Abstract
If the large brains and great intelligence characteristic of primates were favoured by selection pressures associated with life in complex societies, then cognitive abilities and nervous systems with primate-like attributes should have evolved convergently in non-primate mammals living in large, elaborate societies in which social dexterity enhances individual fitness. The societies of spotted hyenas are remarkably like those of cercopithecine primates with respect to size, structure and patterns of competition and cooperation. These similarities set an ideal stage for comparative analysis of social intelligence and nervous system organization. As in cercopithecine primates, spotted hyenas use multiple sensory modalities to recognize their kin and other conspecifics as individuals, they recognize third-party kin and rank relationships among their clan mates, and they use this knowledge adaptively during social decision making. However, hyenas appear to rely more intensively than primates on social facilitation and simple rules of thumb in social decision making. No evidence to date suggests that hyenas are capable of true imitation. Finally, it appears that the gross anatomy of the brain in spotted hyenas might resemble that in primates with respect to expansion of frontal cortex, presumed to be involved in the mediation of social behaviour.
Keywords: hyena, hyaena, intelligence, social cognition, brain morphology
1. Introduction
Primates appear to be endowed with cognitive abilities that are superior to, and qualitatively different from, those observed in most other mammals (reviewed in Byrne & Whiten 1988; Harcourt & de Waal 1992; Tomasello & Call 1997). The social complexity hypothesis suggests that the key selection pressures shaping the evolution of these cognitive abilities have been imposed by complexity associated with the labile social behaviour of conspecific group members (reviewed in Byrne & Whiten 1988). Predictions of this hypothesis have now been confirmed in a number of primate species, suggesting that the evolution of intelligence has been more strongly influenced by social pressures than by non-social aspects of the environment (reviewed in Byrne 1994; Tomasello & Call 1997). Unfortunately, the generality of this hypothesis is severely limited by the current dearth of information about social cognition in animals other than primates (Harcourt & de Waal 1992). The social complexity hypothesis predicts that, if indeed the large brains and great intelligence found in primates evolved in response to selection pressures associated with life in complex societies, then cognitive abilities and nervous systems with primate-like attributes should have evolved convergently in non-primate mammals living in large, elaborate societies in which individual fitness is strongly influenced by social dexterity.
Mammalian carnivores represent an excellent group, outside of the primates, within which to evaluate the relationship between cognitive abilities and social complexity. Carnivores often form social groups that are comparable in size and complexity to those of primates; many species live in large, permanent social units that contain both males and females from multiple, overlapping generations. Recent studies of phylogenetic relationships among the orders of eutherian mammals suggest that Carnivora and Primates are not sister taxa, but rather are members of distinct clades (Laurasiatheria and Euarchontoglires, respectively) that last shared a common ancestor between 90 and 100 Myr ago (Springer et al. 2003, 2005). Therefore, mammalian carnivores offer the opportunity for an independent test of the hypothesis that demands imposed by social living have driven the evolution of both intelligence and nervous systems in mammals. Here, we adopt Kamil's (1987) broad definition of intelligence, and therefore define social intelligence as ‘those processes by which animals obtain and retain information about their social environments, and use that information to make behavioural decisions’.
Gregarious carnivores engage in a variety of behaviours, such as cooperative hunts of large vertebrate prey, that have prompted many observers to infer that these predators must possess extraordinary intellectual powers (e.g. Guggisberg 1962). However, the cognitive abilities of carnivores have seldom been the subject of systematic study, and they are currently poorly understood (e.g. Byrne 1994). In our research, we are examining the perceptual, cognitive and neural mechanisms underlying complex social behaviour in one gregarious carnivore, the spotted hyena (Crocuta crocuta). One of our primary objectives is to determine whether or not these hyenas exhibit some of the same specific cognitive abilities as those found in primates. Evidence for the existence of shared cognitive abilities would suggest convergent evolution in these two distantly related taxa and would strongly support the social complexity hypothesis. Another major objective is to evaluate the possibility that there has been convergent evolution in the gross anatomy of the brain in hyenas and primates, favoured in both groups by the need to predict and interpret the labile social behaviour of conspecifics. After briefly reviewing the relevant aspects of Crocuta's biology below, we summarize our progress to date towards achieving each of these objectives.
2. Relevant biology of spotted hyenas
Spotted hyenas are large terrestrial predators occurring throughout sub-Saharan Africa. Although they occupy a different trophic niche than primates and have various sensory capabilities not shared with primates, hyenas nevertheless exhibit many remarkable similarities to cercopithecine primates with respect to their life histories and to the size and complexity of their social groups. Like macaques and baboons, spotted hyenas are large-bodied mammals with slow life histories. Although Crocuta's diet matches that of other large African carnivores (Kruuk 1972; Schaller 1972; Mills 1990; Caro 1994), the foods of both hyenas and cercopithecine primates generally occur in rich scattered patches appearing unpredictably in space and time. Female hyenas bear litters containing only one or two cubs, and they nurse each litter for up to 24 months. Thus, hyenas, like primates, produce small litters at long intervals, and their offspring require an unusually long period of nutritional dependence on the mother. Both hyenas and primates experience a long juvenile period during which every individual must learn a great deal about its physical and social environments. Males reach reproductive maturity at 24 months of age, and most females start bearing young in their third or fourth year. Like many primates, hyenas have a long lifespan: they are known to live up to 19 years in the wild (Drea & Frank 2003) and up to 41 years in captivity (Jones 1982).
(a) Group size and structure
The complexity of spotted hyena societies is comparable in most respects to that found in societies of cercopithecine primates, and far exceeds that found in the social lives of any other terrestrial carnivore (e.g. Gittleman 1989, 1996; Holekamp et al. 2000). Crocuta live in permanent complex social groups, called clans, that range in size from 6 to 90 individuals. All members of a clan recognize each other, cooperatively defend a common territory and rear their cubs together at a communal den (Kruuk 1972; Henschel & Skinner 1991). Like cercopithecine primates, Crocuta establish enduring relationships with clan mates that often last many years. Clan size and territory size vary with prey abundance across the species' range, but the clans inhabiting the prey-rich plains of eastern Africa are as large as sympatric baboon troops (e.g. Sapolsky 1993), and often contain more than 70 individuals (Kruuk 1972). Like baboon troops, hyena clans contain multiple adult males and multiple matrilines of adult female kin with offspring, including individuals from several overlapping generations. Relatedness is high within matrilines but, on average, clan members are only very distantly related due to high levels of male-mediated gene flow among clans, and mean relatedness declines only slightly across clan borders (Van Horn et al. 2004).
Like many primates, hyenas within each clan can be ranked in a linear dominance hierarchy based on outcomes of agonistic interactions, and priority of resource access varies with social rank (Tilson & Hamilton 1984; Andelman 1985; Frank 1986). As in many cercopithecine primates, dominance ranks in hyena society are not correlated with size or fighting ability; instead, power in hyena society resides with the individuals having the best network of allies. In both hyenas and cercopithecine primates, members of the same matriline occupy adjacent rank positions in the group's hierarchy, and female dominance relations are extremely stable across a variety of contexts and over periods of many years. One interesting difference between hyenas and cercopithecines in regard to rank is that adult female hyenas dominate adult males, whereas male cercopithecines dominate females. However, as in virtually all cercopithecine species, male hyenas disperse voluntarily from their natal groups after puberty, whereas females are usually philopatric (Cheney & Seyfarth 1983; Henschel & Skinner 1987; Mills 1990; Smale et al. 1997; Boydston et al. 2005). Although adult natal male hyenas dominate adult females ranked lower than their own mothers in the clan's dominance hierarchy so long as they remain in the natal clan, when males disperse they behave submissively to all new hyenas encountered outside the natal area. This is the point during ontogenetic development at which females come to dominate males (Smale et al. 1993, 1997). When a male joins a new clan, he assumes the lowest rank in that clan's dominance hierarchy (Smale et al. 1997). Immigrant males rarely fight among themselves; instead, they form a queue in which the immigrant that arrived first in the clan holds the highest rank in the male hierarchy and the most recently arrived male the lowest (Smale et al. 1997; East & Hofer 2001).
(b) Competition and cooperation
In contrast to the social groups of most cercopithecine primates, which tend to be extremely cohesive, Crocuta clans are fission–fusion societies in which individual hyenas spend much of their time alone or in small groups, particularly when foraging (Holekamp et al. 1997a,b). Ungulate carcasses represent extremely rich but ephemeral food resources: a group of hungry hyenas can reduce a large antelope to a few scattered bones in less than half an hour. Competition when feeding at carcasses is therefore extremely intense, and dominant hyenas, which can most effectively displace conspecifics from food, gain access to the choicest bits and largest quantities of food. Crocuta often need kin and other allies to defend a carcass from other clan mates. In addition, Crocuta need allies during cooperative defence of the clan's territory against alien hyenas (e.g. Henschel & Skinner 1991; Boydston et al. 2001). Members of multiple hyena matrilines frequently cooperate to defend their kills against lions or hyenas from other clans, and by doing so risk serious injury or death (Kruuk 1972; Mills 1990; Henschel & Skinner 1991; Hofer & East 1993; Boydston et al. 2001). Help from clan mates is also often required while hunting ungulate prey; the probability of successfully making a kill increases by approximately 20% with the presence of each additional hunter (Holekamp et al. 1997b). Thus, as in cercopithecine primates, the enduring cooperative relationships found among these long-lived carnivores affect survival and reproduction of individual group members.
The many aspects of their social lives and life histories that hyenas share with cercopithecine primates set an ideal stage for comparative analysis of social cognition and nervous system organization. Here, we first review what is known about hyena communication signals, perception of those signals and demonstrated abilities in the domain of social cognition. We then describe work we have recently initiated comparing gross anatomy of the brain in spotted hyenas with that in less gregarious carnivores.
3. Communication signals, perceptual abilities and social cognition
Cercopithecine primates possess well-developed cognitive abilities, making them unusually adept at predicting outcomes of behavioural interactions among their conspecifics (e.g. Kamil 1987; Byrne & Whiten 1988; Cheney & Seyfarth 1990, 2003; Harcourt & deWaal 1992; Byrne 1994). They recognize individual conspecifics based on information acquired via multiple sensory modalities, they remember outcomes of earlier encounters with particular conspecifics, and they modify their social behaviour on the basis of interaction histories. Furthermore, cercopithecines clearly possess knowledge about the social relationships among other group members, and adaptively base their decision-making in social situations upon this knowledge (e.g. Cheney & Seyfarth 1990). Here, we review what spotted hyenas know about their social companions, and how they use that knowledge.
(a) Individual recognition
Social complexity is often reflected in the variety of communication signals emitted by a species and in the ability of receivers to perceive and process that information (Blumstein & Armitage 1997). Furthermore, perceptual mechanisms influence and constrain cognitive abilities (Barrett & Henzi 2005). Spotted hyenas emit a rich repertoire of visual, acoustic and olfactory signals. They use these signals to discriminate clan members from alien hyenas (Kruuk 1972; Mills 1990; Henschel & Skinner 1991), to recognize the other members of their social units as individuals and to obtain information about signallers' affect and current circumstances.
Hans Kruuk (1972) was the first observer to become convinced, based on watching spotted hyenas interact in nature, that they can recognize all their group mates using visual, acoustic or olfactory cues. No systematic analysis has been done of visual recognition. However, den-dwelling cubs in our study populations respond appropriately when their mothers, approaching the den silently from downwind, are still hundreds of metres away, suggesting good visual acuity and its application in individual recognition. In the presence of conspecifics, hyenas attend closely to body postures and visual displays of other animals, and especially while feeding at a carcass, they attend to the relative positions of conspecifics; young hyenas, in particular, attempt to gain access to carcasses by entering each feeding melee next to one or more potential allies.
Recognition of conspecifics using vocal and olfactory cues has been systematically studied in Crocuta. Spotted hyenas emit a rich repertoire of sounds that includes groans, growls, lows, yells, screams, rumbles and giggles (Kruuk 1972). However, the only hyena call that has been analysed to date is the long-distance ‘whoop’ vocalization. A whoop bout, which lasts several seconds, is a loud vocalization containing several brief calls separated by pauses. Whoops are emitted by hyenas of both sexes and all ages, starting a few hours after birth (East & Hofer 1991a,b). A whoop travels up to 5 km, and clearly has a number of different functions depending on which individuals whoop, and the circumstances under which these calls are emitted. Whereas the acoustic structure of whoop vocalizations varies markedly among individual hyenas, the whoops produced by any single hyena are highly consistent, even over periods of up to several years (East & Hofer 1991a). Thus, an acoustic basis exists for individual recognition in Crocuta.
We used playback experiments, modelled after those conducted earlier with vervet monkeys by Cheney & Seyfarth (1980), to determine whether hyenas are capable of identifying individual conspecifics on the basis of their whoop vocalizations (Holekamp et al. 1999). Hyena mothers frequently respond to the whoops of their own cubs by rushing to help them, much like vervet females respond to the distress cries of their offspring (Cheney & Seyfarth 1980). In fact, one of the most important functions of whoops by cubs is to request assistance when they are threatened or frightened (East & Hofer 1991b). Our experiments revealed that a cub's whoops were far more likely to elicit approach and intervention behaviour by its mother than by other listeners (Holekamp et al. 1999).
In addition to coding information about the individual identity of callers, whoops also convey information about the caller's age and sex (East & Hofer 1991a; Holekamp et al. 1999). Whoops by cubs typically contain fewer harmonics, wider spacing between harmonics, shorter durations of low-frequency sections of calls and higher fundamental frequencies than do whoops emitted by adults (East & Hofer 1991a). In addition, Theis et al. (submitted) have recently found that hyenas encode information about their current emotional state by altering the rate at which they produce individual whoops within a whoop bout and by adjusting the length of intervals between these calls. When callers are frightened or upset, they produce calls within bouts at higher rates and reduce inter-whoop interval length. Listeners are significantly more likely to respond to calls with shorter inter-whoop intervals, and from this we infer that listening hyenas monitor the urgency signalled in such calls.
Olfaction plays a similarly important role in the social lives of spotted hyenas. These animals have a keen olfactory sense, and they engage in frequent scent-marking behaviour. Each clan appears to have a unique scent signature (Hofer et al. 2001), and wild hyenas mark the boundaries of their group territories with secretions from their scent glands (Kruuk 1972; Henschel & Skinner 1991; Boydston et al. 2001). Drea et al. (2002a,b) found that captive female hyenas spent more time investigating the scents of male than female conspecifics, and that adult subjects of both sexes investigated scents of familiar conspecifics for shorter amounts of time than they spent investigating scents of unfamiliar individuals. These studies demonstrated clearly that Crocuta can use olfactory cues to discriminate sex and familiarity of conspecifics (Drea et al. 2002a,b).
Recent field experiments by Theis et al. (in press) have shown that wild hyenas can use olfactory cues to acquire additional information as well, and that adults perform differently from cubs in these experiments. Both cubs and older hyenas can distinguish scents of their clan mates from those of hyenas from other clans. Furthermore, cubs express equal interest in scents from males and females, and they also express equal interest in scents from pregnant and lactating females. By contrast, adult females in the wild show clear preferences for scents from females over those from males (the opposite of what was found among captives) and for scents from pregnant over lactating females. The differences in performance between cubs and adults in these olfactory discrimination experiments suggest that these scents and their meanings are learned.
(b) Recognition of kin
As in most primates (e.g. Seyfarth 1980; Seyfarth & Cheney 1984), nepotism is common among Crocuta, kin spend more time together than do non-kin (Holekamp et al. 1997a), and individuals direct affiliative behaviour towards kin more frequently than towards non-kin (Walters 1980; East et al. 1993; Wahaj et al. 2004). Hyenas can distinguish vocalizations of kin from those of non-kin, and in fact the intensity of their responses to whoop vocalizations increases with degree of relatedness between vocalizing and listening animals (Holekamp et al. 1999). Results of our playback experiments suggest that kin recognition may occur among hyenas as distantly related as great-aunts and cousins. Although male hyenas do not participate at all in parental care, Van Horn et al. (2004) found that sires identified by molecular genetic analysis associated more closely with their daughters than with unrelated control females. In addition, these workers found that cubs favoured their fathers by directing less intense aggression at them than at unrelated adult males. Cubs of both sexes associate more closely with their fathers than with control males after cubs become independent of the communal den. All these results indicate that fathers can recognize their offspring as is the case in baboons (Buchan et al. 2003) and that offspring can also recognize their sires.
In an analysis of nepotism between siblings, Wahaj et al. (2004) found that full siblings from twin litters associate more closely, and direct more affiliative behaviour towards each other, than do half-sibling littermates. This, like the ability of offspring to recognize their sires, indicates that spotted hyenas use phenotype matching (Holmes & Sherman 1982) to recognize kin. However, Wahaj et al. (2004) also found that young hyenas associate more closely with maternal half-siblings than with paternal half-siblings, which suggests the operation of an association-based mechanism along with phenotype matching in Crocuta's kin recognition.
(c) Imitation and coordination of behaviour among multiple animals
Understanding how, when and why animals coordinate their behaviour can shed light on the underlying cognitive and neurobiological processes (Barrett & Henzi 2005). Like cercopithecine primates, spotted hyenas often appear to modify their behaviour after observing the goal-directed behaviour of their group mates. However, although we have made no experimental inquiries about this, we have found no evidence to date that hyenas engage in true imitation, defined as emulating a novel act from the repertoire of a conspecific (Byrne 1995). Instead, spotted hyenas appear to engage on a daily basis in simpler forms of social learning that make fewer cognitive demands than true imitation, including observational conditioning (Emery & Clayton 2005) and response facilitation (Byrne 1994). For example, in response to the played-back sound of a cub whooping in distress, hyenas related to that cub orient to the sound, but only start searching for it when the mother does so first (Holekamp et al. 1999). This suggests that response facilitation in this species might be an important proximate mechanism mediating decisions regarding whether or not to aid others. In general, this and other forms of social facilitation appear to play much larger roles in the social lives of spotted hyenas than they do in the lives of cercopithecine primates (e.g. Woodmansee et al. 1991; Yoerg 1991; Glickman et al. 1997; Holekamp et al. 2000). In addition to mediating aiding behaviour, response facilitation strongly affects the behaviour of spotted hyenas engaged in feeding, scent-marking, coalition formation, greeting ceremonies and group hunts (reviewed in Glickman et al. 1997).
Cooperative hunting permits hyenas to capture prey animals many times larger than any individual hunter. Group hunts by spotted hyenas, lions and other gregarious carnivores often appear to involve intelligent coordination and division of labour among hunters (e.g. Guggisberg 1962; Peters & Mech 1973). Group hunts by mammalian carnivores certainly represent more complexly organized phenomena than mere opportunistic grabs at prey (e.g. Stander 1992a,b). However, although myriad observers have claimed that the group hunting activity of large carnivores requires the operation of human-like mental processes, coordinated hunting behaviour by hyenas can in fact most parsimoniously be explained by the operation of a few simple mental rules of thumb, such as ‘Move wherever you need to in order to keep the selected prey animal between you and another hunter’ (Holekamp et al. 2000). Currently, there is no evidence that hyenas use mental algorithms more complex than simple rules of thumb to capture prey during group hunts. Falsification of the simple ‘rules of thumb’ hypothesis will require experimental evidence, not only that individual hyenas monitor both their prey and their fellow hunters (e.g. Stander 1992b), but also that they accurately anticipate the behaviour of the latter based on knowledge of their goals.
(d) Rank acquisition and social memory
Spotted hyenas appear to enter the world prepared (sensu Bolles 1973) to learn their positions in the clan's dominance hierarchy and to remember their histories of interactions with individual conspecifics. During an early period of intensive learning, each hyena comes to understand its own position in a dominance hierarchy that may contain dozens of other individuals (Holekamp & Smale 1993). During the first 2 years of life, juvenile hyenas of both sexes acquire ranks immediately below those of their mothers (Holekamp & Smale 1991, 1993; Smale et al. 1993). This occurs through an elaborate process of associative learning called ‘maternal rank inheritance’ in which the mediating mechanisms are virtually identical to those operating during the period of rank acquisition in many cercopithecine primates (Horrocks & Hunte 1983; Jenks et al. 1995; Engh et al. 2000). In particular, coalitionary aggression plays an important role in acquisition and maintenance of social rank in Crocuta (Mills 1990; Zabel et al. 1992; Holekamp & Smale 1993; Smale et al. 1993), as it does in these primates (e.g. Cheney 1977; Walters 1980; Datta 1986; Chapais 1992).
When hyena cubs first arrive at the clan's communal den, at approximately one month of age, they are just as likely to attack cubs from higher-ranking matrilines as they are to attack offspring of lower-ranking females (Holekamp & Smale 1991, 1993). However, through maternal interventions and coalitionary support from maternal kin and unrelated clan mates, juvenile hyenas learn during early life that they can dominate individuals ranked lower than their mothers (Horrocks & Hunte 1983; Holekamp & Smale 1993; Smale et al. 1993; Engh et al. 2000). By the time they are eight to nine months of age, their attack behaviour directed at higher-born peers has been completely extinguished, and they now restrict their attacks to lower-born individuals. The process of rank acquisition relative to non-peer clan mates appears to be complete by around 18 months of age (Smale et al. 1993). Furthermore, non-littermate hyena siblings assume relative ranks that are inversely related to age in a pattern of ‘youngest ascendancy’ exactly like that seen in cercopithecine primates (Horrocks & Hunte 1983; Holekamp & Smale 1993). Here too, the mechanisms involved appear to be identical to those in primates (Kurland 1977; Chapais & Schulman 1980); mothers assist their youngest cubs during resource competition, even when this forces mothers to behave aggressively towards their older offspring (Holekamp & Smale 1993).
Spotted hyenas appear to remember the identities and ranks of their clan mates throughout their lives. Although we have conducted no formal studies of social memory in this species, anecdotes provide some basic information about it. For example, we have observed hyenas behave as though they remember individuals from whom they have been separated for one to several years. In one case, two females that had been absent from the clan for an entire year were allowed to rejoin it, albeit at the lowest possible rank positions in the female hierarchy, whereas all other females intruding into the clan's territory were inevitably expelled (Holekamp et al. 1993). On another occasion during a border skirmish between members of neighbouring hyena clans, a male that had dispersed from one of these clans several years earlier came racing onto the scene with tail bristled, clearly excited to engage in battle, as were the other immigrant males present at the scene. However, upon orienting towards and recognizing some of its female kin from afar among the opposing combatants, the male immediately desisted, lowering its tail and exhibiting other signs of loss of enthusiasm for battle. From cases like these, we deduce that hyenas can long remember their histories of past interactions with particular conspecifics, and modify their behaviour accordingly.
(e) Application of knowledge about social rank
To see that spotted hyenas adaptively use their knowledge of the social ranks of their clan mates, one needs only spend a few minutes watching them fight over a fresh carcass. Despite the fact that all the hyenas present at a kill are often covered in blood from the prey animal, individual Crocuta are astoundingly good at knowing which conspecifics are safe to attack and displace from the carcass, and which ones are better left alone. Adult hyenas only attack animals lower-ranking than themselves in the clan's dominance hierarchy, and they never attack higher-ranking individuals as to do so would most probably result in counterattack by the target animal and its allies, as well as potentially serious injury. The only ‘mistakes’ we have ever seen under these circumstances have involved young hyenas at kills: here a youngster that had recently become independent of the communal den would inappropriately attack a high-ranking hyena with which the attacker had most probably had very little or no prior experience.
Aside from competing over carcasses, another situation in which it would be adaptive for Crocuta to be able to discriminate among clan mates of different ranks occurs during courtship interactions between adult males and females. Szykman et al. (2001) found that male–female interactions and associations in this species are almost exclusively initiated and maintained by males. These authors also found that the social ranks of both male and female hyenas influenced intersexual patterns of association. Both high- and middle-ranking males associated most closely with the highest-ranking females. Since female reproductive success varies enormously with social rank in Crocuta (Frank et al. 1995; Holekamp et al. 1996; Hofer & East 2003), males should attempt to associate and mate with the highest-ranking females possible if males are able to discriminate among females based on status. Indeed, when female reproductive condition is controlled, high- and middle-ranking males preferentially seek out high-ranking females, suggesting that males can discern relative rank relationships among their prospective mates (Szykman et al. 2001). Interestingly, low-ranking immigrant males, which had only recently arrived in the study clan, failed to exhibit a preference for high-ranking females. One interpretation of this is that these low-ranking males were disadvantaged by their lack of experience in the social group such that they were less adept than males with longer tenure at assessing rank (hence reproductive value) among clan females, and indicating that it may take immigrant males some time to learn the relative ranks of resident females (Szykman et al. 2001).
(f) Partner choice and recognition of relationship value
The value of a relationship reflects the magnitude of social or ecological benefits likely to accrue from it, with highly valuable relationships most worthy of maintenance and protection (Cords 1988). Owing to the strict linear dominance hierarchy that structures every Crocuta clan, an individual's social rank should reflect its value as a social partner, and thus potential social partners should vary greatly in their value to conspecifics in this species. When conspecifics vary in their relative value as social partners, individuals should possess the ability to assess the value of each potential partner, and compete for partners of the highest relative value based on those assessments (Noë & Hammerstein 1994). Primatologists have long known that cercopithecine primates associate most closely with unrelated dominants ranking immediately above them in the social hierarchy (see Cheney et al. 1986; Schino 2001 for reviews), and even assign higher value to information they receive about high- than low-ranking social partners (e.g. Deaner et al. 2005).
Hyenas associate most often with their kin (Holekamp et al. 1997a). Since kin are most often involved in group hunts, coalition formation and cooperative defence of carcasses, kin are highly valuable as social partners. However, as in cercopithecine primates (e.g. Seyfarth 1980), hyenas strongly prefer high-ranking non-kin over lower-ranking non-kin as social companions (Holekamp et al. 1997a). Furthermore, patterns of greeting behaviour in Crocuta follow primate patterns of social grooming (East et al. 1993), in which individuals prefer to spend time with, and direct affiliative behaviour towards, high-ranking non-kin (Seyfarth & Cheney 1984). This indicates that hyenas, like many primates, recognize that some group members are more valuable social partners than others. Smith et al. (2007) recently found that adult Crocuta of both sexes associate most often with non-kin holding ranks similar to their own, and that high-ranking animals are more gregarious than low-ranking individuals. Unrelated female hyenas associate most often with dominant and adjacent-ranking females, as occurs in cercopithecines. Females join subgroups based on the presence of particular conspecifics such that subordinates join focal females at higher rates than do dominants.
Dominant hyenas benefit from association with unrelated subordinates by enjoying priority of access to resources obtained and defended by multiple group members, whereas subordinates benefit because dominants direct less aggression against unrelated females with whom they associate more closely, and they also permit them better access to food at kills (Smith et al. 2007). Thus, there is some evidence that reciprocal exchange of goods and services occurs among hyenas as it does among primates. Our results resemble the positive relationship found between proximity and tolerance at drinking and feeding sites among unrelated adult female rhesus monkeys (de Waal 1986, 1991). Although Crocuta resemble most cercopithecine primates in that kinship fails to protect them from aggression (Wahaj et al. 2004), close association was found by Smith et al. (2007) to reduce rates of aggression received from non-kin. These findings suggest that social relationships among adult females are valued commodities within the biological marketplace of a Crocuta clan; social rank determines the value of social partners, and Crocuta possess the ability to assess relative partner value.
(g) Repair of damaged relationships
Affiliative gestures functioning to repair social relationships damaged during a fight are called reconciliation behaviours (de Waal 1993). Reconciliation is an important behavioural mechanism regulating social relationships and reducing social tension in hierarchical primate societies (Aureli & de Waal 2000). Reconciliation occurs in many primates during friendly reunions between former opponents shortly after aggressive conflicts (reviewed by Aureli & de Waal 2000). Similarly, spotted hyenas use unsolicited appeasement and greeting behaviours to reconcile approximately 15% of their fights (East et al. 1993; Hofer & East 2000; Wahaj et al. 2001). As is also true in many primates (Aureli & van Schaik 1991a,b; Aureli 1992; Kappeler 1993), victims in hyena fights are significantly more likely to reconcile than are aggressors, and male hyenas are more likely to reconcile than females. The latter finding is not surprising in a female-dominated society as males may benefit from information about the state of their relationships with higher-ranking females (Wahaj et al. 2001).
The vast majority of conflicts we observe among wild hyenas occur between unrelated opponents, suggesting that kin are more tolerant of each other than non-kin in Crocuta and that kin may require conciliatory behaviours to repair their relationships less often than do non-kin. Unrelated hyenas exhibit significantly higher rates of reconciliation and are more likely to reconcile their conflicts than are kin. Since related spotted hyenas associate more closely and interact at higher rates than do non-kin (East et al. 1993; Holekamp et al. 1997a), they might be expected to be most ‘forgiving’ of aggressive displays from relatives or to minimize the potential costs of conflicts with relatives (Aureli et al. 1989).
Species differences in reconciliation may reflect the amount of social cohesion necessary to survive in the wild (de Waal & Ren 1988). The conciliatory tendency of 12% found by Wahaj et al. (2001) in spotted hyenas falls relatively low on the conciliatory tendency scale observed in primates, and may reflect the fission–fusion nature of hyena society. Although hyenas depend on cooperation from other clan members for survival and reproduction, they appear to rely more heavily than primates on dispersive rather than non-dispersive mechanisms of conflict resolution.
(h) Quotidian expedience
Barrett & Henzi (2005) recently suggested that, rather than surpassing other mammals with respect to Machiavellian mind-reading or strategic planning abilities, monkeys are more complex than other animals in terms of the number and variety of ways in which they achieve their short-term goals. They referred to this broadly as ‘quotidian expedience’. They argued that monkeys can achieve the same goal in a number of different ways. For example, a monkey might avoid aggression by hiding from the aggressor, using ‘protected threats’, or alarm calling as a distraction. They also suggested that a monkey can achieve a number of different goals in the same way, as when using grooming to achieve access to meat, tolerance, mates, infants or the product of a skilled individual's labour. Barrett & Henzi (2005) further suggested that perhaps monkeys and apes are better than other mammals with respect to their ability to select whatever tactic is necessary to solve an immediate problem, regardless of the possible long-term consequences of such an action. However, it is not clear to us that spotted hyenas differ appreciably from monkeys with respect to the number or variety of ways in which they accomplish their short-term social goals. For example, a hyena can avoid aggression by leaving the aggressor's subgroup, exhibiting appeasement behaviour or distracting the aggressor (Engh et al. 2000; Wahaj et al. 2001). A hyena can potentially use greeting ceremonies to reconcile fights, reintroduce itself to conspecifics from which it has been separated, or increase conspecifics' arousal levels in preparation for a border patrol or group hunt (Holekamp et al. 2000). Whereas the ability to solve the same problem in multiple ways or use one behaviour to solve multiple problems may be a characteristic of complex mammalian societies, Crocuta's social behaviour suggests these traits are not unique to monkeys and apes.
(i) Recognition of third-party relationships
One aspect of social intelligence in which, until recently, primates appeared to differ qualitatively from other gregarious animals was their ability to recognize tertiary, or third-party, relationships among conspecific group members (de Waal 1982; Tomasello & Call 1997). These involve interactions and relationships in which the observer is not directly involved. For example, female vervet monkeys (Cercopithecus aethiops) respond to the distress call of an infant by orienting towards the infant's mother, indicating that they perceive an association between the mother and infant regardless of whether or not they are related to that mother–infant pair (Cheney & Seyfarth 1980). Several primate species have been shown to use information about the social relationships among conspecifics in activities, such as recruiting useful allies, challenging competitors, redirecting aggression and reconciling after fights (Bachmann & Kummer 1980; Cheney & Seyfarth 1989; Silk 1999). Laboratory tests have suggested that macaques can use mental representations to categorize tertiary kin relationships (Dasser 1988), and recent field experiments have shown that baboons (Papio ursinus) categorize information hierarchically about tertiary rank and kin relationships among other group members (Bergman et al. 2003). Tomasello & Call (1997) hypothesized that the ability to recognize third-party relationships is unique to primates and that this distinguishes their mental abilities from those of all other animals, but now this hypothesis has been falsified in both corvids (Paz-y-Miño et al. 2004) and spotted hyenas (Engh et al. 2005).
As in many cercopithecine primates, the ranks of very young hyenas are dependent on the presence or absence of their mothers (Smale et al. 1993; Engh et al. 2000). When the mother is absent, animals lower-ranking than the mother sometimes behave aggressively towards the cub, but when the mother is nearby, lower-ranking animals rarely direct aggression towards its cub. Since hyenas treat these youngsters differently in the presence of their mothers than in the presence of other higher-ranking adults, it appears that they might recognize the association represented by the mother–cub pair. On the other hand, it may be that the hyenas are simply learning to use the mother's presence as a discriminative stimulus. If they distress the cub when its mother is nearby, they are likely to be attacked, whereas bothering the cub in the absence of its mother results in no punishment.
Studies of reconciliation and triadic agonistic interactions in cercopithecine primates have indicated that recognition of third-party relationships occurs in many different species (e.g. Cheney & Seyfarth 1986, 1989; Judge 1991; Sinha 1998; Judge & Mullen 2005). Cercopithecines are known to reconcile after fights, not only with their former opponents, but also with the kin of former opponents (e.g. Cheney & Seyfarth 1989), indicating that the conciliatory monkeys recognize those tertiary relationships. In contrast, we rarely observe hyenas reconciling with any animals but their former opponents (Wahaj et al. 2001). This suggests either that the ability to recognize tertiary relationships under these circumstances does not significantly enhance their fitness or that hyenas lack this ability.
We have conducted two different studies in which we specifically sought to determine whether spotted hyenas exhibit a primate-like ability to recognize tertiary relationships. In our first study, an experiment designed after Cheney & Seyfarth (1980), we played recordings of cub whoops to groups of female hyenas and monitored reactions of the mother and other adult (control) females (Holekamp et al. 1999). In contrast to control monkeys, control hyenas were no more likely to look towards the mother of the whooping cub after the playback than before. At this point, it was unclear whether our results meant that hyenas truly lacked the ability to recognize third-party relationships or that hyenas simply failed to demonstrate this ability in our playback test situation. Therefore, we focused attention in our second study on the behaviour of hyenas during and after fights (Engh et al. 2005).
We expected that, if indeed hyenas can recognize third-party relationships based on the social ranks and kin relationships of other hyenas, then they would be able to use this knowledge adaptively in two ways during and after agonistic interactions. First, we predicted that hyenas would be able to discriminate between the ranks of two individuals engaged in a fight and that they would aid the higher-ranking combatant, regardless of their own social ranks in relation to those of the fighters. Second, we predicted that hyenas would be able to recognize the relatives of their former opponents and that they would increase their rates of aggression towards relatives of their opponents after a fight, as occurs in cercopithecine primates (e.g. Cheney & Seyfarth 1986, 1989).
When aggression between two hyenas escalates, one or more others may join the skirmish by forming a coalition with the attacker against the target individual. Typically, animals joining to form coalitions are all dominant to the victim. Thus, a hyena considering an attack might benefit, for example, when attempting to displace a larger subordinate animal from food, by delaying its attack until the arrival of a potential coalitionary ally that is higher-ranking than the target animal. If hyenas increase their rates of aggression only after higher-ranking hyenas arrive on the scene, then they may be following a simple rule of thumb, such as ‘only attack a larger subordinate when another individual is present who is higher-ranking than yourself’. Alternatively, if the attack rate also increases following the arrival of an individual that is dominant to the victim but subordinate to the attacker, then the attacking hyena must recognize the relative ranks of the other two individuals. In the latter case, the hyenas would be demonstrating that they can indeed recognize tertiary relationships. This assumes the behaviour of the subordinate animal does not change in ways perceived by the dominant when a new hyena arrives on the scene. Although we looked for behavioural changes in the subordinate under these circumstances, we could not see any.
Our results strongly indicated that hyenas can and do recognize third-party relationships (Engh et al. 2005). We found evidence that hyenas which join ongoing disputes do so in a manner consistent with recognition of relative rank relationships. When hyenas joined fights in progress, they almost always joined on the side of the dominant animal, even when that animal was lower-ranking than they were. Zabel et al. (1992) suggested that hyenas have a strong tendency to do what other hyenas are doing and therefore that hyenas often join coalitions as a result of social facilitation (Zajonc 1965) rather than based on an assessment of relative ranks. Since most aggression in hyena society is directed towards lower-ranking individuals, simply joining an aggressor is likely to result in the pattern observed by Engh et al. (2005), in which the dominant animal is aided far more frequently than the subordinate animal. However, when we looked at rare instances of rank reversals, situations in which the initiator of aggression was lower-ranking than the target, animals that intervened in these fights overwhelmingly came to the aid of the dominant animal. Assuming that the winning subordinate behaves like a dominant animal when it wins a fight, this suggests that hyenas recognize third-party rank relationships, and that they are not just following simple rules, such as ‘join in support of aggressors’ or ‘join whichever animal is winning’. Clearly, hyenas will aid the dominant animal even when that individual is losing the fight. Our post-conflict aggression data also strongly supported the notion that hyenas recognize tertiary kin relationships. Aggressors were more likely to attack the relatives of their opponents after a fight than during a matched control period, and after a fight they were more likely to attack relatives of their opponents than to attack other lower-ranking animals unrelated to their opponents (Engh et al. 2005).
(j) Tactical deception, gaze-following and theory of mind
In an effort to replicate with spotted hyenas, Menzel's (1974) classic study of spatial knowledge and non-vocal communication in chimpanzees, Yoerg (in an unpublished study described in Drea & Frank 2003) found that captive hyenas appeared to be deceptive about their knowledge of the environment and that the hyenas' behaviour varied with their immediate social circumstances. When a dominant hyena was informed about the location of food hidden among various potential caches, it approached the baited cache directly, whether alone or accompanied by naive group members. By contrast, a subordinate hyena tested under identical conditions initially led naive group members astray, and later surreptitiously returned to the baited site to claim the prize (Drea & Frank 2003, p 137).
Similarly, we have made anecdotal observations of seemingly deceptive behaviour by wild hyenas. For example, we once observed a low-ranking male, which was travelling with several higher-ranking hyenas, spy a leopard with a young wildebeest it had killed only moments before. The leopard had not yet had time to move its kill to a safe place and was crouching in a creek bed beside the carcass. The group of hyenas crossed the creek bed just upwind of the kill, and none of the other hyenas appeared to note the leopard or its prey. However, four different human observers saw the low-ranking male hyena look directly at the kill as he crossed the creek, but continue past it with the rest of the group until he was well over 100 m beyond the creek. At that point, he turned and loped directly back to the kill and wrangled it away from the leopard without having to compete for it with any higher-ranking hyenas. On other occasions, we have seen low-ranking individuals emit alarm vocalizations in what appeared to be deceptive attempts to gain access to food. Ordinarily, an alarm rumble (Kruuk 1972) emitted by any hyena around an ungulate carcass causes all hyenas present to race off a short distance, then scan for danger (e.g. lions or humans). On each of these particular occasions, however, the low-ranking individual giving the alarm raced directly to the carcass and fed alone until its clan mates realized that there was in fact no danger. On other occasions, we have seen mothers emit alarm rumbles in what appeared to be deceptive efforts to interrupt attacks on their cubs by conspecifics. Although these anecdotes suggest that individual hyenas may sometimes exhibit tactical deception, more systematic work like that of Yoerg (in Drea & Frank 2003) must be done before alternative explanations can be ruled out.
Although gaze-following has never been systematically studied in Crocuta, our observations of wild hyenas suggest that, like canids (Hare & Tomasello 1999), hyenas often follow the gaze cues of conspecifics to locate food or danger. However, we have no evidence that hyenas know anything at all about the current mental state or future intentions of conspecifics unless they directly perceive sensory cues that provide them with such information. Thus, like monkeys (e.g. Cheney & Seyfarth 1990; Povinelli & Preuss 1995), spotted hyenas appear to show no understanding of the thoughts or beliefs of others.
(k) Cultural traditions
Rather than intelligence evolving by natural selection favouring animals that can anticipate and manipulate the behaviour of their social companions, van Schaik (2006) recently suggested that intelligence evolves by selection favouring culture in animal societies. He argued that intelligence is likely to evolve in species in which individual animals generate behavioural innovations and conspecifics are tolerated in close enough proximity sufficiently frequently to permit social learning of these innovations by others, as well as the transmission of these innovations between members of consecutive generations. Although we know that Crocuta engage in extensive social learning early in life, the topic of culture in hyenas is totally unexplored. However, studies have now been conducted on spotted hyenas in many different parts of sub-Saharan Africa and on multiple clans in some locales, without reports of behavioural variants among clans, other than strong preferences for particular prey species, that might be construed as cultural transmission. On the other hand, cultural variants have never been specifically sought in these study populations, researchers seldom work with hyena clans separated by large distances or other significant barriers to dispersal, and no laboratory experiments have yet been conducted on this with captive Crocuta. Thus, it would be premature to rule out the possibility that socially learned behavioural innovations occur in hyenas.
(l) Future directions in the study of hyena cognition
We need to follow up our field studies of cognition in free-living spotted hyenas with more carefully controlled experiments in the laboratory with captive hyenas. For example, although the study by Engh et al. (2005) strongly suggested Crocuta can recognize third-party relationships based on rank and relatedness, we were forced to make certain assumptions in the field that can only be confirmed in laboratory experiments. However, given that birds living in far simpler societies than spotted hyenas have been shown in controlled experiments to be able to recognize tertiary relationships among conspecifics (Bond et al. 2003; Paz-y-Miño et al. 2004), it would certainly surprise us if spotted hyenas could not also perform this cognitive feat when the ability to do so could so strongly affect their fitness.
An ideal test of the social complexity hypothesis would include data documenting the cognitive abilities of hyenas other than the spotted hyena. In particular, evidence that less gregarious hyaenids (e.g. brown hyenas, Hyaena brunnea and striped hyenas, Hyaena hyaena) lack some of the cognitive abilities previously documented in Crocuta would provide further support for the notion that social complexity favours enhancement of intelligence. We are currently initiating a field study of striped hyenas, which are known to be solitary (Wagner in press). We plan to administer simple standardized ‘intelligence tests’ to individuals in our study populations of both spotted and striped hyenas. Although these two species are very closely related and confront many of the same ecological problems, the social complexity hypothesis predicts spotted hyenas should perform far better on such standardized tests than striped hyenas, because Crocuta have been challenged for many thousands of generations by the labile behaviour of conspecifics.
4. Brain organization
Cognitive processes are, of course, mediated by nervous systems; thus the social complexity hypothesis predicts that non-primates living in complex societies should possess brain structures mediating social behaviour that are similar to those in primates. The social complexity hypothesis considered specifically in relation to nervous systems has been dubbed ‘the social brain hypothesis’ (Brothers 1990; Barton & Dunbar 1997). Considered in relation to body size, the brains of primates are relatively large and complex compared with those of other animals, including most non-primate mammals (Jerison 1973; Macphail 1982; Harvey & Krebs 1990). The relatively large brain size noted among primates is due primarily to the unusually large expanse of neocortex, the laminated, almost uniformly thick grey matter covering much of the outer surface of the brain (Dunbar 2003). Such variables as social group size (Dunbar 1992, 1995), number of social partners, grooming clique size (Kudo & Dunbar 2001) and frequency of social play (Lewis 2001) all correlate strongly with neocortical volume in primates.
The mammalian brain comprises a number of functionally distinct systems, and natural selection acting on particular behavioural capacities causes size changes selectively in the systems mediating those capacities (Barton & Harvey 2000). Frontal cortex is known to mediate complex social behaviour in humans and other mammals (Adolphs 2001; Amodio & Frith 2006); therefore, the social brain hypothesis predicts that we should find larger frontal cortex volumes in gregarious species than in closely related solitary species. Among primates, neocortex disproportionately covers the frontal area whereas a similar relationship does not appear to exist among other mammalian species. Dunbar (2003) suggested that the relatively large frontal neocortex in primates is specifically associated with the demands imposed by life in complex social groups. Thus, social complexity in primates appears to be related generally to greater brain volume and specifically to the expansion of frontal cortex (Dunbar & Bever 1998). If the social brain hypothesis is correct, we should find these same patterns in the brains of non-primate mammals that, although closely related to each other, vary with respect to the complexity of their social lives.
Radinsky (1969) noted a moderate expansion of the frontal cortex of dogs that, like hyenas, are gregarious carnivores. Furthermore, Dunbar & Bever (1998) found that neocortex size in carnivores is correlated with group size and lies on the same grade as does neocortex size in primates. However, Bush & Allman (2004) recently evaluated scaling of frontal cortex across a wide array of mammals, and concluded that frontal cortex in non-primate species does not undergo the same expansion as that observed among primates. These authors examined volume of the frontal cortex, neocortical volume and subcortical brain volume in 55 mammalian species and noted significant differences between primates and carnivores in the scaling of frontal cortex. These differences support the hypothesis that frontal cortex in primates is functionally distinct from that in carnivores and suggest that frontal cortex in each taxon may have been shaped by different selection pressures (Preuss 1995; Bush & Allman 2004).
The conflicts between the results obtained by Dunbar & Bever (1998) and those of Bush & Allman (2004) may derive from problems associated with making meaningful comparisons between brains of primates and carnivores. In particular, Bush & Allman (2004) defined frontal cortex as consisting of all cortex anterior to and including motor cortex. Motor cortex is involved in mediating the planning and execution of movement. Electrophysiological mapping studies of motor cortex have demonstrated that the representation of the hand and face is expanded in primate motor cortex relative to the representation of the remainder of the body (Penfield & Rasmussen 1950; Woolsey 1958). In contrast, similar studies in carnivores including cats and dogs have shown no comparable expansion of forelimb representation in motor cortex (Woolsey 1958; Górska 1974). When motor cortex is included in frontal cortical volume, it is likely to inflate the relative volume of frontal cortex in primates while possibly diminishing the relative frontal cortical volume among carnivores. Moreover, the surface of the brain in most primates has a prominent central sulcus, a deep infolding of tissue that separates somatosensory cortex caudally from motor and frontal cortex rostrally. Unfortunately, this important landmark is not present in carnivore brains. The post-cruciate dimple in carnivores (figure 1) is hypothesized to be homologous to the central sulcus in primates in that it demarcates the boundary between motor and somatosensory cortex (Hardin et al. 1968; Górska 1974). However, the post-cruciate dimple is not visible on the brain surface in many carnivore species, so it is not a reliable landmark.
Figure 1.
Dorsal views of the cerebral hemispheres of four carnivore species. The yellow box indicates the approximate area of the frontal cortex, defined as the cortex rostral to the cruciate sulcus (cs). Note the relative volume differences among the species. The relative amount of frontal cortex appears greatest in dog and spotted hyena. Other abbreviations: PR, proreal gyrus; prs, presylvian sulcus; psd, post-cruciate dimple. Whole brain images from the University of Wisconsin Comparative Mammalian Brain Collections (supported by the National Science Foundation) at http://www.brainmuseum.org.
Accurate comparisons of frontal cortical volumes between primates and other mammalian orders are extremely difficult, particularly when the large sulcus that demarcates primate frontal cortex is absent in many taxa of interest. Although different cell types and their distribution can be used to determine the boundary between the rostrally located agranular motor cortex and the caudal granular somatosensory cortex, this determination must be based on microscopic cytoarchitectonic analysis of serial brain sections. Since hyenas lack a central sulcus to delimit the border between motor and somatosensory cortex, we are using cytoarchitectonic analysis in Crocuta to determine the volume of frontal cortex both including and excluding motor cortex. For purposes of comparison with primates, the latter measurement may prove to be the best indicator of frontal cortex volume in carnivores, since this will eliminate the exaggerated representation of certain body parts (e.g. forelimb) within the motor cortex as a variable. Ultimately, we hope to undertake a large-scale comparison of primates and carnivores to determine whether we obtain results more closely resembling those of Bush & Allman (2004) or those of Dunbar & Bever (1998).
Our second goal here is to conduct accurate volumetric assessments of frontal cortex in relation to total brain volume in spotted hyenas, and compare these measurements with those obtained from Crocuta's closest living relatives and other carnivore species that vary with respect to social complexity. The spotted hyena is one of only four extant species in the family Hyaenidae. These four species span a wide spectrum of social complexity. In contrast to the highly social Crocuta, the striped hyena is solitary (Wagner in press), the aardwolf (Proteles cristatus) lives in monogamous pairs (Richardson 1988) and the brown hyena lives in small family groups of up to nine individuals (Mills 1990). Crocuta occur sympatrically with all three of these other species in Africa. The four hyena species last shared a common ancestor approximately 11 Myr ago (Koepfli et al. 2006). Using skeletal material from the four extant hyaenids, we have recently started using computed tomography (CT) to image hyena brains to examine the relationship between frontal cortex volume and social complexity.
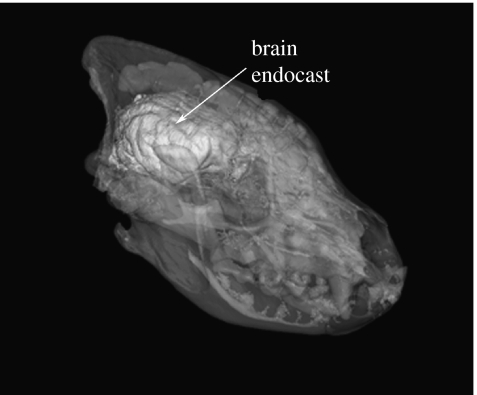
The use of CT technology for addressing comparative questions is a relatively recent phenomenon. The CT scanner makes X-ray slices through an object. These slices display differences in X-ray absorption arising mainly through differences in density within an object. When the slices are put back together, the object can literally be seen, inside and out, in three dimensions. The object itself is untouched. Therefore, high-resolution CT can produce three-dimensional images that permit analyses of deep structures without tissue destruction. Since the CT method can produce detailed images of the interior of a skull, including surface impressions left by the sulcal pattern on the brain's surface, it is a useful technique for generating virtual endocasts of the brain (figure 2). We are using CT imagery to determine whether volume of frontal cortex varies with social complexity among hyena species as it does among primates.
Figure 2.
Rendering of CT images from an adult female spotted hyena skull showing both the skull and virtual brain endocast (arrow).
In contrast to primate brains, carnivore brains exhibit a large cruciate sulcus (figure 1). Based on both anatomical and physiological studies, this prominent sulcus is coincident with much of the rostral extent of motor cortex in cat (Hassler & MühsClement 1964), dog (Górska 1974; Stanton et al. 1986; Tanaka 1987; Sakai et al. 1993) and raccoon (Sakai 1982, 1990). Our current cytoarchitectonic analysis of Crocuta brains will determine whether this is also true in hyenas. If so, then the cruciate sulcus is likely to offer the most reliable landmark for demarcating the boundary between frontal and motor cortex in carnivores. Our preliminary work suggests that the relative amount of cortex rostral to the cruciate sulcus is greater in the spotted hyena than in the other carnivore species we have examined to date (figure 1). Our new CT analysis of virtual brains reconstructed from multiple skulls from each hyena species, combined with our on-going cytoarchitectonic analysis, should offer a strong test of the social brain hypothesis. Specifically, the hypothesis predicts that size of frontal cortex should increase relative to total cortical volume and brain volume in the following order within the family Hyaenidae, as we move from solitary to highly gregarious: striped hyenas; aardwolves; brown hyenas; and spotted hyenas. We anticipate that our current work with the hyena family will set the stage for a larger-scale analysis of the relationship between social complexity and brain structure in other carnivores to determine whether the same relationship between frontal cortex and social complexity found in primates holds within this second large order of mammals.
5. Conclusions
The social complexity hypothesis posits that big brains and great intelligence have been favoured by selection pressures associated with life in challenging social environments (Jolly 1966; Humphrey 1976; Byrne & Whiten 1988). de Waal & Tyack (2003) suggest that the most challenging societies are those in which animals live in stable multi-generational units, group members recognize each other individually, individuals cooperate as well as compete for resource access and a substantial amount of learning occurs during social development. Although some primatologists argue there is already ample evidence that primate societies are more complex than those of other mammals (e.g. Dunbar 2003), we are not entirely convinced this is true. Work to date on spotted hyenas has shown that they live in social groups just as large and complex as those of cercopithecine primates, that they experience an extended early period of intensive learning about their social worlds like primates, that the demand for social dexterity during competitive and cooperative interactions is no less intense than it is in primates, and that hyenas appear to be capable of many of the same feats of social recognition and cognition as are primates.
Much remains to be learned about social cognition in hyenas. For example, we do not yet know whether Crocuta use hierarchical classification of rank and kinship as occurs in baboons (Bergman et al. 2003). Nor do we know to what extent hyenas might be able to ‘keep score’, as tamarins do (Hauser et al. 2003), of earlier altruistic and selfish acts directed at them by conspecifics. Whether hyenas are capable of tactical deception or cultural transmission of behaviour will not be fully revealed until the appropriate controlled experiments can be conducted. However, based on existing information, it appears that Crocuta differ from ‘more intelligent’ species in that they give us no indication that they are capable of true imitation and in that they rely more intensively on simple rules of thumb in social decision-making. In any case, along with odontocete cetaceans and elephants, hyenas continue to offer a useful model system in which to test hypotheses suggesting cognitive abilities that distinguish primates from other mammals. Furthermore, a comparison between the cognitive abilities and brains of spotted hyenas and those of other hyena species with less complex social systems should allow us to determine whether convergent evolution of brain and behaviour has occurred in non-primate mammals in response to social complexity.
Acknowledgments
This work was supported by grant 05IRGP358 from Michigan State University and NSF grants 0343381 and IOB0618022. We thank L. Smale for insightful comments on an earlier draft of this paper, and J. E. Smith and S. Benson-Amram for helpful discussions. Finally, thanks to Andrea Kaiser, Colleen Hammond, Dr Kevin Berger and the Department of Radiology, Michigan State University.
Footnotes
One contribution of 19 to a Dicussion Meeting Issue ‘Social intelligence: from brain to culture’.
References
- Adolphs R. The neurobiology of social cognition. Curr. Opin. Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. doi:10.1016/S0959-4388(00)00202-6 [DOI] [PubMed] [Google Scholar]
- Amodio D.M, Frith C.D. Meeting of the minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. doi:10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Andelman, S. J. 1985 Ecology and reproductive strategies of vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Unpublished Ph.D. dissertation, University of Washington, Seattle, WA.
- Aureli F. Post-conflict behaviour among wild long-tailed macaques. Behav. Ecol. Sociobiol. 1992;31:329–337. doi:10.1007/BF00177773 [Google Scholar]
- Aureli F, de Waal F.B.M. University of California Press; Berkeley, CA: 2000. Natural conflict resolution. [Google Scholar]
- Aureli F, van Schaik C.P. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis): I. The social events. Ethology. 1991a;89:89–100. [Google Scholar]
- Aureli F, van Schaik C.P. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis): II. Coping with uncertainty. Ethology. 1991b;89:101–114. [Google Scholar]
- Aureli F, van Schaik C.P, van Hooff J.A.R.A.M. Functional aspects of reconciliation among captive longtailed macaques (Macaca fascicularis) Am. J. Primatol. 1989;19:39–51. doi: 10.1002/ajp.1350190105. doi:10.1002/ajp.1350190105 [DOI] [PubMed] [Google Scholar]
- Bachman C, Kummer H. Male assessment of female choice in hamadryas baboons. Behav. Ecol. Sociobiol. 1980;6:315–321. doi:10.1007/BF00292774 [Google Scholar]
- Barrett L, Henzi P. The social nature of primate cognition. Proc. R. Soc. B. 2005;272:1865–1875. doi: 10.1098/rspb.2005.3200. doi:10.1098/rspb.2005.3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton R.A, Dunbar R.I.M. Evolution of the social brain. In: Whiten A, Byrne R.W, editors. Machiavellian intelligence II: extensions and evaluations. Cambridge University Press; Cambridge, UK: 1997. pp. 240–263. [Google Scholar]
- Barton R.A, Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. doi:10.1038/35016580 [DOI] [PubMed] [Google Scholar]
- Bergman T.J, Beehner J.C, Cheney D.L, Seyfarth R.M. Hierarchical classification by rank & kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. doi:10.1126/science.1087513 [DOI] [PubMed] [Google Scholar]
- Blumstein D.T, Armitage K.B. Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am. Nat. 1997;150:179–200. doi: 10.1086/286062. doi:10.1086/286062 [DOI] [PubMed] [Google Scholar]
- Bolles R.C. The comparative psychology of learning: the selective association principle and some problems with ‘general’ laws of learning. In: Bermant G, Glenview I.L, editors. Perspectives in animal behaviour. Scott, Foreman & Co; New York, NY: 1973. [Google Scholar]
- Bond A.B, Kamil A.C, Balda R.P. Social complexity and transitive inference in corvids. Anim. Behav. 2003;65:479–497. doi:10.1006/anbe.2003.2101 [Google Scholar]
- Boydston E.E, Morelli T.L, Holekamp K.E. Sex differences in territorial behaviour exhibited by the spotted hyena (Crocuta crocuta) Ethology. 2001;107:369–385. doi:10.1046/j.1439-0310.2001.00672.x [Google Scholar]
- Boydston E.E, Kapheim K.M, Van Horn R.C, Smale L, Holekamp K.E. Sexually dimorphic patterns of space use throughout ontogeny in the spotted hyaena (Crocuta crocuta) J. Zool. 2005;267:271–281. doi:10.1017/S0952836905007478 [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behaviour and neurophysiology in a new domain. Concept. Neurosci. 1990;1:27–251. [Google Scholar]
- Buchan J.C, Alberts S.C, Silk J.B, Altmann J. True paternal care in a multi-male primate society. Nature. 2003;425:179–181. doi: 10.1038/nature01866. doi:10.1038/nature01866 [DOI] [PubMed] [Google Scholar]
- Bush E.C, Allman J.M. The scaling of frontal cortex in primates and carnivores. Proc. Natl Acad. Sci. USA. 2004;101:3962–3966. doi: 10.1073/pnas.0305760101. doi:10.1073/pnas.0305760101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R.W. The evolution of intelligence. In: Slater P.J.B, Halliday T.R, editors. Behaviour and evolution. Cambridge University Press; Cambridge, UK: 1994. pp. 223–265. [Google Scholar]
- Byrne R.W. Oxford University Press; Oxford, UK: 1995. The thinking ape. [Google Scholar]
- Byrne R.W, Whiten A, editors. Machiavellian intelligence. Clarendon Press; Oxford, UK: 1988. [Google Scholar]
- Caro T. University of Chicago Press; Chicago, IL: 1994. Cheetahs of the Serengeti plains. [Google Scholar]
- Chapais B. The role of alliances in the social inheritance of rank among female primates. In: Harcourt A.H, de Waal F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford Science; Oxford, UK: 1992. pp. 29–60. [Google Scholar]
- Chapais B, Schulman S. An evolutionary model of female dominance relations in primates. J. Theor. Biol. 1980;82:47–89. doi: 10.1016/0022-5193(80)90090-9. doi:10.1016/0022-5193(80)90090-9 [DOI] [PubMed] [Google Scholar]
- Cheney D.L. The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behav. Ecol. Sociobiol. 1977;2:303–318. doi:10.1007/BF00299742 [Google Scholar]
- Cheney D.L, Seyfarth R.M. Vocal recognition in free-ranging vervet monkeys. Anim. Behav. 1980;28:362–367. doi:10.1016/S0003-3472(80)80044-3 [Google Scholar]
- Cheney D.L, Seyfarth R.M. Non-random dispersal in free-ranging vervet monkeys: social and genetic consequences. Am. Nat. 1983;122:392–412. doi:10.1086/284142 [Google Scholar]
- Cheney D.L, Seyfarth R.M. The recognition of social alliances by vervet monkeys. Anim. Behav. 1986;34:1722–1731. doi:10.1016/S0003-3472(86)80259-7 [Google Scholar]
- Cheney D.L, Seyfarth R.M. Redirected aggression and reconciliation among vervet monkeys, Cercopithecus aethiops. Behaviour. 1989;110:258–275. [Google Scholar]
- Cheney D.L, Seyfarth R.M. University of Chicago Press; Chicago, IL: 1990. How monkeys see the world. [Google Scholar]
- Cheney D.L, Seyfarth R.M. The structure of social knowledge in monkeys. In: de Waal F.B.M, Tyack P.L, editors. Animal social complexity. Harvard University Press; Cambridge, MA: 2003. pp. 207–229. [Google Scholar]
- Cheney D.L, Seyfarth R.M, Smuts B. Social relationships and social cognition in nonhuman primates. Science. 1986;234:1361–1366. doi: 10.1126/science.3538419. doi:10.1126/science.3538419 [DOI] [PubMed] [Google Scholar]
- Cords M. Resolution of aggressive conflicts by immature long-tailed macaques Macaca fascicularis. Anim. Behav. 1988;36:1124–1135. doi:10.1016/S0003-3472(88)80072-1 [Google Scholar]
- Dasser V. A social concept in Java monkeys. Anim. Behav. 1988;36:225–230. doi:10.1016/S0003-3472(88)80265-3 [Google Scholar]
- Datta S.B. The role of alliances in the acquisition of rank. In: Else J.G, Lee P.C, editors. Primate ontogeny, cognition and social behaviour. Cambridge University Press; Cambridge, UK: 1986. pp. 219–225. [Google Scholar]
- Deaner R.O, Khera A.V, Platt M.L. Monkeys pay per view: adaptive value of social images by rhesus macaques. Curr. Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. doi:10.1016/j.cub.2005.01.044 [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M. Harper and Row Publishers; New York, NY: 1982. Chimpanzee politics. [Google Scholar]
- de Waal F.B.M. Class structure in a rhesus monkey group: the interplay between dominance and tolerance. Anim. Behav. 1986;34:1033–1040. doi:10.1016/S0003-3472(86)80162-2 [Google Scholar]
- de Waal F.B.M. Rank distance as a central feature of rhesus monkey social organization: a sociometric analysis. Anim. Behav. 1991;41:383–395. doi:10.1016/S0003-3472(05)80839-5 [Google Scholar]
- de Waal F.B.M. Reconciliation among primates: a review of empirical evidence and unresolved issues. In: Mason W.A, Mendoza S.P, editors. Primate social conflict. State University of New York Press; New York, NY: 1993. pp. 111–144. [Google Scholar]
- de Waal F.B.M, Ren R.M. Comparison of the reconciliation behaviour of stumptail and rhesus macaques. Ethology. 1988;78:129–142. [Google Scholar]
- de Waal, F. B. M. & Tyack, P. (eds) 2003 Animal social complexity Chicago, IL: University of Chicago Press.
- Drea C.M, Frank L.G. The social complexity of spotted hyenas. In: de Waal F.B.M, Tyack P.L, editors. Animal social complexity. Harvard University Press; Cambridge, MA: 2003. pp. 121–148. [Google Scholar]
- Drea C.M, Vignieri S.N, Cunningham S.B, Glickman S.E. Responses to olfactory stimuli in spotted hyenas (Crocuta crocuta): II. Investigation of environmental odors and the function of rolling. J. Comp. Psychol. 2002a;116:331–341. doi: 10.1037/0735-7036.116.4.331. doi:10.1037/0735-7036.116.4.331 [DOI] [PubMed] [Google Scholar]
- Drea C.M, Vignieri S.N, Kim H.S, Weldele M.L, Glickman S.E. Responses to olfactory stimuli in spotted hyenas (Crocuta crocuta): II. Discrimination of conspecific scent. J. Comp. Psychol. 2002b;116:342–349. doi: 10.1037/0735-7036.116.4.342. doi:10.1037/0735-7036.116.4.342 [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M. Neocortex size as a constraint on group size in primates. J. Hum. Evol. 1992;20:469–493. doi:10.1016/0047-2484(92)90081-J [Google Scholar]
- Dunbar R.I.M. Neocortex size and group size in primates: a test of the hypothesis. J. Hum. Evol. 1995;28:287–296. doi:10.1006/jhev.1995.1021 [Google Scholar]
- Dunbar R.I.M. The social brain: mind, language and society in evolutionary perspective. Annu. Rev. Anthropol. 2003;325:163–181. doi:10.1146/annurev.anthro.32.061002.093158 [Google Scholar]
- Dunbar R.I.M, Bever J. Neocortex size predicts group size in carnivores and some insectivores. Ethology. 1998;104:695–708. [Google Scholar]
- East M.L, Hofer H. Loud-calling in a female-dominated mammalian society: I. Structure and composition of whooping bouts of spotted hyaenas, Crocuta crocuta. Anim. Behav. 1991a;42:637–649. doi:10.1016/S0003-3472(05)80246-5 [Google Scholar]
- East M.L, Hofer H. Loud-calling in a female-dominated mammalian society: II. Behavioural contexts and functions of whooping of spotted hyaenas, Crocuta crocuta. Anim. Behav. 1991b;42:651–669. doi:10.1016/S0003-3472(05)80247-7 [Google Scholar]
- East M.L, Hofer H. Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 2001;12:558–568. doi:10.1093/beheco/12.5.558 [Google Scholar]
- East M.L, Hofer H, Wickler W. The erect ‘penis’ as a flag of submission in a female-dominated society: greetings in Serengeti spotted hyenas. Behav. Ecol. Sociobiol. 1993;33:355–370. doi:10.1007/BF00170251 [Google Scholar]
- Emery N.J, Clayton N.S. Animal cognition. In: Bolhuis J.J, Giraldeau L.A, editors. The behaviour of animals: mechanisms, function and evolution. Blackwell Publishing; Malden, MA: 2005. pp. 170–196. [Google Scholar]
- Engh A.L, Esch K, Smale L. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim. Behav. 2000;60:323–332. doi: 10.1006/anbe.2000.1502. doi:10.1006/anbe.2000.1502 [DOI] [PubMed] [Google Scholar]
- Engh A.L, Siebert E.R, Greenberg D.A, Holekamp K.E. Patterns of alliance formation and post-conflict aggression indicate spotted hyenas recognize third party relationships. Anim. Behav. 2005;69:209–217. doi:10.1016/j.anbehav.2004.04.013 [Google Scholar]
- Frank L.G. Social organization of the spotted hyaena (Crocuta crocuta): II. Dominance and reproduction. Anim. Behav. 1986;35:1510–1527. doi:10.1016/S0003-3472(86)80221-4 [Google Scholar]
- Frank L.G, Holekamp K.E, Smale L. Dominance, demography, and reproductive success of female spotted hyenas. In: Sinclair A.R.E, Arcese P, editors. Serengeti II: conservation, research, and management. University of Chicago Press; Chicago, IL: 1995. pp. 364–384. [Google Scholar]
- Gittleman J.L. Cornell University Press; Ithaca, NY: 1989. Carnivore behaviour, ecology and evolution. [Google Scholar]
- Gittleman J.L. Cornell University Press; Ithaca, NY: 1996. Carnivore behaviour, ecology and evolution II. [Google Scholar]
- Glickman S.E, Zabel C.J, Yoerg S.I, Weldele M.L, Drea C.M, Frank L.G. Social facilitation, affiliation, and dominance in the social life of spotted hyenas. Ann. N. Y. Acad. Sci. 1997;807:175–184. doi: 10.1111/j.1749-6632.1997.tb51919.x. doi:10.1111/j.1749-6632.1997.tb51919.x [DOI] [PubMed] [Google Scholar]
- Górska T. Functional organization of cortical motor areas in adult dogs and puppies. Acta Neurobiol. Exp. 1974;34:171–203. [PubMed] [Google Scholar]
- Guggisberg C.A.W. Bailey Brothers and Swinfen; London, UK: 1962. Simba. [Google Scholar]
- Harcourt A.H, de Waal F.B.M. Coalitions and alliances in humans and other animals. In: Harcourt A.H, de Waal F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford Science; Oxford, UK: 1992. pp. 445–471. [Google Scholar]
- Hardin W.B, Jr, Arumugasamy N, Jameson H.D. Pattern of localization in ‘precentral’ motor cortex of raccoon. Brain Res. 1968;11:611–617. doi: 10.1016/0006-8993(68)90149-2. doi:10.1016/0006-8993(68)90149-2 [DOI] [PubMed] [Google Scholar]
- Hare D, Tomasello M. Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J. Comp. Psychol. 1999;113:173–177. doi:10.1037/0735-7036.113.2.173 [Google Scholar]
- Harvey P.H, Krebs J.R. Comparing brains. Science. 1990;249:140–146. doi: 10.1126/science.2196673. doi:10.1126/science.2196673 [DOI] [PubMed] [Google Scholar]
- Hassler R, MühsClement K. Architektonischer Aufbau des sensorimotorischen und parietalen Cortex der Katze. J. Hirnforsch. 1964;6:377–420. [PubMed] [Google Scholar]
- Hauser M.D, Chen M.K, Chen F, Chuang E. Give unto others: genetically unrelated cotton-top tamarin monkeys preferentially give food to those who altruistically give food back. Proc. R. Soc. B. 2003;270:2363–2370. doi: 10.1098/rspb.2003.2509. doi:10.1098/rspb.2003.2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschel J.R, Skinner J.D. Social relationships and dispersal patterns in a clan of spotted hyaenas Crocuta crocuta in the Kruger National Park. S. Afr. J. Zool. 1987;22:18–24. [Google Scholar]
- Henschel J.R, Skinner J.D. Territorial behaviour by a clan of spotted hyenas (Crocuta crocuta) Ethology. 1991;88:223–235. [Google Scholar]
- Hofer H, East M.L. The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. II. Intrusion pressure and commuter's space use. Anim. Behav. 1993;46:559–574. doi:10.1006/anbe.1993.1223 [Google Scholar]
- Hofer H, East M.L. Conflict management in female-dominated spotted hyenas. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. University of California Press; Berkeley, CA: 2000. pp. 232–234. [Google Scholar]
- Hofer H, East M.L. Behavioural processes and costs of co-existence in female spotted hyenas: a life-history perspective. Evol. Ecol. 2003;17:315–331. doi:10.1023/A:1027352517231 [Google Scholar]
- Hofer H, East M.L, Sammang I, Dehnhard M. Analysis of volatile compounds in scent-marks of spotted hyenas (Crocuta crocuta) and their possible function in olfactory communication. In: Marchlewska-Koj A, Lepri J.J, Muller-Schwarze D, editors. Chemical signals in vertebrates. Kluwer Academic/Plenum; New York, NY: 2001. pp. 141–148. [Google Scholar]
- Holekamp K.E, Smale L. Rank acquisition during mammalian social development: the ‘inheritance’ of maternal rank. Am. Zool. 1991;31:306–317. [Google Scholar]
- Holekamp K.E, Smale L. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with other immature individuals. Anim. Behav. 1993;46:451–466. doi:10.1006/anbe.1993.1214 [Google Scholar]
- Holekamp K.E, Ogutu J, Frank L.G, Dublin H.T, Smale L. Fission of a spotted hyena clan: consequences of female absenteeism and causes of female emigration. Ethology. 1993;93:285–299. [Google Scholar]
- Holekamp K.E, Smale L, Szykman M. Rank and reproduction in the female spotted hyaena. J. Reprod. Fertil. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- Holekamp K.E, Cooper S.M, Katona C.I, Berry N.A, Frank L.G, Smale L. Patterns of association among female spotted hyenas (Crocuta crocuta) J. Mammal. 1997a;78:55–64. doi:10.2307/1382638 [Google Scholar]
- Holekamp K.E, Smale L, Berg R, Cooper S.M. Hunting rates and hunting success in the spotted hyaena. J. Zool. 1997b;242:1–15. [Google Scholar]
- Holekamp K.E, Boydston E.E, Szykman M, Graham I, Nutt K.J, Birch S, Piskiel A, Singh M. Vocal recognition in the spotted hyaena and its possible implications regarding the evolution of intelligence. Anim. Behav. 1999;58:383–395. doi: 10.1006/anbe.1999.1157. doi:10.1006/anbe.1999.1157 [DOI] [PubMed] [Google Scholar]
- Holekamp K.E, Boydston E.E, Smale L. Group travel in social carnivores. In: Boinksi S, Garber P, editors. On the move: how and why animals travel in groups. University of Chicago Press; Chicago, IL: 2000. pp. 587–627. [Google Scholar]
- Holmes W.G, Sherman P.W. The ontogeny of kin recognition in two species of ground squirrels. Am. Zool. 1982;22:491–517. [Google Scholar]
- Horrocks J, Hunte W. Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Anim. Behav. 1983;31:772–782. doi:10.1016/S0003-3472(83)80234-6 [Google Scholar]
- Humphrey N.K. The social function of intellect. In: Bateson P.P.G, Hinde R.A, editors. Growing points in ethology. Cambridge University Press; Cambridge, UK: 1976. pp. 303–317. [Google Scholar]
- Jenks S.M, Weldele M.L, Frank L.G, Glickman S.E. Acquisition of matrilineal rank in captive spotted hyaenas emergence of a natural social system in peer-reared animals and their offspring. Anim. Behav. 1995;50:893–904. doi:10.1016/0003-3472(95)80092-1 [Google Scholar]
- Jerison H.J. Academic Press; New York, NY: 1973. Evolution of the brain and intelligence. [Google Scholar]
- Jolly A. Lemur social behaviour and primate intelligence. Science. 1966;152:501–506. doi: 10.1126/science.153.3735.501. doi:10.1126/science.153.3735.501 [DOI] [PubMed] [Google Scholar]
- Jones M.L. Longevity of captive mammals. Zool. Gart. 1982;52:113–128. [Google Scholar]
- Judge P.G. Dyadic and triadic reconciliation in pigtail macaques (Macaca nemistrina) Am. J. Primatol. 1991;23:225–237. doi: 10.1002/ajp.1350230403. doi:10.1002/ajp.1350230403 [DOI] [PubMed] [Google Scholar]
- Judge P.G, Mullen S.H. Quadratic post-conflict affiliation among bystanders in a hamadryas baboon group. Anim. Behav. 2005;69:1345–1355. doi:10.1016/j.anbehav.2004.08.016 [Google Scholar]
- Kamil A.C. A synthetic approach to the study of animal intelligence. Nebr. Symp. Motiv. 1987;7:257–308. [PubMed] [Google Scholar]
- Kappeler P.M. Reconciliation and post-conflict behaviour in ringtailed lemurs, Lemur catta and redfronted lemurs, Eulemur fulvus rufus. Anim. Behav. 1993;45:901–915. doi:10.1006/anbe.1993.1110 [Google Scholar]
- Koepfli K.P, Jenks S.M, Eizirik E, Zahirpour T, Can Valkenburgh B, Wayne R.K. Molecular systematics of the Hyaenidae: relationships of a relictual lineage resolved by a molecular supermatrix. Mol. Phylogenet. Evol. 2006;38:603–620. doi: 10.1016/j.ympev.2005.10.017. doi:10.1016/j.ympev.2005.10.017 [DOI] [PubMed] [Google Scholar]
- Kruuk H. University of Chicago Press; Chicago, IL: 1972. The spotted hyena: a study of predation and social behaviour. [Google Scholar]
- Kudo H, Dunbar R.I.M. Neocortex size and social network size in primates. Anim. Behav. 2001;62:711–722. doi:10.1006/anbe.2001.1808 [Google Scholar]
- Kurland J.A. Contributions to primatology. vol. 12. S. Karger; Basel, Germany: 1977. Kin selection in the Japanese monkey. [PubMed] [Google Scholar]
- Lewis K. A comparative study of primate play behaviour: implications for the study of cognition. Folia Primatol. 2001;71:417–421. doi: 10.1159/000052740. doi:10.1159/000052740 [DOI] [PubMed] [Google Scholar]
- Macphail E.M. Clarendon Press; Oxford, UK: 1982. Brain and intelligence in vertebrates. [Google Scholar]
- Menzel E.W. A group of chimpanzees in a 1-acre field: leadership and communication. In: Schrier A.M, Stollnitz F, editors. Behaviour of nonhuman primates. Academic Press; New York, NY: 1974. pp. 83–153. [Google Scholar]
- Mills M.G.L. Unwin Hyman; London, UK: 1990. Kalahari hyaenas: the behavioural ecology of two species. [Google Scholar]
- Noë R, Hammerstein P. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism, and mating. Behav. Ecol. Sociobiol. 1994;35:111. [Google Scholar]
- Paz-y-Miño G.C, Bond A.B, Kamil A.C, Balda R.P. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. doi:10.1038/nature02723 [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. Macmillan; New York, NY: 1950. The cerebral cortex of man. A clinical study of localization function. [Google Scholar]
- Peters R, Mech L.D. Behavioural and intellectual adaptations of selected mammalian predators to the problem of hunting large animals. In: Tuttle R.H, editor. Socioecology and psychobiology of primates. Mouten Publishing; The Hague, The Netherlands: 1973. pp. 279–300. [Google Scholar]
- Povinelli D.J, Preuss T.M. Theory of mind: evolutionary history of a cognitive specialization. Trends Neurosci. 1995;18:418–424. doi: 10.1016/0166-2236(95)93939-u. doi:10.1016/0166-2236(95)93939-U [DOI] [PubMed] [Google Scholar]
- Preuss T.M. Do rats have a prefrontal cortex? The Rose–Woolsey–Akert program reconsidered. J. Cogn. Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Radinsky L.B. Outlines of canid and felid brain evolution. Ann. N. Y. Acad. Sci. 1969;167:277–288. doi:10.1111/j.1749-6632.1969.tb20450.x [Google Scholar]
- Richardson P.R.K. Mate desertion in response to female promiscuity in the socially monogamous aardwolf, Proteles cristatus. S. Afr. Tydskr. Dierk. 1988;23:306–308. [Google Scholar]
- Sakai S.T. The thalamic connectivity of the primary motor cortex (MI) in the raccoon. J. Comp. Neurol. 1982;204:238–252. doi: 10.1002/cne.902040304. doi:10.1002/cne.902040304 [DOI] [PubMed] [Google Scholar]
- Sakai S.T. Corticospinal projections from area 6 in the raccoon. Exp. Brain Res. 1990;79:240–248. doi: 10.1007/BF00608232. doi:10.1007/BF00608232 [DOI] [PubMed] [Google Scholar]
- Sakai S.T, Stanton G.B, Isaacson L. Thalamic afferents of areas 4 and 6 in the dog: a multiple retrograde fluorescent dye study. Anat. Embryol. 1993;188:551–559. doi: 10.1007/BF00187010. doi:10.1007/BF00187010 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. Endocrinology alfresco: psychoendocrine studies of wild baboons. Rec. Prog. Horm. Res. 1993;48:437–468. doi: 10.1016/b978-0-12-571148-7.50020-8. [DOI] [PubMed] [Google Scholar]
- Schaller G. University of Chicago Press; Chicago, IL: 1972. The Serengeti lion. [Google Scholar]
- Schino G. Grooming, competition, and social rank among female primates: a metaanalysis. Anim. Behav. 2001;62:265–271. doi:10.1006/anbe.2001.1750 [Google Scholar]
- Seyfarth R.M. The distribution of grooming and related behaviours among adult female vervet monkeys. Anim. Behav. 1980;28:798–813. doi:10.1016/S0003-3472(80)80140-0 [Google Scholar]
- Seyfarth R.M, Cheney D.L. Grooming, alliances, and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. doi:10.1038/308541a0 [DOI] [PubMed] [Google Scholar]
- Silk J.B. Male bonnet macaques use information about third-party rank relationships to recruit allies. Anim. Behav. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. doi:10.1006/anbe.1999.1129 [DOI] [PubMed] [Google Scholar]
- Sinha A. Knowledge acquired and decisions made: triadic interactions during allogrooming in wild bonnet macaques, Macaca radiata. Phil. Trans. R. Soc. B. 1998;353:619–631. doi: 10.1098/rstb.1998.0230. doi:10.1098/rstb.1998.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Frank L.G, Holekamp K.E. Ontogeny of dominance in free-living spotted hyenas: juvenile rank relations with adults. Anim. Behav. 1993;46:467–477. doi:10.1006/anbe.1993.1215 [Google Scholar]
- Smale L, Nunes S, Holekamp K.E. Sexually dimorphic dispersal in mammals: patterns, causes and consequences. Adv. Stud. Behav. 1997;26:181–250. [Google Scholar]
- Smith J.E, Memenis S.K, Holekamp K.E. Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta) Behav. Ecol. Sociobiol. 2007 doi:10.1007/s00265-006-0305-y [Google Scholar]
- Springer M.S, Murphy W.J, Eizirik E, O'Brien S. Placental mammal diversification and the Cretaceous-Tertiary boundary. Proc. Natl Acad. Sci. USA. 2003;100:1056–1061. doi: 10.1073/pnas.0334222100. doi:10.1073/pnas.0334222100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M.S, Murphy W.J, Eizirik E, O'Brien S. Molecular evidence for major placental clades. In: Rose K.D, Archibald J.D, editors. The rise of placental mammals. The Johns Hopkins University Press; Baltimore, MD: 2005. pp. 37–49. [Google Scholar]
- Stander P.E. Foraging dynamics of lions in a semi-arid environment. Can. J. Zool. 1992a;70:8–21. [Google Scholar]
- Stander P.E. Cooperative hunting in lions: the role of the individual. Behav. Ecol. Sociobiol. 1992b;29:445–454. doi:10.1007/BF00170175 [Google Scholar]
- Stanton G.B, Tanaka D, Sakai S.T, Weeks O.I. The thalamic and subcortical afferents of area 6 subdivisions on the anterior sigmoid gyrus in the dog. J. Comp. Neurol. 1986;252:446–467. doi: 10.1002/cne.902520403. doi:10.1002/cne.902520403 [DOI] [PubMed] [Google Scholar]
- Szykman M, Engh A.L, Van Horn R.C, Funk S, Scribner K.T, Holekamp K.E. Association patterns between male and female spotted hyenas reflect male mate choice. Behav. Ecol. Sociobiol. 2001;50:231–238. doi:10.1007/s002650100356 [Google Scholar]
- Tanaka D. Neostriatal projections from cytoarchitecturally defined gyri in the prefrontal cortex of the dog. J. Comp. Neurol. 1987;261:48–73. doi: 10.1002/cne.902610105. doi:10.1002/cne.902610105 [DOI] [PubMed] [Google Scholar]
- Theis K.R, Heckla A.L, Verge J.R, Holekamp K.E. The ontogeny of pasting behaviour in free-living spotted hyenas (Crocuta crocuta) In: Hurst J, Beynon R, editors. Chemical signals in vertebrates. vol. 11. Springer; New York, NY: In press.. [Google Scholar]
- Theis, K. R., Greene, K., Benson-Amram, S. R. & Holekamp, K. E. Submitted. Sources of variation in the long-distance vocalizations of spotted hyenas.
- Tilson R.T, Hamilton W.J. Social dominance and feeding patterns of spotted hyaenas. Anim. Behav. 1984;32:715–724. doi:10.1016/S0003-3472(84)80147-5 [Google Scholar]
- Tomasello M, Call J. Oxford University Press; Oxford, UK: 1997. Primate cognition. [Google Scholar]
- Van Horn R.C, Wahaj S.A, Holekamp K.E. Role-reversed nepotistic interactions between sires and offspring in the spotted hyena. Ethology. 2004;110:1–14. doi:10.1111/j.1439-0310.2004.00984.x [Google Scholar]
- van Schaik C. Why are some animals so smart? Sci. Am. 2006;294:64–71. doi: 10.1038/scientificamerican0406-64. [DOI] [PubMed] [Google Scholar]
- Wagner A. P. In press. Hyaena hyaena (Linnaeus). In The mammals of Africa (eds J. Kingdon, D. Happold & T. Butynski). London, UK: Elsevier Science.
- Wahaj S, Guze K, Holekamp K.E. Reconciliation in the spotted hyena (Crocuta crocuta) Ethology. 2001;107:1057–1074. doi:10.1046/j.1439-0310.2001.00717.x [Google Scholar]
- Wahaj S.A, Van Horn R.C, Van Horn T, Dreyer R, Hilgris R, Schwarz J, Holekamp K.E. Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav. Ecol. Sociobiol. 2004;56:237–247. [Google Scholar]
- Walters J. Interventions and the development of dominance relationships in female baboons. Folia Primatol. 1980;34:61–89. doi: 10.1159/000155948. [DOI] [PubMed] [Google Scholar]
- Woodmansee K.B, Zabel C.J, Glickman S.E, Frank L.G, Keppel G. Scent marking (pasting) in a colony of immature spotted hyenas (Crocuta crocuta): a developmental study. J. Comp. Psychol. 1991;105:1014. doi: 10.1037/0735-7036.105.1.10. doi:10.1037/0735-7036.105.1.10 [DOI] [PubMed] [Google Scholar]
- Woolsey C.N. Organization of somatic sensory and motor areas of the cerebral cortex. In: Harlow H.F, Woolsey C.N, editors. Biological and biochemical bases of behaviour. University of Wisconsin Press; Madison, WI: 1958. pp. 63–81. [Google Scholar]
- Yoerg S.I. Social feeding reverses learned flavour aversions in spotted hyenas (Crocuta crocuta) J. Comp. Psychol. 1991;105:185–189. doi: 10.1037/0735-7036.105.2.185. doi:10.1037/0735-7036.105.2.185 [DOI] [PubMed] [Google Scholar]
- Zabel C.J, Glickman S.E, Frank L.G. Coalition formation in a colony of prebubertal spotted hyaenas. In: Harcourt A.H, de Waal F.B.M, editors. Coalitions and alliances in humans and other animals. Oxford Science; Oxford, UK: 1992. pp. 113–135. [Google Scholar]
- Zajonc R.B. Social facilitation. Science. 1965;149:269–274. doi: 10.1126/science.149.3681.269. doi:10.1126/science.149.3681.269 [DOI] [PubMed] [Google Scholar]