, lifelong monogamous avian species, such as parrots) demonstrate a form of relationship intelligence, rather than a general social intelligence, allowing them to become ‘in tune’ with their partner, and so providing them with the competitive edge to out compete individuals who do not form similar partnerships.
2. The avian social brain?
In early discussions of the SIH, a number of biological, ecological and behavioural preconditions were proposed as essential to the presence of sophisticated social processing in primates. These were a large brain, long developmental period before maturation, individualized social groups and extended longevity (Humphrey 1976; Byrne & Whiten 1988; van Schaik & Deaner 2003). Indeed, in primate species with complex social systems, such as cerecopithecine monkeys and hominoid apes, these preconditions are fulfilled. In addition, primates with large brains (or more precisely, a large neocortex ratio against the rest of the brain) tended to form larger social groups than species with a smaller neocortex ratio (Dunbar 1992; Barton 1996).
We now know that these biological preconditions are not exclusive to primates and have been demonstrated in many social mammals, such as elephants (McComb 2001), hyenas (Holekamp et al. 2007) and cetaceans (Connor 2007). What about birds? Although birds do not possess absolute brain sizes anywhere in the region of most mammals (mean 3.38±0.11 g; range from 0.13 (Cuban Emerald hummingbird) to 46.19 g (Emperor penguin; Iwaniuk & Nelson 2003) compared with the 7800 g brain of a sperm whale), some birds have brains that, relatively speaking, are the same size as those of chimpanzees (after removing the effects of body size; Emery & Clayton 2004).
Have avian brains evolved to solve the same or similar cognitive problems as mammalian brains? Emery (2006) reviewed evidence that some birds use and manufacture tools, possess episodic-like memory (i.e. remember what they cached, where they cached and when they cached it), predict the behaviour of conspecifics and possibly understand their mental states. These traits tended to be found in those birds with a large relatively sized brain, an omnivorous diet, a complex social system and which live in a harsh, changeable environment: traits which are shared with mammals that have been suggested to be the most intelligent. Indeed, Godfrey-Smith (1996), Sterelny (2003) and Potts (2004) have suggested that such environmental complexity presented numerous ecological problems for our hominid ancestors which could only be solved by the evolution of flexible forms of innovative behaviour.
The potential relationship between sociality and brain size in birds is complex. In three studies, no clear relationship was found between group size and brain size across various bird families (Beauchamp & Fernandez-Juricic 2004), cooperative breeding group size and brain size in corvids (Iwaniuk & Arnold 2004) or simple social structure and brain size across many bird families (Emery 2004).
Beauchamp & Fernandez-Juricic (2004) were the first to attempt to evaluate Dunbar's claims with respect to flock size in birds. As their measure of social complexity, Beauchamp & Fernandez-Juricic (2004) recorded the mean and maximum flock size outside the breeding season and flocking propensity; they also used three independent sources for brain volumes. They did not find any significant relationships. One reason for this lack of relationship may have been that group size is not a stable trait in many birds, which tend to form pair bonds during the breeding season and then foraging flocks of various sizes outside the breeding season. The size of these flocks is dependent largely on food availability and quality, rather than the underlying social interactions within the flock. Emery (2004) used a more conservative measure of sociality, categorizing species based on whether they tended to live territorially (solitary except during the breeding season), in pairs, in families, in small non-family groups (10–50), medium groups (greater than 50) or in large flocks (100 s and 1000 s). There was no significant difference in relative forebrain size across the various social categories, except when the different families and/or orders were analysed separately. At that level of evolutionary analysis, the corvids and parrots had much larger brains than other birds, but only those birds found in pairs, small flocks or medium flocks (Emery 2004).
Cooperative breeding birds tend to flock together throughout the year, and so may present a better reflection of the underlying social interactions. Iwaniuk & Arnold (2004) investigated any potential relationship between the brain size of cooperative and non-cooperative corvids, or any correlation between cooperative group size and brain size in corvids. They failed to find any significant relationships at this family level.
As we have already suggested, any comparison between these studies and those on primates (or other mammals) has to be viewed with some caution as the analyses were performed at different neural levels (brain size in birds versus neocortex ratio in mammals). Although not directly comparable to the neocortex, the most extensive dataset in birds for a more specific brain area is the size of the telencephalon (forebrain). Burish et al. (2004) did find a strong relationship between social complexity and size of the avian telencephalon (forebrain); however, this analysis used a strange social category, ‘transactional’, that included those species which demonstrated ‘complex’ forms of behaviour (not necessarily social), such as ceremonial dancing, communal gatherings, fission–fusion societies, memory performance, food sharing, ‘parliaments’, ‘weddings’, aerial acrobatics, social play, milk-bottle opening and problem-solving. This category appears to be completely tautological—selecting complex behaviours likely to require a significant amount of brain power makes a significant relationship with brain size unsurprising. In addition, some of the species included in this category are solitary (e.g. woodpeckers; Winkler et al. 1995), and this does not align well with the SBH.
Perhaps avian sociality is too heterogeneous a concept to be used effectively in studies related to brain size? One problem in attempting to find a simple relationship between brain size and sociality across birds is that the social organization of most birds tends to be very flexible, both temporally and spatially. As such, species may vary in social system based on their geographical location and its different ecological pressures. A harsh environment in which food is scarce or difficult to locate could make raising healthy offspring more challenging. Long-term monogamy may be favoured in these conditions, as parents that cooperate in raising their offspring year-in-year-out may gain an advantage that would not apply in less harsh environments. Similarly, cooperative-breeding, in which the young remain with their parents to help raise the next brood, may be adopted because the environment is so harsh that it cannot provide for an additional family.
Carrion crows, for example, tend to be socially monogamous; however populations in Northern Spain, where conditions are harsh, are cooperative breeders (Baglione et al. 2002b). When crow eggs from Switzerland (non-cooperative breeders) were transferred to the nests of cooperative breeding crows in Spain, some of the chicks developed into helpers (Baglione et al. 2002a). Similarly, Florida scrub-jays are cooperative breeders (McGowan & Woolfenden 1989), whereas their close relatives, Western scrub-jays are typically semi-territorial (Carmen 2004) although they breed co-operatively in parts of Mexico (Curry et al. 2002). Although classified as two separate species, biologically they are almost identical, differing primarily in mating/social system. Young Florida scrub-jays do not necessarily become helpers if the ecological conditions do not require additional aid in raising the brood or prevent them from finding a mate of their own (Woolfenden & Fitzpatrick 1984). Species may also demonstrate different social systems temporally, pair-bonding in the breeding season, and forming family groups once the young have fledged but remain with the parents, with the juveniles then leaving the colony to form larger foraging flocks. Many corvids and parrots appear to form such a variety of social systems within a species (Goodwin 1986; Juniper & Parr 1998).
3. Mating system, brains and social complexity
It is not clear from the previous section that there is any relationship between the avian brain and sociality. We have suggested that may be because avian social systems are flexible, depending on changing season or environmental conditions, and that the earlier analyses were based on factors that may not be good indicators of social complexity, such as group size. We therefore performed two new analyses, the first comparing species based on a simple measure of social network size (based on average group size) and the second comparing species based on mating system, as mating system may be a more robust measure of social complexity than group size (Bennett & Owens 2002).
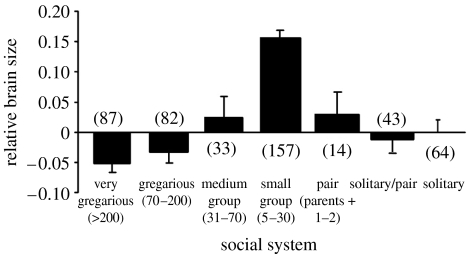
We used an extensive dataset of avian brain volumes (n=1482 species; Iwaniuk & Nelson 2003). Information on social category was collated from the literature from as many avian species as possible (using the following categories: solitary/territorial, except for the breeding season; solitary/pair, typically solitary or in a pair year round; pair, breeding pair+offspring; small group, 5–30 individuals; medium group, 31–70 individuals; gregarious, 70–200 individuals; and very gregarious, greater than 200 individuals). Emery's earlier categorization of social system was based on maximum group size which may not be an accurate reflection of social complexity (Emery 2004). When it comes to interacting with the same individuals over a long time period, it may be quality rather than quantity which is important. For example, rooks live in colonies during the breeding season, forming long-term pair bonds and nesting in close proximity to other pairs, then they form small family groups once the offspring have fledged, and then join large winter foraging flocks and roost with up to 40 000 individuals. Rook pairs remain stable throughout the year (and across years). It is very unlikely that rooks will interact with every other bird in this size group or remember their interactions or their social relationships, in the same way that humans living in London do not interact with everyone they meet on the London Underground, only with a limited set of individuals they may see everyday. This does not mean that the capacity for interacting with a large number of individuals is absent.
Residuals from a regression of brain volume against body weight were used as the index of relative brain size. There was a significant effect of social system on relative brain size (ANOVA; F6,473=23.05, p<0.00001; figure 1), with birds in small groups having larger brains than birds with other social systems (Bonferroni correction, t-test, p<0.008 all comparisons). This was in direct contrast to results from mammals which report a relationship between increasing neocortex size and increasing social group size in mammals.
Figure 1.
Social system and relative brain size across 480 species of birds (raw brain volume data from Iwaniuk & Nelson (2003); social system data from various published sources).
Given that almost 90% of birds are monogamous (Lack 1968), and that birds in small groups tend to have the largest brains, perhaps mating system may be a clearer indication of social complexity, especially if we stress the importance of relationship quality rather than relationship quantity. Monogamy in birds takes different forms, from serially monogamous species which take new partners in every breeding season (e.g. most passerines) to pair-bonded species which mate for life (e.g. albatross). Serially monogamous species are often socially monogamous, which means they remain with the one partner during the breeding season, but often take part in extra-pair copulations (EPC). This compares to species (that are more often than not lifelong bonded species) which can be classified as genetically monogamous, which means they demonstrate mate fidelity with little or no evidence for EPCs (Henderson et al. 2000; Reichard 2003).
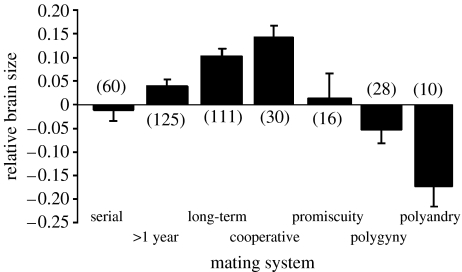
Although mating system can change, especially to reflect changes in climate or other environmental factors, it is perhaps a more stable representation of social structure in birds than social system, which can change quite dramatically across the space of a year for some species. For example, many birds form pairs during the autumn/winter, interacting primarily with their partner during the breeding season and then forming family groups when their offspring become independent, finally forming large roosting and foraging flocks later in the year. Such diversity within a species cannot easily be represented in an analysis of group size and brain size. Therefore, the relationship between brain size and mating system was investigated using the same brain volume dataset as above (Iwaniuk & Nelson 2003). Birds were classified according to whether they were monogamous, cooperative breeders, polygamous, polygynous or polyandrous. Monogamy often refers to the formation of a pair bond between a male and female which lasts throughout the breeding season, however in birds there are three alternative forms of monogamy. Birds which form pairs throughout the breeding season, but which are not exclusive (as determined by DNA paternity tests) and which do not re-form in the next season were classified as ‘1 year’ monogamous. Birds which formed pairs for longer than 1 year were classified as ‘>1 year’ monogamous, and birds which paired for life were classified as ‘long-term’ monogamous. Lifelong monogamy is extremely difficult to prove without the ability to track pairs across the seasons and across years, especially in very long-lived birds. In addition, there may be two forms of lifelong monogamy: those species such as rooks and jackdaws who remain together throughout the year, returning together to the same nest site (Cramp & Perrins 1994), and those species such as albatrosses, which pair during the breeding season and return to the same nesting site and partner every 2 years, but which travel separately from their partner outside of the breeding season (Perrins 2003).
As with the earlier analysis, residuals from a regression of brain volume on body weight were used as an index of brain size. There was a significant relationship between mating system and brain size across birds (ANOVA; F6,373=9.73, p<0.00001; figure 2) with those species forming long-term pair bonds or who are cooperative breeders (which also form long-term bonds) tending to have the largest relative brain size.
Figure 2.
Mating system and relative brain size across 480 species of birds (raw brain volume data from Iwaniuk & Nelson (2003); mating system data from various sources).
As brain size does not increase linearly with social group size, and the largest brains are found in long-term monogamous species rather than in species with a polygamous mating system, this suggests that the original formulation of the social brain hypothesis was primatocentric and should be reassessed for other animals (mammalian and non-mammalian). Even though the relationship between monogamy and brain size is significant, it seems counter-intuitive to suggest that cognitive complexity underlies the relationship between members of a long-term pair, rather than between many different individuals. Certainly, if competition was the only manifestation of social intelligence then this would be a fair conclusion. However, there is another aspect of social intelligence which is the polar opposite of deception and manipulation: cooperation. Cooperation is the key to establishing and maintaining a strong affiliative relationship of which the pair bond is the archetypal example; however competition still remains important for the interactions with conspecifics and the procurement of resources (see later).
According to the traditional formulation of the SBH, it is questionable whether primate brains increased in size in order to process and store information about social relationships. Indeed, the capacity to store and remember individuals, their current relationships to one another as well as previous ones has not been empirically tested. It seems unlikely that brains should function in such a one-to-one relationship, especially in those cases where the store only has a limited space available, such as in the bird brain. Hence, perhaps, the failure to find a linear relationship between group size and brain size in birds is not particularly surprising, especially when the absolute size of the avian brain is constrained by flight. As such, birds would not have the neural space available to put down large stores of social information (Nottebohm 1981). Even though corvids (crows, rooks, jackdaws, magpies) have almost twice the neural density in the forebrain than pigeons (Voronov et al. 1994), it remains unlikely that this could function as a permanent store of social memories of large numbers of individuals.
One speculative mechanism for how birds may circumvent this problem is neuronal plasticity. Bird brains are incredibly plastic, and some regions, such as the song control system and hippocampus, show marked changes in size and these changes correlate with use (e.g. song learning during the breeding season, Nottebohm (1981); or searching for nests during the breeding season in brood parasitic birds, Reboreda et al. 1996; Clayton et al. 1997; or caching food for the winter, Barnea & Nottebohm 1994; Clayton & Krebs 1994). In a similar manner, perhaps those regions of the avian brain which respond to social stimuli (such as the bed nucleus of the stria terminalis and amygdala, Bharati & Goodson 2006; Goodson & Wang 2006) also increase their size in response to seasonal changes in social complexity. These regions may decrease in size or decrease the rate of neurogenesis during the breeding season, when costs for maintaining a big brain are high (e.g. males constantly foraging and provisioning mate and offspring) and when social information processing is largely restricted to the mate when compared with increases in size and neurogenesis outside the breeding season when individuals form large social groups, when such social information may be important.
Some evidence in support of this hypothesis comes from studies of zebra finches raised in groups with different social complexity. Finches were raised in family groups and then either housed singly or transferred to groups of 40–45 strangers. Different sub-groups were then allowed to survive in these social conditions for various durations (40, 60 or 150 days) after the injection of [3H]-thymidine (a marker for neurogenesis). At the end of these time points, the birds were sacrificed and the number of new neurons counted in either the caudal nidopallium (NC) or the hippocampal complex (HC). There were a greater number of new neurons in the NC of communally housed birds than of singly housed birds, and the number of new neurons was highest after 40 days and lowest after 150 days (Barnea et al. 2006). By contrast, there were a higher number of new neurons in the HC in the communally housed birds than in the singly housed birds, but only at 40 days after [3H]-thymidine injection. In a corresponding study (Lipkind et al. 2002), an increase of new neurons was found in the NC, higher vocal centre (HVC) and Area X (both areas involved in song learning) 40 days after injection of [3H]-thymidine. This increase was specific to those birds housed in a more complex social environment (large heterosexual group rather than male–female pairs or singly housed).
Based on this data, we propose two neural systems in monogamous species which also form large groups outside the breeding season. The first would be a core neural system which may function as a permanent store of information about their bonded partner. Certainly, in lifelong pair-bonded species, the pair should be able to recognize their partner and ideally the subtleties of their relationship. The HC is a likely candidate for a core neural system as described in the study above (Barnea et al. 2006), in which new neurons did not appear to increase dramatically when housed in larger social groups for long periods. The second would be a more plastic secondary neural system which would function in processing and retaining information about transient social relationships, such as with individuals forming ephemeral flocks outside the breeding season. An example of this might be the NC described in the study above (Barnea et al. 2006), in which the number of new neurons increased significantly when housed in larger groups, across all time periods. This high turnover may reflect a high number of transient relationships with many individuals. As such, the social memories in the NC will be recent, and need to be updated frequently, whereas the social memories stored in the HC will be relatively older, representing both past events and declarative knowledge (e.g. a partner's typical responses in social situations, their preferences, their past history, etc.).
Although this suggestion is speculative these recent studies on the relationship between changes in sociality and neurogenesis provide a tantalizing glimpse of how such a system might be implemented in the avian brain. Indeed, the question of how mating system could affect the structure and function of avian brains is ready for comparative analysis along the same lines as studies on the distribution of receptors implicated in affiliation (e.g. oxytocin and vasopressin) in monogamous and promiscuous voles (Insel et al. 1994; Young et al. 2001). The avian brain may possess a similar ‘social behaviour network’ to the mammalian brain (Goodson 2005). Indeed, an analysis of arginine vasotocin (the non-mammalian equivalent to arginine vasopressin and oxytocin) distribution in the brains of various species of Estrildidae (grass finches and waxbills), which are all monogamous but which differ in species-typical group size, found patterns of receptor binding in the ‘social behaviour network’ which could be explained by social system (Goodson et al. 2006). It remains to be seen whether similar results would be found with corvids of different mating/social systems.
4. Can lifelong pair bonds be cognitively complex?
If the size of the avian brain is related to mating system, with especially large brains found in those species which form lifelong pair bonds or cooperative breeding groups, we need to address the following question, ‘Which aspects of the pair bond may require this additional brain processing power?’ Certainly, a problem arises, as this finding is counter intuitive to our thoughts about large brains and social complexity when they are formulated around the traditional primate view of the SBH. If we did not know something about the socio-cognitive abilities of birds (especially the cache protection strategies of corvids, see Clayton et al. 2007), we might be inclined to suggest that, perhaps, sociality was not an important factor for the evolution of the avian brain and intelligence.
It seems premature to discard the SIH with respect to birds without first thinking of alternative explanations for the relationship between brains and pair bonds. Hence, returning to the original question, ‘why do lifelong pair-bonded avian species have the largest brains?’, is there something special about retaining an exclusive partner over many years compared with mating with one individual during one breeding season and then taking a different partner the next or even mating with multiple partners during the same breeding season? Does lifelong monogamy require more than just reproductive collaboration? Are all lifelong pair-bonded species the same?
With respect to brain size, if we chose a random group of distantly related, lifelong monogamous species, and compared their brain sizes, would we expect any differences between them? We therefore chose five Procellariformes (white-capped albatross, grey-headed albatross, wandering albatross, black-browed albatross and black-footed albatross); five Anseriformes (snow goose, white-fronted goose, Brant goose, Bewick's swan & whooper swan); three Corvidae (jackdaw, rook and raven) and six Psittaciformes (white cockatoo, blue & yellow macaw, lorikeet, black-capped lorry, kea and African grey) from the Iwaniuk & Nelson (2003) dataset, and plotted brain volume against body mass. Interestingly, the data split into two grades; the swans, geese and albatrosses grouped around a low sloped regression line, whereas the corvids and parrots grouped around a steeper regression line (figure 3). Such a difference is unlikely to be due to ancestry as the groups are all distantly related; or to differences in diet, given that geese are herbivorous and albatrosses eat fish (higher protein value), yet have similar sized brains, and the corvids tend to be omnivorous, whereas the parrots eat fruit, nuts and seeds. The differences in brain size could be due to finding and processing food rather than the type of food per se, but again this does not explain the similarity in brain size for the geese and albatrosses (although the albatrosses brains are all above the regression line for that grade). It also seems unlikely that these differences are due to processing information about home range, as geese migrate over long-distances, and albatrosses range for thousands of miles within a year; and hence perhaps, we would predict that they would have the largest brains if range size was the crucial factor. One difference may be amount of innovative behaviour, particularly with respect to novel foods and novel feeding techniques (Lefebvre et al. 1997) or extractive foraging and tool use (Lefebvre et al. 2002). However, we would like to suggest an alternative explanation for this neural difference (though not a mutually exclusive one), namely that the pair bond is more socially and cognitively complex in corvids and parrots than geese and albatrosses.
Figure 3.
Brain/body relationships in long-term, pair-bonded birds (geese, swans, albatrosses, corvids and parrots). Body size and brain volume data are from Iwaniuk & Nelson (2003). The lines represent two grade shifts between corvids and parrots, and geese, swans and albatrosses, but are do not represent actual regression statistics.
In an attempt to determine whether this is a plausible explanation, the remainder of this section will briefly review the social and mating system of three lifelong pair-bonded species: greylag geese, rooks and jackdaws. We appreciate that the choice of these species is rather arbitrary, indeed, many other species could have provided similar information (e.g. ravens; Heinrich 1990, 1999). However, these species were chosen because a lot is known about their reproductive and social behaviour from long-term field studies (see table 1), and owing to our own personal knowledge from working with the two corvid species.
Table 1.
Comparison between jackdaws, rooks and greylag geese of various socio-ecological variables which are relevant to lifelong monogamy.
| jackdaws | rooks | greylag geese | |
|---|---|---|---|
| pair for life? | ✓a | ✓b | ✓c |
| pairs associate throughout the year? | ✓a,d,e | ✓f,b | ✓c |
| extra pair copulations? | ×g | ✓b,h | ✓? |
| non-sexual relationships? | ✓ | ✓i | ✓ |
| social grouping | pairs in breeding season, large flocks throughout year | colonial in breeding season, large flocks throughout yearj,k | pairs whilst incubating, large flocks throughout yearl–n |
| retain smaller units (pairs, families) within larger groups? | ✓ (pairs only) | ✓f | ✓ |
| social support | ✓o | ✓i | ✓p–r |
| age at maturity | 2 yearsd,f | 2 yearss | 2–3 yearsl |
| paired affiliative behaviour | ✓preening, food-sharing, displayinga,d,e,t | ✓preening, food-sharing, displayingu | ×(except family triumph ceremony)v |
| altricial young? | ✓f | ✓f | ×(precocial)l |
| joint activities? | ✓nest site establishment, territory defence, provisioning younga,e,w | ✓nest site establishment, territory defence, provisioning youngx,y | ✓territory defencec,l |
| diet | omnivorous, largely insectsf | omnivorous, largely cereals and earthwormsb | herbivorousl |
see current paper.
A. von Bayern, unpublished observations.
All three species form lifelong partnerships; however there are striking similarities and differences in their socio-ecology. One clear difference is that infant geese are precocial (fully developed at hatching, and able to move unaided, finding food for themselves, imprinting on their mother and following her around), whereas infant corvids are altricial (hatch at a much earlier stage of development, where they are completely dependent on being fed by their parents, and cannot move out of the nest until they fledge). As such, altricial offspring are completely dependent on, at least, one parent (usually the mother), hence it becomes critical that the other parent takes the role of finding food for both his mate and his offspring, as all the mother's efforts are directed towards the needs of her offspring: feeding them when she receives food from the male and protecting them from predators. Therefore, it has been suggested that parents of altricial offspring have to cooperate in the raising of their brood (i.e. bi-parental care), at least until they become independent (Reichard 2003). Such close collaboration leads not only to joint care of the offspring, but also to extensive physical contact within the mated pair. Rook pairs collaborate in finding a nest site and build nests together (Coombs 1978), whereas jackdaw pairs collaborate to acquire a nest hole, and then cooperate in defending this new nest site from other pairs and predators, as these are rare commodities (Roell 1978).
By comparison, the parents of precocial offspring do not need to cooperate in raising their offspring as they can provide for themselves. Food is abundant because geese are herbivorous, therefore goslings can find their own food and do not have to travel far to find it. Geese build simple nests on the ground, which are easily abandoned, but high risk from ground predators, therefore parents do cooperate in defending the goslings from predators.
It is clear that there are differences in both the ecology and life history of these species (table 1). What then is the role for lifelong monogamy in the evolution of intelligence? One possibility is that lifelong monogamy, once evolved, provides a platform for the partnership between mated pairs to become a synchronized, cooperative, year-round solution to the challenges of social and environmental complexity, for those species faced with such challenges.
Consistent with this notion is one further difference between geese, rooks and jackdaws: the quality of the relationship between the bonded male and female. In greylag geese, the pair will continue to associate throughout the year, even when forming large foraging flocks. There is no evidence of any prolonged physical contact between the male and female; there is no allofeeding, no allopreening, with the only real indication of a bond being the reduced proximity between the partners (when compared with other individuals), lack of aggression between them, behavioural synchrony, mate guarding and reciprocity in the triumph display (demonstrated primarily by the male after an aggressive encounter or used as a greeting when being re-introduced to the partner). Although geese provide social support to their partner, most examples are of passive social support, in which the mere presence of a partner has an effect on subsequent aggression. Active social support is less frequent, in which the partner actively interferes in a fight between their partner and a second individual (Scheiber et al. 2005).
It is the purpose of the next section to describe new data on rook partnerships and use this data to investigate the quality of their relationship. We suggest that pairs may become established through reciprocal acts of physical affiliative contact, such as food-sharing (allofeeding, possibly as a display of good parenting skills and the ability to provision the female when brooding chicks), bill twining and allopreening. Once pairs have become established, their behaviour resembles the alliances of many primates, with members of the pair aiding one another in fights (either ganging together against a common victim or passive social support or intervening in a current dispute), attacking the aggressor of their partner or their aggressor's partner, and directing affiliative behaviour towards their partner after they had been the victim or aggressor in a fight. Similar studies are being conducted on jackdaws (A. von Bayern 2004-2006, unpublished observations); however similar patterns are being reported, such as active food-sharing as an essential component for the development of the pair bond (von Bayern et al. 2005). Jackdaw pairs have been suggested to demonstrate similar forms of affiliative behaviour in other studies (e.g. Wechsler 1989).
5. Primate-like social complexity in rooks
Although relative brain size is a useful measure for predicting an animal's intellectual capacity (especially when comparing across a large number of species), it is also extremely limited. Only detailed behavioural observations and experiments can answer questions of social complexity in non-human animals. For individuals living within in a social group, it pays to develop selective relationships with others, to aid in the acquisition of resources and to receive protection against the threat of those who are intent on accessing your resources (Cords 1997). Monkeys, apes and dolphins either form temporary coalitions or occasionally more long-term alliances (Connor 2007); however it is not clear whether these relationships are more complex than in other animals, or only different (Harcourt 1992; Marler 1996). It is assumed that these relationships are fostered through reciprocal altruism and tactical manipulation (Seyfarth & Cheney 1984; de Waal & Lutrell 1988), mechanisms which may require sophisticated cognitive processes (Stevens & Hauser 2004). Although most previous studies of social complexity have focused on mammals, there is no reason to assume that the same processes are not important for birds, possibly occurring through a process of convergent evolution (Emery & Clayton 2004). Marler (1996) suggested that this lack of data was due to the research questions of field ornithologists rather than lack of data per se. As rooks have relatively large brains and form lifelong pair bonds, we chose them as a model corvid in which to investigate the formation of social relationships, and primate-like social knowledge. We therefore hand raised a group of rooks and followed their social development, including the development of affiliative relationships and pair bonds, until they were ten months old.
Twelve nestling rooks were taken from four different nests in Cambridgeshire and hand-raised in three separate groups until they fledged (approx. 32 days old). The birds' sex was determined through analysis of genetic material in their breast feathers. At fledging, the juveniles were released into an aviary. For the initial observations which occurred between July and October 2002, the rooks were observed once per day for approximately 1 h in the morning for five blocks (1 block=1 week). During every trial, each rook was presented with small pieces of cheese in turn. The birds who begged first tended to receive the first pieces of cheese, but each bird received approximately similar amounts and in a semi-random order.
Observers made ad lib observations of the rooks' social behaviour (affiliative and aggressive), feeding, caching, recovery and vocalizations. Examples of aggressive behaviour included actual physical aggression (e.g. pecking and jabbing), displacements (i.e. one bird flies to the exact spot in which another bird is sitting and supplants that bird from its spot), chases and submissions. Examples of affiliative behaviour included food offering (e.g. active giving, begging, stealing and tolerated theft; de Kort et al. 2003, 2006), dual caching (i.e. two individuals cache the same piece of food together), bill-twining, play, allopreening and providing agonistic aid (i.e. two individuals either gang together against a third party or one individual intervenes in an ongoing fight).
Two additional blocks of trials (Blocks 6–7) were performed from December 2002 to January 2003 to determine whether the rooks' social behaviour had stabilized. A dominance index comparing number of wins minus number of losses over the total number of aggressive encounters was calculated for each individual for each block.
(a) The alliance as a valuable relationship
Valuable relationships come in different forms, from parent–offspring relationships to pair bonds and adult friendships. Such valuable relationships form owing to some mutual benefit for the two parties involved in the relationship. van Schaik & Aureli (2000) defined a valuable relationship by the presence of a number of properties. Individuals which spend more time in proximity, more friendly behaviours (e.g. grooming or preening) between two individuals, lower rates of agonistic conflict (e.g. aggression and submission) between two individuals and more agonistic support against a third party could be classified as forming a valuable relationship. This pattern of behaviours could be used to describe many forms of social bond, in which individuals are likely to derive considerable value from their relationship (Kummer 1978), such as parents and offspring, ‘friends’ and long-term mated pairs. To determine whether our group of juvenile rooks had formed valuable relationships, these properties will be examined in turn.
(i) Affiliative behaviour
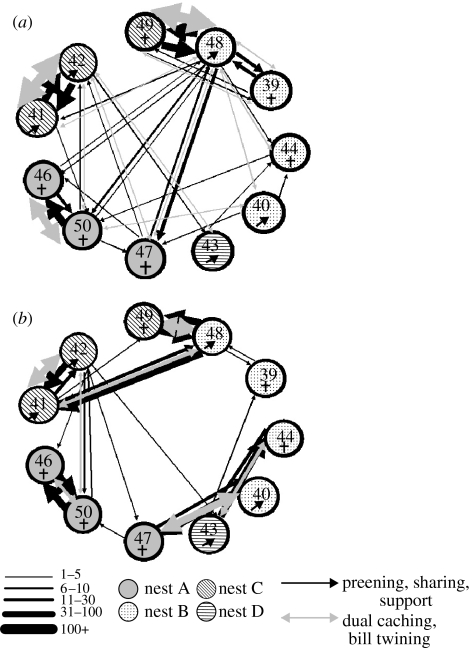
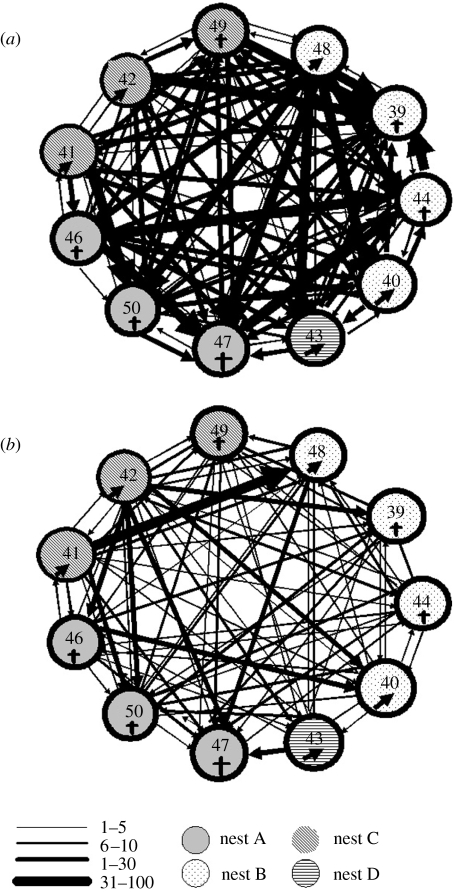
The total frequency of affiliative behaviour in which one individual either directs their behaviour towards another (e.g. food sharing, allopreening, social support) or both individuals take part in a collaborative behaviour (e.g. dual caching, bill twining) was recorded for each potential dyad and the frequencies presented as sociograms. The thickness of the lines on the sociogram reflected the frequency of the behaviour and therefore the strength of the bond. In Blocks 1–5, the frequency of affiliative behaviour suggested that three pairs had formed based on the total amount of friendly behaviour directed towards a specific individual (pairs 41 & 42, 46 & 50 and 48 & 49; figure 4a). At this stage (i.e. six months old), these relationships were not exclusive, as there was evidence of some affiliative behaviour directed towards individuals outside the pair (figure 4a). These pairs did not appear to be based on sex, as 41 & 42 was a male–male pair, 46 & 50 was a female–female pair, and 48 & 49 was a male–female pair. Indeed, at six months old, the rooks would not have reached sexual maturity.
Figure 4.
Sociogram displaying the total frequency of affiliative behaviour between 11 hand-raised rooks (a) during Blocks 1–5, and (b) during Blocks 6–7. Numbers refer to individual birds (39, 40, 41, 42, 43, 44, 46, 47, 48, 49 & 50). The thickness of the lines represents the total frequency of affiliative behaviour across the time period represented. Black line represent unidirectional behaviours (food sharing, social support and allopreening), and the grey lines represent bidirectional behaviours (dual caching and bill-twining). The different patterned circles represent the nest from which the nestling was taken (A–D).
In the later observation period, when the rooks were nine months old (Blocks 6 & 7), the three pairs had stabilized, and two new pairs had formed (pairs 40 & 47 and 43 & 44; figure 4b). Both of the new pairs were male–female pairs, but still unlikely to have been sexually mature (Cramp & Perrins 1994). By this stage, the pairs appeared to be relatively exclusive, with little evidence of affiliation directed towards individuals outside the pair (figure 4b). There were exceptions to this, including a developing ‘friendship’ between 42 and 50, and between 41 and 48. This last affiliative relationship is particularly interesting, as 41 and 48 were two of the most dominant rooks (see a similar development for chimpanzees; de Waal 1982).
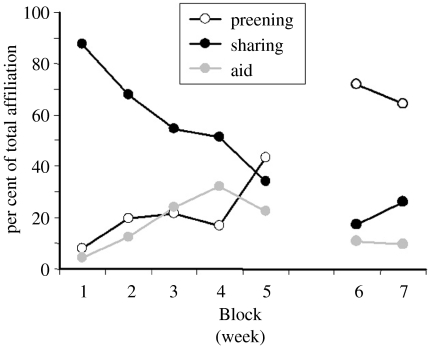
In the early stages of pair formation, food sharing appeared to be the most important affiliative behaviour, particularly the unsolicited transfer of highly valuable food from one individual to another (active giving; de Kort et al. 2003). The frequency of active giving appeared to be high at the initiation of the study when the rooks were approximately 3–4 months old (Block 1), but had significantly tailed off by the end of the first observation period (Block 5; figure 5). During Blocks 6 and 7, active giving was vastly reduced and largely restricted to the two new pairs. A similar pattern of results was found during the development of jackdaw socialization using the same food provisioning and observational methods (von Bayern et al. 2005; de Kort et al. 2006). By comparison with food sharing, allopreening was relatively infrequent during Block 1, but steadily increased during Block 5, and began to level off during Blocks 6 and 7 (figure 5). Agonistic (social) support occurred at a relatively stable but low level across this whole period (figure 5). This suggests that active giving may be essential for the formation of the pair bond, but that allopreening takes over its affiliative role once the bond has become established, and so may be used to maintain the bond. We suggest that food sharing functions in this early role in bond formation owing to its similarity to parental feeding (the first individuals the young rooks and jackdaws bond with), and the fact that in hand-raised birds the parents are not around, and so the ‘givers’ may be taking on parental duties. However, this does not explain why some birds take on this role and why their food giving is so exclusive. Bond development in older birds is likely to be a consequence of reproduction, with the high frequency of food sharing as an example of courtship feeding (however in the young rooks described here, both partners give one another food).
Figure 5.
Proportion of the total frequency of food sharing, allopreening and agonistic (social) support across Blocks 1–7 averaged across all 11 rooks.
(ii) Aggressive behaviour
Aggression in this particular group of rooks was extremely frequent during Blocks 1–5 (figure 6a), with various acts of aggression directed towards other individuals (from displacements to physical aggression to chases). Of course, this may have been exaggerated due to the nature of the observation sessions in which food was provisioned to the birds and an effect of being in captivity where space was somewhat restricted; however each bird was in the same situation and received the same amount of food. Of particular interest to the issue of valuable relationships was the fact that little or no acts of aggression were recorded between individuals in a pair (figure 6a). Aggression was less frequent in Blocks 6 and 7, but again little or no aggression was seen between partners (figure 6b).
Figure 6.
Sociogram displaying the total frequency of aggressive behaviour between 11 hand-raised rooks (a) during Blocks 1–5, and (b) during Blocks 6–7. All symbols and lines are the same as figure 5.
(iii) Social support
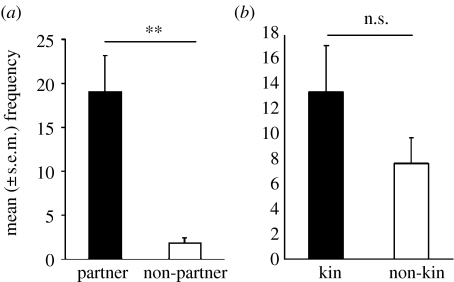
Individuals within a pair should be more likely to provide social support (agonistic aid) to one another than non-partners, so that their partner has a better chance of winning a contest, and possibly gaining access to a resource which both individuals in the pair can benefit (Cords 1997). In this group of juvenile rooks, individuals were more likely to provide agonistic support to their partner than a non-partner, and to a member of their peer group (i.e. who shared their cat box during hand-raising), but not to their kin (figure 7). Although two of the pairs were kin (41 & 42 and 46 & 50), choice was not significant. Interestingly, at sexual maturity these two pairs exchanged partners, so that 41 & 46 and 42 & 50 became male–female pairs who were not kin. This result suggests that the coalitions within a long-term alliance or partnership maybe akin to the pair bonds which develop at sexual maturity as they only choose non-kin as long-term partners.
Figure 7.
Mean ±s.e.m. frequency with which (a) a social partner provided social support during an agonistic encounter with another individual when compared with a non-partner, (b) kin provided social support compared with non-kin. **p<0.01 (t-test).
(b) The benefits of being in a partnership
This data suggest that young hand-raised rooks form selective partnerships with a specific individual at a very early age, which is not due to sexual reproduction. What are the benefits of forming a partnership so early in life? Although rooks do not become sexually mature until the second year of life, they have to procure a nest site which is not occupied by an established pair, and which may be suitable for the life of the pair. This does not explain why same sex pairs form, especially when there are sufficient members of the opposite sex with whom to form a partnership. Perhaps, the benefits of being in a pair, such as gaining access to resources usually unavailable to a singleton, outweigh the importance of choice of partner, especially when a more appropriate partner may be chosen later on. This is not the case for jackdaws who become sexually mature earlier than rooks (in the first breeding season) and which have to locate a nest site, and guard it throughout most of the year. There is little evidence for divorce or EPC in jackdaws, even after multiple instances of reproductive failure; therefore, the choice of the right partner is a more crucial decision than in rooks. It is not clear why jackdaws have to make this decision so early on in life, and why their choice of partner is so permanent.
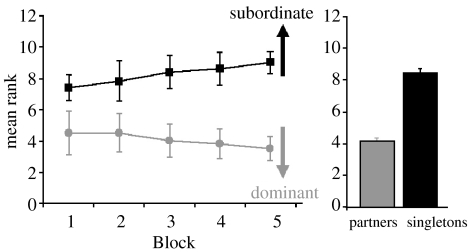
The most obvious benefit of being in a pair is to increase dominance, and the cascade of additional benefits this brings, which is the case for rooks. Figure 8 displays the mean rank of individual rooks in Blocks 1–5 based on whether they were in a pair or were singletons. Although the paired birds had a higher dominance than singletons throughout the observation period, the rank difference did increase over time suggesting the increase in status was a direct effect of becoming a pair.
Figure 8.
Mean ±s.e.m. social rank of partners compared with singletons (i.e. those individuals without a partner) across Blocks 1–5, and the same data collapsed across blocks. Blocks 6–7 are not represented as only one bird remained a singleton during this period.
(c) Reciprocity, interchange and the maintenance of pair bonds
Once a non-sexual partnership has become established, even though there are direct benefits by remaining in the pair (see above), the relationship needs to be maintained if it is to survive till sexual maturity. This is particularly important for the types of social partnerships described here, as the requirements for being in a pair after sexual maturity are relatively transparent (e.g. sexual fidelity, paternal certainty, help with parental care, etc.). Breaking a partnership too prematurely causes a loss of social status, loss of nest site, but also the search for a new partner. Although we suggested earlier that allopreening may be important for the maintenance of a partnership, studies of primate behaviour have proposed that it is the ‘trade’ between different behavioural commodities (such as grooming, sharing, social support, etc.) which forms the basis for a solid partnership (Seyfarth & Cheney 1984; de Waal & Lutrell 1988). We therefore examined the frequency with which three different affiliative behaviours (allopreening, food sharing and provision of social support) were provided to others or received from others across the different blocks. This resulted in a number of actor–receiver matrices, which were then analysed using correlational methods. As these social behaviours may be influenced by dominance status of the two individuals or their time spent in proximity, we used permutation procedures (Kr-test) to remove the effect of social status (Hemelrijk 1990, 1994; Hemelrijk & Ek 1991). Proximity could not be controlled as it was not directly recorded in our data; however informal observations suggest that pairs spend almost all their time in proximity.
As seen in table 2, there were significant correlations between giving food and receiving food (reciprocity) and receiving preening (interchange), providing social support and receiving food or preening (interchange), and initiating preening and receiving food (interchange). There were also significant correlations between dual caching, bill twining and mutual social support (i.e. the direction of behaviour cannot be determined). Surprisingly, this pattern of results was not repeated during Blocks 6–7 (table 2). One interpretation of these results is that a complex suite of interrelated affiliative behaviours are used exclusively by both members of a partnership in order to solidify their social bond. The resultant correlations are therefore an inevitable outcome of being in close proximity to, and interacting exclusively with one other individual. This pattern was not repeated in Blocks 6–7 possibly because these behaviours are used in different frequencies (figure 5). An alternative explanation is that each individual keeps a score of each food transfer, assistance in a fight or bout of preening, and reciprocates to a same degree, even with different behaviours of ‘equal value’. This mechanism would require some of the cognitive abilities discussed earlier (such as complex memory). We favour the first explanation, as being in an exclusive partnership is a mutual enterprise, in which the benefits cannot easily be quantified (such as increase in social status and therefore resources). This is not to say that reciprocity and interchange are outside of the repertoire of corvids, but that the social organization of rooks does not provide a stable platform for its evolution. We therefore need to collect similar kinds of data to this in other social corvids, which do not demonstrate a rook-like social organization.
Table 2.
Correlations between various affiliative behaviours (unidirectional—give food, receive food, initiate allopreening, receive allopreening, provide social support; bidirectional—dual caching, bill-twining) during Blocks 1–5 & Blocks 6–7; p<0.05.
| Blocks 1–5 | Blocks 6–7 | |||||||
|---|---|---|---|---|---|---|---|---|
| r2 | F | d.f. | p | r2 | F | d.f. | p | |
| give food–receive food | 0.45 | 7.48 | 1,9 | 0.023 | 0.03 | 0.3 | 1,9 | 0.6 |
| initiate preen–receive preen | 0.32 | 4.26 | 1,9 | 0.07 | 0.22 | 2.51 | 1,9 | 0.15 |
| provide support–receive food | 0.77 | 30.93 | 1,9 | 0.0004 | 0.2 | 2.20 | 1,9 | 0.17 |
| give food–receive preen | 0.45 | 7.5 | 1,9 | 0.023 | 0.004 | 0.03 | 1,9 | 0.86 |
| preen–receive food | 0.52 | 9.59 | 1,9 | 0.01 | 0.07 | 0.7 | 1,9 | 0.42 |
| provide support–receive preen | 0.68 | 18.75 | 1,9 | 0.002 | 0 | 0 | 1,9 | 1 |
| dual caching–bill-twining | 0.81 | 38.27 | 1,9 | 0.0002 | 0.1 | 0.98 | 1,9 | 0.35 |
| dual caching–sharing | 0.56 | 11.65 | 1,9 | 0.008 | 0.004 | 0.004 | 1,9 | 0.95 |
| dual caching–support | 0.76 | 28.49 | 1,9 | 0.0005 | 0.31 | 4.13 | 1,9 | 0.07 |
(d) Understanding third-party relationships
Evidence that rooks may understand third party relationships was provided by an analysis of ‘redirected aggression’ (RA). This usually takes the form of selective aggression against the kin of a previously aggressive individual in monkeys and apes. In monkeys, this type of behaviour has been recorded up to 1 h after the initial aggressive act had taken place and may form the basis for a ‘revenge’ system (Aureli et al. 1992). We recorded all occurrences of aggression (inc. displacement) towards third parties not involved in the fight for the immediate period after the fight (less than 3 s), and also recorded all occurrences in the hour after the fight. We recorded three forms of RA; the aggressor (X1) attacked the victim (Y1), then either (i) the victim's partner (Y2) attacked the aggressor (X1), (ii) the victim (Y1) attacked the aggressor's partner (X2) or (iii) the victim's partner (Y2) attacked the aggressor's partner (X2). We recorded a total of 19 acts of RA in the immediate period after a fight across all the blocks. Four acts (21.05%) appeared to be random acts of ‘frustration’ which did not include the partners of those involved; however a significantly greater number of acts (n=15; 78.95%) were partner-oriented (Binomial, p<0.01). There were differences in the frequency of the different forms of RA. The majority of acts, 9 (60%), followed the Y2→X1 form, 4 acts (26.67%) followed the Y1→X2 form and 2 acts (13.33%) followed the Y2→X2 form.
The number of aggressive acts which occurred in the hour after a fight (where possible, due to the total length of each data collection session) was 106. We need to be cautious about the causal role of the targeted fight in the subsequent acts of RA, especially in such a long period after the fight. However, we need to use the same caution when discussing data from monkeys and apes. Across all the blocks, 29 acts (27.4%) were of the Y2→X1 form, 47 acts (44.3%) were of the Y1→X2 form and 30 acts (28.3%) were of the Y2→X2. This data tentatively suggests (with the above caveats) that rooks (i) remember past interactions (and the protagonists of those interactions), (ii) recognize the affiliative partners of others, and (iii) may use this information to act upon previous acts of aggression in which they were the victims, possibly as a form of ‘revenge’.
(e) Managing valuable relationships
Post-conflict affiliation is unlikely to occur between individuals who do not have a valuable relationship (van Schaik & Aureli 2000; Aureli et al. 2002). Such a relationship does not necessarily have to be with only one individual; indeed, the only criteria should be that each individual benefits from maintaining the relationship (Silk 2002). As we have already seen, rooks form close partnerships with another individual, not necessarily of the same sex, which is categorized by the amount of time spent in close proximity, amount of affiliative behaviour directed exclusively towards their partner and level of support provided during agonistic encounters. Such partnerships may therefore be classified as the strongest form of valuable relationship (and particularly so when the individuals in the partnership become sexually mature). Frequency of aggression towards other non-affiliated members of the social group is relatively high (figure 6), but does not usually lead to sustained acts of physical aggression, rather taking the form of displacements and minor pecks. There is little or no aggression between affiliative partners (figure 6). Therefore, rooks in partnerships only form valuable relationships with one individual, their affiliative partner. The Valuable Relationship Hypothesis (van Schaik & Aureli 2000; Aureli et al. 2002) suggests that ‘post-conflict reunions should occur more often when the opponents are mutually valued social partners, because disturbance of a more valuable relationship entails a larger loss of benefits for both opponents’ (Aureli et al. 2002, p. 334). Therefore, if no aggression occurs between affiliative partners, then we would not expect to see reconciliation. As yet, we have not witnessed any occurrence of reconciliation within our colony of rooks (Seed et al. in press).
By contrast, aggression does occur between non-affiliated members of the social group (figure 6). Consolation has been defined as ‘contact between a recipient of aggression and an uninvolved bystander not long after the former was involved in a conflict’ (de Waal & Aureli 1996, p. 93), however this is a rather anthropomorphic, functional term, which may not represent what is actually going on inside the animal's mind. Furthermore, there is evidence that affiliative contacts between actors and third-parties does not in fact serve to reduce stress levels in chimpanzees and long-tailed macaques (Koski & Sterck 2007; Das 2000). We therefore use the less loaded term ‘third-party post-conflict affiliation’. We predicted that such affiliation should occur more frequently between affiliated partners post-conflict, either initiated by an individual involved in aggression or by an affiliated bystander, than in matched control conditions in which aggression did not occur. Indeed, in a study of rooks using the PC-MC method (post-conflict-matched control; de Waal & Yoshihara 1983), both initiators and targets of aggression engaged in third-party affiliation with a social partner at higher levels during the post-conflict period than in the matched control. Both former aggressors and uninvolved third-parties initiated affiliative contacts (Seed et al. in press). The function of third-party affiliation remains to be explicitly tested, both for rooks and most primates, although dampening the stress response and strengthening or signalling alliances seem to be likely candidates. Rooks, like chimpanzees (de Waal & Aureli 1996), appear to use a specific affiliative behaviour during the post-conflict period, which may aid in reducing stress. Like the embracing and kissing of chimpanzees, rooks employ a special form of physical contact, bill twining, with their partner in the first minute after a fight, and almost exclusively within this period and this context (Seed et al. in press).
Observations of the rooks' social interactions demonstrate a very rapid formation of selective long-term partnerships with individuals of either sex, normally a member of the same peer group, not kin. Membership of a partnership appeared to increase the social status of both partners. Food sharing was found within each partnership, and was both initiated and reciprocated. Sharing also increased the probability of receiving other affiliative behaviours, such as social support and preening. Partners also demonstrated RA towards the partners of those individuals that had aggressed against them or their partners in the immediate past and possibly up to 1 h after the fight.
These results suggest that rooks display many of the complex social traits so far only reported for hyenas, elephants, cetaceans, monkeys and apes (de Waal & Tyack 2003; Connor 2007; Holekamp et al. 2007; Silk 2007), but almost certainly dependent on different mechanisms. Certainly, the fact that rooks form strong bonds very early in development, which can be lifelong, suggests a different scenario from mammals that do not form such strong, long-term pair-bonds.
6. Cooperation and coordination
One important factor for the formation, maintenance and longevity of the rooks' partnerships is their ability to cooperate. This most likely occurs through mutualism, rather than reciprocity. Mutualism is what Dugatkin (1997) has termed ‘no cost cooperation’, in which both individuals receive an equal gain from cooperating, with little potential for cheating because if they do not cooperate, both individuals will lose as there can never only be one benefactor. This makes sense for long-term partnerships in which the benefits, such as increased dominance and access to resources, are accrued by both members of the pair. During pair formation, both individuals demonstrate their willingness to cooperate by offering food to their chosen partner (de Kort et al. 2003; 2006; von Bayern et al. 2005), allopreening, and offering assistance during agonistic encounters (social support) or affiliating post-conflict.
Once a pair has become established, the partners provide one another with resources or ‘commodities’, thereby establishing a biological market which maintains the partnership and the resultant mutual benefits (Nöe & Hammerstein 1995). In such biological markets, commodities tend to be exchanged (same commodity) or interchanged (different commodity). For example, Rook X preens his partner Rook Y, and subsequently Rook Y aids her partner Rook X in a fight at a later time. In this form of cooperation, there is no need for mental score-keeping as both individuals (by the fact that they have formed a long-term affiliative relationship) will benefit from these actions equally. The primary benefit is the stability of the relationship, and the resultant effects on dominance status and resource acquisition.
Similarly to cooperation, individuals in long-term partnerships become coordinated in their behaviour, such as synchronizing their body movements, social displays and vocalizations. Individual vocalizations may contain a ‘signature’ which is unique to the caller. For example, interindividual variation in the peak frequency, maximum frequency, duration, energy, bandwidth and minimum frequency was found in the contact calls of spectacled parrotlets (Wanker & Fischer 2001). Importantly, individuals within a particular social class shared the same call structure and preferentially responded to calls made by individuals within the same social class (i.e. adults discriminated their mate's call, sub-adults responded to their sibling's call; Wanker et al. 1998). Spectacled parrotlets also discriminate between the contact calls of a family member versus a non-family member and use different contact calls for different social companions (e.g. mate, offspring; Wanker et al. 2005).
It has been suggested that this form of vocal categorization occurs through socialization, with individuals of a particular social class with convergence upon the same call structure with increasing socialization (Brown & Farabaugh 1997). By this process, individuals which are either strongly bonded, such as monogamous pairs, or siblings or parents to their offspring spend a long time in proximity and rapidly converge on the same call structure (vocal imitation). For example, in budgerigars, pair-bonded individuals converge on the same contact call within two weeks of their initial pairing (Hile et al. 2000). Similar examples of vocal sharing occur, with the warble vocalizations within groups of Australian magpies where magpies in the same group shared more warble syllables than magpies in different groups (Brown et al. 1988), the non-territorial song of American crows (Brown 1985) and the contact calls of budgerigars (Farabaugh et al. 1994).
Although we did not explicitly record whether the partners in our rook colony coordinated their actions, our casual observations of the pairs (now 4+ years old) have recorded many examples of motor imitation (figure 9; see electronic supplementary material, video file 1 for an example). One striking example is the bowing and tail-fanning display in which one rook (usually the male) bows their head while loudly cawing (Coombs 1978). After bowing, their tail becomes raised and is then fanned out, with the wings kept close to the body. The rook then slowly raises its head and repeats this pattern, with renewed cawing vocalizations. Interestingly, paired individuals either alternate which element of the display they produce (usually if facing one another while greeting) or produce the elements in concert if directing the display towards a rival or a predator. It has been suggested that such a display may function in reinforcing the bond within the pair if they come together after being separated or it may function as a display of the bond for others in the social group (Armstrong 1965; Wickler 1976; Zahavi 1976; Wachtmeister 2001).
Figure 9.
Still frames ***(a–i) from a short film clip of one rook pair synchronizing or co-ordinating their behaviour. Frames (a–c), bowing; Frames (d–f), bill-wiping; and Frames (g–i), head-turning. See electronic supplementary material, video file 1.
7. A reassessment of social complexity
What does this new data on rooks tell us about social complexity and its relationship to the evolution of the avian brain? Perhaps the concept of social complexity as proposed by the traditional SBH needs to be reassessed, at least for birds. Even Dunbar himself suggested that ‘the SBH implies that constraints on group size arise from the information-processing capacity of the primate brain, and that the neocortex plays a role in this. However, even this proposal is open to several interpretations as to how the relationship is mediated’ (Dunbar 1998, p. 184, our italics). Dunbar (1998) suggested that the constraint on group size could be mediated by the ability to recognize individuals and the unique pattern of their behaviour, the ability to remember who is in a relationship with whom, the ability to manipulate this information (e.g. in managing relationships or using social information in a deceptive manner) or the ability to recognize and respond appropriately to others' emotional and mental states. In assessing the evidence for primates, Dunbar (1998) stated that the only ability which appeared to explain the relationship between brain and social complexity was the ability to manage social information. We would concur with this assessment, and expand the suggestion to include birds in long-term monogamous relationships.
In long-term monogamous birds, there may be little requirement to remember the individual characteristics of hundreds of individuals, even when forming large foraging flocks or roosts. Rather, keeping track of the accumulating, subtle behavioural characteristics of a bonded partner over the course of a relationship requires a different kind of processing: a form of relationship intelligence, which enables them to accurately read the social signals of their partner, respond appropriately to them, thereby predicting their behaviour and hence resulting in the stability of a successful partnership with mutual benefits for both parties. This suggestion does not eliminate processing social information altogether, as monogamous pairs do not live in a social vacuum (as suggested by the rook data presented earlier). On the contrary, it is likely that ‘relationship intelligence’ is a response to environmental complexity, and living in a social environment may provide additional challenges (such as competition over resources and nest sites) to which the maintenance of a strong pair bond throughout the year provides a cooperative solution. Similarly, ecological pressures may favour the evolution of this type of social intelligence in long-term monogamous birds.
This form of social intelligence has not been readily addressed in social primates, or other social mammals for that matter, largely because most adult primates do not form the same strong attachments to one individual (except monogamous mammals). Future studies need to address whether monogamous birds, such as rooks, really are ‘in tune’ with their partner when compared with other members of their social group as we suggest, and whether this requires cognitive abilities which are not found in polygamous species.
8. Conclusions
Although rooks (and possibly other large-brained birds) may have evolved similar socio-cognitive abilities to primates, such forms of social knowledge appear to be used primarily within the context of the pair bond, rather than applied to a larger social network, such as found in primates. Long-term monogamy depends on different forms of social information processing compared with polygyny (the most common mammalian mating system). For example, recognizing the subtle social signals produced by a partner and using such information to predict their future behaviour suggests different social skills than remembering who did what to whom. Indeed, long-term pair-bonded species, including those which form cooperative breeding groups, appear to have the largest brains within birds. There are differences between long-term monogamous species, in both brain size and the complexity of their partnerships, something which is highlighted by the social relationships of young rooks. The question of whether these partnerships are cognitively sophisticated, particularly whether pairs have an advantage in behaviour-reading (especially when their partner is the one providing the social cues), remains to be tested.
Acknowledgments
The importance of relationship intelligence in pair bonding is supported by the fact that the first and last authors are married, and were still together by the end of writing. The original research described in this paper was supported by grants from the BBSRC, Royal Society and University of Cambridge. Nathan Emery was supported by a Royal Society University Research Fellowship, Amanda Seed by a BBSRC studentship and an ASAB summer studentship, and Auguste von Bayern by a BBSRC studentship, the German Academic Exchange Service (DAAD), Cambridge European Trust and the Balfour Fund. Many thanks to Selvino de Kort for the help in hand raising and collecting some of the rook social behaviour data, and Richard Broughton for collecting data on avian social and mating systems. We also thank Kurt Kotrschal for useful discussion of geese sociality. All research conformed to UK Home Office and University of Cambridge regulations governing animal research.
Footnotes
One contribution of 19 to a Dicussion Meeting Issue ‘Social intelligence: from brain to culture’.
Supplementary Material
Frames i.–iii., bowing; Frames iv.–vi., bill-wiping and Frames vii.–ix., head-turning
References
- Armstrong E.A. Bird display and behaviour. Dover Publications Inc; New York: 1965. [Google Scholar]
- Aureli F, Cozzolino R, Cordischi C, Scucchi S. Kin-oriented redirection among Japanese macaques: an expression of a revenge system? Anim. Behav. 1992;44:283–291. doi:10.1016/0003-3472(92)90034-7 [Google Scholar]
- Aureli F, Cords M, van Schaik C.P. Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 2002;64:325–343. doi:10.1006/anbe.2002.3071 [Google Scholar]
- Baglione V, Canestrari D, Marcos J.M, Greisser M, Ekman J. History, environment and social behaviour: experimentally induced cooperative breeding in the carrion crow. Proc. R. Soc. B. 2002a;296:1247–1251. doi: 10.1098/rspb.2002.2016. doi:10.1098/rspb.2002.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglione V, Marcos J.M, Canestrari D. Cooperatively breeding groups of carrion crow, Corvus corone corone in northern Spain. Auk. 2002b;119:790–799. doi:10.1642/0004-8038(2002)119[0790:CBGOCC]2.0.CO;2 [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadee. Proc. Natl Acad. Sci. USA. 1994;91:11 217–11 221. doi: 10.1073/pnas.91.23.11217. doi:10.1073/pnas.91.23.11217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Mishal A, Nottebohm F. Social and spatial regimes for new neurons in two regions of the adult brain: an anatomical representation of time? Behav. Brain Res. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. doi:10.1016/j.bbr.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Barton R.A. Neocortex size and behavioural ecology in primates. Proc. R. Soc. B. 1996;263:173–177. doi: 10.1098/rspb.1996.0028. doi:10.1098/rspb.1996.0028 [DOI] [PubMed] [Google Scholar]
- Barton R.A, Dunbar R.I.M. Evolution of the social brain. In: Whiten A, Byrne R.W, editors. Machiavellian intelligence II. Cambridge University Press; Cambridge, UK: 1997. pp. 240–263. [Google Scholar]
- Beauchamp G, Fernandez-Juricic E. Is there a relationship between forebrain size and group size in birds? Evol. Ecol. Res. 2004;6:833–842. [Google Scholar]
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life-history, mating system and extinction. [Google Scholar]
- Bharati L.S, Goodson J.L. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. doi:10.1016/j.neuroscience.2006.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.D. Social relationship as a variable affecting responses to mobbing and alarm calls of common crows (Corvus brachyrhynchos) Zeitschrift fur Tierpsychologie. 1985;70:45–52. [Google Scholar]
- Brown E.D, Farabaugh S.M. What birds with complex social relationships can tell us about vocal learning: vocal sharing in avian groups. In: Snowdon C.T, Hausberger M, editors. Social influences on vocal development. Cambridge University Press; Cambridge, UK: 1997. pp. 98–127. [Google Scholar]
- Brown E.D, Farabaugh S.M, Veltman C.J. Song sharing in a group-living songbird, the Australian magpie, Gymnorhina tibcen. I. Vocal sharing within and among groups. Behaviour. 1988;104:1–28. [Google Scholar]
- Burish M.J, Kueh H.Y, Wang S.S.-H. Brain architecture and social complexity in modern and ancient birds. Brain Behav. Evol. 2004;63:107–124. doi: 10.1159/000075674. doi:10.1159/000075674 [DOI] [PubMed] [Google Scholar]
- Byrne R.W, Whiten A, editors. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes and humans. Clarendon Press; Oxford, UK: 1988. [Google Scholar]
- Carmen W.J. Non-cooperative breeding in the California scrub-jay. Stud. Avian Biol. 2004:28. [Google Scholar]
- Clayton N.S, Krebs J.R. Hippocampal growth and attrition in birds affected by experience. Proc. Natl Acad. Sci. USA. 1994;91:7410–7414. doi: 10.1073/pnas.91.16.7410. doi:10.1073/pnas.91.16.7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N.S, Reboreda J.C, Kacelnik A. Seasonal changes of hippocampal volume in parasitic cowbirds. Behav. Process. 1997;41:237–243. doi: 10.1016/s0376-6357(97)00050-8. doi:10.1016/S0376-6357(97)00050-8 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Dally J.M, Emery N.J. Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Phil. Trans. R. Soc. B. 2007;362:507–522. doi: 10.1098/rstb.2006.1992. doi:10.1098/rstb.2006.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor R.C. Dolphin social intelligence: complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Phil. Trans. R. Soc. B. 2007;362:587–602. doi: 10.1098/rstb.2006.1997. doi:10.1098/rstb.2006.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs C.F.G. Observations on the rook, Corvus frugilegus, in southwest Cornwall. Ibis. 1960;102:394–419. [Google Scholar]
- Coombs C.F.G. Batsford; London, UK: 1978. The crows. [Google Scholar]
- Cords M. Friendships, alliances, reciprocity and repair. In: Whiten A, Byrne R.W, editors. Machiavellian Intelligence II: extensions and evaluations. Cambridge University Press; Cambridge, UK: 1997. pp. 24–49. [Google Scholar]
- Cramp S. Ostrich to ducks. vol. I. Oxford University Press; Oxford, UK: 1977. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic. [Google Scholar]
- Cramp S, Perrins C. Crows to finches. vol. VIII. Oxford University Press; Oxford, UK: 1994. Handbook of the birds of Europe, the Middle East and North Africa. The birds of the Western Palearctic. [Google Scholar]
- Curry R.L, Towsend Peterson A, Langen T.A. Western Scrub-Jay. In: Poole A, Gill F, editors. The birds of North America, no. 712. The Birds of North America, Inc; Philadelphia, PA: 2002. pp. 1–36. [Google Scholar]
- de Kort S.R, Emery N.J, Clayton N.S. Food offering in jackdaws (Corvus monedula) Naturwissenschaften. 2003;90:238–240. doi: 10.1007/s00114-003-0419-2. [DOI] [PubMed] [Google Scholar]
- de Kort S.R, Emery N.J, Clayton N.J. Food sharing in jackdaws, Corvus monedula: what, why and with whom? Anim. Behav. 2006;72:297–304. doi:10.1016/j.anbehav.2005.10.016 [Google Scholar]
- de Waal F.B.M. John Hopkins University Press; Baltimore, MD: 1982. Chimpanzee politics. [Google Scholar]
- de Waal F.B.M, Aureli F. Consolation, reconcilation, and a possible cognitive difference between macaques and chimpanzees. In: Russon A.E, Bard K.A, Parker S.T, editors. Reaching into thought: the minds of the great apes. Cambrisge University Press; Cambridge, UK: 1996. pp. 80–110. [Google Scholar]
- de Waal F.B.M, Lutrell L.M. Mechanisms of social reciprocity in three primate species: symmetrical relationship characteristics or cognition? Ethol. Sociobiol. 1988;9:101–118. doi:10.1016/0162-3095(88)90016-7 [Google Scholar]
- de Waal F.B.M, Tyack P.L, editors. Animal social complexity: intelligence, culture and individualised societies. Harvard University Press; Cambridge, MA: 2003. [Google Scholar]
- de Waal F.B.M, Yoshihara D. Reconcilation and redirected affection in rhesus monkeys. Behaviour. 1983;85:224–241. [Google Scholar]
- Das M. Conflict management via third-Parties: post-conflict affiliation of the aggressor. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. University of California Press; Berkeley, CA: 2000. [Google Scholar]
- Dugatkin L.A. Cooperation among animals. Oxford University Press; New York: 1997. [Google Scholar]
- Dunbar R.I.M. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;20:469–493. doi:10.1016/0047-2484(92)90081-J [Google Scholar]
- Dunbar R.I.M. The social brain hypothesis. Evol. Anthropol. 1998;6:178–190. doi:10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8 [Google Scholar]
- Dunbar R.I.M, Bever J. Neocortex size determines group size in insectivores and carnivores. Ethology. 1998;104:695–708. [Google Scholar]
- Emery N.J. Are corvids ’feathered apes’? Cognitive evolution in crows, jays, rooks and jackdaws. In: Watanabe S, editor. Comparative analysis of minds. Keio University Press; Tokyo, Japan: 2004. pp. 181–213. [Google Scholar]
- Emery N.J. Cognitive ornithology: the evolution of avian intelligence. Phil. Trans. R. Soc. B. 2006;361:23–43. doi: 10.1098/rstb.2005.1736. doi:10.1098/rstb.2005.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery N.J, Clayton N.S. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–1907. doi: 10.1126/science.1098410. doi:10.1126/science.1098410 [DOI] [PubMed] [Google Scholar]
- Farabaugh S.M, Linzenbold A, Dooling R.J. Vocal plasticity in budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. J. Comp. Psychol. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. doi:10.1037/0735-7036.108.1.81 [DOI] [PubMed] [Google Scholar]
- Fischer H. Das Triumphgeschrei der Graugans (Anser anser) Zeitschrift fur Tierpsychologie. 1965;22:247–304. [PubMed] [Google Scholar]
- Frigerio D, Weiss B.M, Kotrschal K. Spatial proximity among adult siblings in greylag geese (Anser anser): evidence for female bonding? Acta Ethologica. 2001;3:121–125. doi:10.1007/s102110000028 [Google Scholar]
- Frigerio D, Weiss B.M, Dittami J, Kotrschal K. Social allies modulate corticosterone excretion and increase success in agonistic interactions in juvenile hand-raised greylag geese (Anser anser) Can. J. Zool. 2003;81:1746–1754. doi:10.1139/z03-149 [Google Scholar]
- Godfrey-Smith P. Cambridge University Press; Cambridge, UK: 1996. Complexity and the function of mind in nature. [Google Scholar]
- Goodson J.L. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. doi:10.1016/j.yhbeh.2005.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J.L, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc. Natl Acad. Sci. USA. 2006;103:17 013–17 017. doi: 10.1073/pnas.0606278103. doi:10.1073/pnas.0606278103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson J.L, Evans A.K, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm. Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. doi:10.1016/j.yhbeh.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. British Museum (Natural History) Press; London, UK: 1986. Crows of the world. [Google Scholar]
- Harcourt A.H. Coalitions and alliances: are primates more complex than non-primates? In: Harcourt A.H, de Waal F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford University Press; Oxford, UK: 1992. pp. 445–471. [Google Scholar]
- Harrison C.J.O. Allopreening as agonistic behaviour. Behaviour. 1967;24:161–209. [Google Scholar]
- Heinrich B. Barrie & Jenkins; London, UK: 1990. Ravens in winter. [Google Scholar]
- Heinrich B. Harper Collins; New York, NY: 1999. Mind of the raven. [Google Scholar]
- Hemelrijk C. Models of, and tests for, reciprocity, unidirectionality and other social interaction patterns at a group level. Anim. Behav. 1990;39:1013–1029. doi:10.1016/S0003-3472(05)80775-4 [Google Scholar]
- Hemelrijk C. Support for being groomed in long-tailed macaques, Macaca fascicularis. Anim. Behav. 1994;48:479–481. doi:10.1006/anbe.1994.1264 [Google Scholar]
- Hemelrijk C, Ek A. Reciprocity and interchange of grooming and ’support’ in captive chimpanzees. Anim. Behav. 1991;41:923–935. doi:10.1016/S0003-3472(05)80630-X [Google Scholar]
- Henderson I.G, Hart P.J.B. Reproductive success in jackdaws, Corvus monedula, provisioning as a constraint on the mating system. Ornis Scand. 1993;24:142–148. [Google Scholar]
- Henderson I.G, Hart P.J.B, Burke T. Strict monogamy in a semi-colonial passerine: the jackdaw Corvus monedula. J. Avian Biol. 2000;31:177–182. doi:10.1034/j.1600-048X.2000.310209.x [Google Scholar]
- Hile A.G, Plummer T.K, Striedter G.F. Male vocal imitation produces call convergence during pair bonding in budgerigars, Melopsittacus undulatus. Anim. Behav. 2000;59:1209–1218. doi: 10.1006/anbe.1999.1438. doi:10.1006/anbe.1999.1438 [DOI] [PubMed] [Google Scholar]
- Holekamp K.E, Sakai S.T, Lundrigan B.L. Social intelligence in the spotted hyena (Crocuta crocuta) Phil. Trans. R. Soc. B. 2007;362:523–528. doi: 10.1098/rstb.2006.1993. doi:10.1098/rstb.2006.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey N.K. The social function of intellect. In: Bateson P.P.G, Hinde R.A, editors. Growing points in ethology. Cambridge University Press; Cambridge, UK: 1976. pp. 303–317. [Google Scholar]
- Insel T.R, Wang Z.X, Ferris C.F. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J. Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk A.N, Arnold K.E. Is cooperative breeding associated with bigger brains? A comparative test in the corvida (Passeriformes) Ethology. 2004;110:203–220. doi:10.1111/j.1439-0310.2003.00957.x [Google Scholar]
- Iwaniuk A.N, Nelson J.E. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 2003;81:1913–1928. doi:10.1139/z03-190 [Google Scholar]
- Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153:501–507. doi: 10.1126/science.153.3735.501. doi:10.1126/science.153.3735.501 [DOI] [PubMed] [Google Scholar]
- Juniper T, Parr M. Yale University Press; New Haven, CT: 1998. Parrots: a guide to parrots of the world. [Google Scholar]
- Katzir G. Relationships between social structure and response to novelty in captive jackdaws, Corvus monedula. II. Response to novel palatable foods. Behaviour. 1983;83:183–208. [Google Scholar]
- Koski S.E, Sterck E.H.M. Triadic postconflict affiliation in captive chimpanzees: does consolation console? Anim. Behav. 2007;73:133–142. doi:10.1016/j.anbehav.2006.04.009 [Google Scholar]
- Kummer H. On the value of social relationships to nonhuman primates: a heuristic scheme. Soc. Sci. Inf. 1978;17:687–705. [Google Scholar]
- Lack D. Methuen; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Lamprecht J. Factors influencing leadership: a study of goose families (Anser indicus) Ethology. 1991;89:265–274. [Google Scholar]
- Lefebvre L, Whittle P, Lascaris E, Finkelstein A. Feeding innovations and forebrain size in birds. Anim. Behav. 1997;53:549–560. doi:10.1006/anbe.1996.0330 [Google Scholar]
- Lefebvre L, Nicolakakis N, Boire D. Tools and brains in birds. Behaviour. 2002;139:939–973. doi:10.1163/156853902320387918 [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav. Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. doi:10.1016/S0166-4328(01)00416-8 [DOI] [PubMed] [Google Scholar]
- Lorenz K. Studies in animal and human behaviour. Methuen & Co Ltd; London, UK: 1970. Contributions to the study of the ethology of social Corvidae (1931) pp. 1–56. [Google Scholar]
- Lorenz K. Harper Collins; London, UK: 1991. Here I am—where are you? The behaviour of the greylag goose. [Google Scholar]
- Marino L. Convergence of complex cognitive abilities in cetaceans and primates. Brain Behav. Evol. 2002;59:21–32. doi: 10.1159/000063731. doi:10.1159/000063731 [DOI] [PubMed] [Google Scholar]
- Marler P. Social cognition: are primates smarter than birds? In: Nolan V, Ketterson E.D, editors. Current ornithology. Plenum Press; New York, NY: 1996. pp. 1–32. [Google Scholar]
- Marshall A.J, Coombs C.F.J. The interaction of environmental, internal and behavioural factors in the rook, Corvus frugilegus. Proc. Zool. Soc. 1957;128:545–589. [Google Scholar]
- McComb K. Matriarchs as repositories of social knowledge in African elephants. Science. 2001;292:491–494. doi: 10.1126/science.1057895. [DOI] [PubMed] [Google Scholar]
- McGowan K.J, Woolfenden G.E. A sentinel system in the Florida scrub jay. Anim. Behav. 1989;37:1000–1006. doi:10.1016/0003-3472(89)90144-9 [Google Scholar]
- Noe R, Hammerstein R. Biological markets. Trends Ecol. Evol. 1995;10:336–339. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. doi:10.1126/science.7313697 [DOI] [PubMed] [Google Scholar]
- Patterson I.J, Grace E.S. Recruitment of young rooks, Corvus frugilegus, into breeding populations. J. Appl. Ecol. 1984;53:559–572. [Google Scholar]
- Perrins C. Oxford University Press; Oxford, UK: 2003. The new encyclopedia of birds. [Google Scholar]
- Potts R. Paleoenvironmental basis of cognitive evolution in great apes. Am. J. Primatol. 2004;62:209–228. doi: 10.1002/ajp.20016. doi:10.1002/ajp.20016 [DOI] [PubMed] [Google Scholar]
- Reboreda J.C, Clayton N.S, Kacelnik A. Species and sex differences in hippocampus size between parasitic and non-parasitic cowbirds. NeuroReport. 1996;7:505–508. doi: 10.1097/00001756-199601310-00031. doi:10.1097/00001756-199601310-00031 [DOI] [PubMed] [Google Scholar]
- Reichard U.H. Monogamy: past and present. In: Reichard U.H, Boesch C, editors. Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press; Cambridge, UK: 2003. pp. 3–25. [Google Scholar]
- Richards P.R. Pair formation and pair bond in captive rooks. Bird Study. 1976;23:207–212. [Google Scholar]
- Roell A. Social behaviour of the jackdaw, Corvus monedula, in relation to its niche. Behaviour. 1978;64:1–124. [Google Scholar]
- Røskaft E. Reactions of rooks, Corvus frugilegus, during the breeding season to intrusions by other birds and mammals. Fauna norv. Ser. C. Cinclus. 1980;3:56–59. [Google Scholar]
- Røskaft E. The daily activity pattern of the rook, Corvus frugilegus, during the breeding season. Fauna norv. Ser. C. Cinclus. 1981;4:76–81. [Google Scholar]
- Røskaft E. Male promiscuity and female adultery by the rook Corvus frugilegus. Ornis Scand. 1983;14:175–179. [Google Scholar]
- Scheiber I.B.R, Weiss B.M, Frigerio D, Kotrschal K. Active and passive social support in families of greylag geese (Anser anser) Behaviour. 2005;142:1535–1557. doi: 10.1163/156853905774831873. doi:10.1163/156853905774831873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed, A. M., Clayton, N. S. & Emery, N. J. In press. Third-party, post-conflict affiliation in rooks (Corvus frugilegus). Curr. Biol [DOI] [PubMed]
- Seyfarth R.M, Cheney D.L. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. doi:10.1038/308541a0 [DOI] [PubMed] [Google Scholar]
- Shultz S, Dunbar R.I.M. Both social and ecological factors predict ungulate brain size. Proc. R. Soc. B. 2006;273:207–215. doi: 10.1098/rspb.2005.3283. doi:10.1098/rspb.2005.3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. Using the ’F’-word in primatology. Behaviour. 2002;139:421–446. doi:10.1163/156853902760102735 [Google Scholar]
- Silk J.B. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. doi:10.1098/rstb.2006.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterelny K. Blackwell; New York, NY: 2003. Thought in a hostile world. [Google Scholar]
- Stevens J.R, Hauser M.D. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. doi:10.1016/j.tics.2003.12.003 [DOI] [PubMed] [Google Scholar]
- van Schaik C.P, Aureli F. The natural history of valuable relationships in primates. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. University of California Press; Berkeley, CA: 2000. pp. 307–333. [Google Scholar]
- van Schaik C.P, Deaner R.O. Life history and cognitive evolution in primates. In: de Waal F.B.M, Tyack P.L, editors. Animal social complexity. Harvard University Press; Cambridge, MA: 2003. pp. 5–25. [Google Scholar]
- von Bayern, A. M. P., de Kort, S. R., Clayton, N. S. & Emery, N. J. 2005 Frequent food- and object-sharing during jackdaw (Corvus monedula) socialization. Poster presented at the XXIX International Ethological Conference, Budapest, Hungary.
- Voronov L.N, Bogoslovskaya L.G, Markova E.G. A comparative study of the morphology of forebrain in corvidae in view of their trophic specialization. Zool. Z. 1994;73:82–96. [Google Scholar]
- Wachtmeister C.A. Display in monogamous pairs: a review of empirical data and evolutionary explanations. Anim. Behav. 2001;61:861–868. doi:10.1006/anbe.2001.1684 [Google Scholar]
- Wanker R, Fischer J. Intra- and inter-individual variation in the contact calls of spectacled parrotlets (Forpus conspicillatus) Behaviour. 2001;138:709–726. doi:10.1163/156853901752233361 [Google Scholar]
- Wanker R, Apcin J, Jennerjahn B, Waibel B. Discrimination of different social companions in spectacled parrotlets (Forpus conspicillatus): evidence for individual vocal recognition. Behav. Ecol. Sociobiol. 1998;43:197–202. doi:10.1007/s002650050481 [Google Scholar]
- Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Anim. Behav. 2005;70:111–118. doi:10.1016/j.anbehav.2004.09.022 [Google Scholar]
- Wechsler B. Measuring relationships in jackdaws. Ethology. 1989;80:307–317. [Google Scholar]
- Whiten A, Byrne R.W. Cambridge University Press; Cambridge, UK: 1997. Machiavellian intelligence II: extensions and evaluations. [Google Scholar]
- Wickler W. The ethological analysis of attachment: sociometric, motivational and sociophysiological aspects. Zeitschrift für Tierpsycholgie. 1976;42:12–28. [PubMed] [Google Scholar]
- Winkler H, Christie D.A, Nurney D. Houghton Mifflin; Boston, MA: 1995. Woodpeckers: an identification guide to the woodpeckers of the world. [Google Scholar]
- Woolfenden G.E, Fitzpatrick J.W. Princeton University Press; Princeton, NJ: 1984. The Florida scrub jay. [Google Scholar]
- Young L.J, Lim M.M, Gingrich B, Insel T.R. Cellular mechanisms of social attachment. Horm. Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. doi:10.1006/hbeh.2001.1691 [DOI] [PubMed] [Google Scholar]
- Zahavi A. The testing of a bond. Anim. Behav. 1976;25:246–247. doi:10.1016/0003-3472(77)90089-6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frames i.–iii., bowing; Frames iv.–vi., bill-wiping and Frames vii.–ix., head-turning