Abstract
Bilateral subthalamic nucleus deep brain stimulation (STN DBS) can reduce working memory while improving motor function in Parkinson disease (PD), but findings are variable. One possible explanation for this variability is that the effects of bilateral STN DBS on working memory function depend in part on functional or disease asymmetry. The goal of this study was to determine the relative contributions of unilateral DBS to the effects seen with bilateral DBS. Motor (Unified Parkinson Disease Rating Scale Part III, UPDRS) and working memory function (Spatial Delayed Response, SDR) were measured in 49 PD patients with bilateral STN DBS while stimulators were Both-off, Left-on, Right-on and Both-on in a randomized, double-blind manner. Patients were off PD medications overnight. Effects of unilateral DBS were compared to effects of bilateral STN DBS. Mean UPDRS and SDR responses to Left-on vs. Right-on conditions did not differ (p>.20). However, improvement in contralateral UPDRS was greater and SDR performance was more impaired by unilateral DBS in the more affected side of the brain than in the less affected side of the brain (p=.008). The effect of unilateral DBS on the more affected side on contralateral UPDRS and SDR responses was equivalent to that of bilateral DBS. These results suggest that motor and working memory function respond to unilateral STN DBS differentially depending on the asymmetry of motor symptoms.
Keywords: Parkinson’s disease, Executive Function, Basal Ganglia, Deep Brain Stimulation, Working Memory
Working memory, the ability to maintain, monitor and use internal information to guide behavior, is a fundamental cognitive skill underlying other more complex “executive” functions (Baddeley, 1992), and is known to be affected by Parkinson disease (PD). Individuals with PD are particularly impaired on spatial working memory tasks, even at the early stages of the disease (Gabrieli, Singh et al., 1996; Lewis, Slabosz et al., 2005), perhaps due to altered basal ganglia output or changes in mesocortical dopaminergic pathways (Carbon and Marie, 2003). The degree of impairment in spatial working memory may depend in part on which hemisphere of the brain is more affected by PD, since worse left-sided motor dysfunction in PD is associated with worse spatial (right hemisphere) tasks and worse right-sided motor dysfunction is associated with more impairment on verbally mediated (left-hemisphere) tasks (Taylor, Saint-Cyr et al., 1986; Starkstein, Leiguarda et al., 1987; Spicer, Roberts et al., 1988; Blonder, Gur et al., 1989; Huber, Miller et al., 1992; Amick, Grace et al., 2006).
Recent work has suggested that bilateral deep brain stimulation of the subthalamic nucleus (STN DBS), apart from any effects of the surgical procedure itself, can impair spatial working memory below already suboptimal levels of function while simultaneously improving motor symptoms of PD (Hershey, Revilla et al., 2004). For example, STN DBS decreases response inhibition performance under conditions of strong response conflict challenges (Jahanshahi, Ardouin et al., 2000; Schroeder, Kuehler et al., 2002; Hershey, Revilla, Wernle, Schneider-Gibson, Dowling, and Perlmutter, 2004; Witt, Pulkowski et al., 2004; Temel, Weber et al., 2004). Similar findings have been reported for working memory performance, particularly under conditions with higher demand on cognitive control processes (Hershey, Revilla, Wernle, Schneider-Gibson, Dowling, and Perlmutter, 2004). In contrast, there have also been reports of improved working memory with STN DBS (Rivaud-Pechoux, Vermersch et al., 2000; Pillon, Ardouin et al., 2000) . However, neither study withdrew levodopa before testing, although levodopa is known to influence working memory and possibly other cognitive skills (Gotham, Brown et al., 1988). Other studies have reported that STN DBS improves extinction task and non-declarative memory performance, worsens declarative memory and causes no changes in a gambling task (Halbig, Gruber et al., 2004; Funkiewiez, Ardouin et al., 2004). The emerging pattern of results suggests that tasks with greater cognitive control demands are most susceptible to the negative effects of STN DBS. These effects may be mediated through the STN’s connections to prefrontal cortex (Nakano, Kayahara et al., 2000; Baunez, Humby et al., 2001; Chudasama, Baunez et al., 2003).
Despite the overall trends observed, findings are variable both across studies and across individuals, driving some controversy in the field. One possible explanation for some of this variability is that clinical factors such as disease asymmetry may modify the effect of bilateral STN DBS on working memory and other cognitive functions. To determine the influence of disease asymmetry on bilateral STN DBS-induced changes in working memory, responses to unilateral STN DBS must be examined. We measured working memory and motor responses to unilateral and bilateral STN DBS in a within-subjects design to address whether responses differed depending on which hemisphere (left vs. right; more affected vs. less affected side of the brain) was stimulated. This information may shed light on the neuropathophysiology of cognitive dysfunction in PD, and help us understand the contributing factors to effects of bilateral STN DBS on working memory.
Materials and Methods
Subjects
This study was approved by the Human Research Protection Office at Washington University School of Medicine and participants gave informed consent. These procedures are in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association. Individuals with PD with previously implanted bilateral stimulators in the STN region were studied. Prior to implantation, all met diagnostic criteria for clinically definite PD (Racette, Rundle et al., 1999) and had a Hoehn and Yahr score of between 2 and 4 in the on medication state. Exclusionary criteria included a history of neurological events or diagnoses other than PD, or dementia on clinical exam prior to surgery. The surgical implantation of stimulators (Medtronic model 3389 DBS leads) targeted STN using T2 weighted magnetic resonance imaging (MRI) with microelectrode recording (Starr, Christine et al., 2002). Intraoperative test stimulation confirmed adequate location of electrodes, similar to other published methods (Starr, Christine, Theodosopoulos, Lindsey, Byrd, Mosley, and Marks, Jr., 2002). The clinical benefit achieved by stimulation in our center, as measured by change in UPDRS motor scores, is comparable to other centers (Tabbal, Revilla et al., 2007).
Fifty-one patients completed the study, but 2 were excluded because their data points were greater than 3 SDs from the mean. On average, the remaining 49 subjects (18 female, 31 male) were 58.7 years old (SD=9.3), had been diagnosed with PD for 13.7 years (SD=4.9) and were tested 16.2 months following STN stimulator implantation (SD=20). At the time of the study, in the off medication, off stimulation state, patients had Hoehn and Yahr scores between 2 and 5 (mean=3.4, SD=1.2). All subjects except 3 were taking levodopa/carbidopa daily and all except 5 were taking other PD medications (amantadine, tolcapone, trihexyphenidyl, pergolide, pramipexole, ropinirole, entacapone, selegiline). All subjects except 3 were right handed. Subjects used their dominant hands to respond on the computer.
Protocol
On the study day, subjects refrained from taking any PD medications for at least 12 hours prior to testing. Working memory testing and motor function rating was performed in four stimulator conditions: both stimulators off (Both-off), both stimulators on (Both-on), left stimulator on (Left-on) and right stimulator on (Right-on). The order of these conditions was randomly assigned for each subject, and patients and examiners were not informed of the order. Stimulator settings were changed at least 60 minutes prior to testing for each condition.
Motor function was rated by a trained movement disorders clinician using the UPDRS III rating scale. Categorization of the “more affected” versus “less affected” side of the brain for each participant was based on the lateralized UPDRS motor score from the Both-off condition. For example, if the left side of the body had more motor symptoms than the right side of the body in the Both-off condition, then we considered the (contralateral) right side of the brain “more affected.”, i.e. right hemisphere.
The SDR Task (Hershey, Craft et al., 1998) measured working memory. This task is an experimentally derived spatial working memory task that has been closely linked to lateral prefrontal cortex functioning in animals and humans (Goldman-Rakic, Funahashi et al., 1990; Luciana, Depue et al., 1992; Funahashi, Bruce et al., 1993). In this task, subjects focused on a central fixation cross on a computer screen placed approximately 40 cm away. While fixated, either one or two cues (each 1 cm in diameter) appeared for 150 msec in any of 32 locations at an 11.5 cm radius from the central fixation. A delay period (5 or 15 sec) was then imposed. During the delay, subjects performed a continuous performance task in which a series of geometric shapes (triangle, square and diamond) appeared in place of the fixation cross (1000 msec duration, 750–1250 msec inter-trial interval). Subjects pressed the spacebar whenever the diamond shape appeared. After the delay, the fixation cue returned, and subjects pointed to where they remembered seeing the cue(s). Responses were measured in X and Y coordinates and compared to the actual location of the cue. Delay trials and trials with no mnemonic load (cue-present trials) were presented in random order. On the cue-present trials the cue (dot) was present during the response phase. This set of trials gave an indication of subjects’ pointing accuracy. Mean error in mm (distance between recall and actual target) was calculated for each subject for each type of trial. Either 1 or 2 cues had to be remembered on each trial. In the two-cue condition, both locations were presented simultaneously, and in the recall phase, subjects pointed to both locations, in any order desired. Forty experimental trials were presented, 20 with one cue presented and 20 with two cues presented. Trials were blocked by number of cues and the order of blocks was counterbalanced across subjects. Subjects performed 4 cue-present trials and 8 trials per delay for each block. The task took approximately 15 minutes to complete.
Analysis
Motor symptoms
Lateralized UPDRS motor scores were summed for each side of the body separately (upper extremity: rest tremor, action or postural tremor, rigidity, finger taps, hand pronation/supination repetitive movements and hand opening/closing repetitive movements; lower extremity: foot tapping, rest tremor and rigidity; total possible for each side = 36 points). Percent change from the Both-off condition was calculated for the left and right sides of the body for each stimulator condition. To compare motor responses across unilateral and bilateral conditions, repeated measures general linear model analyses were performed (Left-on, Right-on and Both-on). A between-subjects variable was added (right hemisphere more affected vs. left hemisphere more affected) to determine if disease asymmetry further modulated the effect of stimulation condition. Univariate analyses and t-tests were performed to follow up significant main effects. Paired t-tests were used to compare responses across conditions and one-sample t-tests were used to determine if a response was significantly different from 0. The threshold for statistical significance was set at p<.05.
Working memory
Percent change in recall error from the Both-off condition was calculated for SDR performance variables for the Left-on, Right-on and Both-on conditions. To compare SDR responses across unilateral and bilateral conditions, repeated measures general linear models analyses were performed on the hardest condition of the SDR task (15 sec delay) with number of cues (1 vs. 2) and stimulator condition (Left-on, Right-on, Both-on) as repeated measures. A between-subjects variable was added (right hemisphere more affected vs. left hemisphere more affected) to determine if disease asymmetry further modulated the effect of stimulation condition. Univariate analyses and t-tests were performed to assess significant main effects. Paired t-tests were used to compare responses across conditions and one-sample t-tests were used to determine if a response was significantly different from 0. The threshold for statistical significance was set at p<.05.
Results
Subjects
Subjects had good clinical benefit from bilateral stimulation (mean improvement in UPDRS scores=49%, SD=23; t=13.7, p<.001). The more affected side of the brain was the right hemisphere in 28 patients and the left hemisphere in 21 patients. These two groups did not differ in age, gender distribution or duration of disease (p values > .07). The average absolute difference between UPDRS motor scores on the more affected vs. less affected sides across the entire sample was 4 points (SD=3). Degree of asymmetry of symptoms across sides of the body did not correlate with the degree of overall PD severity (Both-off, off medication UPDRS total motor score; r=.10, p=.53) or the duration of symptoms (r=.03, p=.86). Stimulation voltage, rate, pulse width and impedance were comparable across sides (left v. right; more affected v. less affected; paired t-tests, p values>.10; Table 1).
Table 1.
Means (SEM) for stimulator, motor and cognitive variables across bilateral and unilateral stimulation conditions.
| Both-on | Left-on | Right-on | More affected side on | Less affected side on | |
|---|---|---|---|---|---|
| Amplitude, V | 3.0 (0.6) | 2.9 (.1) | 3.0 (0.1) | 3.0 (0.1) | 2.9 (0.1) |
| Pulse Width, μs | 66.0 (2.1) | 67.9 (1.7) | 66.1 (1.7) | 66.5 (1.8) | 65.5 (1.7) |
| Rate, Hz | 184.8 (0.2) | 185 (0) | 184.7 (0.3) | 184.7 (0.3) | 185 (0) |
| Impedance, ohms | 1076 (35) | 1040 (42) | 1098 (40) | 1032 (36) | 1106 (45) |
| UPDRS lateralized motor score % change | −36.2 (4.0) | −26.7 (4.8) | −29.4 (4.2) | −33.9 (3.5) | −22.1 (5.2) |
UPDRS
The main effect of DBS condition on lateralized UPDRS responses was significant (Left-on v. Right-on v. Both-on, F(2,46)=4.3, p=.02). All conditions produced significant improvement in lateralized UPDRS scores (p<.001), but the Both-on condition produced significantly greater improvement than either unilateral condition (p<.04). The main effect of group (right hemisphere more affected v. left hemisphere more affected) was not significant (F(1,47)=.009, p=.93). However, the interaction between group and condition on UPDRS response was significant (F(2,46)=3.8, p=.03). UPDRS mean values per condition were: Both-on, 16.6 (SD=6.9); Left-on, 23.0 (SD=8.3); Right-on, 22.1 (SD=7.6); Both-off, 29.4 (SD=10.6) (Figure 1). DBS on the more affected side of the brain and the Both-on condition improved contralateral UPDRS motor scores to the same extent (p=.52), and both conditions improved motor scores to a greater extent than DBS on the less affected side of the brain (p values<.01) (Figure 3).
Figure 1. Effects of unilateral and bilateral STN DBS on motor function by group.
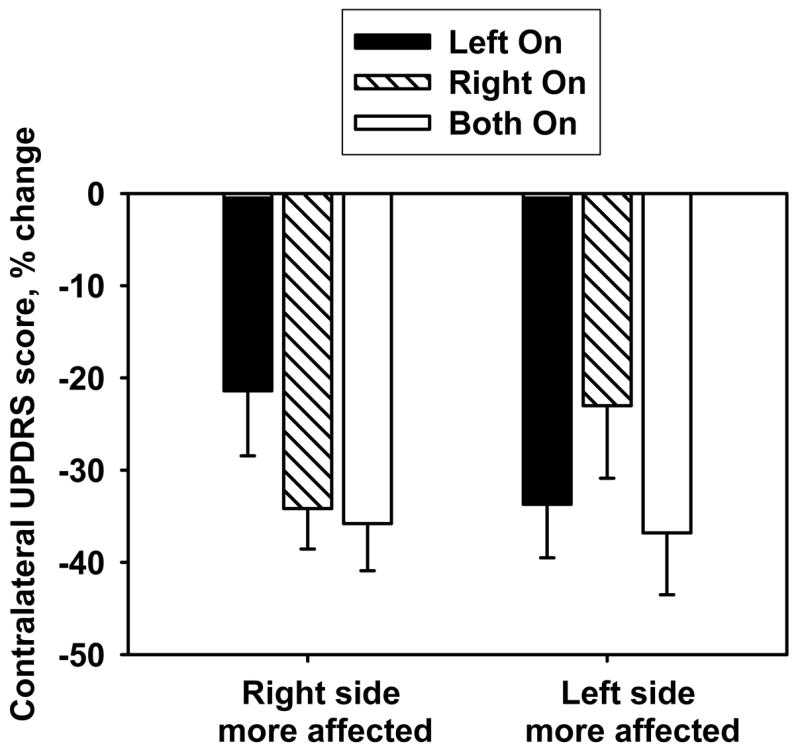
Mean (±SEM) percent change in motor scale score (UPDRS) with unilateral stimulation compared to Both-off condition. Positive values indicate worsening with stimulation; negative values indicate improvement with stimulation. There was a significant interaction between stimulation condition and group for both motor function (p=.03).
Figure 3.

Effects of stimulation on the more affected and less affected sides of the brain compared to bilateral stimulation. Mean (±SEM) percent change in working memory performance (SDR) and motor function (UPDRS) with unilateral stimulation compared to Both-off condition. Positive values indicate worsening with stimulation; negative values indicate improvement with stimulation. Stimulation on the more affected side of the brain was associated with significantly greater decrement in working memory performance and significantly greater improvement in motor function compared to stimulation on the less affected side of the brain. * Different from Both On condition, p<.05; ** Different from less affected side condition, p<.05.
SDR Test
The main effects of DBS condition (Left-on vs. Right-on vs. Both-on) and group (right hemisphere more affected vs. left hemisphere more affected) on SDR change were not significant (Stimulation condition: F(2,46)=0.8, p=.44; Group: F(1,47)=.008, p=.93). However, the interaction between group and condition on SDR response was significant (F(2,46)=3.8, p=.03) (Figure 2). No other interactions were significant (p values>.17). SDR mean values per condition were: Both-off, 22.1 (SD=8.0); Both-on, 22.7 (SD=8.1); Left-on, 21.5 (SD=5.9); Right-on, 22.9 (SD=7.3). The Right-on and Both-on conditions produced significant decrement in SDR performance (15 sec delay, average of 1 and 2 cue trials, all p values<.05), but the Left-on condition did not (p=.23). One-sample t-tests within each group show significant SDR change with more affected side stimulation in the right hemisphere group (p=.03) but not the left hemisphere group (p=.11). Notably, power is reduced due to dividing the total sample in half. However, in a categorical analyses, both groups had more individuals that performed worse with more affected side stimulation than with less affected side stimulation (Chi-sq test; right hemisphere group, p=.01; left hemisphere group, p=.03).
Figure 2. Effects of unilateral and bilateral STN DBS working memory performance by group.
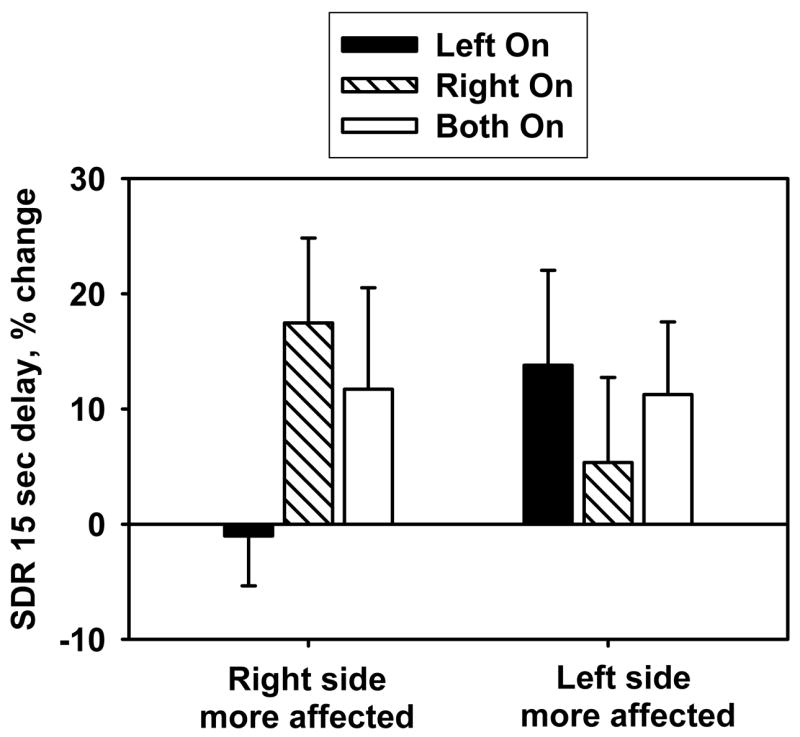
Mean (±SEM) percent change in working memory performance (SDR) with unilateral stimulation compared to Both Off condition. Positive values indicate worsening with stimulation; negative values indicate improvement with stimulation. There was a significant interaction between stimulation condition and group for SDR performance (p=.03).
DBS on the more affected side of the brain and bilateral DBS were associated with significant decrement in SDR performance (15 sec delay, average of 1 and 2 cue trials, one sample t-tests, More affected side DBS, p=.005; bilateral DBS, p=.047). DBS on the less affected side of the brain produced a negligible effect on SDR performance (one sample t-test, p=.67). This change was less than the change induced by bilateral DBS (paired t-test; p=.06) and less than the change induced by DBS in the more affected side of brain (paired t-test; p=.008)(Figure 3). To further test this finding, we categorized individuals as having improved or worsened in performance in response to unilateral DBS. Analyses revealed that there were significantly more individuals with impaired performance in response to DBS on the more affected side than expected by chance (67% of patients had worse performance, Chi-sq=5.9, p=.01), but not in response to DBS on the less affected side (45% of patients had worse performance, Chi-sq=0.5, p=.58). In addition, these two distributions were significantly different from each other (Chi-sq=10.1, p=.001).
To determine if pointing ability was also altered by DBS condition, we performed similar analyses on the cue present trials. There were no significant main effects of conditions or interactions with other variables on pointing accuracy (group, number of cues; p values>.21).
Discussion
In this study, unilateral STN DBS differentially affected working memory and motor function depending primarily on disease asymmetry. Motor function improved more with DBS on the more affected side of the brain than with DBS on the less affected side of brain. In contrast, DBS on the more affected side of the brain impaired working memory, whereas DBS on the less affected side did not. These results suggest that clinical asymmetry interacts with STN DBS to determine behavioral responses.
There are two main points that can be derived from these findings. First, motor and cognitive function can be differentially affected by both unilateral and bilateral STN DBS. This type of dissociation is consistent with our previous work (Hershey, Revilla, Wernle, Schneider-Gibson, Dowling, and Perlmutter, 2004) and other studies. For example, in humans with PD, high frequency STN DBS (130-Hz) improved motor function and decreased verbal fluency, while low frequency STN DBS (10-Hz) worsened motor function and improved verbal fluency, particularly for higher demand conditions of the task (Wojtecki, Timmermann et al., 2006). Likewise, STN DBS at different amplitudes differentially affected cognitive (choice reaction time) and motor function in rats (Temel, Blokland et al., 2005). These differential motor and cognitive responses to STN DBS support the idea that circuits subserving cognitive and motor functions have separable physiologic characteristics (Temel, Blokland, Steinbusch, and Visser-Vandewalle, 2005).
The circuitry underlying working memory skills likely includes ventromedial and rostral portions of STN. These regions receive projections from prefrontal regions (Brodmann areas 6, 8 and 9) which are associated with working memory. These STN regions also send projections to associative areas of caudate, putamen and globus pallidus (Nakano, Kayahara, Tsutsumi, and Ushiro, 2000). The STN as a whole projects to GPi and substantia nigra pars reticulata (SNr), which in turn projects to dorsolateral prefrontal cortex (DLPFC; Brodmann areas 9 and 46) (Middleton and Strick, 1994; Mink, 1996) one of the most critical areas for working memory function (Miller and Cohen, 2001). In PET studies, bilateral STN DBS has been shown to decrease resting brain blood flow in widespread cortical areas, including regions associated with working memory function (e.g. Brodmann areas 9 and 10) (Pochon, Levy et al., 2002; Hershey, Revilla et al., 2003). More specifically, STN DBS decreased activation in the anterior cingulate cortex during a response inhibition (Stroop) task. This decreased activity correlated with decreases in Stroop interference performance in these STN DBS patients (Schroeder, Kuehler, Haslinger, Erhard, Fogel, Tronnier, Lange, Boecker, and Ceballos-Baumann, 2002). Thus, the STN is linked, both neuroanatomically and neurophysiologically, to prefrontal cortical regions responsible for working memory. Stimulation of these circuits could thus feasibly influence working memory function.
A hypothesized mechanism for STN DBS efficacy for motor function is that it forces the “regularization” of the irregular bursting firing of STN output neurons, present in PD, that project through motor circuits (Vitek, 2002). Although regularization may be beneficial for STN output related to motor function, it may not be optimal for STN output related to working memory. A forced regular rate of firing could interfere with the mnemonic representation of information, by eliminating, overriding, or adding interference to important signals, thus making it more difficult to hold and manipulate transient, task-related information on-line. The more affected side of the brain may be less able to compensate for this interference in information representation, particularly when there is a higher demand on the system. It should be noted that the changes we observe occur after approximately an hour of DBS. However, there may be some aspects of motor, cognitive or mood responses to STN DBS that evolve over hours, months or even years. These aspects are not well understood and may involve many other complicated factors (e.g. medication changes, progression of disease).
The second conclusion from our study is that there is a greater effect of disease asymmetry than hemisphere (e.g. left vs. right) on motor and spatial working memory responses to unilateral STN DBS. Both groups (left side of brain more affected and right side of brain more affected) had greater negative responses to more affected than less affected side stimulation. However, the right hemisphere group (left side of the body more affected) did have a more pronounced difference in responses than the left hemisphere group. This result could indicate some influence of the connection between right hemisphere function and spatial working memory (McCarthy, Puce et al., 1996). Ideally, using a similarly designed verbal working memory task in the same conditions would be able to convincingly distinguish these effects.
Asymmetry of disease severity has been shown to influence responses to treatments previously. For example, levodopa-induced dyskinesias and off-period dystonia appear earliest and more severely in the more affected side of the body and in more advanced patients, presumably through levodopa’s interactions with the disease process (Mones, Elizan et al., 1971; Nutt, 1990; Burkhard, Shale et al., 1999). In addition, levodopa can produce the greatest benefit in motor symptoms on the worse side of the body with good responses to levodopa usually preceding development of dopa-induced dyskinesias (Mones, Elizan, and Siegal, 1971; Nutt, 1990). Similarly, unilateral and bilateral STN DBS can induce dyskinesias (Limousin, Pollak et al., 1996; Kumar, Lozano et al., 1999) that have similar characteristics to levodopa-induced dyskinesias (Krack, Pollak et al., 1999; Benabid, Benazzouz et al., 2000) including the tendency to occur in patients with better response (Houeto, Welter et al., 2003). We observe here that STN DBS, like levodopa, may interact with disease asymmetry to produce adverse cognitive effects despite improved motor function.
Although disease asymmetry may be an important factor in the cognitive and motor responses from unilateral STN DBS, there are also variables related to the stimulation itself that could modulate responses. For example, the precise location of the stimulating electrode and field of stimulation delivered by the active contact could modulate STN DBS’s effects on motor and cognitive functions. Stimulation in or near ventral STN may have more adverse effects on cognition than stimulation in or near dorsal STN (Temel, Blokland, Steinbusch, and Visser-Vandewalle, 2005). If disease asymmetry influences the regions of STN affected by unilateral stimulation this could explain some differences in behavioral responses. It is unlikely that the surgical process of electrode placement would be systematically altered by which side of the brain was more affected. However, the active contact chosen during programming conceivably could be influenced by severity of symptoms, since these clinical decisions are based on observed motor responses to different stimulator settings. Future studies that incorporate the exact location of contacts and the degree and strength of current spread may be useful in understanding the physiological characteristics of the anatomical pathways underlying adverse cognitive response and optimal motor response to stimulation.
Acknowledgments
Supported by: the Greater St. Louis Chapter of the American Parkinson Disease Association (APDA), NIH (NS41248; NS41509), American Academy of Neurology, APDA Advanced Center for PD Research at Washington University, and the Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund and the Jack Buck Fund for PD Research).
Disclosure: Dr. Karimi received partial fellowship funding from Medtronic, Inc, the manufacturer of the implanted stimulators. There are no other conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amick MM, Grace J, Chou KL. Body side of motor symptom onset in Parkinson’s disease is associated with memory performance. J Int Neuropsychol Soc. 2006;12:736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: the interface between memory and cognition. J Cogn Neurosci. 1992;4:281–288. doi: 10.1162/jocn.1992.4.3.281. [DOI] [PubMed] [Google Scholar]
- Baunez C, Humby T, Eagle DM, Ryan LJ, Dunnett SB, Robbins TW. Effects of STN lesions on simple vs choice reaction time tasks in the rat: preserved motor readiness, but impaired response selection. Eur J Neurosci. 2001;13:1609–1616. doi: 10.1046/j.0953-816x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Benazzouz A, Limousin P, Koudsie A, Krack P, Piallat B, Pollak P. Dyskinesias and the subthalamic nucleus. Ann Neurol. 2000;47:S189–S192. [PubMed] [Google Scholar]
- Blonder LX, Gur RE, Gur RC, Saykin AJ, Hurtig HI. Neuropsychological functioning in hemiparkinsonism. Brain Cogn. 1989;9:244–257. doi: 10.1016/0278-2626(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Burkhard PR, Shale H, Langston JW, Tetrud JW. Quantification of dyskinesia in Parkinson’s disease: validation of a novel instrumental method. Mov Disord. 1999;14:754–763. doi: 10.1002/1531-8257(199909)14:5<754::aid-mds1007>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Carbon M, Marie RM. Functional imaging of cognition in Parkinson’s disease. Curr Opin Neurol. 2003;16:475–480. doi: 10.1097/01.wco.0000084225.82329.3c. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Baunez C, Robbins TW. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: evidence for corticosubthalamic interaction. J Neurosci. 2003;23:5477–5485. doi: 10.1523/JNEUROSCI.23-13-05477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13:1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkiewiez A, Ardouin C, Krack P, Dubois B, Benabid A-L, Pollak P. Effects of levodopa and STN stimulation on decision making in Parkinson’s disease. Mov Disord. 2004;19:S402–403. [Google Scholar]
- Gabrieli JDE, Singh J, Stebbins GT, Goetz CG. Reduced working memory span in Parkinson’s disease: evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology. 1996;10:322–332. [Google Scholar]
- Goldman-Rakic PS, Funahashi S, Bruce CJ. Neocortical memory circuits. Cold Spring Harbor Symposia on Quantitative Biology. 1990;55:1025–1038. doi: 10.1101/sqb.1990.055.01.097. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Halbig TD, Gruber D, Kopp UA, Scherer P, Schneider GH, Trottenberg T, Arnold G, Kupsch A. Subthalamic stimulation differentially modulates declarative and nondeclarative memory. Neuroreport. 2004;15:539–543. doi: 10.1097/00001756-200403010-00031. [DOI] [PubMed] [Google Scholar]
- Hershey T, Craft S, Glauser TA, Hale S. Short-term and long-term memory in early temporal lobe dysfunction. Neuropsychology. 1998;12:52–64. doi: 10.1037//0894-4105.12.1.52. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla F, Wernle A, Schneider-Gibson P, Dowling J, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62:1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle A, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Welter ML, Bejjani PB, Tezenas du MS, Bonnet AM, Mesnage V, Navarro S, Pidoux B, Dormont D, Cornu P, Agid Y. Subthalamic stimulation in Parkinson disease: intraoperative predictive factors. Arch Neurol. 2003;60:690–694. doi: 10.1001/archneur.60.5.690. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Miller H, Bohaska L, Christy JA, Bornstein RA. Asymmetrical cognitive differences associated with hemiparkinsonism. Arch Clin Neuropsychol. 1992;7:471–480. [PubMed] [Google Scholar]
- Jahanshahi M, Ardouin CM, Brown RG, Rothwell JC, Obeso J, Albanese A, Rodriguez-Oroz MC, Moro E, Benabid AL, Pollak P, Limousin-Dowsey P. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain. 2000;123(Pt 6):1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- Krack P, Pollak P, Limousin P, Benazzouz A, Deuschl G, Benabid AL. From off-period dystonia to peak-dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain. 1999;122(Pt 6):1133–1146. doi: 10.1093/brain/122.6.1133. [DOI] [PubMed] [Google Scholar]
- Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Limousin P, Pollak P, Hoffmann D, Benazzouz A, Perret JE, Benabid AL. Abnormal involuntary movements induced by subthalamic nucleus stimulation in parkinsonian patients. Mov Disord. 1996;11:231–235. doi: 10.1002/mds.870110303. [DOI] [PubMed] [Google Scholar]
- Luciana M, Depue RA, Arbisi P, Leon A. Facilitation of working memory in humans by a D2 dopamine receptor agonist. J Cogn Neurosci. 1992;4:58–68. doi: 10.1162/jocn.1992.4.1.58. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mones RJ, Elizan TS, Siegal GJ. Analysis of L-dopa induced dyskinesia in 51 patients with parkinsonism. J Neurol Neurosurg Psychiatry. 1971;34:668–673. doi: 10.1136/jnnp.34.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(Suppl 5):V1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology. 1990;40:340–345. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- Pillon B, Ardouin C, Damier P, Krack P, Houeto JL, Klinger H, Bonnet AM, Pollak P, Benabid AL, Agid Y. Neuropsychological changes between “off” and “on” STN or GPi stimulation in Parkinson’s disease. Neurology. 2000;55:411–418. doi: 10.1212/wnl.55.3.411. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: an fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- Rivaud-Pechoux S, Vermersch A, Gaymard B, Ploner CJ, Bejjani BP, Damier P, Demeret S, Agid Y, Peirrot-Deseilligny C. Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2000;68:381–384. doi: 10.1136/jnnp.68.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder U, Kuehler A, Haslinger B, Erhard P, Fogel W, Tronnier VM, Lange KW, Boecker H, Ceballos-Baumann AO. Subthalamic nucleus stimulation affects striato-anterior cingulate cortex circuit in a response conflict task: a PET study. Brain. 2002;125:1995–2004. doi: 10.1093/brain/awf199. [DOI] [PubMed] [Google Scholar]
- Spicer KB, Roberts RJ, LeWitt PA. Neuropsychological performance in lateralized parkinsonism. Arch Neurol. 1988;45:429–432. doi: 10.1001/archneur.1988.00520280079019. [DOI] [PubMed] [Google Scholar]
- Starkstein S, Leiguarda R, Gershanik O, Berthier M. Neuropsychological disturbances in hemiparkinson’s disease. Neurology. 1987;37:1762–1764. doi: 10.1212/wnl.37.11.1762. [DOI] [PubMed] [Google Scholar]
- Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, Marks WJ., Jr Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- Tabbal SD, Revilla F, Mink JW, Schneider-Gibson P, Wernle A, De Erausquin GA, Perlmutter JS, Rich KM, Dowling JL. Safety and Efficacy of Subthalamic Nucleus Deep Brain Stimulation Performed With Limited Intra-Operative Mapping for Treatment of Parkinson Disease. Neurosurgery. 2007;61(3 Suppl):119–27. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain. 1986;109(Pt 5):845–883. doi: 10.1093/brain/109.5.845. [DOI] [PubMed] [Google Scholar]
- Temel Y, Blokland A, Steinbusch HW, Visser-Vandewalle V. The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol. 2005;76:393–413. doi: 10.1016/j.pneurobio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Temel Y, Weber WE, Blokland A, Acermans L, Boon P, Visser-Vandewalle V. Improved motor responding, but central slowing, after bilateral subthalamic nucleus stimulation in patients with Parkinson’s disease. Mov Disord. 2004;19:S287–288. [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: Excitation or inhibition. Mov Disord. 2002;17:S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, Krack P. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61:697–700. doi: 10.1001/archneur.61.5.697. [DOI] [PubMed] [Google Scholar]
- Wojtecki L, Timmermann L, Jorgens S, Sudmeyer M, Maarouf M, Treuer H, Gross J, Lehrke R, Koulousakis A, Voges J, Sturm V, Schnitzler A. Frequency-dependent reciprocal modulation of verbal fluency and motor functions in subthalamic deep brain stimulation. Arch Neurol. 2006;63:1273–1276. doi: 10.1001/archneur.63.9.1273. [DOI] [PubMed] [Google Scholar]


