Abstract
CD4+CD25+ regulatory T lymphocytes play a crucial role in inhibition of autoimmune pathology. In accordance with this physiological role, it is now well established that the repertoire of these lymphocytes is strongly enriched in autospecific cells. However, despite extensive investigation, the thymic mechanisms involved in development of regulatory T cells remain incompletely defined. To address the issue of selection of regulatory T cell-precursors in mice with a naturally diverse TCR-repertoire, we have analyzed development of superantigen-specific regulatory T cells in hematopoietic chimeras in which endogenous superantigens are exclusively presented by thymic epithelial cells. Our results demonstrate that recognition of agonist ligands expressed by thymic epithelium does not lead to deletion but substantially enhances development of mature regulatory T cells. Interestingly, also development of a small subpopulation of CD25-expressing T cells lacking Foxp3, thought to be autospecific, is enhanced by expression of agonist ligand on thymic epithelium. Based on quantitative arguments, we propose that commitment to the regulatory T cell lineage is not dictated by the specificity of the precursor, but that recognition of agonist ligand expressed by thymic epithelium substantially enhances their positive selection.
Keywords: Animals; Antigen Presentation; genetics; Antigens; CD4; biosynthesis; CD4-Positive T-Lymphocytes; immunology; metabolism; Cell Differentiation; genetics; immunology; Epithelial Cells; immunology; metabolism; Epitopes; T-Lymphocyte; biosynthesis; immunology; Forkhead Transcription Factors; biosynthesis; Hematopoietic Stem Cells; cytology; immunology; metabolism; Ligands; Mice; Mice; Inbred C57BL; Mice; Inbred DBA; Mice, Knockout; Radiation Chimera; Receptors; Antigen; T-Cell; biosynthesis; genetics; Receptors; Interleukin-2; biosynthesis; Superantigens; biosynthesis; immunology; metabolism; T-Lymphocytes; Regulatory; cytology; immunology; metabolism; Thymus Gland; cytology; immunology; metabolism
Keywords: T cells, Cell differentiation, Thymus, Repertoire development, Tolerance, Suppression, Anergy
Introduction
T-lymphocyte tolerance to self-antigens is induced in the thymus during the process of negative selection (1). Despite the quantitatively impressive nature of this process (2), significant numbers of auto-specific T cells migrate to the periphery (3). In the periphery, autospecific T cells are kept silent by recessive (induction of apoptosis or anergy) and dominant tolerance mechanisms (4). Dominant or “active” tolerance is assured by regulatory/suppressor T lymphocytes. The best characterized regulatory T cell (Treg) subset consists of CD4+ T cells expressing high levels of CD25, GITR, CTLA-4, and the forkhead/winged helix transcription factor Foxp3 (5–7). While for practical purposes (i.e. isolation of Treg) the best marker is CD25, Foxp3 expression appears to correlate best with regulatory function (8). CD4+CD25+ Treg play a major role in the prevention of autoimmunity (5, 6) and inflammatory bowel disease (9), in regulating immunity to viral and parasite infections (10), in maintenance of maternal tolerance to the fetus (11), and in inhibition of anti-tumor immunity (12).
Interestingly, the normally diverse CD4+CD25+ regulatory thymocyte population selected on naturally expressed ligands, has higher avidity for self than CD4+CD25− cells (13), resulting in a peripheral Treg repertoire highly enriched in self-reactive cells (14–16). These observations raise important questions concerning the selection of these cells in the thymus. In this organ, CD4+CD25+ Treg are positively selected by cortical epithelial cells, as are conventional CD4+ lymphocytes (17). Surprisingly, Treg precursors have been shown to be susceptible to thymic negative selection (14, 17, 18). However, while autospecific Treg precursors are negatively selected by APC of bone marrow origin (14), they appear relatively resistant to negative selection induced by thymic epithelium (TE, refs. (13, 19). Therefore, TE plays an important role in development of dominant tolerance, as previously postulated based on the observation that TE can induce dominant transplantation tolerance (20).
The precise role of positive selection in development of Treg remains unclear. It has been proposed that interaction with agonist ligands directs developing thymocytes to the Treg lineage. However, studies with mice doubly transgenic for TCR and agonist ligand have led to conflicting results. In some cases, evidence of increased selection of CD4+CD25+ Treg by agonist ligand was observed (21–25). In other TCR/ligand doubly transgenic mice, the relatively increased percentage of CD4+CD25high Treg has been attributed to deletion of CD25− cells rather than increased development of CD4+CD25+ cells (19). The potential differences reported in the distinct doubly transgenic mice may be due to the particular avidity of the chosen TCR/ligand pair and/or to variations in the expression pattern of the ligand. Therefore, it remains unclear 1) if TCR-mediated signals are involved in Treg lineage choice, and 2) if differences exist between positive selection of conventional and regulatory T lymphocytes.
To gain insight in the mechanisms of thymic Treg differentiation, we analyzed the role of agonist ligands expressed by TE in development of Treg-precursors with a naturally diverse TCR-repertoire. We generated bone-marrow chimeras in which superantigen (sAg)-presentation is limited to TE, and analyzed development of sAg-specific Treg. Our results indicate that agonist ligands considerably enhance positive selection of Treg.
Materials and Methods
Mice
All mice were used at 6 to 10 weeks of age. C57BL/6 and DBA/2 mice were purchased from Janvier (Le Genest St. Isle, France). B10.D2 mice were originally obtained from Harlan France (Gannat, France) and maintained in our SPF animal facilities. C57BL/6 mice deficient in MHC expression (MHC°) because of targeted deletions in the β2-microglobulin (26) and IAβb genes (27) were obtained from the “Centre de Développement des Techniques Avancés- Centre National de la Recherche Scientifique” (Orléans, France) and were maintained in our SPF animal facility. All experiments involving animals were performed in compliance with the relevant laws and institutional guidelines (INSERM; approval no. 31–13) and have been approved by the local ethics committee (Midi-Pyrénees, France; ref MP/01/31/10/03).
Antibodies
The following antibodies were used for phenotypic analysis: FITC-labeled anti-TCR Vβ 3, -4, -5, -6, -14, -17a, PE-labeled anti-CD25, PE-Cy7-labeled anti-CD4 (BD Pharmingen, San José, CA), PE-labeled anti-Foxp3, APC-labeled anti-CD8 and anti-CD25 antibodies (eBioscience, San Diego, CA).
Flow Cytometry
Thymi were homogenized, washed in medium, and resuspended in 2.4G2 (anti-FcγR mAb, (28)) hybridoma supernatant. After incubation of 30 rain on ice, saturating concentrations of antibody were added. 20 minutes later, cells were washed in PBS, 2.5% PCS and 0.02% NaN3. Labeled cells were analyzed using a FACSCalibur and CellQuest software (BD Biosciences, San Jose, CA). Dead cells were excluded using appropriate FSC/SSC gates.
For TCR Vβ analysis, thymi of three mice were pooled, depleted of CD8+ cells by treatment with anti-CD8 mAb 31.M (29) and complement (Saxon Europe, Suffolk, U.K.), followed by Lympholyte-M gradient (Cederlane Laboratories, Hornby, Canada). The efficiency of CDS depletion was verified in every experiment and was routinely >99%.
Bone marrow chimeras
Irradiation bone marrow chimeras were generated by lethally irradiating (8.5 Gy γ) C57B1/6 hosts using a 137Cs source (7 Gy/min). Next day, irradiated mice were reconstituted by i.v. injection of 107 bone marrow cells. Chimeras were kept on antibiotic containing water (0.2% Bactrim, Roche, Basel, Switzerland) for the complete duration of the experiment.
Statistical analysis
Statistical significance of differences between subpopulations were assessed using Student’s t test and is indicated as: ns, not significant (p≥0.05); * p<0.05; ** p<0.01; *** p<0.001.
Results
Superantigens presented by TE enhance differentiation of CD4+CD8−CD25high regulatory T cells
To study the role of naturally expressed agonist ligands in thymic selection of Treg-precursors with a normally diverse TCR-repertoire, we generated irradiation chimeras in which sAg are exclusively presented by TE. As hosts we used (lethally irradiated) DBA/2 mice which present endogenous sAg encoded by mouse mammary tumor viruses 1, 6, 7, 8, 11, and 13. These sAg are high affinity ligands for Vβ3, Vβ5 and Vβ6 while they do not interact with Vβ4 and Vβ14 (30). MHC-deficient (MHC°) bone marrow was used to prevent thymic deletion of Treg precursors induced by APC of hematopoietic origin (14). These MHC°→DBA/2 chimeras were compared to MHC°→C57BL/6 (B6) mice, in which no sAg-mediated thymic deletion is known to occur (30). Controls consisted of B6→B6 and DBA/2→DBA/2 chimeras.
Chimeras were analyzed by flow-cytometry six weeks after reconstitution. The percentage of thymocytes of donor origin was always superior to 99%. In the spleen, more than 99% of T and B lymphocytes were of donor origin. Less than 7% of host CD11c+CD11b− thymic DC remained in the chimeras. Moreover, the ratio of CD8low to CD8high cells among remaining host DC was identical to that found in unmanipulated animals (data not shown). In the thymus of DBA/2→DBA/2 chimeras, significantly higher percentages of CD25high Treg among CD4+CD8− (CD4SP) thymocytes were observed than in B6→B6 chimeras (Figs. 1A and B). This result confirms genetically determined quantitative differences in Treg development, due to thymocyte-intrinsic factors, that we have reported previously (31). Significantly reduced percentages of sAg-specific Vβ3, Vβ5 and Vβ6 expressing CD4SP CD25− and CD25high cells were found in DBA/2→DBA/2 as compared to B6→B6 chimeras (Figs. 1C and D). No difference in percentages of Vβ4 and Vβ14 T cells (which do not react with sAg presented in DBA/2 mice) was observed between the two types of chimeras. These data confirm that Treg precursors are sensitive to thymic deletion, as we and others have previously reported (14, 18).
Figure 1. SAg presented by TE enhance generation of CD4SP CD25high regulatory T cells.

(A) Analysis of CD25-expression by electronically gated CD4SP thymocytes. (B) Total numbers of thymocytes (left panel), percentage of CD4SP cells among total thymocytes (middle panel), and percentage of CD25high cells among CD4SP thymocytes (right panel), in indicated chimeras. (C) Flow-cytometry analysis of TCR Vβ expression by electronically gated CD4SP CD25− and CD25high thymocytes in indicated chimeras. (D) Percentages of CD4SP CD25− and CD25hi thymocytes expressing indicated Vβ in the distinct chimeras. ***, p < 0.001; **, p < 0.01; ns, not significant; Student’s t test. Error bars indicate SD (B6→B6 and DBA/2→DBA/2: n = 3, MHC°→B6 and MHC°→DBA/2: n = 6).
We next analyzed irradiation chimeras in which bone marrow derived cells did not express MHC molecules and therefore could not present sAg. As compared to B6→B6 and DBA/2→DBA/2 chimeras, in MHC°→B6 and MHC°→DBA/2 chimeras significantly increased percentages of CD4SP thymocytes were found (Fig. 1B). These results are due to substantially reduced induction of apoptosis of autospecific cells in these chimeras, as previously reported (2).
Interestingly, two-fold higher percentage of CD25− (but not CD25+) CD4SP Vβ5+ (but not Vβ3, Vβ6, Vβ4, or Vβ14) cells were found in MHC°→B6 than in B6→B6 chimeras (Fig. 1D). These results show that Vβ5 specific deletion of CD25− but not CD25+ thymocytes by bone-marrow derived APC occurs in B6→B6 chimeras.
In contrast to chimeras in which TE and APC express sAg, in MHC°→DBA/2 mice sAg-specific Vβ3+ cells were not deleted and only partial deletion of sAg-specific Vβ5 and Vβ6 CD4SP CD25− cells was observed. These results are consistent with previous work documenting the limited role of thymic (medullary) epithelium in deletion of autospecific precursors (32–34). Control Vβ4+ and Vβ14+ thymocytes were not deleted.
As compared to MHC°→B6 chimeras, in MHC°→DBA/2 mice a substantial increase in the percentage of CD4SP CD25high regulatory thymocytes expressing sAg specific Vβ3, Vβ5, and Vβ6 was observed (Figs. 1C and D). Since in the MHC°→B6 and MHC°→DBA/2 chimeras the total number of thymocytes and the percentage of CD4SP cells were similar, and in MHC°→DBA/2 chimeras the percentage of CD25high Treg among CD4SP cells is increased (Fig. 1B), the increase in percentage corresponded to increase in absolute cell numbers of sAg-specific Treg in MHC°→DBA/2 chimeras. Comparable percentages of Vβ4 and Vβ14 CD4SP CD25high Treg developed in both types of chimeras. These results indicate that natural agonist ligands presented by TE substantially enhance the generation of CD4SP CD25high Treg.
SAg enhance generation of CD4+Foxp3+ Treg and a subpopulation of CD4+Foxp3− thymocytes
CD25 is widely used as marker for regulatory T cells (35). However, it is also expressed on activated conventional CD4+ and CD8+ lymphocytes, and does not identify all regulatory T cells. A better marker for Treg is Foxp3 (7, 8, 36), a forkhead/winged helix transcription factor. We therefore assessed if sAg presented by TE also enhanced differentiation of CD4+Foxp3+ regulatory T cells. As compared to MHC°→B6 chimeras, in MHC°→DBA/2 chimeras substantially increased percentages and numbers of Foxp3+ CD4+ thymocytes expressing sAg specific Vβ3, Vβ5 and Vβ6 (but not control Vβ4 and Vβ14) were observed (Figs. 2A, B, and C). In contrast, sAg-specific Foxp3− CD4SP thymocytes were either partially deleted (Vβ5 and 6) or not affected (Vβ3) by sAg presented by TE (Fig. 2C).
Figure 2. SAg-specific CD4SP Foxp3+ T cell-differentiation is enhanced by endogenous sAg presented by TE.

(A) Analysis of Foxp3-expression by electronically gated CD4SP thymocytes. (B) Percentage of Foxp3-expressing cells among CD4SP thymocytes in indicated chimeras. (C) Percentages of thymocytes expressing indicated Vβ among CD4SP Foxp3− and Foxp3+ cells in indicated chimeras. ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, not significant; Student’s t test. Error bars indicate SD (n≥4).
The MHC° mice we used as bone-marrow donors were on the C57BL/6 background in which very few mouse mammary tumor virus genomes are present (30). Since hematopoietic cells are known to produce sAg, sAg-presentation in MHC° → DBA/2 and DBA/2 → DBA/2 chimeras is not only qualitatively but also quantitatively different. One could therefore argue that enhanced positive selection of sAg-specific regulatory T cells in MHC° → DBA/2 chimeras might be due to the lower overall avidity of the interaction of thymocytes with stromal cells, independently of the nature (DC or TEC) of the presenting cell. To assess this possibility we generated MHC° → DBA/2 and B10.D2 → DBA/2 chimeras in which the expression-levels of sAg were the same but the nature of the cells presenting them differed. As shown in Fig. 2C, in contrast to MHC° → DBA/2 chimeras, in B10.D2 → DBA/2 chimeras sAg-specific Vβ3+, Vβ5+, and Vβ6+ Foxp3− CD4SP thymocytes were deleted. Importantly, also substantially less sAg-specific Foxp3+ CD4SP thymocytes were observed in B10.D2 → DBA/2 than in MHC°→ DBA/2 chimeras. These data unequivocally show that the nature of the stromal cell-type presenting the sAg determines the outcome of selection in our experimental model: DC induce deletion and TEC induce positive selection of regulatory T cell precursors.
Foxp3+ CD4SP thymocytes express varying levels of CD25 (Fig. 3A), as previously reported (37). We therefore next assessed if sAg differentially enhanced development of CD25−, CD25int, or CD25high Foxp3+ thymocytes (Fig. 3B). SAg presented by TE substantially enhanced differentiation of all three Foxp3+ subpopulations (Fig. 3B, compare MHC°→DBA/2 with MHC°→B6 chimeras).
Figure 3. TE-expressed sAg differentially affect CD4+ T cell development.
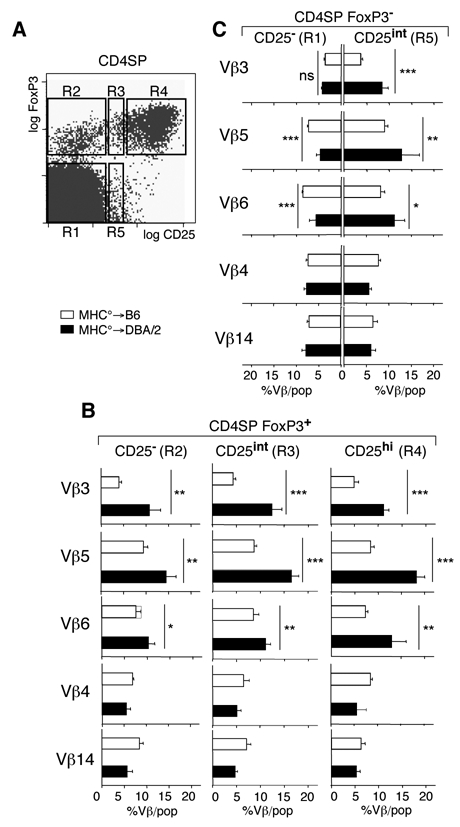
(A) Electronically gated CD4SP thymocytes were subdivided into five different populations according to their CD25 and Foxp3 expression levels. (B). Percentages of thymocytes expressing indicated Vβ among the CD4SP Foxp3+ thymocyte subpopulations indicated in the distinct chimeras. (C) Percentages of thymocytes expressing indicated Vβ among CD25− and CD25+ CD4SP Foxp3− thymocyte subpopulations in the distinct chimeras. ***, p<0.001; **, p<0.01; *, p < 0.05; ns, not significant; Student’s t test. Error bars indicate SD (n≥4).
We also analyzed selection of a small population of CD4SP CD25int Foxp3− thymocytes (Figs. 3A and C). These cells may be thymic precursors for recently described peripheral T lymphocytes with the same phenotype (8, 38). Interestingly, as compared to MHC°→B6 chimeras, in MHC°→DBA/2 chimeras a statistically significant increase in the percentage of CD4SP CD25int Foxp3− thymocytes expressing sAg reactive Vβ3, Vβ5 and Vβ6 (but not control Vβ4 and Vβ14) was observed (Fig. 3C). The CD4SP CD25int Foxp3− population therefore appears to be enriched in self-antigen specific T lymphocytes, as previously suggested (8, 38).
SAg do not enhance positive selection of precursors for conventional T cells
The presented data indicate that generation of regulatory T cells is substantially enhanced by interaction with agonist ligand. We next investigated if thymic positive selection of conventional (i.e. non-regulatory) T cells can also be increased by interaction with agonist ligand. To this end we analyzed Vβ-expression by CD69-expressing CD4+CD8+ (DP) thymocytes. Whereas it is impossible at this very early stage of development to distinguish between precursors for conventional and regulatory T cells, since the vast majority of mature thymocytes are conventional cells, analysis of the whole population in essence reflects analysis of conventional T cell precursors. Analysis of Vβ-expression by CD69DP revealed two levels of Vβ-expression: low and high (Fig. 4A). Vβlow thymocytes are recently activated (positively selected) cells that are precursors for mature CD4SP and CD4 CD8+ (CD8SP) thymocytes (39). Using a TCR-transgenic mouse-system, it has previously been shown that these cells have not yet been submitted to thymic deletion (34). Vβhigh cells are more mature, have been submitted to thymic deletion, and are precursors for CD8SP thymocytes (34, 39, 40). We therefore analyzed the percentage of recently positively (but not yet negatively) selected Vβlow thymocytes in MHC°→B6 and MHC°→DBA/2 chimeras (Fig. 4B). No difference between the percentages of sAg-specific Vβ5low and Vβ6low and control Vβ4low and Vβ14low thymocytes in sAg presenting vs. non-presenting chimeras was observed. We therefore conclude that positive selection of precursors for conventional T cells is not enhanced by agonist ligands.
Figure 4. Positive selection of conventional T cells is not modulated by TE-expressed sAg.

(A) Thymocytes were analyzed by flow-cytometry using antibodies specific for CD4, CDS, CD69, and indicated Vβ. FACS plots are electronically gated as indicated. (B) Percentages of CD69+ DP thymocytes expressing intermediate levels of indicated Vβ in the distinct chimeras.
Discussion
This is the first study on the role of natural agonist ligands on Treg development in mice with a normally diverse TCR-repertoire. We found that sAg-specific Treg precursors were deleted in mice in which both TE and APC of bone-marrow origin presented sAg, as we have previously reported (14). In contrast, in bone marrow chimeras in which sAg are exclusively expressed by TE, we found that development of sAg-specific Treg (but not conventional T cells) was substantially enhanced. Therefore, our data unambiguously show that agonist ligands substantially enhance development of Treg.
Specificity for sAg is determined by the Vβ-region expressed by T lymphocytes. In mice containing endogenous mouse mammary tumor viruses, sAg-specific thymocytes are deleted during T cell development in the thymus (reviewed in ref. (30). We have previously reported that precursors for Treg are not an exception and are efficiently deleted during development (14). When sAg are only presented by TE, deletion of precursors for conventional T cells is much less efficient and tolerance is mediated by clonal anergy (41). In our MHC°→DBA/2 chimeras, in which sAg are exclusively presented by TE, we indeed found that deletion of sAg-specific Foxp3− thymocytes was much less efficient than in control DBA/2→DBA/2 chimeras in which sAg can also be presented by APC. However, depending on the Vβ region expressed, partial deletion clearly took place, confirming earlier reports showing that TE can directly induce deletion of precursors for CD4SP thymocytes (32–34).
Importantly, agonist ligand expressed by TE did not induce any deletion of Treg precursors. In contrast, it substantially enhanced development of Treg. By studying MHC class II transfer from thymic stroma to developing thymocytes, we have previously observed that precursors for CD25−, but not CD25+, CD4SP thymocytes are sensitive to negative selection induced by TE (13). In TCR/ligand doubly transgenic mice in which antigen is expressed by TE, significant deletion of CD25− but not of CD25+ CD4SP precursors has previously been observed (19, 21, 22, 25). However, in these studies it was unclear if deletion was due to presentation of antigen directly by TE or indirectly by APC. In these and other transgenic mouse models, agonist ligand induced development of increased percentages of CD25+ Treg among CD4SP thymocytes (19, 21, 22, 24, 25, 42). Whether or not expression of agonist-ligand led to development of more Treg, in terms of absolute cell-numbers, was unclear in most (21, 22, 24, 42) but not all (25) of these reports, and this was formally dismissed in another (19). However, even if numerically more Treg developed in TCR/antigen doubly transgenic mice, this would not necessarily be due to agonist ligand-mediated recruitment into the Treg lineage or selection of these cells. In this light, we have previously reported that development of CD8SP thymocytes is limited by homeostatic mechanisms (43). When development of CD4SP thymocytes is strongly inhibited by negative selection, as in the TCR/antigen doubly transgenic mice, more Treg might develop due to homeostatic mechanisms. Such complications are related to the fact that in TCR-transgenic mice practically all precursors express the same TCR. In contrast, in the experimental model we presented here a substantially smaller fraction of precursors was involved. Indeed, the total thymocyte-numbers and percentages of CD4SP were similar in the two types of irradiation-chimeras we compared. As compared to chimeras in which no sAg was presented, in chimeras in which sAg were presented only by TE substantially higher numbers of sAg-specific Treg developed. Since in our experimental model it is difficult to imagine how results could be due to homeostatic mechanisms, we conclude that recognition of agonist ligand substantially enhanced development of Treg.
An unexpected observation merits particular attention. It has previously been reported that in B6 thymi Vβ5 expression is skewed to the CD8SP population (44). This result was attributed to a less efficient positive selection of Vβ5+ cells into the CD4SP than the CD8SP population. Also peripheral Vβ5 expression in I-E− (C57BL/10) mice is skewed to the CD8+ population (45). With age the CD4/CD8 ratio of Vβ5 expressing T cells gradually decreases, and this is known to be due to Mtv-8 and Mtv-9 mediated peripheral deletion of CD4+, but not CD8+ Vβ5+ T cells (46). We observed that two-fold more CD4SP Vβ5+ thymocytes developed in absence than in presence of thymic deletion by bone-marrow derived APC. Vβ5+ thymocytes are therefore partially deleted, probably by Mtv-8 and 9. Interestingly, also two-fold more Vβ5+ CD8SP thymocytes developed in MHC°→B6 than in B6→B6 chimeras (data not shown). Therefore, thymic skewing of Vβ5 expression towards the CD8-lineage cannot be explained by differences in thymic deletion mediated by APC of bone-marrow origin. In MHC°→B6 chimeras, the percentage of Vβ5+ cells among freshly positively selected CD69+ DP thymocytes is similar to that among CD4SP cells but significantly lower than that among CD8SP cells (not shown). Together, these data indicate that the thymic skewing of Vβ5 expression towards the CD8-lineage is indeed most probably explained by more efficient positive selection into the CD8-lineage. Moreover, since Mtv-8 and 9 presentation by I-Ab leads to only partial deletion of Vβ5+ thymocytes, clearly low avidity interactions are involved.
Whereas the TCR repertoires of CD25+ regulatory T cells and CD25− conventional T cells show only very limited overlap (16), their TCR Vβ repertoires are very similar (14, 18, 47). An exception to this rule is Vβ5, which, in B6 mice is approximately two-fold more represented in the CD25+ than in the CD25− population. In absence of negative selection by APC (in MHC°→B6 chimeras), the percentage of Vβ5+ cells among CD25−, but not CD25+ CD4SP thymocytes was increased. As compared to conventional T cell precursors, precursors for Treg appear therefore less susceptible to APC-mediated deletion by low avidity ligands. This conclusion is consistent with our earlier observation that the magnitude of deletion of Treg-precursors by APC seemed slightly lower than that of conventional T cell-precursors (14).
Interestingly, no more Vβ5+ Treg developed in MHC°→B6 than in B6→B6 chimeras. Therefore, in contrast to high avidity I-Ed/sAg ligands, low avidity I-Ab/sAg ligands did not induce enhanced positive selection of Treg. This result is consistent with earlier data showing that high avidity, but not low avidity interactions allow for Treg development in TCR/ligand doubly transgenic mice (24).
The precise mechanism involved in sAg-mediated enhanced development of Treg is uncertain. However, it appears unlikely that recognition of agonist ligand on TE by uncommitted precursors recruits them to the Treg lineage. If this were the case, one would expect an even higher increase in development of sAg-specific Treg in MHC°→DBA/2 (as compared to MHC°→B6) chimeras. For example, TE expression of sAg led to deletion of approximately 5×l05 CD25− Vβ5+ CD4SP, while only 2×l04 more CD25high Vβ5+ cells developed. Our data are more compatible with the hypothesis that recognition of agonist ligand allows for positive selection of precursors already committed to the Treg lineage. Alternatively, recognition of agonist ligand expressed in a thymic niche specialized in Treg development may allow for simultaneous positive selection and Treg-lineage commitment. This view is consistent with the observation that thymocytes expressing Treg-derived TCR preferentially, but not exclusively, develop into Treg (16). The second scenario is also consistent with the observation that expression of Foxp3, a master switch in Treg development, requires TCR-ligand interaction (8).
To evaluate the effect of agonist ligand expression by TE on positive selection of conventional T cells, we analyzed Vβ expression by TCRVβlowCD69+DP thymocytes in MHC°→B6 and MHC°→DBA/2 chimeras. These cells have initiated positive selection, but have not yet been submitted to negative selection (34). Comparable percentages of sAg-specific cells were found in the two types of chimeras. We conclude therefore that positive selection of conventional T cells is not enhanced by recognition of agonist ligand. Differentiation of conventional T cells has been shown to require continued TCR-mediated signaling occurring after the initial phase of positive selection (48). Therefore, enhanced positive selection of regulatory T cells by interaction with agonist ligand may very well occur at later stages of development (e.g. in the medulla). In contrast, continued interaction of conventional T cell precursors with agonist ligands does not enhance positive selection but leads to negative selection.
Under physiological conditions, peripheral T lymphocytes of CD4+ CD25int Foxp3− phenotype produce IL-2, required for in vivo maintenance of Treg (38). It has previously been proposed that this population is enriched in self-reactive T cells (8, 38). Intriguingly, we found that recognition of agonist ligand expressed by TE enhanced development of CD4SP CD25int Foxp3− thymocytes. Precursors for these cells therefore appear resistant to negative selection induced by ligands expressed by TE and their positive selection is enhanced by recognition of agonist ligand. Our results strongly suggest that the autospecific CD4+CD25intFoxp3− T cell repertoire is, at least in part, generated in the thymus. These data therefore indicate that at least two apparently distinct T cell lineages, Treg and CD4+CD25intFoxp3−T cells, use identical modes of thymic selection.
In conclusion, natural agonist ligands expressed by TE do not induce deletion of Treg precursors but substantially enhance their positive selection. Importantly, medullary thymic epithelium (but not bone-marrow derived DC) expresses a large variety of “tissue-specific” antigens (49). Therefore, TE will enhance development of Treg specific for ubiquitously expressed as well as for “tissue-specific” antigens, but DC will induce deletion of Treg precursors specific for ubiquitously expressed antigens. Thus, a repertoire of Treg exquisitely appropriate for protection against auto-immune aggression appears to develop.
Acknowledgments
The authors would like to thank the staff of the IFR30 animal facility, and in particular Maryline Calise, for expert animal husbandry, and Fatima Fatima-Ezzahra L’Faqihi-Olive for help in flow cytometry. We thank Drs. Sylvie Guerder and Rob MacDonald for critical reading of the manuscript, and Hans Acha-Orbea for very helpful advice and suggestions.
Abbreviations used in this paper
- Treg
regulatory T lymphocyte
- CD4SP
CD4+CD8− single positive
- CD8SP
CD4−CD8+ single positive
- DP
CD4+CD8+ double positive
- TE
thymic epithelium
- sAg
superantigen(s)
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.van Meerwijk JPM, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 4.Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–265. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 6.Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374–380. doi: 10.1016/j.it.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 10.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 11.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16:157–162. doi: 10.1016/j.coi.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Romagnoli P, Hudrisier D, van Meerwijk JPM. Molecular signature of recent thymic selection events on effector and regulatory CD4+ T lymphocytes. J Immunol. 2005;175:5751–5758. doi: 10.4049/jimmunol.175.9.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romagnoli P, Hudrisier D, van Meerwijk JPM. Preferential recognition of self-antigens despite normal thymic deletion of CD4+CD25+ regulatory T cells. J Immunol. 2002;168:1644–1648. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 15.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous Activation of Autoreactive CD4+ CD25+ Regulatory T Cells in the Steady State. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major Histocompatibility Complex Class II-positive Cortical Epithelium Mediates the Selection of CD4+25+ Immunoregulatory T Cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pacholczyk R, Kraj P, Ignatowicz L. Peptide specificity of thymic selection of CD4+CD25+ T cells. J Immunol. 2002;168:613–620. doi: 10.4049/jimmunol.168.2.613. [DOI] [PubMed] [Google Scholar]
- 19.van Santen HM, Benoist C, Mathis D. Number of T Reg Cells That Differentiate Does Not Increase upon Encounter of Agonist Ligand on Thymic Epithelial Cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaun J. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki J-i, Yamamoto K. Generation of CD4+CD25+ Regulatory T Cells from Autoreactive T Cells Simultaneously with Their Negative Selection in the Thymus and from Nonautoreactive T Cells by Endogenous TCR Expression. J Immunol. 2002;168:4399–4405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 22.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MS, Riley MP, von Boehmer H, Caton AJ. Anergy and suppression regulate CD4(+) T cell responses to a self peptide. Eur J Immunol. 2000;30:136–144. doi: 10.1002/1521-4141(200001)30:1<136::AID-IMMU136>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 25.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 26.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in 62M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 27.Chan SH, Cosgrove D, Waltzinger C, Benoist C, Mathis D. Another view of the selective model of thymocyte selection. Cell. 1993;73:225–236. doi: 10.1016/0092-8674(93)90225-f. [DOI] [PubMed] [Google Scholar]
- 28.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarmiento M, Dialynas DP, Lancki DW, Wall KA, Lorber MI, Loken MR, Fitch FW. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–169. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 30.Luther SA, Acha-Orbea H. Mouse mammary tumor virus: immunological interplays between virus and host. Adv Immunol. 1997;65:139–243. [PubMed] [Google Scholar]
- 31.Romagnoli P, Tellier J, van Meerwijk JPM. Genetic control of thymic development of CD4+CD25+FoxP3+ regulatory T lymphocytes. Eur J Immunol. 2005;35:3525–3532. doi: 10.1002/eji.200535225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramsdell F, Lantz T, Fowlkes BJ. A nondeletional mechanism of thymic self tolerance. Science. 1989;246:1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- 33.Klein L. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur J Immunol. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Gallegos AM, Bevan MJ. Central Tolerance to Tissue-specific Antigens Mediated by Direct and Indirect Antigen Presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. JImmunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 36.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas B, Germain RN. Unexpectedly complex regulation of CD4/CD8 coreceptor expression supports a revised model for CD4+CD8+ thymocyte differentiation. Immunity. 1996;5:461–477. doi: 10.1016/s1074-7613(00)80502-6. [DOI] [PubMed] [Google Scholar]
- 40.van Meerwijk JPM, O’Connell EM, Germain RN. Evidence for lineage commitment and initiation of positive selection by thymocytes with intermediate surface phenotypes. J Immunol. 1995;154:6314–6324. [PubMed] [Google Scholar]
- 41.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy: the role of the thymus in inducing self tolerance. Science. 1990;248:1342–1348. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 42.Walker LSK, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent Proliferation of CD4+ CD25+ Regulatory T Cells In Vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Meerwijk JP, Marguerat S, MacDonald HR. Homeostasis limits the development of mature CD8+ but not CD4+ thymocytes. J Immunol. 1998;160:2730–2734. [PubMed] [Google Scholar]
- 44.Bill J, Kanagawa O, Linten J, Utsunomiya Y, Palmer E. Class I and class II MHC gene products differentially affect the fate of V beta 5 bearing thymocytes. J Mol Cell Immunol. 1990;4:269–279. [PubMed] [Google Scholar]
- 45.Liao NS, Maltzman J, Raulet DH. Expression of the V beta 5.1 gene by murine peripheral T cells is controlled by MHC genes and skewed to the CD8+ subset. J Immunol. 1990;144:844–848. [PubMed] [Google Scholar]
- 46.Blish CA, Gallay BJ, Turk GL, Kline KM, Wheat W, Fink PJ. Chronic modulation of the TCR repertoire in the lymphoid periphery. J Immunol. 1999;162:3131–3140. [PubMed] [Google Scholar]
- 47.Kasow KA, Chen X, Knowles J, Wichlan D, Handgretinger R, Riberdy JM. Human CD4+CD25+ regulatory T cells share equally complex and comparable repertoires with CD4+CD25- counterparts. J Immunol. 2004;172:6123–6128. doi: 10.4049/jimmunol.172.10.6123. [DOI] [PubMed] [Google Scholar]
- 48.Kisielow P, Miazek A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]


