Abstract
Food-caching corvids hide food, but such caches are susceptible to pilfering by other individuals. Consequently, the birds use several counter strategies to protect their caches from theft, e.g. hiding most of them out of sight. When observed by potential pilferers at the time of caching, experienced jays that have been thieves themselves, take further protective action. Once the potential pilferers have left, they move caches those birds have seen, re-hiding them in new places. Naive birds that had no thieving experience do not do so. By focusing on the counter strategies of the cacher when previously observed by a potential pilferer, these results raise the intriguing possibility that re-caching is based on a form of mental attribution, namely the simulation of another bird's viewpoint. Furthermore, the jays also keep track of the observer which was watching when they cached and take protective action accordingly, thus suggesting that they may also be aware of others' knowledge states.
Keywords: experience projection, food caching birds, mental attribution, social intelligence, theory-of-mind, western scrub-jay
1. Introduction
A number of animals live in social groups, and indeed most primates do so. But according to the social function of intellect hypothesis (Humphrey 1976a), it is the ability to survive the political dynamics of a complex social world that has been the primary driving force shaping primate intelligence. Humphrey (1976b, p. 313) likened the dynamics of social life to a game of chess, but one in which the players are not purely selfish because ‘the selfishness of social animals is tempered by sympathy…a tendency on the part of one's social partner to identify himself with the other and so make the other's goals to some extent his own’. This hypothesis about social intelligence was also described independently by Jolly (1966, p. 506), who pointed out that ‘primate social life provided the evolutionary context of primate intelligence’.
Field observations of groups of lemurs (Lemur catta), gorillas (Gorilla gorilla gorilla) and macaques (Macaca mulatta) led both Humphrey and Jolly to suggest that the physical problems that primates encounter are not very different from those of other animals; what makes primates special is the complexity of their social lives. One critical aspect of this social complexity or ‘primate politics’ is the ability to keep track of who did what to you, where and when, and to use this information to predict the actions and intentions of other individuals in your social network (Humphrey 1980), as well as understanding how these relationships change over time (Barrett et al. 2003). Consequently, the need for effective competition and cooperation with conspecifics may have provided the main selective advantage for the evolution of primate intelligence (Byrne & Whiten 1988; Dunbar 1998). Indeed, Humphrey (1980) argued that self-consciousness evolved to enable primates to attribute mental states to other conspecifics and thereby anticipate the actions of those individuals, and that mental attribution is essential for coping with the trials and tribulations of complex social life. This in turn sets the stage for the development of Machiavellian intelligence, the ability to manipulate and deceive competitors (Byrne & Whiten 1988; Whiten & Byrne 1997).
In an extension of the social intelligence hypothesis, Dunbar (1992) suggested that the complexities of primate social life also led to a dramatic expansion of the neocortex to support this cognitive demand. Evidence in support of this social brain hypothesis comes from the finding that the ‘neocortex ratio’ (i.e. the volume of the neocortex divided by the volume of the rest of the brain) correlates positively with average group size in the primate genera. Dunbar (1992, 1998) argued that group size is a good indicator of social complexity in primates because the larger the group the more information conspecifics within that group have to remember in order to keep track of the dynamics of their social world.
Furthermore, there is an exponential increase in the amount of possible interactions and thus the information to be processed when individuals experience polyadic encounters than when individuals only interact with one other individual. Owing to the increased complexity of social networks in larger groups, Dunbar (1992) argued that the relative size of the neocortex served as a constraint on the evolution of group size. Subsequent work has established that relative neocortex size correlates with at least three other indices of sociality, namely the size of the grooming clique (Kudo & Dunbar 2001), the amount of social play (Lewis 2000) and with the frequency of tactical deception (Byrne & Corp 2004).
Although this increase in intelligence in primates was thought to have evolved with the social domain, it is considered to have led to conceptual and inferential processes that transcend a purely social setting. Indeed, Humphrey (1976b, p. 316) has argued that ‘styles of thinking which are primarily suited to social problem solving colour the behaviour of man and other primates even towards the inanimate world’. For example, many primates live in social groups that involve dominance hierarchies between group members and it is argued that primates are capable of observing dyadic relations and inferring complete dominance hierarchies from these observations.
To do so, the animals must be capable of making transitive inferences, using information about known dyadic relationships and applying the rule to novel pairs of relationships. For example, if A is known to be dominant over B and B is known to be dominant over C, then it follows that A must also be dominant over C. Primates can solve abstract transitive inference tests using symbols. Individuals are first trained on an ordered set of various pairwise comparisons (e.g. A+B−, B+C−, etc.). On test, they must transfer information about the dyadic relationships to novel pairs (e.g. B versus D) to solve the task (McGonigle & Chalmers 1977; Treichler & van Tilburg 1996). What is common to these various transfer tasks is the ability to abstract general rules or relationships that transcend the basic learning experience.
Although the social intelligence hypothesis was developed for primates, it is based on general evolutionary principles and consequently it should apply to other groups of large brained animals that face similar ecological challenges (Marino 2002; Emery & Clayton 2004a). Some support for this argument comes from the finding that relative neocortex size correlates with group size in other mammalian groups (e.g. Barton & Dunbar 1997). In principle, this relationship could also apply to more phylogenetically diverse groups of animals provided they possess a neural structure functionally analogous to the mammalian six-layered neocortex, such as the nidopallium of birds, the so-called avian prefrontal cortex (Avian Brain Consortium 2005; Emery & Clayton 2005).
As Emery et al. (2006) discuss, the relationship between brain size and social complexity is far from clear in birds (e.g. Burish et al. 2004, cf. Beauchamp & Fernández-Juricic 2004; Iwaniuk & Arnold 2004), perhaps because not all species that live in large groups form complex social networks and some species that are semi-territorial, such as the western scrub-jay (Aphelocoma californica), are thought to have quite complex social cognition (e.g. Emery & Clayton 2001; Dally et al. 2006b). Furthermore, the size and composition of the social group often changes seasonally, and may differ between individuals within a species. In western scrub-jays, for example, the breeding adults form selective pairbonds, and these pairs are territorial, but there are also variable numbers of floaters and flocked non-breeders. As Curry et al. (2002, p. 18) point out ‘frequent interactions with neighbouring territory holders and prolonged associations with some floaters may result in more complicated social networks than appears superficially’.
A further prediction arising from the social intelligence hypothesis is that animals which show high degrees of general intelligence, however phylogenetically diverse from primates, should also be socially astute. In this article, we shall focus on the cognitive capacities of corvids and argue that this family of food-caching birds provides a compelling case for studying social cognition owing to their high degree of general intelligence and their relatively large brains with expanded avian prefrontal cortex (nidopallium). In order to do so, we shall begin with a discussion of the general biology of corvids, and what features they share in common with primates.
2. Corvid biology and brain
The corvids are a family of songbirds that includes not only the black plumaged crows, ravens and rooks, but also the more brightly and variably coloured jays, magpies and nutcrackers. Like primates, many corvids have complex social lives and are among the most social groups of birds (Goodwin 1986). For example, in the cooperatively breeding Florida scrub-jay (Aphelocoma coerulescens), several closely related family members share the responsibility of raising the young with the parents (Woolfenden 1975; Woolfenden & Fitzpatrick 1984, 1996). A closely related species, the western scrub-jay, typically shows unassisted pair-breeding, although populations in southern Mexico breed cooperatively just as the Florida scrub-jays do (Burt & Peterson 1993; Curry et al. 2002). The breeding behaviour of carrion crows (Corvus corone) is also flexible, as a single population may comprise both unassisted pair-breeders and cooperatively breeding birds (Richner 1990; Baglione et al. 2002).
Rooks and jackdaws are probably the most social among the corvids, congregating in large colonies during the breeding season (Goodwin 1976). Emery (2004) has argued that the social societies in which these birds live share several features in common with chimpanzees. As Emery et al. (in press) discuss, they live in a fission–fusion society, form long-term alliances with other members of their group, and understand ‘third-party’ relationships (i.e. those among other individuals), an ability that is thought to be one of the pinnacles of primate cognition (Tomasello & Call 1997). Another similarity between primates and corvids is that the young experience a long developmental period in which the juveniles associate with many non-relatives as well as kin, and this provides increased opportunities for learning from many different group members (Joffe 1997).
Like primates, and in common with most other groups of birds, the corvids are also highly visual. Brothers (1990, p. 29) argued that, in primates, the evolutionary switch to a diurnal lifestyle ‘was probably accompanied by two developments: (i) a greater reliance on visual social communication and (ii) more complex social structures. That these two developments should go together is not surprising, given that vision permits a high degree of temporal sequencing and brevity of signals compared to olfaction.’ These principles also apply to corvids, given that they are highly visual and diurnal, as well as having complex social lives.
Another commonality between corvids and primates is the relative size of their brains. As Emery et al. (2006) discuss in considerable detail, corvids have the largest brains for their body size of any family of birds, and the same relative size as that of apes (Emery & Clayton 2004b). Corvids also have the largest nidopallium (avian prefrontal cortex), relative to overall brain size, of any group of birds (Emery & Clayton 2004b), and this large expansion of the crow nidopallium mirrors the increase in size of the frontal cortex in the apes (Semendeferi et al. 2002). With these similarities between primates and corvids in mind, notably in their social lives and enlarged brain size, let us now turn to the question of corvid intelligence.
3. Corvid general intelligence
Laboratory tests of cognition also support the notion that corvids are highly intelligent. Like primates, they are particularly good at solving laboratory tasks that rely on the ability to abstract a general rule to solve the task and then transfer the learned rule to new tasks (Wilson et al. 1985; Mackintosh 1988). Indeed, this ability to solve transfer problems by abstracting general rules is what distinguishes rule learners from rote learners. In learning sets, for example, the animals are presented with a series of different discriminations to learn. Like primates, corvids are able to solve these learning set tasks by extracting the general rule such as win–stay, lose–shift rather than having to learn each new discrimination afresh, whereas pigeons cannot solve the transfers. Indeed, pigeons appear to be rote learners, solving the task eventually by learning each of the discriminations individually (reviewed by Mackintosh 1988). Recent work by Paz-y-Miño et al. (2004) suggests that one member of the corvid family, the pinyon jay (Gymnorhinus cyanocephalus), can solve transitive inference tasks in a social setting and use transitive inference to predict dominance. So this may reflect another similarity between primates and corvids in their cognitive capacities.
Another classic feature of intelligence is the ability to devise novel solutions to problems, and one of the most dramatic examples of this is the manufacture of special tools to acquire otherwise unobtainable foods (Beck 1980). Chimpanzees (Pan troglodytes) have been observed to manufacture a range of different tools that are used for specific purposes (Beck 1980), and different geographical populations of chimpanzees use different tools for different uses, suggesting that there may be cultural variations in tool use (Whiten et al. 1999). However, great apes are not the only animals to display diversity and flexibility in tool use and tool manufacture. New Caledonian crows (Corvus moneduloides) also manufacture different types of tool that have different functions (Hunt 1996). Furthermore, crows from different geographical areas have different designs of tool (Hunt & Gray 2003).
In the laboratory, when presented with a variety of sticks of different lengths and food positioned in a tube such that a stick was required in order to reach the food, New Caledonian crows correctly chose the appropriate length and diameter of stick to push out the piece of food (Chappell & Kacelnik 2002, 2004). Even more intriguingly, Weir et al. (2002) have shown that these crows can manipulate novel man-made objects to solve a problem. Two birds, Betty and Abel, were presented with the problem of reaching food in a bucket that was only accessible by using a hook to pull the bucket up. Unfortunately, Abel stole the bent wire and dropped it out of Betty's reach. Betty found a piece of straight wire that was lying on the floor, bent this wire into a hook and used it to lift up the bucket and reach the food. Betty proceeded to retrieve the food using the wire on 9 out of 10 test trials.
Evidence of tool use and manufacture suggests that animals can sometimes combine past experiences to produce novel solutions to problems. However, careful experimentation is required to establish whether the animal can flexibly exploit the tool in a way which suggests that they can understand and reason about the causal relations between the tool and the problem. One of the benchmark tests that has been used to test physical understanding is the trap-tube, which consists of a transparent horizontal tube with a trap along its length (Visalberghi & Trinca 1989). To solve the task, an animal must insert a tool into the tube and use it to push the piece of food out, without it falling into the trap from which it is no longer accessible. Both monkeys and apes can learn to solve this task, although typically only some of the individuals being tested do so, and they generally take somewhere between 60 and 100 trials to solve the task (capuchins, Visalberghi & Limongelli 1994; chimpanzees, Limongelli et al. 1995). However, this task can be solved in at least two ways: using a simple associative rule based on the position of the food in relation to the trap or by an understanding of how the task works. Previous studies have found no evidence that animals understand how the task works (Povinelli 2000). Indeed, Povinelli (2000, p. 7) concluded that ‘chimpanzees do not represent abstract causal variables as explanations for why objects interact in the ways that they do’.
In a recent study by Seed et al. (2006), this classic design was modified to test a non-tool-using species of corvid, the rook (Corvus frugilegus). To do so, the experimenters inserted the tool into the tube, so it needed only to be pushed or pulled. A second trap-like structure was added to the tube, but one that would not trap the food, to test whether the birds could distinguish between a functional and non-functional trap. A variety of transfer tasks were used to determine what successful birds might have understood about the task's causal properties. For example, in one task, the non-functioning trap was bottomless, allowing food to fall through. In the visually distinct transfer test, the food could pass over the top. If the birds' solution was based simply on the arbitrary appearance of the task, they should have failed these transfers. Seven out of eight rooks rapidly solved the task and six of them the visually distinct transfer task. One bird transferred two additional transfer tasks that were impossible to solve using a simple associative rule based on the position of the food in relation to the traps, suggesting that this particular rook may have abstracted a general rule about the causally relevant features common to all four designs of the two-trap tube. Exactly what the rooks understand about physical cognition poses exciting questions for future research, as is also the question of how other species, particularly primates, might perform on this modified version of the trap-tube.
In using tools to obtain otherwise inaccessible food, animals are clearly acting to fulfil a current need state. One feature of human intelligence is the ability to reminisce about the past (episodic memory) and plan for the future. In their mental time travel hypothesis, Suddendorf & Corballis (1997) have argued that mental time travel is unique to humans, and thus animals are incapable of mentally travelling backwards in time to recollect specific past events about what happened where and when or forwards to anticipate future needs. Tulving (2005, p. 47) has also endorsed this view: ‘…makes it possible for people to engage in a conscious activity, mental time travel, that is beyond the reach of living creatures who do not possess episodic memory. Mental time travel takes the form of remembering personally experienced and thought-about events, occasions, and situations that occurred in the past, together with imagining (pre-experiencing) personal happenings in the subjectively felt future’.
However, recent experiments on one species of corvid, the western scrub-jay, challenge the assumption that animals are incapable of episodic recall and planning for the long-term future. Like most corvids, these birds cache food, i.e. they hide food items for future consumption, and rely on memory to recover their caches at a later date (see Shettleworth (1995) for a review). In a series of experiments, it has been shown that western scrub-jays recall specific past caching episodes by forming integrated memories of what they cached, and where and when they hid it (Clayton & Dickinson 1998; Clayton et al. 2003c), and who was watching when they did so (Dally et al. 2006a). As Clayton and colleagues have argued, this ability to remember the what-where-and-when of specific past caching episodes fulfils the behavioural criteria for episodic memory (Clayton et al. 2003a). Of course, in the absence of agreed behavioural markers of conscious experience in non-linguistic animals, the question of whether the jays travel back in their own mind's eye to reminisce about the past remains an open one (Clayton et al. 2003b; Suddendorf & Busby 2003). There is a similar difficulty with assessing whether or not animals can travel forward in the mind's eye to think about the future. However, the fact that the jays can adjust their caching behaviour in anticipation of future needs (Emery & Clayton 2001; Clayton et al. 2005; Dally et al. 2006a) suggests that they may also possess some elements of future planning.
The ability to remember the ‘what-where-and-when’ of a particular episode has yet to be demonstrated in non-human primates. In a foraging paradigm, Hampton et al. (2005) found no evidence that Rhesus monkeys could remember the ‘when’ or previous past episodes, although they could remember which foods were hidden where. Although there is no evidence to suggest that monkeys have episodic memory, the past few years has seen increasing evidence that apes may do so. Studies on chimpanzees and gorillas suggest that they can recall ‘what-where’ and even ‘what-where-and-who’ memories of unique events (Menzel 1999; Schwartz & Evans 2001; Schwartz et al. 2002). To date, there is no evidence that apes can remember the temporal component that is central to episodic-like memory, but absence of evidence is not evidence of absence. Very recently, Mulcahy & Call (2006) have demonstrated that apes appear to select, transport and save tools for use in the future, ones they do not currently need, suggesting that they have some elements of future planning. In humans, episodic memory and future planning appear to go hand in hand; thus, patients who are unable to episodically encode and recall the past but whose semantic memories appear intact, are also unable to plan for the future (see review by Tulving 2005). If mental time travel is the cognitive feat that makes episodic memory special and distinct from other memory systems, as Tulving suggests, then based on the finding that apes do show elements of prospective mental time travel, it follows that they should also be capable of retrospective mental time travel.
Taken together, the results of studies of rule abstraction (e.g. learning sets and transitive inference), problem solving and physical causality, and mental time travel, present a compelling case for corvid cognition. Indeed, elsewhere we have argued that the general intelligence of corvids is comparable to that of chimpanzees and that these two very distantly related families face similar challenges (Emery & Clayton 2004a,b). But what of their social cognition?
4. Social cognition by corvids
Theory of mind, the ability to impute and reason about the mental states of other individuals, is thought to be the pinnacle of social cognition. The term was first introduced by Premack & Woodruff (1978) to describe the behaviour of their language-trained chimpanzee, Sarah, who appeared to impute intentions to a human experimenter. Sarah was shown video sequences of an experimenter in various predicaments. After each sequence, Sarah was presented with a number of photographs showing possible solutions to the problems. Sarah was highly accurate in her selection of the appropriate photographs, and it was argued that she therefore understood the actor's intentions (‘he wanted to get out of the cage’). Sarah had more than 10 years of experience in laboratory tests of cognition, however, as well as extensive experience of watching human experimenters doing these everyday tasks, so the possibility that she selected the photograph that completed a familiar sequence cannot be ruled out.
Subsequent studies suggested that the evidence for theory of mind in primates was at best ambivalent. Indeed, the capacity for non-human animals to attribute others with mental states has been the subject of considerable debate (Heyes 1998; Povinelli et al. 2000; Karin-D'Arcy & Povinelli 2002; Povinelli & Vonk 2004). Traditional studies relied on the use of human trainers, and the chimpanzees were tested in a paradigm in which they were expected to cooperate with the trainers for food (e.g. Povinelli et al. 1990; Call & Tomasello 1998).
However, the most convincing studies of social cognition by chimpanzees have stemmed from the work by Hare et al. (2000, 2001), which exploited the competitive nature of chimpanzee social life and used conspecifics. Hare et al. (2000) placed a subordinate and a dominant individual in competition with one another for two food rewards, one of which was positioned such that it was visible only to the subordinate. Subordinates selectively retrieved those food items that only they could see, suggesting that they may understand the dominant's visual perspective. Subsequent experiments, in which the knowledgeable dominant who had witnessed the baiting of food was switched with a naive one who had not seen this event, suggested that the subordinate chimpanzees keep track of which dominant chimpanzee has seen which particular baiting of the cup. As a result of these experiments, Hare et al. (2001) argued and that chimpanzees are capable not only of understanding what others can and cannot see, but also of what conspecifics do and do not know.
The success of the food-competition paradigm in providing insight into the socio-cognitive abilities of chimpanzees can be credited both to the exploitation of a naturally occurring competitive behaviour, and to the use of conspecifics as protagonists (Hare 2001). Competition for resources is not an aspect of animal social life that is unique to primates, however. Indeed, as we shall go on to describe, it is possible to draw parallels between food competition by chimpanzees, and food caching by corvids such as the western scrub-jay.
(a) Caching and cache protection as a competitive foraging paradigm
Food-caching corvids compete not only for access to available food resources, as chimpanzees do, but also for access to hidden caches of food. Field observations suggest that cachers engage in a number of strategies to reduce this competition with other individuals at the time of caching (Clayton & Emery 2004). For example, they tend to cache in areas where the density of conspecifics is lowest and ideally zero (e.g. rooks, Kalländer 1978; magpies, Pica pica: Clarkson et al. 1986; ravens, Bugnyar & Kotrschal 2002). When other individuals are present, the cachers will wait until the potential pilferers are distracted or cannot see because a barrier obscures their view (e.g. ravens, Corvus corax: Heinrich & Pepper 1998; Heinrich 1999). Competition may also arise once the cache has been hidden. Indeed, returning to cache sites does not guarantee a cacher's recovery success: up to 30% of caches are lost each day to pilfering by competitors (Vander Wall & Jenkins 2003).
Cache theft is particularly problematic for scrub-jays and other members of the corvid family, where potentially pilfering conspecifics use observational spatial memory to accurately steal another's caches that they saw being made (Bednekoff & Balda 1996a,b; Heinrich & Pepper 1998; Clayton et al. 2001; Watanabe & Clayton 2007). Consequently, pilfering jays can wait until the cacher has left the scene and then steal its caches at will, whenever they are hungry, and without relying on successfully displacing a possibly more dominant cacher.
Bugnyar & Kotrschal (2002) suggested that the capacity for observational spatial memory in corvids represented the catalyst for an ‘evolutionary arms race’ between cachers and pilferers, such that pilferers should develop methods for observing cachers as unobtrusively as possible, and cachers develop strategies to counter the risk of cache pilferage. Critical to a cacher's use of tactics to counter the risk of cache theft, however, should be the risk that potential thieves pose to their caches, i.e. whether or not they witnessed the cacher caching and are therefore knowledgeable as to cache locations. Consequently, just as Hare et al.'s food-competition paradigm provided a competitive, and naturalistic, forum with which to probe the socio-cognitive abilities of apes, experimental paradigms based on food-caching behaviour have the potential to do the same with respect to the cognitive abilities of corvids. Furthermore, Dally et al. (2006b) have argued that, because corvids such as the western scrub-jay act as both cacher and pilferer, this role-taking has led to a refinement of increasingly more sophisticated, cognitively based cache protection and pilfering strategies.
(b) Anticipating pilferage when observers are present
Jays use a number of cache protection strategies at the time of caching, all of which appear to reduce the amount of visual information available to the observer. In the first experiment, we tested whether scrub-jays, like chimpanzees, are sensitive to what competitors can and cannot see (Dally et al. 2005a). Rather than competing directly for access to food, some of which was in view and some of which was out of view, in our ‘barrier’ experiment, the cachers were given the opportunity to cache food in two different, visuospatially distinct trays that were placed in the bird's home cage. A second bird, the observer, was placed in a cage opposite to that of the cacher such that it could easily see the caching bird. One of the trays was placed behind a barrier such that it was out of view of the observer, but the other was in full view so that the observer could clearly see the location of caches hidden in the full-view tray. On some trials, the cacher consequently cached while being observed by an observer (observed condition), whereas on other trials the observer's view of the entire caching event was obscured by a screen so that the cache was able to hide its caches in privacy (in private condition). The design of this experiment is shown in figure 1.
Figure 1.
The caching and recovery conditions for ‘barrier’ experiment. Storers cached in a single trial when ‘observed’ and when ‘in private’. An opaque divider was attached to one side of the back of the storer's cage in the observed caching condition, such that the observer could only see one (full-view) tray, and to both sides of the cage during the in private caching condition preventing the observer from seeing either tray.  full-view tray in the observed condition,
full-view tray in the observed condition,  out of view tray in the observed condition. Recovery always took place in private.
out of view tray in the observed condition. Recovery always took place in private.
Perhaps not surprisingly, in the observed caching condition, the jays cached preferentially in sites located behind barriers which observers could not see, whereas there was no preference to cache behind barriers when they cached in private. However, when an observer was present during caching, not all the caches were hidden behind the barrier. Indeed, figure 2 shows that the cacher placed approximately 25% of its caches in high-risk cache sites, namely in the full-view tray.
Figure 2.
The mean number of items (+s.e.m.) cached in the full-view and out of view trays during the observed condition of the ‘barrier’ experiment, and the mean number of items cached in private (Friedman's ANOVA, Χ22=2.57, n=8, p=0.03). As depicted on the figure, post hoc tests revealed that the birds cached significantly more items in the out of view tray compared to the full-view tray (Dunnett's test, q=2.9, n=8, p<0.05) and the mean of the private trays (Dunnett's test, q=4.1, n=7, p<0.01). No statistical difference was identified between the number of items cached in the full-view tray and the mean of the private trays (Dunnett's test, q=1.2, n=7, p>0.05). *, p<0.05.
This pattern of results suggests that, like chimpanzees, scrub-jays prefer to take the food that is out of view of the competitor. One interpretation of these results is that the cachers are sensitive to what an observer can and cannot see, and that is why they place the majority of the caches in the tray behind the barrier. However, a simpler explanation is that the cachers are responding to what they themselves can see. When they cache behind the barrier, the observer is effectively out of sight, and consequently perhaps out of mind.
One way in which it might be possible to determine whether scrub-jays are sensitive to what others can and cannot see, as opposed to basing their cache protection strategies solely on their own visual perspective, is to investigate whether they are able to exploit relative differences in the level of visual access that observers have to cache sites. By caching in sites that observers might find hard to see, cachers might reduce the quality and transfer of visual information to observers, thereby making the location of cache sites less certain. Critically, the ‘out of sight, out of mind’ hypothesis would be unable to explain a preference to cache in hard-to-see sites, as potential thieves would still be in view of the caching bird.
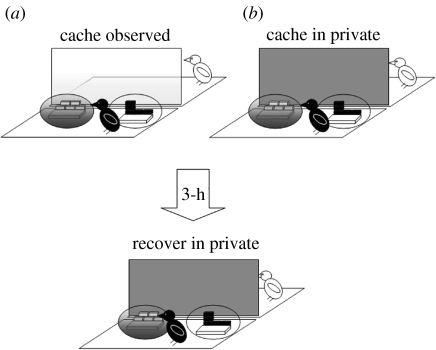
To determine whether scrub-jays would exploit hard-to-see sites, we examined the effects of sun and shade on a scrub-jay's choice of where to cache (Dally et al. 2004). We hypothesized that scrub-jays might prefer to cache in shady sites, especially when another bird was watching them, because shady sites would reduce the visual information available to the potential pilferer and thus reduce the chance that the pilferer could accurately relocate the caches. Similarly, in the ‘barrier’ experiment, the birds were able to cache in two trays in each of two conditions, observed by a conspecific or in private. The critical difference, however, was that a lamp was placed close to each of the two caching trays. By switching one of the lamps on, and leaving the other switched off, we were able to create two sites that were identical except for the level to which they were lit such that ratio of luminance between the two sites was 4 : 1. The experimental design is shown in figure 3.
Figure 3.
The caching and recovery conditions of the ‘sun/shade’ experiment.  shade tray,
shade tray,  well-lit tray. Storers cached in a single trial in each of two conditions (a) separated from the observer by a transparent divider (observed condition) and (b) separated from the observer by an opaque divider which obscured the observer's view (in private condition). Cache recovery always occurred in private.
well-lit tray. Storers cached in a single trial in each of two conditions (a) separated from the observer by a transparent divider (observed condition) and (b) separated from the observer by an opaque divider which obscured the observer's view (in private condition). Cache recovery always occurred in private.
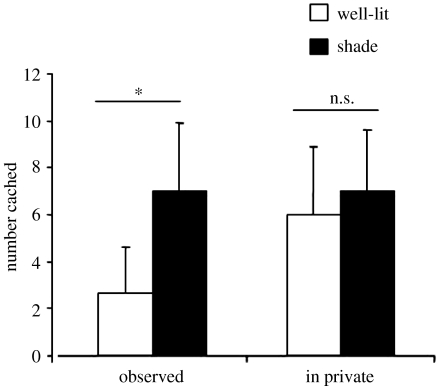
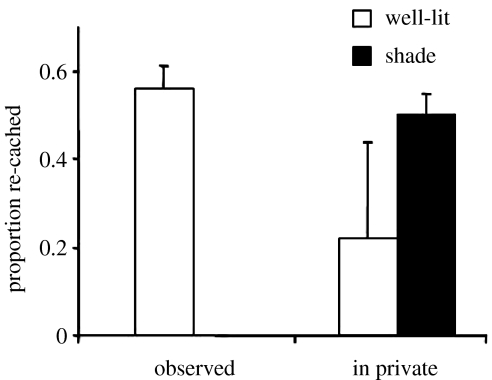
Figure 4 provides support for this hypothesis about where the birds should cache when observed by another jay. When another jay is present at the time of caching, the cachers do prefer to hide most of their items in shady areas compared to well-lit areas, whereas they are not selective about cache location when caching in private. Note, however, that not all the items were cached in the shady sites when an observer was watching: approximately 20% of the items were placed in the well-lit sites, a point we shall return to presently.
Figure 4.
The mean number of items (+s.e.m.) cached in the well-lit and shady trays during the observed (Wilcoxon's paired test, T=21, n=6, p=0.016) and in private caching conditions (Wilcoxon's paired test, T=21, n=8, p=0.38). *, p<0.05; ns, non-significant.
Subsequent experiments established that the jays also use distance as a cache protection strategy (Dally et al. 2005a). In the ‘distance’ experiment, the birds were given the opportunity to cache worms in each of two trays. The trays were positioned approximately 60 cm apart from one another, such that one tray was near to and one tray far from the observer's cage. As in the previous experiments, the birds cached either while observed or in private. The experimental design is shown in figure 5.
Figure 5.
The caching and recovery conditions of the ‘distance’ experiment. 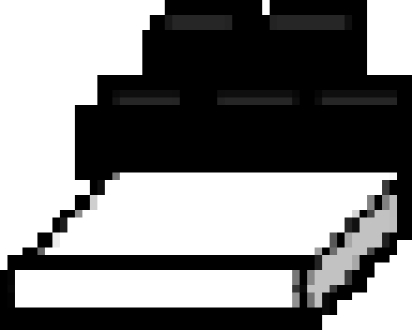 far tray,
far tray, 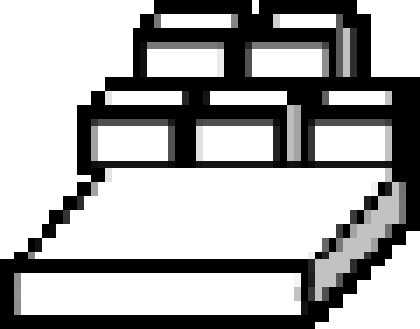 near tray,
near tray,  storer,
storer,  observer. Storers were given four trials in each of two conditions (a) when a transparent divider separated the storer and the observer (observed condition), (b) when opaque dividers separated the storer and observer (in private condition). Irrespective of condition, recovery occurred in private.
observer. Storers were given four trials in each of two conditions (a) when a transparent divider separated the storer and the observer (observed condition), (b) when opaque dividers separated the storer and observer (in private condition). Irrespective of condition, recovery occurred in private.
Figure 6 shows that the birds had a preference to hide most of their caches in the tray located far from the observer, as opposed to the other tray placed near to the observer. However, they did not show a preference for distance when caching in private and instead cached equal amounts in both trays.
Figure 6.
The mean number of items (+s.e.m.) cached in near and far trays during the observed and in private caching conditions of the ‘distance’ experiment. The number of items cached by the birds did not vary across trials in either the observed (Friedman's ANOVA, Χ22=6.0, p=0.09) or in private condition (Friedman's ANOVA, Χ22=0.3, p=0.96). Birds cached predominantly in the far tray when observed (Wilcoxon's paired test, T=4, n=8, p=0.027), whereas no significant difference was identified between the number of items cache in the near and far trays during the private condition (Wilcoxon's paired test, T=16, n=8, p=0.67). *, p<0.05; ns, non-significant.
It therefore appears that the birds are able to exploit a range of environmental variables, such as the level of ambient light and the relative distance of cache sites to the observer, to reduce the transfer of visual information to potential pilferers. As in the previous two experiments, note that they did not cache exclusively in the far tray when observed, but that they cached approximately 25% of their caches in the near tray.
When competing with others for access to resources, an animal might not only adjust its behaviour as a result of the presence of a competitor, but also as a function of the competitor's social status. Consider Hare and colleagues' competitive paradigm. The finding that chimpanzees preferentially recovered food that competitors cannot see was specific to subordinate individuals, because their dominant competitors would be able to physically monopolize known food sources. The relative dominance of a competitor might also affect the use of cache protection tactics by food-cachers. Like Hare et al.'s chimps, the capacity for cachers to physically compete with other birds for access to cache sites is also specific to dominant scrub-jays (Dally et al. 2005b). Similarly, only dominant birds are able to physically defend cache sites against potential pilferers. We might therefore expect cachers to be most likely to engage in protective behaviours when caching in view of dominant birds, thereby reducing the need to actively defend cache sites and negating the need for aggressive interaction which carries a risk of injury or even death.
Field observations suggest that observer dominance might not be the only factor affecting the use of cache protection tactics by corvids. Instead, it appears that the social relationship between a cacher and an observer might affect the use of protective behaviours. For example, while ravens preferentially cache out of view of conspecifics (Bugnyar & Kotrschal 2002), there is evidence to suggest that this is not the case when the cacher's partner is the sole witness to a caching event (Heinrich & Pepper 1998). Moreover, caching jays commonly tolerate cache theft by their partner, but direct aggression towards other individuals that approach cache sites (Goodwin 1956; Dally et al. 2005b).
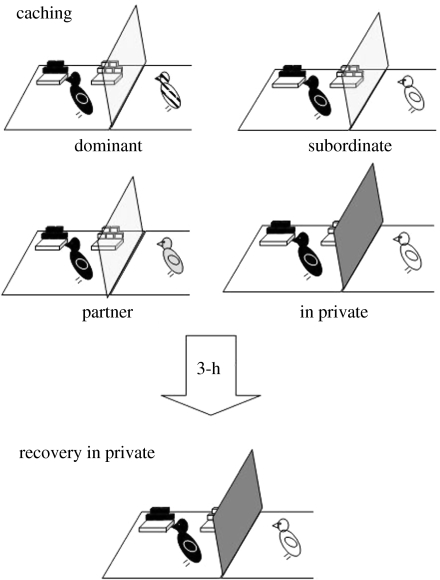
If corvids do not perceive their mate to represent a threat to cache safety, then the cachers should abstain from investing energy in the implementation of cache protection strategies when caching in their presence. To determine whether this is the case, we replicated the ‘distance’ experiment we described previously, such that jays were able to cache in sites that were either near to or far from a conspecific. As shown in figure 7, in this experiment the birds cached either observed by a dominant bird, a subordinate, their partner, or in private (Dally et al. 2006a). Based on the findings of our earlier experiments, we predicted that cachers should hide food preferentially in the distant sites in the presence of potential thieves, but cache in both trays in private. The question is whether, in the partner condition, cachers will cache preferentially in far sites just as they had done in the previous experiments when another bird was watching at the time of caching, or whether they differentiate between mates and other individuals and thus will refrain from engaging in protective behaviours in the presence of their mate.
Figure 7.
The caching and recovery conditions of the ‘distance’ experiment in which storers ( ) cached in a single trial in each of four conditions: observed by a dominant (
) cached in a single trial in each of four conditions: observed by a dominant ( ), subordinate (
), subordinate ( ), their partner (
), their partner ( ) or in private.
) or in private.  far tray,
far tray,  near tray. Transparent dividers separated the storer and the observer in the dominant, subordinate and partner conditions. Opaque dividers separated the storer and observer during the in private caching condition. Irrespective of condition, recovery occurred in private.
near tray. Transparent dividers separated the storer and the observer in the dominant, subordinate and partner conditions. Opaque dividers separated the storer and observer during the in private caching condition. Irrespective of condition, recovery occurred in private.
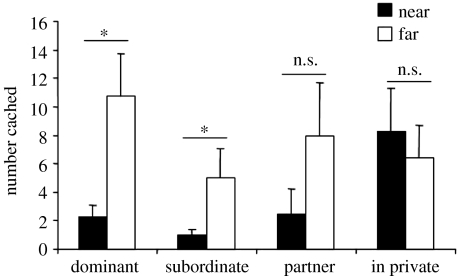
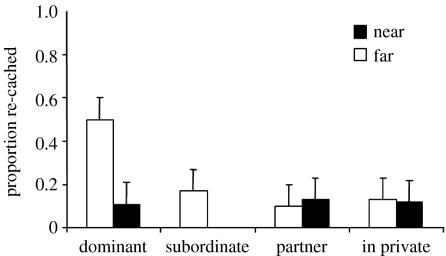
As predicted, cachers cached equally in both the near and far trays when in private (figure 8). Intriguingly, however, although the birds cached chiefly in the far tray in the dominant and subordinate conditions, they did not differentiate between the two trays when watched by their partner, suggesting that they did not perceive their mate as a competitor.
Figure 8.
The mean number of items (+s.e.m.) cached in near and far trays during each caching condition: dominant, subordinate, partner, in private. Birds cached predominantly in the far tray in the dominant (Sign test; S=7/7, p=0.02) and subordinate conditions (Sign test; S=7/7, p=0.02), but not in the partner condition (Sign test; S=5/7, p=0.13) or in private (Sign test; S=3/7, p>0.05). *, p<0.05; ns, non-significant.
This finding suggests that, at least in some corvids, the specific risk that an observer poses, not just the presence or absence of an observer, governs whether or not cache protection strategies are employed.
(c) Multiple moves
In the wild, it may not be possible for cachers to cache in sites that afford hidden items a degree of visual protection. For example, if cache sites are equidistant to observers, then the cachers would be unable to use a distance strategy. Furthermore, if observers were not only present, but also non-stationary, it would be unlikely that any cache site would be consistently out of view of potential thieves. In this situation, a cacher's best chance of minimizing cache theft might be to reduce the accuracy with which competitors are able to relocate hidden items, perhaps by moving items around multiple times. In the wild, it is reported that Eurasian jays do just that (Goodwin 1956).
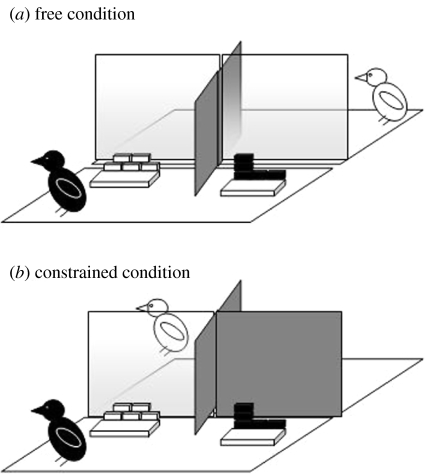
In a recent experiment (Dally et al. 2005a), western scrub-jays were given the opportunity to cache in two trays in each of two conditions (figure 9). In the ‘constrained’ condition, the position of observers relative to cache sites was reliable, such that one tray was constantly in view and one constantly out of view of a potential thief. By contrast, in the ‘free’ condition, whether or not a specific cache site was in view of a potential thief was dependant on the current position of that same observer. To elucidate, when an observer was on the left-hand side of its cage, the left-hand caching tray would be in view, whereas the right-hand tray would be hidden by an opaque barrier. If the observer moved to the right-hand side of its cage, however, the right-hand tray would be in view, and the barrier would block the observer's view of the left-hand tray.
Figure 9.
The experimental set-up for (a) the free condition and (b) the constrained condition. In the free condition, the cages of both the storer and observer were partially divided with opaque dividers which restricted the visual access of both birds to one side of the opposite cage. In the constrained condition, the storer's cage was partially divided, and the observer's cage fully divided, with opaque screens. The observer therefore had visual access to only one side of the storer's cage. All storers received three caching trials in the free condition and two trials in the constrained condition. Irrespective of condition, recovery took place in private (solid dividers attached to both sides of the storer's cage).
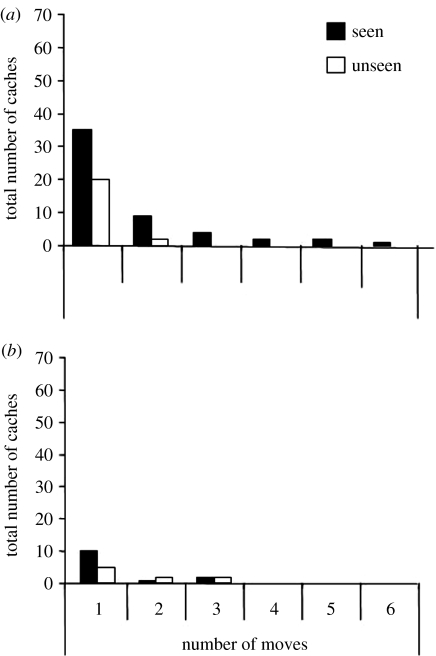
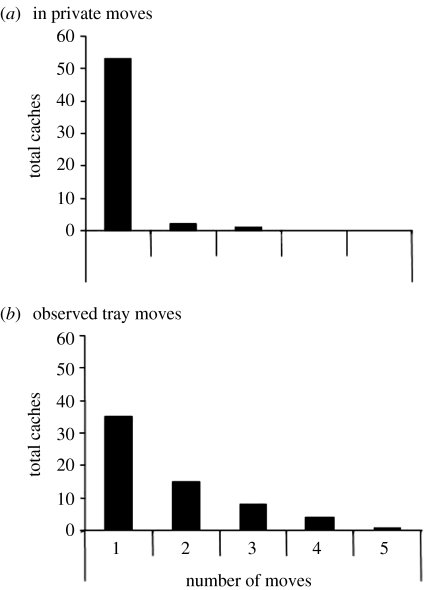
As predicted by the results of the ‘barrier’ experiment we described previously, cachers predominantly cached out of view of the observer in both the constrained and free conditions. When caching in the free condition, however, cachers often recovered cached items and re-hid them elsewhere. As shown in figure 10, the cachers moved items the observer had seen being cached up to six times, movements that occurred specifically in view of their competitor. By contrast, when cache sites were consistently in view or out of view of potential thieves (constrained condition), few items were moved. The repeated movement of caches in the free condition appears to be a response to the unpredictability of the observer's position, and consequently what they could and could not see.
Figure 10.
The total number of times individual caches from seen and unseen sites were moved during the caching period of (i) the free condition and (ii) the constrained condition. Caches were moved significantly more often during the free condition than in the constrained condition (Wilcoxon's paired test, T=2, n=8, p=0.01). *, p<0.05; ns, non-significant.
(d) Cache protection at recovery when observers have left the scene
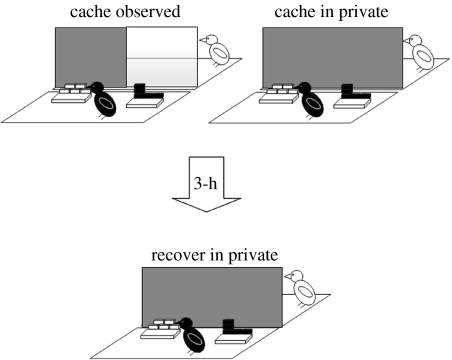
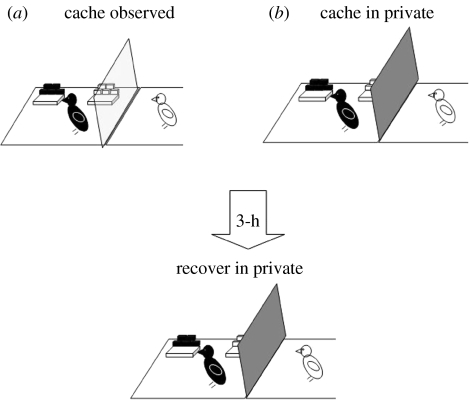
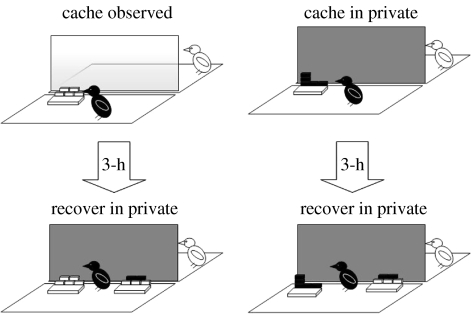
The use of tactics to reduce cache theft is not limited to the initial period of caching. In the wild, corvids have been observed to return alone to caches hidden in the presence of conspecifics, and to re-hide them in new places unbeknownst to potential thieves (Goodwin 1955; Heinrich 1999). To determine whether this ‘re-caching’ behaviour could be attributed to the presence of potential thieves at the time of caching, we allowed our jays to cache either in private or while a conspecific was watching, and then recover their caches 3 h later (figure 11). Irrespective of caching condition, recovery always occurred in private, preventing the cacher's behaviour from being influenced by any cues provided by a prior observer. As a result, any differences in the birds' recovery behaviour after caching observed or in private must depend on a memory of the social context of a previous caching event (i.e. the absence or presence of an observer jay).
Figure 11.
The experimental set-up of the caching and recovery conditions. Storers cached either watched by a conspecific (observed) or when the observer's view was obscured by an opaque divider (in private). In each condition, storers cached in a single tray ( observed tray,
observed tray,  in private tray). At recovery, which occurred in private, storers received the tray in which they had previously cached plus one ‘new’ tray (
in private tray). At recovery, which occurred in private, storers received the tray in which they had previously cached plus one ‘new’ tray ( ).
).
We predicted that the jays would be much more likely to re-cache if they had been observed by a conspecific while they were caching than after caching in private. Furthermore, these caches should be moved to new sites unbeknown to the observer, if this re-caching is indeed a cache protection strategy.
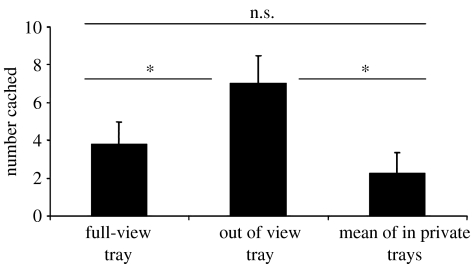
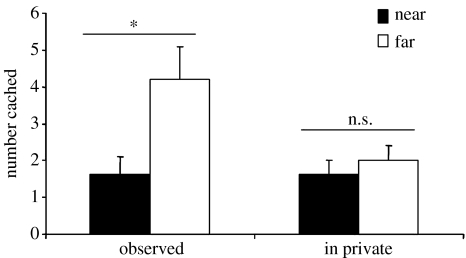
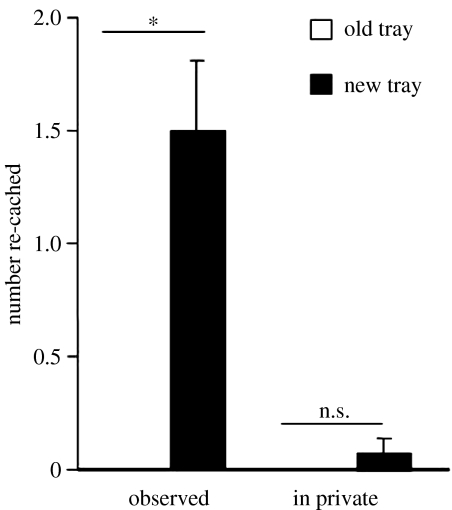
Figure 12 shows that, as predicted, the jays were much more likely to re-cache if they had been observed by a conspecific while they were caching than when they had cached in private. By re-caching items that the observer had seen them cache, the cachers significantly reduce the chance of cache theft, as observers would be unable to rely on memory to facilitate accurate cache theft.
Figure 12.
The mean number of items (+s.e.m.) re-cached in the new and old trays after the observed and in private caching conditions. Storers re-cached significantly more in new sites after being observed during caching (Wilcoxon's paired test, Z=2.4, n=7, p<0.05), but did not discriminate between re-caching in old or new sites during the in private condition (no statistical comparison possible). *, p<0.05; ns, non-significant.
At this juncture, it is perhaps worth describing how this re-caching behaviour differs from the multiple movement behaviour we described earlier. First, whereas re-caching occurs during recovery in private, the multiple movement of caches occurs at caching. Moreover, while re-cached items are moved once to new sites, items moved multiple times are commonly moved to sites that have been used previously. Presumably, the principal benefit of re-caching at recovery is that items are moved from sites that the observer knows about to new sites of which the observer is ignorant. Repeatedly moving items around, however, might serve a very different function. By moving items the observer witnessed being cached multiple times, the observer's memory for each new cache site would suffer from interference from the memory of old cache sites. Consequently, the accuracy with which potential thieves are able to steal hidden items may be reduced. Critically, however, cachers are able to recover moved and unmoved caches with equal precision.
While re-caching behaviour is a cache protection behaviour in its own right, the results of our experiments suggest that it is often used in conjunction with protective behaviours at the time of caching. Let us look again at the results of the sun and shade and distance experiment. As predicted by our first re-caching experiment, after being observed during caching, the birds engaged in re-caching behaviour during a private recovery period. By contrast, they engaged in much less re-caching when they had cached in private. Note, however, that it was the items that had been placed in the well-lit tray during the observed caching condition that were moved to new sites, even though the cacher had placed only approximately 20% of its caches in that tray (figure 13; Dally et al. 2004). The same pattern of results was identified for the distance and barrier experiments: re-caching occurred almost exclusively after the birds had cached in the observed condition, and was directed specifically at those caches that had been placed in risky sites (Dally et al. 2005a). This selectivity to re-cache items at the greatest risk of cache theft acts to confers them with a degree of cache protection, as prior observers would no longer be able to rely on memory to steal them.
Figure 13.
The mean proportion of caches (+s.e.m.) re-cached from the well-lit and shady trays after the observed and in private caching conditions. The data could not be analysed statistically as only three birds recovered their caches in the observed condition and two birds in the in private condition.
The pattern of re-caching exhibited by cachers at recovery is clearly influenced by the location in which items were cached. A second point of interest, however, is whether a cacher's recovery behaviour is influenced by who was watching at the time of caching. Remember that at the time of caching, cachers engage in protective tactics only when observed by non-partners. It therefore follows that cachers might refrain from using cache protection tactics at recovery after caching in view of their partner, and this is indeed an accurate reflection of the cacher's behaviour. As shown in figure 14, after caching in view of a subordinate or a dominant bird, cachers re-cached hidden items specifically from the sites to which the observer had the best visual access (near tray). However, no such preference was exhibited if the cacher hid the caches in view of their partner. Cachers also appeared to differentiate between the relative risk the subordinate and dominant birds posed to their caches, as re-caching levels were highest after caching in view of a dominant bird (Dally et al. 2006a).
Figure 14.
The mean proportion of caches (+s.e.m.) re-cached at recovery after each caching condition: dominant, subordinate, partner, in private. The data could not be analysed statistically as several birds only cached in one tray (two in the dominant condition, four in the partner condition, two in private).
(e) Keeping track of who was watching when
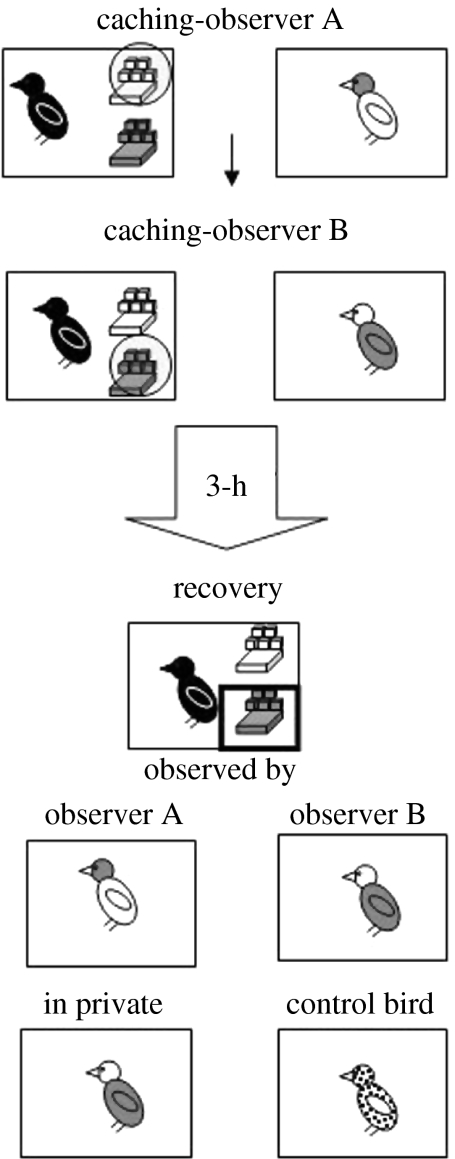
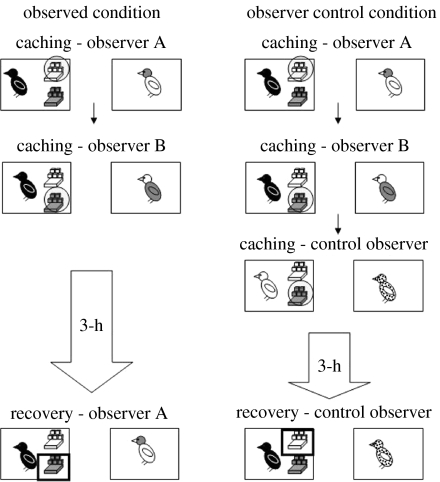
Until now, we have focused on the capacity for corvids to remember whether or not they were observed during a specific caching event, and, during a private recovery period, to adjust their cache recovery behaviour accordingly. In a naturalistic situation, however, cachers may not be able to recover their caches unobserved. It might be the case that observers do not leave the area in which the cacher has cached, or that while the same observers are not always present, there is always another bird in the immediate vicinity. This situation was mimicked in one of our most recent experiments (Dally et al. 2006a) in which, although observers were often present upon recovery, they differed as to the specific caches they had witnessed the cacher hide. To elucidate, cachers were given the opportunity to cache in the presence of two different observers in two consecutive caching events, and then to recover their caches either when in private, watched by a prior observer (observed condition), or watched by a ‘control’ bird that had not witnessed either caching event. The experimental design is shown in figure 15.
Figure 15.
The caching and recovery conditions of the ‘who was watching’ experiment. Storers received eight trials in which they ( ) cached in one tray in the presence of observer A (
) cached in one tray in the presence of observer A ( ) and in a different tray in the presence of observer B (
) and in a different tray in the presence of observer B ( ). At recovery, storers were able to retrieve their caches in one of four conditions: in the presence of observer A, observer B, a control bird that had not been present when the storer cached (
). At recovery, storers were able to retrieve their caches in one of four conditions: in the presence of observer A, observer B, a control bird that had not been present when the storer cached ( ) or in private (opaque dividers occluding a conspecifics view of the storer's recovery behaviour). Each bird received two trials in each recovery condition.
) or in private (opaque dividers occluding a conspecifics view of the storer's recovery behaviour). Each bird received two trials in each recovery condition.  Perspex strips,
Perspex strips,  ‘observed’ tray.
‘observed’ tray.
In line with our previous findings, cachers re-cached hidden items in new sites when cache recovery occurred in private. By contrast, in the presence of a prior observer, cachers repeatedly moved caches the observer had seen them make. This propensity for birds to repeatedly move caches around in the presence of a prior observer is analogous to the multiple movement of caches we described previously, a behaviour that is apparent when cachers are unable to engage in a behaviour that affords their caches some form of visual protection (e.g. caching out of view). By moving caches repeatedly, cachers might reduce the accuracy with which the observer would be able to steal their caches. As shown in figure 16, while the cachers moved caches they had been observed to make multiple times, caches that the observer at recovery had not seen them hide were rarely moved. Similarly very few caches were moved during the control condition. In essence, moving these items would only have provided observers, who were currently ignorant to their existence, with observational information that might have been used to facilitate future cache theft.
Figure 16.
The total number of times individual caches were moved from (a) the in private trays and (b) the observed tray. Caches in the observed tray were moved significantly more often than caches in the in private trays (Friedman's test, Χ12=8.0, p=0.01).
Although the results of this experiment suggest that scrub-jays remember who was present when specific caches were being made, it is possible that the cacher was simply reacting to cues provided by the observer. For example, observers may spend more time attending to a tray they have seen a bird caching in. In order to determine whether the jays adjust their behaviour as a function of what the bird at recovery knows, or whether they rely on differences in the behaviour of the observers towards sites in which they have or have not watched caches being hidden, we ran a further experiment (Dally et al. 2006a). As shown in figure 17, we repeated the observed condition of our previous experiment, such that cachers cached successively in two trays, each in view of a different observer, and contrasted it with a second observer control condition. Critical to the observer control condition was that after the two caching periods, a control observer was given the opportunity to observe a control cacher caching in one of the two trays. In this way, both the original observers and the control observer witnessed a cacher hiding the food in one of the two trays. At recovery, both observer and control observer saw an original cacher recover its caches. Consequently, in the observed condition, the cacher was observed by the same observer at caching and recovery, whereas in the observer control condition, the cacher was observed by an observer at caching and the control observer at recovery.
Figure 17.
The experimental set-up of the observed and observer control conditions. Storers ( ) received two caching trials in each of the observed and observer control conditions. In each condition, storers cached in one tray in the presence of observer A (
) received two caching trials in each of the observed and observer control conditions. In each condition, storers cached in one tray in the presence of observer A ( ) and in a different tray in the presence of observer B (
) and in a different tray in the presence of observer B ( ). In the observer control condition, an additional storer (
). In the observer control condition, an additional storer ( ) also cached in one of the two trays in the presence of a control observer (
) also cached in one of the two trays in the presence of a control observer ( ). At recovery, in the observed condition, storers recovered their caches in the presence of observer A or observer B. In the observer control condition, storers recovered their caches in the presence of the control observer.
). At recovery, in the observed condition, storers recovered their caches in the presence of observer A or observer B. In the observer control condition, storers recovered their caches in the presence of the control observer.  Perspex strips, (
Perspex strips, ( ) observed tray.
) observed tray.
If the caching scrub-jay remembers who was present during caching, then its behaviour should differ between the observed and observer control conditions. Based on the previous experiment, cachers in the observed condition should re-cache items predominantly from the tray the observer at recovery had seen them cache in (observed tray) and not from the other tray. By contrast, cachers should re-cache few items from either tray in the observer control condition, because the control observer was not present when the cacher cached. If, however, the birds attend primarily to trays in which they have observed caching, and cachers use this information to guide cache recovery, cachers should re-cache items from the observed tray in both conditions, as although the control observer was not present when the cacher cached, it would be attending the tray in which it had previously observed the control cacher cache.
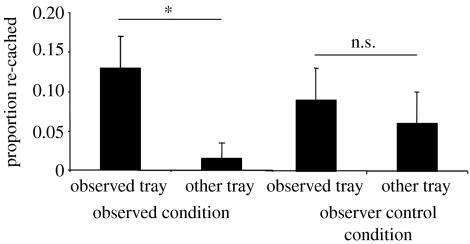
As shown in figure 18, birds in the observed condition re-cached a significantly greater proportion of caches from the tray in which the observer at recovery had seen them cache. By contrast, in the observer control condition, items were re-cached from both trays without selectivity. Consequently, it seems rather unlikely that cacher's use of cache protection tactics is cued by the observers' behaviour. The results of these two experiments therefore support the hypothesis that the jays remember which individuals watched them cache during specific events, and use this information to combat the future risk that particular observers pose to their caches.
Figure 18.
Mean proportion (+s.e.m.) of items re-cached from the observed and other trays in the observed (sign test; S=6/6, p=0.03) and observer control conditions (Sign test; S=4/6, p=0.69). *, p<0.05; ns, non-significant.
Of course a more elaborate behaviour cueing account could be proposed given that in the observed condition, the observer saw the cacher hide food in tray A, whereas in the observer control condition the observer saw a different bird cache food in tray A. Consequently at recovery, the observer's behaviour may have differed between the two conditions, but to do so requires the observer to remember which particular cacher was present during the previous caching episode as well as which tray the cacher had stored the food in. Note that this more elaborate behavioural cueing account requires of the observer what we have claimed of the storer, namely an ability to recognize particular individuals and to remember which individuals were present and where they cached during specific past caching episodes. Consequently we argue that by either account, western scrub-jays keep track of who was watching when.
These findings raise questions about whether the scrub-jay has a ‘theory of mind’, given that the jays appear to be sensitive to what particular individuals have and have not seen when deciding which caches to protect. Furthermore, since the birds differentiate between dominant and subordinate observers, and between mate and other birds, even when these birds are not present at the time of recovery when they are re-caching the food items, they must recognize different individuals and use this social memory to take protective action accordingly. Finally, the fact that they can do so even when those observers are not present at the time of recovery means not only that the birds must have remembered who was present at the time of caching, but also that we can rule out a behaviour-reading account of re-caching behaviour.
Of course, as we have pointed out in the original paper (Dally et al. 2006a), we caution that these abilities do not necessarily require a human-like theory of mind. For one thing, it is hard to imagine how a non-linguistic subject could theorize about the minds of other individuals. Clearly, it would be informative to develop a model of how the jays might achieve this seemingly complex behaviour (see Emery & Clayton (in press) for our attempt to construct a cognitive architecture of mind-reading in scrub-jays). However, the selectivity of the cache protection behaviours does appear to depend on a sensitivity to what others have and have not seen, and who is and is not a threat. In short, these studies show that scrub-jays keep an eye on the competition and protect their caches accordingly. Such behaviour would appear to meet the behavioural criteria for one form of theory of mind, namely knowledge attribution, if by the term we mean the ability to attribute different informational states to particular individuals.
(f) The role of experience
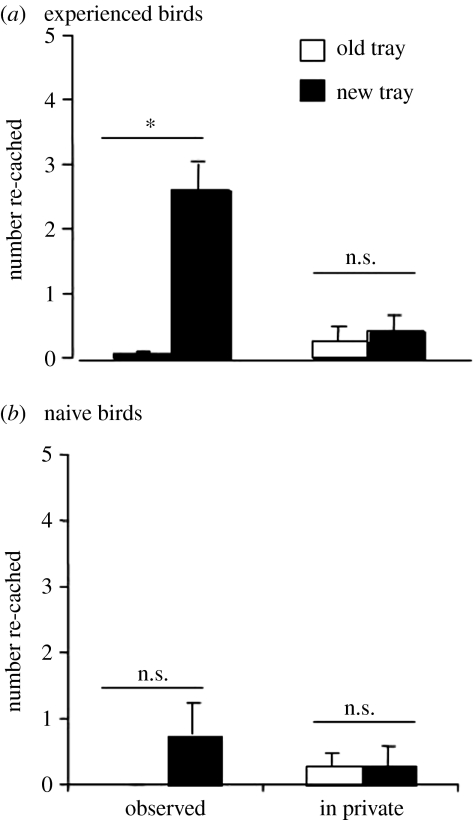
There is one particularly striking finding about the re-caching behaviour of these birds, i.e. not all western scrub-jays engage in it. Emery & Clayton (2001) found that re-caching behaviour depends not only on whether or not the cacher was observed by another jay during caching, but that it also depends upon experience of being a pilferer. Whereas experienced thieves engaged in high levels of re-caching at recovery when they were observed during the previous caching episode, control birds, who had not been thieves in the past and therefore had no prior experience of stealing other birds' caches, showed hardly any re-caching at all, as shown in figure 19.
Figure 19.
The mean number (+s.e.m.) of items re-cached in new and old sites at recovery after the observed and in private caching conditions for (a) birds that had previously stolen the caches of others (experienced birds; Wilcoxon's paired test, Z=2.2, n=7, p<0.05), and (b) birds that had not experienced stealing others' caches (naive birds; Wilcoxon's paired test, Z=1.6, n=7, p>0.05). *, p<0.05; ns, non-significant.
The fact that only experienced birds re-cache has a number of important implications. The first is that this behaviour cannot be innate, otherwise all scrub-jays should re-cache. Importantly, we can also rule out a simple conditioning explanation because the birds never received any positive reinforcement or any punishment for re-caching, given that they never had the opportunity to learn about the fate of the caches that they had re-cached. As a consequence, we can make the inference that the jay used information gained during the previous caching event to anticipate whether or not its caches were likely to be stolen, and thus engaged in the appropriate cache protection strategy at recovery, namely whether or not to re-cache and, if so, to re-cache those that had previously been placed in high-risk sites. The fact that they appear to anticipate cache risk in the future lends further credence to the idea that they can plan for the future, as discussed in §3. Emery & Clayton (2004b) have argued that the fact that experienced birds differ so dramatically from control birds who lack the experience of being a thief suggests that the experienced jays are not only capable of future planning, but also experience projection. Experience projection refers to a second form of theory of mind, namely the ability to use one's own experiences, in this case of having been a thief, to predict how another individual might think or behave, in this case what the potential pilferer might do. Experience projection has yet to be demonstrated in any of the great apes, other than humans. Consequently, most people have assumed that experience projection is a uniquely human trait. The jay studies challenge this assumption, though it should be reiterated that we do not suppose that their abilities need to be akin to human theory of mind, and of course simply using such terminology offers no mechanistic explanation of how the jays might express these abilities.
5. Conclusions
According to the social function of intellect hypothesis (Humphrey 1976a,b), it is the need to survive the trials and tribulations of social life that has selected for increased socio-cognitive skills. Indeed, Humphrey (1980, p. 59) argued that ‘If a social animal is to become—as it must become—one of “Nature's psychologists” it must somehow come up with the appropriate ideology for doing psychology’ and that they do so by introspection, modelling the behaviour of other individuals by reasoning by analogy of their own experiences.
Although this hypothesis was formulated with primates in mind, in principle it could be applied to other organisms. In this paper, we have argued that at least one member of the corvid family fulfils these criteria. Consequently, we conclude that the western scrub-jay is by Humphrey's definition one of ‘nature's psychologists’.
There are two important implications that arise from this conclusion. The first is that elements of complex social cognition appear to have evolved independently in at least two very disparate groups of animals, namely the apes and corvids (Emery & Clayton 2004a). The second is that since birds do not have the typical six-layered cortex found in humans and other mammals, this divergence in structural organization of the brain raises the question of whether these cognitive abilities are achieved by similar or different neurocognitive mechanisms in the avian and mammalian brain (Emery & Clayton 2005).
Footnotes
One contribution of 19 to a Dicussion Meeting Issue ‘Social intelligence: from brain to culture’.
References
- Avian Brain Nomenclature Consortium. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglione V, Marcos J.M, Canestrari D. Cooperatively breeding groups of carrion crow (Corvus corone corone) in Northern Spain. Auk. 2002;119:790–799. doi:10.1642/0004-8038(2002)119[0790:CBGOCC]2.0.CO;2 [Google Scholar]
- Barrett L, Henzi P, Dunbar R. Primate cognition: from ‘what now?’ to ‘what if?’. Trends Cogn. Sci. 2003;7:494–497. doi: 10.1016/j.tics.2003.09.005. doi:10.1016/j.tics.2003.09.005 [DOI] [PubMed] [Google Scholar]
- Barton, R. A. & Dunbar, R. I. M 1997 Evolution of the social brain. In Machiavellian intelligence II: extensions and evaluations (eds A. Whiten & R. W. Byrne), pp. 240–263. Cambridge, UK: Cambridge University Press.
- Beauchamp G, Fernández-Juricic E. Is there a relationship between forebrain size and group size in birds? Evol. Ecol. Res. 2004;6:833–842. [Google Scholar]
- Beck B. Garland Press; New York, NY: 1980. Animal tool behavior: the use and manufacture of tools by animals. [Google Scholar]
- Bednekoff P, Balda R. Social caching and observational spatial memory in pinyon jays. Behaviour. 1996a;133:807–826. [Google Scholar]
- Bednekoff P, Balda R. Observational spatial memory in Clark's nutcrackers and Mexican jays. Anim. Behav. 1996b;52:833–839. [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Bugnyar T, Kotrschal K. Observational spatial learning and the raiding of food caches in ravens, Corvus corax: is it tactical deception? Anim. Behav. 2002;64:185–195. doi:10.1006/anbe.2002.3056 [Google Scholar]
- Burish M.J, Kueh H.Y, Wang S.S. Brain architecture and social complexity in modern and ancient birds. Brain Behav. Evol. 2004;63:107–124. doi: 10.1159/000075674. doi:10.1159/000075674 [DOI] [PubMed] [Google Scholar]
- Burt D.B, Peterson A.T. Biology of cooperative-breeding scrub jays (Aphelocoma coerulescens) of Oaxaca, Mexico. Auk. 1993;110:207–214. [Google Scholar]
- Byrne R.W, Corp N. Neocortex size predicts deception rate in primates. Proc. R. Soc. B. 2004;271:1693–1699. doi: 10.1098/rspb.2004.2780. doi:10.1098/rspb.2004.2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W, Whiten A. Clarendon Press; Oxford, UK: 1988. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes and humans. [Google Scholar]
- Call J, Tomasello M. Distinguishing intentional from accidental actions in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes) and human children (Homo sapiens) J. Comp. Psychol. 1998;112:192–206. doi: 10.1037/0735-7036.112.2.192. doi:10.1037/0735-7036.112.2.192 [DOI] [PubMed] [Google Scholar]
- Chappell J, Kacelnik A. Tool selectivity in a non-primate, the New Caledonian crow (Corvus moneduloides) Anim. Cogn. 2002;5:71–76. doi: 10.1007/s10071-002-0130-2. doi:10.1007/s10071-002-0130-2 [DOI] [PubMed] [Google Scholar]
- Chappell J, Kacelnik A. Selection of tool diameter by New Caledonian crows Corvus moneduloides. Anim. Cogn. 2004;7:121–127. doi: 10.1007/s10071-003-0202-y. doi:10.1007/s10071-003-0202-y [DOI] [PubMed] [Google Scholar]
- Clarkson K, Eden S.F, Sutherland W.J, Houston A.I. Density dependence and magpic food hoarding. J. Anim. Ecol. 1986;55:111–121. [Google Scholar]
- Clayton N.S, Dickinson A. Episodic-like memory during cache recovery by scrub-jays. Nature. 1998;395:272–274. doi: 10.1038/26216. doi:10.1038/26216 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Emery N.J. Cache robbing. In: Bekoff M, Goodall J, editors. Encyclopedia of animal behaviour. Greenwood Publishing Group; Westport, CT: 2004. pp. 251–252. [Google Scholar]
- Clayton N.S, Griffiths D.P, Emery N.J, Dickinson A. Elements of episodic-like memory in animals. Phil. Trans. R. Soc. B. 2001;356:1483–1491. doi: 10.1098/rstb.2001.0947. doi:10.1098/rstb.2001.0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N.S, Bussey T.J, Dickinson A. Can animals recall the past and plan for the future? Nat. Rev. Neurosci. 2003a;4:685–691. doi: 10.1038/nrn1180. doi:10.1038/nrn1180 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Bussey T.J, Emery N.J, Dickinson A. Prometheus to Proust: the case for behavioural criteria for ‘mental time travel’. Trends Cogn. Sci. 2003b;7:436–437. doi: 10.1016/j.tics.2003.08.003. doi:10.1016/j.tics.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Clayton N.S, Yu K.S, Dickinson A. Interacting cache memories: evidence of flexible memory use by scrub jays. J. Exp. Psychol. Anim. Behav. Proc. 2003c;29:14–22. [PubMed] [Google Scholar]
- Clayton N.S, Dally J.M, Gilbert J.D.J, Dickinson A. Food caching by western scrub-jays (Aphelocoma californica) is sensitive to conditions at recovery. J. Exp. Psychol. Anim. Behav. Proc. 2005;31:115–124. doi: 10.1037/0097-7403.31.2.115. [DOI] [PubMed] [Google Scholar]
- Curry R.L, Towsend Peterson A, Langen T.A. Western scrub-jay. In: Poole A, Gill F, editors. The birds of North America. vol. 712. Academy of Natural Sciences, American Ornithologists Union; Philadelphia, PA; Washington, DC: 2002. pp. 1–36. [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. Cache protection strategies by western scrub-jays (Aphelocoma californica): hiding food in the shade. Proc. R. Soc. B. 2004;271(Suppl.6):S387–S390. doi: 10.1098/rsbl.2004.0190. doi:10.1098/rsbl.2004.0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. Cache protection strategies by western scrub-jays: implications for social cognition. Anim. Behav. 2005a;70:1251–1263. doi:10.1016/j.anbehav.2005.02.009 [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. The social suppression of caching in western scrub-jays (Aphelocoma calfornica) Behaviour. 2005b;142:961–977. doi:10.1163/1568539055010084 [Google Scholar]
- Dally J.M, Emery N.J, Clayton N.S. Food-caching western scrub-jays keep track of who was watching when. Science. 2006a;312:1662–1665. doi: 10.1126/science.1126539. doi:10.1126/science.1126539 [DOI] [PubMed] [Google Scholar]
- Dally J.M, Clayton N.S, Emery N.J. The behaviour and evolution of cache protection and pilferage. Anim. Behav. 2006b;72:13–23. doi:10.1016/j.anbehav.2005.08.020 [Google Scholar]
- Dunbar R.I.M. Neocortex size as a constraint on group size in primates. J. Human Evol. 1992;20:469–493. doi:10.1016/0047-2484(92)90081-J [Google Scholar]
- Dunbar R.I.M. The social brain hypothesis. Evol. Anthropol. 1998;6:178–190. doi:10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8 [Google Scholar]
- Emery N.J. Are corvids ‘feathered apes’? Cognitive evolution in crows, jays, rooks and jackdaws. In: Watanabe S, editor. Comparative analysis of minds. Keio University Press; Tokyo, Japan: 2004. pp. 181–213. [Google Scholar]
- Emery N.J, Clayton N.S. Effects of experience and social context on prospective caching strategies in scrub jays. Nature. 2001;414:443–446. doi: 10.1038/35106560. [DOI] [PubMed] [Google Scholar]
- Emery N.J, Clayton N.S. The mentality of crows. Convergent evolution of intelligence in corvids and apes. Science. 2004a;306:1903–1907. doi: 10.1126/science.1098410. doi:10.1126/science.1098410 [DOI] [PubMed] [Google Scholar]
- Emery N.J, Clayton N.S. Comparing the complex cognition of birds and primates. In: Rogers L.J, Kaplan G.S, editors. Comparative vertebrate cognition. Kluwer Academic Press; The Hague, The Netherlands: 2004b. pp. 3–55. ch. 1. [Google Scholar]
- Emery N.J, Clayton N.S. Evolution of avian brain and intelligence. Curr. Biol. 2005;15:R1–R5. doi: 10.1016/j.cub.2005.11.029. doi:10.1016/j.cub.2005.11.029 [DOI] [PubMed] [Google Scholar]
- Emery, N. J. & Clayton, N. S. In press. How to build a scrub-jay that reads minds. In: Origins of the social mind: evolutionary and developmental perspectives (eds S. Itakura & K. Fujita). Tokyo, Japan: Springer.
- Emery N.J, Seed A.M, von Bayern A.M.P. Cognitive adaptations of social bonding in birds. Phil. Trans. R. Soc. B. 2006;362:489–505. doi: 10.1098/rstb.2006.1991. doi:10.1098/rstb.2006.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Jays and crows recovering hidden food. Br. Birds. 1955;48:181–183. [Google Scholar]
- Goodwin D. Further observations on the behaviour of the jay (Garrulus glandarius) Ibis. 1956;98:186–219. [Google Scholar]
- Goodwin D. British Museum (Natural History); Bury St Edmunds, UK: 1976. Crows of the world. [Google Scholar]
- Goodwin, D. 1986 Crows of the world, 2nd edn. London, UK: British Museum (Natural History).
- Hampton R.R, Hampstead B.M, Murray E.A. Rhesus monkeys (Macaca mulatta) demonstrate robust memory for what and where, but not when, in an open-field test of memory. Learn. Motiv. 2005;36:245–259. doi:10.1016/j.lmot.2005.02.004 [Google Scholar]
- Hare B. Can competitive paradigms increase the validity of experiments on primate social cognition? Anim. Cogn. 2001;4:269–280. doi: 10.1007/s100710100084. doi:10.1007/s100710100084 [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Agnetta M, Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim. Behav. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. doi:10.1006/anbe.1999.1377 [DOI] [PubMed] [Google Scholar]
- Hare B, Call J, Tomasello M. Do chimpanzees know what conspecifics know? Anim. Behav. 2001;61:139–151. doi: 10.1006/anbe.2000.1518. doi:10.1006/anbe.2000.1518 [DOI] [PubMed] [Google Scholar]
- Heinrich B. Harper Collins; New York, NY: 1999. Mind of the raven. [Google Scholar]
- Heinrich B, Pepper J. Influence of competitors on caching behaviour in the common raven (Corvus corax) Anim. Behav. 1998;56:1083–1090. doi: 10.1006/anbe.1998.0906. doi:10.1006/anbe.1998.0906 [DOI] [PubMed] [Google Scholar]
- Heyes C. Theory of mind in non-human primates. Behav. Brain Sci. 1998;21:101–148. doi: 10.1017/s0140525x98000703. doi:10.1017/S0140525X98000703 [DOI] [PubMed] [Google Scholar]
- Humphrey N.K. The social function of intellect. In: Byrne R.W, Whiten A, editors. Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes and humans. Clarendon Press; Oxford, UK: 1976a. pp. 285–305. [Google Scholar]
- Humphrey N.K. The social function of intellect. In: Bateson P.P.G, Hinde R.A, editors. Growing points in ethology. Cambridge University Press; Cambridge, UK: 1976b. pp. 303–317. [Google Scholar]
- Humphrey N.K. Nature's psychologists. In: Josephson B.D, Ramachandran V.S, editors. Consciousness and the physical world. Pergamon Press; Oxford, UK: 1980. pp. 57–80. [Google Scholar]
- Hunt G.R. Manufacture and use of hook-tools by New Caledonian crows. Nature. 1996;379:249–251. doi:10.1038/379249a0 [Google Scholar]
- Hunt G.R, Gray R.D. Diversification and cumulative evolution in New Caledonian crow tool manufacture. Proc. R. Soc. B. 2003;270:867–874. doi: 10.1098/rspb.2002.2302. doi:10.1098/rsbl.2003.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk A.N, Arnold K. Is cooperative breeding associated with bigger brains? A comparative test in the Corvids (Passeriformes) Ethology. 2004;10:203–220. doi:10.1111/j.1439-0310.2003.00957.x [Google Scholar]
- Joffe T.H. Social pressures have selected for an extended juvenile period in primates. J. Human Evol. 1997;32:593–605. doi: 10.1006/jhev.1997.0140. doi:10.1006/jhev.1997.0140 [DOI] [PubMed] [Google Scholar]
- Jolly A. Lemur social behaviour and intelligence. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. doi:10.1126/science.153.3735.501 [DOI] [PubMed] [Google Scholar]
- Kalländer H. Hoarding in the rook (Corvus frugilegus) Anser Suppl. 1978;3:124–128. [Google Scholar]
- Karin-D'Arcy R, Povinelli D. Do chimpanzees know what others see? A closer look. Int. J. Comp. Psychol. 2002;15:21–54. [Google Scholar]
- Kudo H, Dunbar R.I.M. Neocortex size and social network in primates. Anim. Behav. 2001;62:711–722. doi:10.1006/anbe.2001.1808 [Google Scholar]
- Lewis K.P. A comparative study of primate play behaviour: implications for the study of cognition. Folia Primatol. 2000;71:417–421. doi: 10.1159/000052740. [DOI] [PubMed] [Google Scholar]
- Limongelli L, Boysen S.T, Visalberghi E. Comprehension of cause–effect relations in a tool-using task by chimpanzees (Pan troglodytes) J. Comp. Psychol. 1995;109:18–26. doi: 10.1037/0735-7036.109.1.18. doi:10.1037/0735-7036.109.1.18 [DOI] [PubMed] [Google Scholar]
- Mackintosh N.J. Approaches to the study of animal intelligence. Br. J. Psychol. 1988;79:509–525. [Google Scholar]
- Marino L. Convergence in complex cognitive abilities in cetaceans and primates. Brain Behav. Evol. 2002;59:21–32. doi: 10.1159/000063731. [DOI] [PubMed] [Google Scholar]
- McGonigle B.O, Chalmers M. Are monkeys logical? Nature. 1977;267:694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Menzel C.R. Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. J. Comp. Psychol. 1999;113:426–434. doi: 10.1037/0735-7036.113.4.426. doi:10.1037/0735-7036.113.4.426 [DOI] [PubMed] [Google Scholar]
- Mulcahy N, Call J. Apes save tools for future use. Science. 2006;312:1038–1040. doi: 10.1126/science.1125456. doi:10.1126/science.1125456 [DOI] [PubMed] [Google Scholar]
- Paz-y-Miño G.C, Bond A.B, Kamil A.C, Balda R.P. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Povinelli D. Oxford University Press; Oxford, UK: 2000. Folk physics for apes. The chimpanzee's theory of how the word works. [Google Scholar]
- Povinelli D.J, Vonk J. We don't need a microscope to explore the chimpanzee's mind. Mind Lang. 2004;19:1–28. [Google Scholar]
- Povinelli D.J, Nelson K.E, Boysen S.T. Inferences about guessing and knowing by chimpanzees (Pan troglodytes) J. Comp. Psychol. 1990;104:203–210. doi: 10.1037/0735-7036.104.3.203. doi:10.1037/0735-7036.104.3.203 [DOI] [PubMed] [Google Scholar]
- Povinelli D.J, Bering J.M, Giambrone S. Towards a science of other minds: escaping the argument by analogy. Cogn. Sci. 2000;24:509–541. doi:10.1016/S0364-0213(00)00023-9 [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;1:515–526. [Google Scholar]
- Richner H. Helpers at the nest in carrion crows (Corvus corone corone) J. Anim. Ecol. 1990;58:427–440. [Google Scholar]
- Schwartz B.L, Evans S. Episodic memory in primates. Am. J. Primatol. 2001;55:71–85. doi: 10.1002/ajp.1041. doi:10.1002/ajp.1041 [DOI] [PubMed] [Google Scholar]
- Schwartz B.L, Colon M.R, Sanchez I.C, Rodriguez I.A, Evans S. Single-trial learning of “what” and “who” information in a gorilla (Gorilla gorilla gorilla): implications for episodic memory. Anim. Cogn. 2002;5:85–90. doi: 10.1007/s10071-002-0132-0. doi:10.1007/s10071-002-0132-0 [DOI] [PubMed] [Google Scholar]
- Seed A.M, Tebbich S, Emery N.J, Clayton N.S. Investigating physical cognition in rooks (Corvus frugilegus) Curr. Biol. 2006;16:697–701. doi: 10.1016/j.cub.2006.02.066. doi:10.1016/j.cub.2006.02.066 [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002;5:272–276. doi: 10.1038/nn814. doi:10.1038/nn814 [DOI] [PubMed] [Google Scholar]
- Shettleworth S.J. Comparative studies of memory in food storing birds: from the field to the Skinner box. In: Alleva E, Fasolo A, Lipp H.-P, Nadel L, Ricceri L, editors. Behavioural brain research in naturalistic and semi-naturalistic settings. Academic Press; Dordrecht, The Netherlands: 1995. pp. 159–194. [Google Scholar]
- Suddendorf T, Busby J. Mental time travel in animals? Trends Cogn. Sci. 2003;7:391–396. doi: 10.1016/s1364-6613(03)00187-6. doi:10.1016/S1364-6613(03)00187-6 [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis M.C. Mental time travel and the evolution of the human mind. Genet. Soc. Gen. Psychol. Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- Treichler F.R, Van Tilburg D. Concurrent conditional discrimination tests of transitive inference by macaque monkeys: list linking. J. Exp. Psychol. Anim. Behav. Proc. 1996;22:105–117. [PubMed] [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York, NY: 1997. Primate cognition. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: uniquely human? In: Terrace H, Metcalfe J, editors. The missing link in cognition: evolution of self-knowing consciousness. Oxford University Press; Oxford, UK: 2005. pp. 3–56. [Google Scholar]
- Vander Wall S.B, Jenkins S.H. Reciprocal pilferage and the evolution of food-hoarding behaviour. Behav. Ecol. 2003;14:656–667. doi:10.1093/beheco/arg064 [Google Scholar]
- Visalberghi E, Limongelli L. Lack of comprehension of cause–effect relations in tool-using capuchin monkeys (Cebus apella) J. Comp. Psychol. 1994;108:15–22. doi: 10.1037/0735-7036.108.1.15. doi:10.1037/0735-7036.108.1.15 [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Trinca L. Tool use in capuchin monkeys: distinguishing between performing and understanding. Primates. 1989;30:511–521. doi:10.1007/BF02380877 [Google Scholar]
- Watanabe, S. & Clayton, N. S. 2007 Observational visuospatial encoding of the cache locations of others by western scrub-jays (Aphelocoma californica). J. Ethol (doi:10.1007/s10164-006-0023-y)
- Weir A.A.S, Chappell J, Kacelnik A. Shaping of hooks in New Caledonian crows. Science. 2002;297:981. doi: 10.1126/science.1073433. doi:10.1126/science.1073433 [DOI] [PubMed] [Google Scholar]
- Whiten, A. & Byrne, R. W. 1997 Machiavellian intelligence II Cambridge, UK: Cambridge University Press.
- Whiten A, Goodall J, McGrew W.C, Nishida T, Reynolds V, Sugiyama Y, Tutin C.E.G, Wrangham R.W, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. doi:10.1038/21415 [DOI] [PubMed] [Google Scholar]
- Wilson B.J, Mackintosh N.J, Boakes R.A. Transfer of relational rules in matching and oddity learning by pigeons and corvids. Q. J. Exp. Psychol. 1985;37B:313–332. [Google Scholar]
- Woolfenden G.E. Florida scrub jay helpers at the nest. Auk. 1975;92:1–15. [Google Scholar]
- Woolfenden G.E, Fitzpatrick J.W. Monographs of population biology. vol. 20. Princeton University Press; Princeton, NJ: 1984. The Florida scrub-jay; demography of a cooperative breeding bird. [Google Scholar]
- Woolfenden G.E, Fitzpatrick J.W. Florida scrub-jay (Aphelocoma coerulescens) In: Poole A, Gill F, editors. The birds of North America. vol. 228. The Academy of Natural Sciences, American Ornithologists' Union; Philadelphia, PA; Washington, DC: 1996. [Google Scholar]