Abstract
The Psychosis Proneness Scales developed by the Chapmans and colleagues (Chapman et al., 1995) are widely used to identify nonpatient individuals who are hypothesized to possess heightened vulnerability to schizophrenia and related psychopathology. Yet surprisingly little is known about whether schizophrenia patients themselves show abnormalities on these scales across different clinical states, as would be expected for vulnerability indicators. Scores on four of the Psychosis Proneness Scales were evaluated at three assessment points over a 15-month period in healthy controls (n = 54) and in recent-onset schizophrenia patients (n = 72) who experienced symptom fluctuations across assessments. Patients showed steady elevations on the Physical Anhedonia Scale across time and clinical state, consistent with a stable vulnerability indicator. Patients had higher scores on the Perceptual Aberration and Magical Ideation Scales than controls throughout the follow-up period but scores also changed across clinical states, consistent with a mediating vulnerability indicator. Patients had higher scores on the Impulsive Non-Conformity Scale than controls only during a psychotic state, reflecting an episode indicator. The longitudinal characteristics of these scales in people who are actually diagnosed with schizophrenia provide key evidence for the validity of three commonly used psychometric indicators of vulnerability to psychosis.
Introduction
Vulnerability indicators for schizophrenia refer to anomalous traits that are present prior to, during, and following periods of clinical symptom exacerbations (Nuechterlein and Dawson 1984; Nuechterlein et al. 1992). They are presumed to reflect on-going proneness to develop the symptoms of schizophrenia and are thus believed to be more central to core etiological processes than clinical symptoms themselves. The concept of vulnerability factor is closely related to that of an “intermediate endophenotype” (Gottesman and Gould 2003), although the concept of vulnerability has sometimes been associated with a somewhat broader range of causal factors, including genetic influences as well as neurodevelopmental and other environmental factors (Nuechterlein 1990). Candidate vulnerability indicators include certain basic neurophysiological and information processing anomalies, as well as subjective experiences or overt behaviors that may be less severe variants of characteristic clinical symptoms of schizophrenia. This report focuses on the latter level of analysis by longitudinally evaluating whether widely used “Psychosis Proneness” scales display the characteristics of vulnerability indicators in recent-onset schizophrenia patients.
Based largely upon Meehl’s (Meehl 1962) theory of schizotypy, the Chapmans and their colleagues developed five self-report scales to assess a broad range of experiential and behavioral features of schizotypy, a personality organization proposed to reflect vulnerability to schizophrenia or, more generally, psychosis-proneness (Chapman et al. 1995). The Perceptual Aberration Scale (PerAb) and the Magical Ideation Scale (MagId) measure aspects of positive schizotypy, including distorted perceptions of one’s own body and of other objects, and a tendency to accept unconventional forms of causality. The Revised Social Anhedonia Scale (SocAnh) and the Physical Anhedonia Scale (PhysAnh) assess aspects of negative schizotypy, including diminished experience of pleasure from social and physical stimuli. Finally, the Impulsive Non-Conformity Scale (ImpNon) measures impulsivity and failure to conform to social expectations about the rights of others. These scales have been used in varying degrees across the hypothesized spectrum of schizophrenia-related psychopathology.
The primary use of the Psychosis Proneness Scales has been to identify putatively schizotypal individuals in non-clinical samples. Individuals with elevated scores on the PerAb, MagId, SocAnh, and PhysAnh demonstrate psychological, neurocognitive, and psychophysiological disturbances resembling those of schizophrenia patients, while the presence of such disturbances is less clear in individuals with elevated ImpNon (Edell 1995; Fernandes and Miller 1995; Horan et al. 2004). In the few prospective studies, PerAb and MagId predicted the development of symptoms of psychotic as well as other psychiatric disorders (Chapman et al. 1994; Gooding et al. 2005), SocAnh more specifically predicted symptoms of schizophrenia spectrum disorders (Gooding et al. 2005; Kwapil 1998), and PhysAnh and ImpNon did not predict later psychosis (Chapman et al. 1994).
The status of these scales as vulnerability indictors has been directly evaluated in studies of unaffected biological relatives of schizophrenia patients and, to a very limited extent, in individuals who themselves have schizophrenia spectrum personality disorders (schizotypal, schizoid, and paranoid personality disorders) or schizophrenia. In biological relatives, elevations have most commonly been reported for PhysAnh and SocAnh (Edell 1995; Horan et al. 2006a). PerAb and MagId have typically not shown elevations in biological relatives ((Edell 1995) but see (Lenzenweger and Loranger 1989)). To our knowledge, no published reports have examined ImpNon in biological family members. The scant research in patients diagnosed with schizophrenia spectrum personality disorders suggests cross-sectional elevations on the Psychosis Proneness Scales ((Camisa et al. 2005; Thaker et al. 1993); also see (Bailey et al. 1993)), though their specificity to these disorders is unclear.
Given the intent of the Psychosis Proneness scales to measure traits associated with vulnerability to schizophrenia, it is somewhat surprising that so few studies have examined them in individuals who are actually diagnosed with schizophrenia spectrum disorders. It is true that these scales were developed primarily for use in non-clinical samples, yet all definitions of vulnerability factors indicate that abnormalities should be present both prior to and following the emergence of clinical symptoms. Thus, if the scales are indeed valid vulnerability indicators, abnormalities should be detectable across time and clinical symptom status in people who meet diagnostic criteria for spectrum disorders.
Nuechterlein and Dawson (Nuechterlein and Dawson 1984) delineated two criteria for evaluating candidate vulnerability indicators in schizophrenia patients: 1) patients should show abnormality from the general population, and 2) continued abnormality should be present throughout both symptomatic and asymptomatic periods. Studies of these scales in schizophrenia patients have been almost exclusively cross-sectional and therefore address only the first criterion. Patients have repeatedly shown elevated PhysAnh and SocAnh (Horan et al. 2006c) and less frequently, but consistently, elevated PerAb (Edell 1995). The remaining scales have been examined much less frequently, though elevated MagId (George and Neufeld 1987) and ImpNon (Chapman et al. 1984) have been reported in inpatient samples.
To evaluate the second criterion for a vulnerability indicator, it is essential to study schizophrenia patients longitudinally across different clinical states. To distinguish among indicators that reflect traits, clinical state, or combinations of traits and states, Neuchterlein and Dawson (Nuechterlein and Dawson 1984) proposed the following tripartite model:
Stable vulnerability indicators refer to abnormalities that are highly stable and independent of symptom fluctuations. This pattern would be expected for traits that are linked to vulnerability and not directly linked to development of psychotic episodes.
Mediating vulnerability factors are abnormalities that are present during symptomatic and asymptomatic states, but are more deviant during and possibly somewhat before symptomatic periods. These vulnerability factors are more likely to be involved in subclinical processes leading to formation of clinical symptoms than stable vulnerability indicators.
Episode indicators, on the other hand, are abnormal during symptomatic periods and normalize in remitting periods, and therefore reflect clinical state rather than enduring characteristics associated with proneness to schizophrenia.
To date, only the PhysAnh and SocAnh have been evaluated longitudinally in schizophrenia patients. During the more chronic stages of the illness, scores on PhysAnh and or SocAnh show good temporal stability across periods of three-months to 20 years (Blanchard et al. 1998; Herbener and Harrow 2002; Herbener et al. 2005) and across acutely symptomatic versus remitted states (in contrast to state-related changes found in depressed patients (Blanchard et al. 2001)). These findings suggest that anhedonia reflects an enduring trait in schizophrenia, at least among chronically ill patients.
This 15-month study evaluated scores on four Psychosis Proneness scales (PhysAnh, PerAb, MagId, ImpNon) at three time points in recent-onset patients and in healthy controls. The fluctuating early stage of schizophrenia, which includes periods of full remission more frequently than later stages of the illness (Nuechterlein et al. 2006), provides an excellent opportunity to clarify the extent to which candidate vulnerability indicators represent state or trait phenomena, and minimizes confounds associated with prior treatment or chronicity. Based on the evidence reviewed above, we expected that PhysAnh would demonstrate the characteristics of a stable vulnerability indicator and that ImpNon would reflect an episode indicator. We also expected that PerAb and MagId would demonstrate characteristics of vulnerability indicators, but we did not have strong predictions as to whether they would more likely reflect stable or mediating vulnerability factors.
Methods
Overview
Participants included 72 patients and 54 nonpsychiatric controls from Sample 1 of the Developmental Processes in Schizophrenic Disorders project (Nuechterlein et al. 1992), a two-phase longitudinal study of schizophrenia patients who had recently (within 2 years) had a first episode of psychosis. This report focuses on Phase 1 of the project during which patients completed major testing batteries at three standardized time points: Assessment 0, psychotic period at study entry; Assessment 1, following an outpatient medication stabilization period of approximately 3 months; Assessment 2, at the conclusion of a 1 year follow-through period during which patients received injectable antipsychotic medication [standardized starting dosage of 12.5 mg fluphenazine (Prolixin) decanoate every 2 weeks] and psychosocial treatment. During the follow-through period, the medication dosage was increased if significant symptoms returned or reduced if intolerable side effects occurred, and patients were treated with adjunctive medications (e.g. antidepressants) as dictated by clinical need. At each assessment, patients completed clinical symptom assessments and the Psychosis Proneness scales. Healthy controls were administered three major test batteries that included the Psychosis Proneness scales at the same time intervals as the patient sample.
Participants
All patients met Research Diagnostic Criteria (Spitzer et al. 1978) for schizophrenia or schizoaffective, mainly schizophrenic subtype, as well as criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM-III; (Association 1987)) as determined by an expanded version of the Present State Examination (Wing et al. 1974). Additional inclusion criteria for patients included: a recent onset of a psychotic disorder with symptoms lasting at least two weeks and a first psychotic episode starting not more than two years before project entry; age 18 to 45 years; Anglo-American, Native American, or acculturated Hispanic or Asian background (including fluency in English).
Controls met the following inclusion criteria: age 18 to 45 years, no evidence of schizophrenia spectrum disorder or major mood disorders based on an expanded PSE adapted for a lifetime perspective; Sc scale on the Minnesota Multiphasic Personality Inventory (Hathaway and McKinley 1967) within normal limits; self-report of no prior treatment for psychiatric disorder; no evidence of a first degree relative having been treated for a major psychotic, mood, or substance use disorder; Anglo-American, Native American, or acculturated Hispanic or Asian background (including fluency in English). Exclusion criteria for both groups were: evidence of organic central nervous system disorder (e.g. epilepsy, encephalitis); evidence of significant and habitual drug abuse or alcoholism in the previous 6-month period; mental retardation (i.e. premorbid IQ less than 70).
Demographic data are presented in Table 1. The groups did not differ in age, ethnicity, marital status, or sex. Patients had less education than controls, t(124) = 2.72, p < 0.01, but the groups did not differ in parental education levels. Individuals in the patient group were young adults with a typical age of onset, were primarily living in the community outside of institutional settings, and had a very low rate of re-hospitalization during the study period.
Table 1.
Demographic Information for the schizophrenia (n = 72) and control (n = 54) groups
| Schizophrenia | Controls | |
|---|---|---|
| Age at study entry (Mean, SD) | 23.4 (4.6) | 24.0 (3.9) |
| Sex (% Male) | 80% | 81% |
| Ethnicity (% Caucasian) | 90% | 90% |
| Education (Mean, SD) | 12.4 (2.1) | 13.3 (1.6) |
| Parental education (Mean, SD) | 14.5 (2.6) | 14.9 (2.9) |
| Marital status (% Never married) | 90% | 90% |
| Living situation (%) | ||
| Independent or with family | 91% | 100% |
| Board and care facility | 9% | -- |
| Age of onset (Mean, SD) | 22.2 (4.6) | -- |
| Re-hospitalized during follow-through (%) | 3% | -- |
Measures
Clinical symptoms
At each assessment, patients were rated on the expanded 18-item UCLA version of the Brief Psychiatric Rating Scale (BPRS; (Lukoff et al. 1986; Overall and Gorham 1962)) by a trained rater who was also the patient’s case manager. Each BPRS rater achieved a median intra-class correlation coefficient of 0.80 or higher across all items compared with the criterion ratings (Ventura et al. 1993). Each item is rated on a scale ranging from 1 (not present) to 7 (extremely severe). Following established procedures (Guy 1976), an 18-item total score (possible score range: 18 - 128) and the following five empirically derived subscales scores (based on the mean rating of the items in parentheses) were calculated: Thought disturbance (Conceptual disorganization, Grandiosity, Hallucinatory behavior, Unusual thought content, Bizarre behavior), Anxiety/Depression (Somatic concern, Anxiety, Guilt feelings, Depressive mood, Suicidality), Anergia (Emotional withdrawal, Motor retardation, Blunted affect, Disorientation, Self-neglect), Hostility (Hostility, Suspiciousness, Uncooperativeness), Activation (Tension, Mannerism and posturing, Excitement).
Psychosis Proneness Scales
At each assessment, all participants completed four of the five true-false, self-report Psychosis Proneness Scales. Unfortunately, SocAnh was not administered in this study due to prevailing beliefs at the time this study was initiated that the PhysAnh was a more promising indicator of vulnerability (Chapman et al. 1976) and to reduce the assessment burden on patients. PhysAnh typically correlates in the moderate range with SocAnh in non-clinical (Edell 1995) and schizophrenia samples (Blanchard et al. 1998).
The Revised Physical Anhedonia Scale (PhysAnh; (Chapman and Chapman 1978a)) is a 61-item scale that inquires about the sensory and aesthetic pleasures of eating, touching, feeling, sex, temperature, movement, smell, sight, and sound. The Perceptual Aberration Scale (PerAb; (Chapman et al. 1978; Chapman and Chapman 1978b)) is a 35-item scale comprised of 28 items designed to tap gross distortions in the perception of one’s own body and 7 items for other perceptual distortions. The 30-item Magical Ideation Scale (MIS; (Eckblad and Chapman 1983)) inquires about beliefs in superstitious or magical forms of causation that are regarded as invalid by conventional standards. Finally, the Impulsive Nonconformity Scale (ImpNon; (Chapman et al. 1984)) consists of 51-items designed to tap a failure of incorporation of societal norms, a lack of empathy for the pain of others, and an unrestrained yielding to impulse and self-gratification. An extensive body of research has documented the reliability of the Chapman scales and reviews of this literature can be found elsewhere (Chapman et al. 1995; Edell 1995). Items from all four scales, as well as the Chapman Infrequency Scale (Chapman and Chapman 1983), were interspersed in a single questionnaire. Scales with infrequency scores of three or greater were excluded from analyses.
In the patient group, there were 72 valid Psychosis Proneness Scales at Assessment 0, 69 (2 invalid, 1 not completed) at Assessment 1, and 59 at Assessment 2 (3 invalid, 10 not completed). There were no significant differences between patients who did or did not complete Assessment 2 on any demographic demographics or on symptom ratings at Assessments 0 or 1. For controls, there were 54 valid Psychosis Proneness Scales at Assessment 0, 54 at Assessment 1, and 48 at Assessment 2. There were no significant differences between controls that did or did not complete Assessment 2 on any demographic characteristics. Internal consistency estimates (coefficient alpha) were .75 or greater for all of the Scales in both groups.
Data analysis
Two complementary data analytic strategies were used. In the first strategy, all available data points across the three assessments were used in a series of random effects multilevel modeling regression analyses (MLM; using HLM-6.02 (Raudenbush et al. 2000)). The advantages of MLM for examining longitudinal data with a hierarchical structural (e.g., testing occasions nested within subjects) have been described elsewhere (for in-depth treatments see (Nezlek 2001; Raudenbush and Bryk 2002; Reise and Duan 1999)). A key advantage is that MLM explicitly accounts for the non-independence of such data by simultaneously modeling both within-subject variance components (level-1 or “occasion-level”) and between-subjects variance components (level-2 or “subject-level”). In the current research context, the within-subjects variance reflects how much each subject’s scores fluctuate around his/her own overall mean score from occasion-to-occasion. For example, a variable such as height would have little variation across occasions, whereas a variable like mood would be expected to show significant variation within a person over time. In contrast, the between-subjects variance reflects how much the overall mean scores of individuals across occasions differ from subject-to-subject. In other words, to what extent does a variable reflect stable individual differences?
The MLM analyses assessed three main issues to determine the type of indicator that each of the Psychosis Proneness scales reflected. First, we evaluated the extent to which BPRS symptom ratings (in patients only) and scores on the Psychosis Proneness scales (in both groups) measured trait-like individual differences. This was accomplished by computing the intraclass coefficient (ICC, range 0.0 – 1.0) for each scale, which indexes the proportion of between-subjects or trait-like variance to total variance. As the ICC increases, this indicates that the relative ordering of subjects remains more stable across assessments (i.e., a person who is relatively high on a scale at Occasion 0 is also relatively high at Occasions 1 and 2). Low ICC’s indicate that a variable is more state-like, such that the relative ordering of people changes across occasions. Second, we evaluated whether the patient and control groups differed on the Psychosis Proneness scales at baseline and across testing occasions. This was done by evaluating intercept (i.e., mean scores at Occasion 0) and slope differences between the groups on each scale. The slopes in MLM analyses are reflected in regression coefficients, whose statistical significance and magnitude are evaluated similarly to unstandardized beta weights in typical regression analyses. Third, within the patient group, we evaluated whether changes on the Psychosis Proneness scales across occasions were associated with concurrent changes in BPRS symptom ratings by examining the covariance between scores on the these measures.
A stable vulnerability indicator would be expected to demonstrate a substantial between-subjects (i.e., trait-like) variance component, elevated scores in patients at baseline that persist across assessments, and low, if any, covariation with psychotic symptoms. A mediating vulnerability factor would still have a notable between-subjects (trait-like) variance component, elevated scores in patients at baseline that persist across assessments, and moderate covariation with psychotic symptoms. Finally, an episode indicator would be expected to show elevated scores in patients at baseline that decrease to normal levels at subsequent assessments, and moderate to high covariation with psychotic symptoms (the issue of trait-like variance is not central to this type of indicator).
Some of the schizophrenia patients did not achieve a full remission of symptoms after the baseline assessment. It is therefore possible that any elevations on the Psychosis Proneness Scales in the patients at later assessments could merely reflect persistent psychotic symptoms. To address this possibility, the second data analytic approach evaluated the stability of scores between the first two assessment points in a subset of patients who satisfied stringent operational criteria for being in an acutely psychotic state at Assessment 0 followed by full clinical remission at Assessment 1 (i.e., the end of the medication stabilization period). Following established procedures (Nuechterlein et al. 2006), a psychotic state was defined as being rated 4 or greater on one or more of the BPRS Thinking Disturbance factor items (unusual thought content, hallucinations, conceptual disorganization). Clinical remission was defined as being rated in the nonpathological range (3 or less) on all of the standard 18 items of the BPRS for at least one month. Scores on the Psychosis Proneness scales were evaluated in this subset of patients using 2 (group: Patients versus Controls) × 2 (Assessment: 0 versus 1) Repeated-Measures ANOVA’s.
Results
Multilevel modeling analyses
Documentation of symptom changes in the schizophrenia group
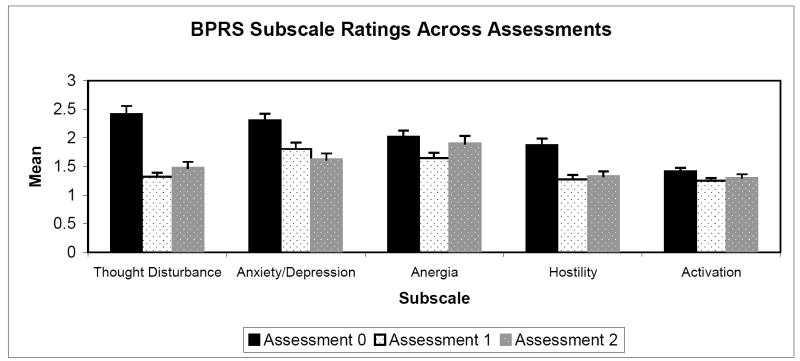
The schizophrenia group’s mean ratings on the BPRS subscales at each assessment are presented graphically in Figure 1. Means on the total BPRS scores (sum across all 18 items) at each assessment were: Assessment 0: 36.8 (SD = 10.4); Assessment 1: 26.7 (SD = 7.7); Assessment 2: 27.94 (SD = 8.6).
Figure 1.
BPRS Subscale Ratings Across Assessments
We first evaluated the extent to which ratings on the BPRS reflect fluctuating, within-subject variance versus stable, between-subjects variance within the schizophrenia group. A series of total unconditional multilevel models that used each BPRS subscale as an outcome variable enabled us to calculate ICC’s for each scale. The ICC’s were both extremely low for Thinking Disturbance and Total BPRS scores at .01, indicating that the variance is almost entirely within-subject. The ICC’s were also quite low for Anxiety/Depression: .12, Hostility: .20, and Activation: .16. Thus, with the exception of the Anergia subscale, for which about half of the variance reflected between-subjects variance (ICC = .48), the BPRS subscales primarily measured state-related variance.
We then evaluated whether the patients’ mean BPRS ratings at Assessments 1 and 2 significantly differed from initial ratings at Assessment 0. A series of conditional models used each BPRS subscale as an outcome variable with two time factors entered as level-1 predictors. The time factors were dummy coded variables that reflected contrasts between the means at Assessments 0 versus 1, and at Assessments 0 versus 2. As summarized in Table 2, the intercepts for all of the BPRS subscales were significantly greater than zero, reflecting the acutely symptomatic status of patients at Assessment 0. With the exception of a non-significant difference between Anergia scores from Assessment 0 to Assessment 2, mean scores on each of the BPRS subscale scores was lower at Assessments 1 and 2 as compared to Assessment 0, indicating that symptoms significantly decreased after the baseline assessment.
Table 2.
Multi-level Modeling Analyses of BPRS Ratings in the Schizophrenia Group
| BPRS Scale | Parameter | Coefficient | SE | t |
|---|---|---|---|---|
| Thought Disturbance | Intercept (β00) | 2.39 | .15 | 16.45*** |
| Contrast 1 (β10) | -.1.06 | .15 | -7.02*** | |
| Contrast 2 (β20) | -.88 | .18 | -4.81*** | |
| Anxiety/Depression | Intercept (β00) | 2.27 | .11 | 19.94*** |
| Contrast 1 (β10) | -.48 | .15 | -3.24** | |
| Contrast 2 (β20) | -.66 | .14 | 4.72*** | |
| Anergia | Intercept (β00) | 2.00 | .11 | 18.91*** |
| Contrast 1 (β10) | -.32 | .10 | -3.32*** | |
| Contrast 2 (β20) | -.18 | .13 | -1.36 | |
| Hostility | Intercept (β00) | 1.86 | .11 | 16.39** |
| Contrast 1 (β10) | -.59 | .11 | -5.15*** | |
| Contrast 2 (β20) | -.58 | .12 | -4.69*** | |
| Activation | Intercept (β00) | 1.43 | .06 | 24.21*** |
| Contrast 1 (β10) | -.14 | .07 | -2.17* | |
| Contrast 2 (β20) | -.17 | .08 | -2.21* | |
| Total Score | Intercept (β00) | 45.72 | 1.41 | 32.36*** |
| Contrast 1 (β10) | -11.65 | 1.70 | -6.83*** | |
| Contrast 2 (β20) | -10.77 | 2.01 | -5.37*** |
Notes: The full model used in these analyses was: BPRS scale = β00 + β10 [Contrast 1] + β20 [Contrast 2] + r0 + e. β00 (df = 71) reflects the grand mean at Occasion 0; β10 (df = 195) indicates the extent to which scores at Occasion 1 differ from scores at Occasion 0; β20 (df = 195) indicates the extent to which scores at Occasion 2 differ from scores at Occasion 0.
p < .05;
p < .005;
p < .001.
Stability of the Psychosis Proneness Scales in patients and controls
The next set of analyses evaluated the variance components for the Psychosis Proneness Scales separately in each group. In the control group, the ICC’s were: PhysAnh = .76; ImpNon = .84; PerAb = .70; MagId = .83, indicating that most of the variance on these scales reflected trait-like, between-subject differences.
In the schizophrenia group, the ICC’s were more variable: PhysAnh = .67; ImpNon = .66; PerAb = .32; MagId = .53. While the patients’ ICC’s for PhysAnh and ImpNon are somewhat lower than controls they are still quite substantial, with about two-thirds of their variance reflecting stable between-subject differences. About half of the variance in MagId scores reflected between-subjects variance, which is also substantial albeit lower than controls. However, most of the variance in PerAb reflected within-subject changes, though its ICC is larger than most of those found for the BPRS subscales reported above.
Group differences on the Psychosis Proneness scales
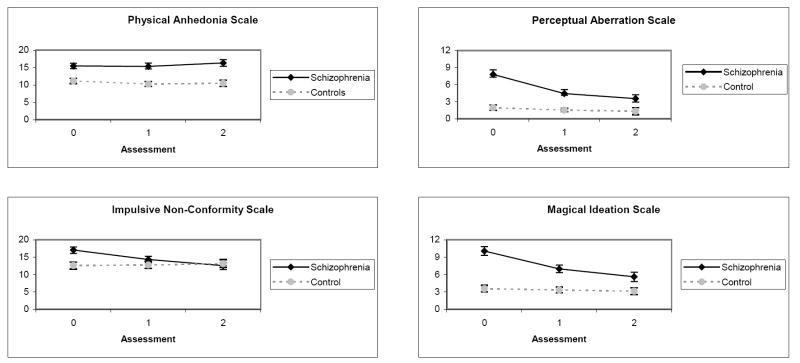
Group means on the Psychosis Proneness scales are presented graphically in Figure 2. Baseline differences and longitudinal changes in mean scores were evaluated with a series of conditional models that used each of the Psychosis Proneness scales as an outcome variable, a Time factor (Assessment 0, 1, 2) as a level-1 predictor, and Group as a level-2 predictor. In addition, a Group X Time interaction term was included in the models to evaluate whether group membership was associated with different slopes on the Psychosis Proneness scales.
Figure 2.
Means Scores On Psychosis Proneness Scales Across All Assessments in the Schizophrenia and Control Groups
Note: Error bars reflect standard errors.
As shown in Table 3, the group effect was significant for each scale, indicating that patients had higher mean scores than controls at Assessment 0 on all four scales. For PhysAnh, neither the Time nor the Group X Time factors were significant, indicating that the between group differences at Assessment 0 remained stable across the subsequent assessments. This pattern, in conjunction with the Scale’s relatively high ICC’s for both groups, is characteristic of a stable vulnerability indicator.
Table 3.
Multi-level Modeling Analyses of Group Differences on the Psychosis Proneness Scales
| Psychosis Proneness Scale | Parameter | Coefficient | SE | t |
|---|---|---|---|---|
| Physical Anhedonia | Intercept (β00) | 11.00 | .70 | 15.66**** |
| Time (β10) | -.43 | .29 | -1.45 | |
| Group (β01) | 4.37 | 1.10 | 3.98**** | |
| Group X Time (β11) | .73 | .51 | 1.42 | |
| Impulsive Non-Conformity | Intercept (β00) | 12.54 | .98 | 12.76**** |
| Time (β10) | .35 | .31 | 1.13 | |
| Group (β01) | 4.29 | 1.32 | 3.25*** | |
| Group X Time (β11) | -2.31 | .51 | -4.54**** | |
| Perceptual Aberration | Intercept (β00) | 1.90 | .41 | 4.67**** |
| Time (β10) | -.24 | .17 | -1.36 | |
| Group (β01) | 5.48 | .87 | 6.28**** | |
| Group X Time (β11) | -1.77 | .51 | -3.49**** | |
| Magical Ideation | Intercept (β00) | 3.56 | .53 | 6.71**** |
| Time (β10) | -.17 | .16 | -1.05 | |
| Group (β01) | 6.18 | .89 | 6.95**** | |
| Group X Time (β11) | -1.88 | .41 | -4.62**** |
Notes: Degrees of freedom: level-1 = 351, level-2 = 124. The full model used in these analyses was: Psychosis Proneness Scale = β00 + β10 [Time] + β01 [Group] + β11 [Group X Time] + u0 + r. β00 indicates the grand mean on the Psychosis Proneness scale at Occasion 0; β10 indicates, within subjects, the extent to which scores change from Occasion 0 to Occasions 1 and 2; β01 indicates the degree to which the mean score in the patient group differs from the mean score in the control group at Occasion 0; The interaction term, β11, indicates the extent to which mean differences between the groups differ across occasions. Level-1 and level-2 predictors were uncentered.
p < .005;
p < .001.
In contrast, the Time and the Group X Time factors were significant for the remaining scales, indicating that scores in the patient group significantly decreased while scores in the control group remained stable across the subsequent assessments. For ImpNon, the patient group did not significantly differ from controls by Assessment 2 (independent samples t-test at Assessment 2 (df = 104) = .40, p > .05), a pattern that is characteristic of an episode indicator. Despite significantly decreasing over time, patients continued to have higher scores than controls on MagId (t [104] = 2.37, p < .05) and PerAb (t[104] = 2.40, p < .05) at Assessment 2. This pattern is characteristic of mediating vulnerability factors.
Covariation of Psychosis Proneness scales and symptoms in the schizophrenia group
The final stage of MLM analyses assessed whether scores on the Psychosis Proneness scales significantly covaried with symptoms at within-subject and between-subject levels in the patient group. BPRS subscales were used as outcome variables with each Psychosis Proneness scale simultaneously entered as a level-1 and a level-2 predictor variable. At the within-subject level (level-1), this addresses the question, “Do scores on the Psychosis Proneness Scales significantly covary with BPRS symptoms from occasion-to-occasion?” At the between-subjects level (level-2), this addresses the question, “Are patients’ overall mean scores across all three assessments on the Psychosis Proneness scales associated with overall mean scores on the BPRS scales?”
As shown in Table 4, the Psychosis Proneness scales differed in their patterns of covariation with symptoms at the within-subject level. PhysAnh showed a selective pattern of covariation with BPRS Anergia. In contrast, the ImpNon, PerAb, and MagId significantly covaried with BPRS Total Scores, Thinking Disturbance, and Hostility ratings, and, to a lesser extent, with Anxiety/Depression and Anergia ratings. Thus, these three scales were more sensitive to changes in psychotic and general psychiatric symptoms from occasion to occasion than PhysAnh.
Table 4.
Multilevel Modeling Analyses of Within-Subject Relationships for the Psychosis Proneness Scales and BPRS Subscales Within the Schizophrenia Group
| BPRS Scale | Physical Anhedonia
|
Impulsive Non-Conformity
|
Perceptual Aberration
|
Magical Ideation
|
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | t | Coefficient | t | Coefficient | t | Coefficient | t | |
| Total BPRS | .23 | 1.21 | .52 | 2.66* | .65 | 3.91**** | .79 | 3.97**** |
| Thought Disturbance | -.01 | -.48 | .05 | 2.45* | .05 | 2.99*** | .07 | 3.16*** |
| Anxiety/Depression | .02 | .96 | .03 | 1.90+ | .05 | 2.96*** | .06 | 3.18*** |
| Anergia | .04 | 3.11*** | -.01 | -1.14 | .04 | 2.74* | .03 | 1.66 |
| Hostility | .001 | .08 | .05 | 3.17** | .05 | 4.40*** | .05 | 3.80**** |
| Activation | .01 | 1.00 | -.001 | -.14 | -.01 | -.81 | -.01 | -.57 |
Note: Degrees of freedom for Level-1 predictors = 194. Each BPRS scale was included as an outcome variable and each Psychosis Proneness scale was simultaneously included as a level-1 and a level-2 predictor variable (e.g., BPRS Total = β00 + β10 [PhysAnh at each occasion] + β01 [PhysAnh mean across occasions] + r0 + e). The level-1 coefficients reported in this table (β10) represent the within-subject relationship between the changes on the Psychosis Proneness Scales and changes on the BPRS Subscales across occasions. Group mean centering was used for level-1 predictors.
p < .05,
p < .01,
p < .005;
p < .001;
p < .10.
As shown in Table 5, Psychosis Proneness scales showed only one significant relationship with the BPRS subscales at the between-subjects level (i.e., means across assessments). Higher mean MagID scores were associated with higher mean scores on the BPRS Thought Disturbance scale. The other Psychosis Proneness scales showed only a few trend-level relationships with symptoms. Overall, mean Psychosis Proneness scale scores did not demonstrate selective patterns of association with different types of mean symptom levels, after accounting for the effects of within-subject variance1.
Table 5.
Multilevel Modeling Analyses of Between-Subject Relationships for the Psychosis Proneness Scales and BPRS Subscales within the Schizophrenia Group
| BPRS Scale | Physical Anhedonia
|
Impulsive Non-Conformity
|
Perceptual Aberration
|
Magical Ideation
|
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | t | Coefficient | T | Coefficient | t | Coefficient | t | |
| Total BPRS | .16 | 1.46 | .11 | .98 | .15 | .97 | .16 | 1.12 |
| Thought Disturbance | -.01 | -.89 | .02 | 1.65 | .02 | 1.66 | .04 | 2.91*** |
| Anxiety/Depression | .20 | 1.80+ | .01 | .70 | .01 | .71 | .001 | .05 |
| Anergia | .01 | 1.22 | -.02 | -1.41 | -.02 | -1.51 | -.02 | -.97 |
| Hostility | .01 | 1.39 | .01 | 1.33 | .01 | .74 | .01 | .93 |
| Activation | .01 | 1.70+ | .01 | 1.76+ | .02 | 1.75+ | .01 | .94 |
Notes: Degrees of freedom for level-2 predictors = 70. Each BPRS scale was included as an outcome variable and each Psychosis Proneness scale was simultaneously included as a level-1 and a level-2 predictor variable (e.g., BPRS Total = β00 + β10 [PhysAnh at each occasion] + β01 [PhysAnh mean across occasions] + r0 + e). The level-2 coefficients reported in this table (β01) reflect the relationship between mean levels on the BPRS scales across occasion and mean levels on each of the Psychosis Proneness scales across occasions. Level-2 predictors were grand mean centered.
p < .005;
p < .10.
Psychosis Proneness scale scores across BPRS-defined psychotic and remitted states
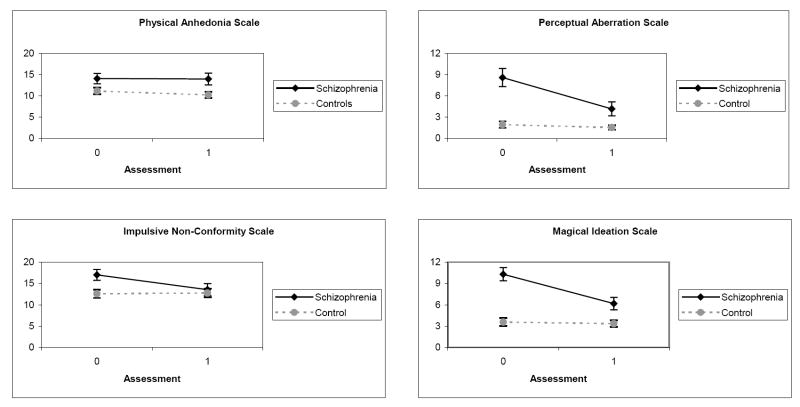
The second phase of analyses sought to corroborate the above findings by examining the stability of Psychosis Proneness scale scores from Assessment 0 to Assessment 1 in a subset of patients who achieved full clinical remission by Assessment 1. According to the criteria described above, 35 patients experienced a psychotic state at Assessment 0 followed by symptom remission at Assessment 1. There were no significant differences on any demographic characteristics between this subset of 35 patients and the remaining 37 patients or the control group.
Group means for each of the Psychosis Proneness scales are presented graphically in Figure 3. For PhysAnh, there was a significant main effect for Group, F(1,85) = 6.39, p < .05, while the Assessment and Group X Assessment interaction effects were not significant (p’s > .25). Patients showed similar elevations on PhysAnh compared to controls across both assessments, a pattern consistent with a stable vulnerability indicator.
Figure 3.
Mean Scores on Psychosis Proneness Scales Across Assessments 1 and 2 in the Subset of Schizophrenia Patients (n = 35) Who Achieved Clinical Remission and Controls
Note: Error bars reflect standard errors.
For ImpNon, there were significant effects for Assessment, F = 8.54, p < .005, and the Group X Assessment interaction, F = 10.83, p < .001, while the main effect for Group was not significant, F = 2.40, p > .05, (all df = 1,85). Patients reported higher ImpNon than controls at Assessment 0 which decreased to normal levels by Assessment 1, consistent with an episode indicator.
The patterns of results for PerAb and MagId were similar to each other. For PerAb, there were significant effects for Group, F = 28.32, p < .001, Assessment, F = 16.75, p < .001, and the interaction of Group X Assessment, F = 11.38, p < .001 (all df = 1,85). For MagId, there were also significant effects for Group, F = 28.32, p < .001, Assessment, F = 25.36, p < .001, and the interaction of Group X Assessment, F = 20.23, p < .001 (all df = 1,85). On both scales, patients reported higher scores than controls at both assessments, while scores within the schizophrenia group were significantly higher during the symptomatic period at Assessment 0 than the remitted period at Assessment 1, patterns that are characteristic of mediating vulnerability factors.
Discussion
Across two complementary data analytic approaches, results provided clear and consistent evidence that PhysAnh scores in recent-onset schizophrenia patients demonstrate characteristics of a stable vulnerability indicator, while PerAb and MagId scores reflect mediating vulnerability factors. ImpNon demonstrated characteristics of an episode indicator rather than a vulnerability indicator. These different patterns are highly consistent with studies in other clinical and high-risk samples, and provide key evidence for the validity of PhysAnh, PerAb, and MagId as indicators of vulnerability to schizophrenia. The differences in their longitudinal associations with psychotic symptoms suggest that these characteristics play different roles in the developmental processes that lead to psychotic symptoms.
The stable elevations in PhysAnh reported by the recent-onset patients converge with findings from chronically ill patients (Blanchard et al. 2001; Herbener and Harrow 2002; Herbener et al. 2005) to suggest that elevated anhedonia is not merely attributable to factors associated with chronicity, such as prolonged exposure to antipsychotic medications or environmental deprivation. In addition, PhysAnh demonstrated minimal covariation with clinical symptoms and remained persistently elevated even in a subsample of patients who achieved a fully remitted state, consistent with prior reports that PhysAnh is relatively independent of symptom state (Blanchard et al. 1994; Katsanis et al. 1992). Thus, anhedonia appears to be insensitive to clinical state fluctuations and present throughout the course of illness, similar to certain neurocognitive and neurophysiological deficits that have been identified as candidate endophenotypes for genetic studies of vulnerability to schizophrenia (Gur et al. 2007).
Several additional lines of evidence from various high-risk populations are consistent with the notion that anhedonia demonstrates the expected characteristics of an intermediate endophenotype (Gottesman and Gould 2003). Anhedonia measured by the Psychosis Proneness scales and conceptually related scales is a substantially heritable trait (Berenbaum and McGrew 1993; Berenbaum et al. 1990; Kendler et al. 1991; Linney 2003; MacDonald et al. 2001), is elevated in subjects with schizotypal personality disorder (Camisa et al. 2005), and specifically predicts the later development of schizophrenia spectrum disorders in psychometric high-risk samples (Gooding et al. 2005; Kwapil 1998). In addition, elevated anhedonia is frequently reported in patients’ biological relatives (Clementz et al. 1991; Franke et al. 1993; Grove et al. 1991; Katsanis et al. 1990; Kendler et al. 1996).
Although there is growing support for the notion that anhedonia and other emotion processing deficits reflect an intermediate endophenotype (Delawalla et al. 2006; Gur et al. 2007), several issues require additional research. Anhedonia and reward processing are complex, multifaceted constructs and further specification of the precise nature of the hedonic deficit in schizophrenia is needed. For example, it is not yet clear whether anhedonia in schizophrenia involves the appetitive or consummatory components of hedonic experience (Horan et al. 2006c). In addition, not all schizophrenia patients experience substantial anhedonia. In the current sample, about half of the patients reported PhysAnh scores that were less than one standard deviation above the control group’s mean at each assessment (52.8%, 52.2%, and 50.0%, respectively). It thus remains unclear if anhedonia is associated with vulnerability to a particular subtype of schizophrenia (Blanchard et al. 2005; Schurhoff et al. 2003).
In contrast to the patients’ stable elevations on the PhysAnh, the PerAb and MagId demonstrated the characteristics of mediating vulnerability indicators. That is, patients demonstrated abnormalities during symptomatic and asymptomatic states, but these were more deviant during symptomatic periods and significantly covaried with general psychotic, mood, and hostility symptoms across assessments (also see (Katsanis et al. 1992). The key difference between stable and mediating vulnerability indicators is that the latter are believed to be more proximal in the chain of causal events that lead to symptom exacerbations than are stable vulnerability indicators. Mediating vulnerability indicators would be expected to worsen somewhat earlier than frank psychotic symptom exacerbations. For example, increases in non-specific electrodermal activity precede clinical symptom exacerbations (Hazlett et al. 1997). Thus, the current findings may help to clarify the roles of MagId and PerAb in the development of psychotic symptoms.
Evidence that elevations on the PerAb and MagId scales are associated with genetic vulnerability to schizophrenia per se is not as strong as for anhedonia. The traits measured by these and conceptually related scales appear to be substantially heritable (Berenbaum and McGrew 1993; Jang et al. 2005; Linney 2003) and these traits do appear in schizotypal personality disorder (Camisa et al. 2005). However, while elevations on these scales are associated with later development of psychotic disorders in psychometric high-risk subjects, they also predict various mood and substance use disorders (Chapman et al., 1994; Gooding et al., 2005). In addition, these scales are typically not elevated in patients’ unaffected relatives (Clementz et al. 1991; Franke et al. 1993; Katsanis et al. 1990; Kendler et al. 1996); but see (Lenzenweger and Loranger 1989)). Thus, while some studies suggest that MagId and PerAb are associated with vulnerability to psychosis, it is less clear whether these traits are specifically related to vulnerability for schizophrenia.
This first longitudinal study of the Psychosis Proneness Scales in recent-onset schizophrenia has several methodological advantages. Assessing the stability of personality characteristics near the time of illness onset allows stronger inferences about the validity of vulnerability indicators than chronically ill patients (Nuechterlein et al. 1992). All patients received a standardized psychosocial and pharmacological treatment package, which included an injectable form of antipsychotic medication that facilitated treatment compliance. In addition, strict operational criteria were used for defining symptomatic and remitted states in the analyses that focused on two specific clinical states.
This study is limited by uncertainty about whether the first generation antipsychotic medication that patients were taking influenced their self-reports on the Psychosis Proneness Scales. Typical antipsychotics like fluphenazine have relatively high dopamine D2 receptor occupancy (Kapur and Mam 2003) and dopamine plays a critical role in certain aspects of hedonic experience and reward processing (Berridge and Robinson 1998; Bressan and Crippa 2005; Voruganti and Awad 2004). However, as noted above, the medicated patients in this study did not invariably report elevated anhedonia. Research in non-medicated high-risk populations also supports the notion that anhedonia is a core feature of vulnerability to schizophrenia. Furthermore, elevated anhedonia and abnormal neural reward processing have been reported in patients taking second generation antipsychotics with lower D2 receptor affinities (e.g., (Horan et al. 2006b)) and in unmedicated patients (Juckel et al. 2006; Zhang et al. 2001). Nevertheless, the potential effects of antipsychotic medications on patients’ reports of anhedonia cannot be definitively determined in the absence of random assignment to different treatment conditions. Despite this limitation, we believe the current findings provide critical evidence for the validity of PhysAnh, MagId and PerAb as vulnerability indicators.
Acknowledgments
This research was supported by Institutional NRSA MH14584 (P.I.: Keith H. Nuechterlein, PhD) and research grants MH066286-05, MH37705 (P.I.: Keith H. Nuechterlein, PhD) and MH30911 (P.I.: Robert P. Liberman, M.D.) from the National Institute of Mental Health. The authors wish to thank George Bartzokis, M.D., Craig Childress, M.A., Rosemary Collier, M.S., Rhonda Daily, B.A., David Fogelson, M.D., Sally Friedlob, M.S.W., Debbie Gioia-Hasick, M.S.W., Michael Gitlin, M.D., Sandy Rappe, M.S.W., Margie Stratton, M.A., and the patients of the Aftercare Research Program for their contributions to this project.
Footnotes
In light of previous findings that positive and negative schizotypy traits are typically not significantly related to each other in non-clinical samples (Horan et al. 2004), we examined whether similar relationships exist in schizophrenia patients. In these analyses, each Psychosis Proneness Scale was used as an outcome variable with separate models that included each of the other three scales as both level-1 and level-2 predictors (e.g., PhysAnh = β00 + β01 (PerAb at each assessment) + β10 (PerAb mean across assessments) + r0 + e). The pattern of results was very clear and will only be summarized (full results available upon request). There were no significant or trend-level associations between PhysAnh and any of the other Psychosis Proneness scales at either the within- or between-subject level. In contrast, ImpNon, PerAb, and MagId were each highly and significantly associated with each other at both the within- and between-subject levels. Thus, PhysAnh demonstrated minimal associations with the other Psychosis Proneness scales.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association, AP. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed., rev. Washington, DC: Author; 1987. [Google Scholar]
- Bailey B, West K, Widiger TA, Freiman K. The convergent and discriminant validity of the Chapman scales. Journal of Personality Assessment. 1993;61(1):121–135. doi: 10.1207/s15327752jpa6101_9. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, McGrew J. Familial resemblance of schizotypic traits. Psychological Medicine. 1993;23(2):327–333. doi: 10.1017/s0033291700028427. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF, Gottesman II. Hedonic capacity in schizophrenics and their twins. Psychological Medicine. 1990;20(2):367–74. doi: 10.1017/s0033291700017682. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Bellack AS, Mueser KT. Affective and social-behavioral correlates of physical and social anhedonia in schizophrenia. Journal of Abnormal Psychology. 1994;103(4):719–728. doi: 10.1037//0021-843x.103.4.719. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Brown SA. Diagnostic differences in social anhedonia: a longitudinal study of schizophrenia and major depressive disorder. Journal of Abnormal Psychology. 2001;110(3):363–71. doi: 10.1037//0021-843x.110.3.363. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: Is there a distinct subtype of negative symptom schizophrenia? Schizophrenia Research. 2005;77:151–165. doi: 10.1016/j.schres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin. 1998;24(3):413–24. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour - review of data from preclinical research. Acta Psychiatrica Scandinavica. 2005;111(Suppl 427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Camisa KM, Bockbrader MA, Lysaker P, Rae LL, Brenner CA, O’Donnell BF. Personality traits in schizophrenia and related personality disorders. Psychiatry Research. 2005;133(1):23–33. doi: 10.1016/j.psychres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal Personality. New York: Cambridge University Press; 1995. pp. 79–109. [Google Scholar]
- Chapman LJ, Chapman JP. Revised Physical Anhedonia Scale. 1978a. Unpublished test. [Google Scholar]
- Chapman LJ, Chapman JP. Infrequency Scale. Madison, WI: 1983. Unpublished test. [Google Scholar]
- Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. Journal of Abnormal Psychology. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Numbers JS, Edell WS, Carpenter BN, Beckfield D. Impulsive nonconformity as a trait contributing the the prediction of psychotic-like and schizotypal symptoms. Journal of Nervous and Mental Disease. 1984;172:681–691. doi: 10.1097/00005053-198411000-00007. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–82. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. Journal of Abnormal Psychology. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, R ML. Perceptual Aberration Scale. University of Wisconsin; Madison: 1978b. Unpublished manuscript. [Google Scholar]
- Clementz BA, Grove WM, Katsanis J, Iacono WG. Psychometric detection of schizotypy: Perceptual aberration and physical anhedonia in relatives of schizophrenics. Journal of Abnormal Psychology. 1991;100:607–612. doi: 10.1037//0021-843x.100.4.607. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher-Eastep J, Thomason ES, Hanewinkel M, Thompson PA, Csernansky JG. Factors Mediating Cognitive Deficits and Psychopathology Among Siblings of Individuals with Schizophrenia. Schizophrenia Bulletin. 2006;32(3):525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Edell WS. The psychometric measurement of schizotypy using the Wisconsin Scales of Psychosis Proneness. In: Miller GA, editor. The Behavioral High-Risk Paradigm in Psychopathology. New York: Springer-Verlag; 1995. pp. 3–46. [Google Scholar]
- Fernandes LOL, Miller GA. Compromised performance and abnormal psychophsiology associated with the Wisconsin Scales of Psychosis Proneness. In: Miller GA, editor. The Behavioral High-Risk Paradigm in Psychopathology. New York: Springer-Verlag; 1995. pp. 47–87. [Google Scholar]
- Franke P, Maier W, Hardt J, Hain C. Cognitive functioning and anhedonia in subjects at risk for schizophrenia. Schizophrenia Research. 1993;10(1):77–84. doi: 10.1016/0920-9964(93)90079-x. [DOI] [PubMed] [Google Scholar]
- George L, Neufeld RW. Magical ideation and schizophrenia. Journal of Consulting and Clinical Psychology. 1987;55(5):778–779. doi: 10.1037//0022-006x.55.5.778. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Tallent KA, Matts CW. Clinical status of at-risk individuals 5 years later: Further validation of the psychometric high-risk strategy. Journal of Abnormal Psychology. 2005;114(1):170–175. doi: 10.1037/0021-843X.114.1.170. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grove WM, Lebow BS, Clementz BA, Cerri A, Medus C. Familial prevalence and coaggregation of schizotypy indeicators: A multitrait family study. Journal of Abnormal Psychology. 1991;100:115–121. doi: 10.1037//0021-843x.100.2.115. [DOI] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The Consortium on the Genetics of Schizophrenia (COGS): Neurocognitive Endophenotypes. Schizophrenia Bulletin. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Hathaway SR, McKinley JC. Minesota Multiphasic Personality Inventory Manual, Revised. New York: Psychological Corporation; 1967. [Google Scholar]
- Hazlett H, Dawson ME, Schell AM, Nuechterlein KH. Electrodermal activity as a prodromal sign in schizophrenia. Biological Psychiatry. 1997;41(1):111–113. doi: 10.1016/s0006-3223(96)00351-4. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M. The course of anhedonia during 10 years of schizophrenic illness. Journal of Abnormal Psychology. 2002;111(2):237–48. [PubMed] [Google Scholar]
- Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophrenia Research. 2005;75(1):97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Gangestad SW, Kwapil TR. The psychometric detection of schizotypy: Do putative schizotypy indicators identify the same latent class? Journal of Abnormal Psychology. 2004;113:339–357. doi: 10.1037/0021-843X.113.3.339. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Kring AM. Anhedonia in schizophrenia: A review of assessment strategies. Schizophrenia Bulletin. 2006a;32(2):359–373. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol. 2006b;115(3):496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophrenia Bulletin. 2006c;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Woodward TS, Lang D, Honer WG, Livesley WJ. The genetic and environmental basis of the relationship between schizotypy and personality: A twin study. Journal of Nervous and Mental Disease. 2005;193:153–159. doi: 10.1097/01.nmd.0000154842.26600.bd. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mam D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Beiser M. Anhedonia and perceptual aberration in first-episode psychotic patients and their relatives. Journal of Abnormal Psychology. 1990;99(2):202–206. doi: 10.1037//0021-843x.99.2.202. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, Beiser M, Lacey L. Clinical correlates of anhedonia and perceptual aberration in first-episode patients with schizophrenia and affective disorder. Journal of Abnormal Psychology. 1992;101(1):184–191. doi: 10.1037//0021-843x.101.1.184. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ochs AL, Gorman AM, Hewitt JK, Ross DE, Mirsky AF. The structure of schizotypy: A pilot multitrait twin study. Psychiatry Research. 1991;36:19–36. doi: 10.1016/0165-1781(91)90114-5. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thacker L, Walsh D. Self-report measures of schizotypy as indices of familial vulnerability to schizophrenia. Schizophrenia Bulletin. 1996;22(3):511–20. doi: 10.1093/schbul/22.3.511. [DOI] [PubMed] [Google Scholar]
- Kwapil TR. Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. Journal of Abnormal Psychology. 1998;107(4):558–65. doi: 10.1037//0021-843x.107.4.558. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, Loranger AW. Detection of familial schizophrenia using a psychometric measure of schizotypy. Archives of General Psychiatry. 1989;46(10):902–907. doi: 10.1001/archpsyc.1989.01810100044008. [DOI] [PubMed] [Google Scholar]
- Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham P. A quantitative genetic analysis of schizotypal personality traits. Psychological Medicine. 2003;33(5):803–816. doi: 10.1017/s0033291703007906. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the expanded Brief Psychiatric Rating Scale. Schizophrenia Bulletin. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: a community-based twin study. Schizophrenia Bulletin. 2001;27(1):47–58. doi: 10.1093/oxfordjournals.schbul.a006859. [DOI] [PubMed] [Google Scholar]
- Meehl P. Schizotaxia, schizotypy, schizophrenia. American Psychology. 1962;17:827–838. [Google Scholar]
- Nezlek JB. Multilevel random coefficient analyses of event- and interval-contingent data in social and personality psychology research. Personality and Social Psychology Bulletin. 2001;27(7):771–785. [Google Scholar]
- Nuechterlein KH. Methodological considerations in the search for indicators of vulnerability to severe psychopathology. In: Rohrbaugh JW, Parasuraman R, Johnson R, editors. Event-Related Brain Potentials: Basic Issues and Applications. New York: Oxford University Press; 1990. pp. 397–433. [Google Scholar]
- Nuechterlein KH, Dawson ME. A heuristic Vulnerability/Stress model of schizophrenic episodes. Schizophrenia Bulletin. 1984;10(2):300–312. doi: 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, S KS, Yee CM, Mintz J. Developmental processes in schizophrenic disorders: Longitudinal studies of vulnerability and stress. Schizophrenia Bulletin. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Miklowitz DJ, Ventura J, Gitlin MJ, Stoddard M, Lukoff D. Classifying episodes in schizophrenia and bipolar disorder: Criteria for relapse and remission applied to recent-onset samples. Psychiatry Research. 2006;144:153–166. doi: 10.1016/j.psychres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong Y, Congdon RT. HLM 5: Hierarchical Linear and Nonlinear Modeling. Chicago: Scientific Software International; 2000. [Google Scholar]
- Reise SP, Duan N. Multilevel modeling and its application to counseling psychology research. The Counseling Psychologist. 1999;27:528–551. [Google Scholar]
- Schurhoff F, Szoke A, Bellivier F, Turcas C, Villemur M, Tignol J, Rouillon F, Leboyer M. Anhedonia in schizophrenia: a distinct familial subtype? Schizophrenia Research. 2003;61(1):59–66. doi: 10.1016/s0920-9964(02)00237-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: Rationale and reliability. Archives of General Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Thaker G, Moran M, Adami H, Cassady S. Psychosis Proneness Scales in schizophrenia spectrum personality disorders: Familial vs. nonfamilial samples. Psychiatry Research. 1993;46(1):47–57. doi: 10.1016/0165-1781(93)90007-4. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance on the Brief Psychiatric Rating Scale: the “drift busters”. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Voruganti L, Awad AG. Neuroleptic dysphoria: towards a new synthesis. Psychopharmacology. 2004;171:121–132. doi: 10.1007/s00213-003-1648-y. [DOI] [PubMed] [Google Scholar]
- Wing JK, Cooper JE, Sartorius N. The Measurement and Classification of Psychiatric Symptoms: An Instruction Manual for the PSE and Catego Program. London: Cambridge University Press; 1974. [Google Scholar]
- Zhang ZJ, Peet M, Ramchand CN, Shah S, Reynolds GP. Plasma homovanillic acid in untreated schizophrenia--relationship with symptomatology and sex. Journal of Psychiatric Research. 2001;35(1):23–28. doi: 10.1016/s0022-3956(01)00008-5. [DOI] [PubMed] [Google Scholar]