Abstract
Excitation of muscle often leads to a net loss of cellular K+ and a rise in extracellular K+ ([ K+ ]o), which in turn inhibits excitability and contractility. It is important, therefore, to determine how this K+ is cleared by diffusion into the surroundings or by reaccumulation into the muscle cells. The inhibitory effects of the rise in [K+ ]o may be assessed from the time course of changes in tetanic force in isolated muscles where diffusional clearance of K+ is eliminated by removing the incubation medium and allowing the muscles to contract in air. Measurements of tetanic force, endurance, and force recovery showed that in rat soleus and extensor digitorum longus (EDL) muscles there was no significant difference between the performance of muscles contracting in buffer or in air for up to 8 min. Ouabain-induced inhibition of K+ clearance via the Na+,K+ pumps markedly reduced contractile endurance and force recovery in air. Incubation in buffer containing 10 mM K+ clearly inhibited force development and endurance, and these effects were considerably reduced by stimulating Na+,K+ pumps with the β2-agonist salbutamol. Following 30–60 s of continuous stimulation at 60 Hz, the amount of K+ released into the extracellular space was assessed from washout experiments. The release of intracellular K+ per pulse was fourfold larger in EDL than in soleus, and in the two muscles, the average [K+ ]o reached 52.4 and 26.0 mM, respectively, appreciably higher than previously detected. In conclusion, prevention of diffusion of K+ from the extracellular space of isolated working muscles causes only modest interference with contractile performance. The Na+,K+ pumps play a major role in the clearance of K+ and the maintenance of force. This new information is important for the evaluation of K+-induced inhibition in muscles, where diffusional clearance of K+ is reduced by tension development sufficient to suppress circulation.
INTRODUCTION
The vast majority of in vitro experiments with isolated muscles are performed in an incubation medium, ascertaining a stable environment with respect to temperature, oxygenation, osmolarity, ionic milieu, and metabolizable substrates. It is difficult, however, in studies of contractile performance to know to which extent Na+, K+, or other ions are exchanged between the interstitial water space and the often relatively large volume of incubation medium. During contractile activity, there is often a considerable efflux of K+ across the sarcolemma, leading to a net loss of cellular K+ and a rise in the interstitial concentration of K+. This exercise-induced rise in extracellular K+ ([K+]o) has repeatedly been proposed to interfere with excitability and thus to be considered a major cause of fatigue (Fenn, 1940; Bigland-Ritchie et al., 1979; Jones, 1981; Sjøgaard, 1990, 1996; Sejersted and Sjøgaard, 2000; Clausen, 2003; for recent reviews see McKenna et al., 2007; Clausen, 2008). The inhibitory effect of elevated [K+]o on excitability may be related to slow inactivation of the Na+ channels (Ruff et al., 1988). In recent studies, the excitation-induced loss of K+ from isolated rat soleus and extensor digitorum longus (EDL) muscles was quantified and the ensuing rise in [K+]o was calculated (Clausen et al., 2004). The inhibitory effects of comparable increases in the K+ concentration of the incubation medium were characterized, leading to the conclusion that acute increases in [K+]o cause predictable and defined reductions in tetanic force as well as contractile endurance (Clausen and Nielsen, 2007). It is generally assumed that the K+ lost from working muscle cells is cleared by washout into the surrounding buffer (in vivo into the extracellular space and the capillaries) or by transport across the sarcolemma into the cytoplasm. The relative contribution of these processes and their importance for contractility, however, is unknown and has to my knowledge not been analyzed. This is important because during strong isometric muscle contractions, where it can be assumed that the excitation-induced efflux of K+ is large, circulation may be occluded (Barcroft and Millen, 1939; Humphreys and Lind, 1963). Under these physiological conditions, therefore, K+ clearance cannot take place by diffusion into the capillaries. The only pathway open for K+ clearance is transport into the muscle cells. The strategy of the present study has been to eliminate the diffusion of K+ into a surrounding incubation medium by letting the contractions of isolated rat muscles take place in air. This approach is combined with a subsequent washout of the Na+ and K+ from the extracellular space into Na+-free ice-cold buffer, allowing quantification of the excitation-induced gain of Na+ and loss of K+ from the muscle cells, using previously established procedures (Everts and Clausen, 1992, 1994; Buchanan et al., 2002). The functional importance of thus restricting the space available for K+ clearance by diffusion is assessed by comparing the time course of contraction, fatigue, and force recovery in buffer and in air. To explore the significance of the rate of the excitation-induced increase in passive K+ efflux, these experiments are performed using muscles (rat soleus and EDL) with widely different excitation-induced Na+ influx and K+ efflux (Clausen et al., 2004). It is reasonable to assume that during continued stimulation in air, extracellular K+ will increase, causing progressive inhibition of excitability and contractility. The rate of decrease in tetanic force is likely to reflect the accumulation of K+ in the extracellular space and this may be assessed from recordings of the time course of force decline during continuous stimulation. The clearance of K+ from the extracellular space is mediated by membrane transport systems, primarily the Na+,K+ pumps. The role of the Na+,K+ pumps was assessed using pretreatment with ouabain, which blocks the Na+,K+ pumps or with the β2 agonist salbutamol, which stimulates the Na+,K+ pumps (Clausen, 2003). Finally, the importance of the length of the diffusional pathways was assessed in incubation experiments in buffer with soleus muscles split so as to reach a size down to one third of the cross-sectional area of the intact muscle.
The following working hypotheses were tested:
(a) In isolated rat soleus and EDL muscles exposed to electrical stimulation, contractile performance depends on the excitation-induced efflux of K+ from the muscle cells and the clearance of extracellular K+ by diffusion into the surrounding buffer.
(b) Force, endurance, and force recovery depend on the activity of the Na+,K+ pumps.
(c) During incubation in buffer, the rate of force decline is reduced by decreasing the size of the muscle preparations and thus favoring K+ clearance by diffusion.
(d) Using muscle contractions in air combined with a subsequent washout of the extracellular space in ice-cold buffer it is possible to quantify the excitation-induced increase in [K+]o.
MATERIALS AND METHODS
Animals, Preparation, and Incubation of Muscles
All handling and use of rats complied with Danish animal welfare regulations. All experiments were performed using 4-wk-old Wistar rats of own breed, weighing 65–75 g. The animals were fed ad libitum and kept in a thermostated environment at 21°C with a 12/12 h light/dark cycle. Animals were killed by cervical dislocation, followed by decapitation. Intact soleus and EDL muscles weighing 20–30 mg were dissected out during wash with 154 mM NaCl at room temperature and then equilibrated at 30°C in standard Krebs-Ringer bicarbonate buffer (pH 7.4 at 30°C), containing the following (in mM): 120.1 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2, and 5.0 d-glucose (K.R.). In a few experiments, the concentration of K+ was increased from 4 to 10 mM. Isoosmolarity was maintained by an equivalent reduction in NaCl. In some experiments, soleus muscles were divided into two portions starting by splitting the proximal tendon and gently tearing the two strips apart, a preparation previously used in our laboratory. By repeating this, a number of muscle strips weighing from 8 to 16 mg, corresponding to 29–58% of the wet wt of the intact muscles were obtained. All incubations took place at 30°C under continuous gassing with a mixture of 95% O2 and 5% CO2 via a cannula with its tip at the bottom of the incubation chamber, thus allowing vigorous stirring of the buffer around the muscle.
Measurements of Na+,K+ Contents and 14C-sucrose Space
In some experiments, the Na+,K+ contents of the muscles were determined after rest or stimulation in air. This was primarily done by washing the muscles during continuous gassing with air for 60 min in ice-cold Na+-free Tris-sucrose buffer, followed by blotting on dry filter paper and wet wt determination. The Na+-free Tris-sucrose had the following composition (in mM): 263 sucrose, 10 Tris-HCl, 2.7 KCl, 1.3 CaCl2, 1.2 MgSO4, 1.2 KH2PO4 (pH 7.4). The muscles were then soaked overnight in 0.3 M trichloroacetic acid (TCA) and samples of this extract were taken for flame photometry. We have previously shown that during wash in ice-cold Na+-free Tris-sucrose buffer, K+ contents of soleus and EDL muscles remain constant for 150 min, whereas the intracellular Na+ content undergoes a slow decrease that can be corrected for by multiplying with a constant. Thus by removing extracellular Na+, as well as the K+ released from the working muscle cells, without causing further loss of intracellular K+, this procedure allows the determination of the total cellular contents of Na+ and K+. The excitation-induced release of intracellular K+ is calculated from the difference between the K+ contents of stimulated and resting muscle. The values are expressed as micromol/g wet wt (Everts and Clausen, 1992) and converted to nmol/stimulation pulse/g wet wt.
14C-sucrose space was determined by preincubating muscles for 90 min in K.R. buffer containing 1 mM sucrose and 14C-sucrose (3.7 kBq/ml). After removal of the buffer, the muscles were either resting or stimulated at 60 Hz, blotted, weighed, soaked overnight in TCA, and the extract taken for counting of 14C. The 14C-sucrose space was calculated on the basis of the 14C-sucrose activity in the tissue and in the K.R. buffer (Clausen and Gissel, 2005). The 14C-sucrose space represents the extracellular water space and can be used to calculate the average extracellular concentration of K+, using the values obtained for K+ washed out in the ice-cold Na+-free buffer.
Electrical Stimulation and Measurement of Force
Muscles were mounted vertically with their tendons intact in force transducers in thermostated chambers containing K.R. with 4 or 10 mM K+, adjusted to optimal length for measurement of isometric contractions, and exposed to direct field stimulation via platinum wire electrodes placed on either side of the central region. In the first series of experiments, the nerve was aspirated into a suction electrode, allowing indirect stimulation with 1-ms pulses of 5 nA. Contractile performance was assessed using chambers filled with 8 ml K.R. buffer or chambers where the buffer had been aspirated. The buffer in the chambers was continuously bubbled with a mixture of humidified 95% O2 and 5% CO2. Following the aspiration of buffer, drops of buffer still adhering to the muscle were gently wiped off before the onset of stimulation and the empty chambers were gassed with a mixture of humidified 95% O2 and 5% CO2. In all instances, the temperature of the chambers was maintained at 30°C by perfusing the hollow glass walls with distilled water circulating from a thermostated water bath. In the majority of the experiments, direct stimulation was applied via platinum wire electrodes using trains of 0.2-ms pulses at supramaximal voltage (10–12 V) applied at 60 Hz. Isometric force development was measured with force displacement transducers (Grass FTO3, Grass-Telefactor) and recorded with a chart recorder and/or a computer using Chart 5.4 software (ADInstruments) and expressed in mN. At a stimulation frequency of 60 Hz, maximum force in soleus is obtained after 1 s of stimulation (Murphy and Clausen, 2007). Therefore, to ensure the development of peak absolute tetanic force, stimulation durations of 2 s were used to test contractile performance of soleus muscles. In EDL muscles, maximum force was reached after 0.5 s of stimulation at 60 Hz, and this duration of stimulation was therefore used in the experiments with EDL muscles. In EDL muscles, stimulation at 90 Hz induced 22% higher force than 60 Hz (n = 4 vs. 4). However, to allow direct comparison with the measurements of force during continuous stimulation of soleus muscles, all experiments were performed using 60 Hz.
Chemicals and Isotope
All chemicals used were of analytical grade. Salbutamol and ouabain were obtained from Sigma-Aldrich, 14C-sucrose (22. 2 GBq/mmol) was from Amersham.
Statistics
All data are presented as means with SEM. The statistical significance of a difference between two groups was ascertained using Student's two-tailed t test for nonpaired observations.
RESULTS
Contractile Force, Endurance, and Recovery
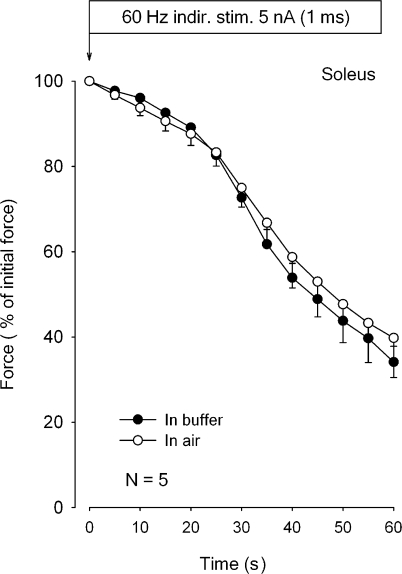
The first experiments were performed using indirect stimulation of the nerve applied via a suction electrode. This was done to ascertain that excitation elicited via the nerve fibers and motor end plates gave the same force development in buffer and in air. Fig. 1 shows the time course of force decline in five isolated intact rat soleus muscles exposed to continuous indirect stimulation at 60 Hz for 60 s in air or in K.R. buffer. There is no significant difference between the time course of the relative force decline of the same five muscles in air or in buffer. Since the setup used for indirect stimulation sometimes gave rise to interruptions of the connection between the nerve and the suction electrode, all the following experiments were performed using direct stimulation. There was no significant difference between the initial force development induced by indirect (393 ± 15 mN) and direct stimulation (376 ± 10 mN; n = 5 vs. 4, P = 0.42).
Figure 1.
Time course of force decline in rat soleus during 60 Hz indirect stimulation in buffer or in air. Soleus muscles were mounted in force transducers, adjusted to optimal length for measurement of isometric contractions, and equilibrated for 30 min in thermostated chambers containing 8 ml K.R. buffer at 30°C. The nerves were aspirated into suction electrodes allowing indirect stimulation as previously described in detail (Overgaard et al., 1999). All muscles were then stimulated for 60 s at 60 Hz using 1-ms pulses at 5 nA before and after aspiration of the buffer from the incubation chamber. Each curve represents the mean of observations on five muscles incubated in K.R. buffer (filled symbols) compared with contralateral muscles from the same rats mounted in air (open symbols) with vertical bars denoting SEM of values recorded at the times indicated.
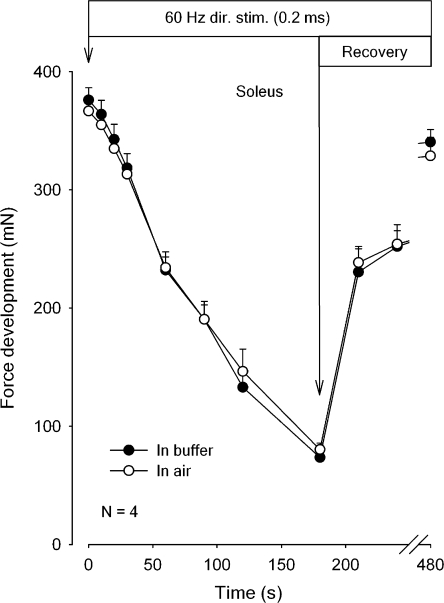
Fig. 2 shows the time course of force decline and recovery in isolated rat soleus exposed to continuous direct stimulation at 60 Hz for 180 s, followed by 300 s of rest, where the recovery was tested at the indicated intervals using 2-s trains of 60-Hz stimulation. There is no significant difference between the absolute force development or force recovery in air or in buffer. After 300 s of rest, the force had recovered to ∼90% of the initial level. In other experiments the stability of force development in air was tested. In soleus muscles maintained for 40 min in air, 30 s of continuous stimulation at 60 Hz gave the same force development when repeated four times at 10-min intervals (unpublished data).
Figure 2.
Time course of force decline and recovery in rat soleus during 60 Hz direct stimulation in buffer or in air. Experimental conditions as described in the legend to Fig. 1, but muscles were exposed to direct stimulation via platinum wires in contact with the muscles. 0.2-ms pulses of 10 V were applied at a frequency of 60 Hz for 180 s, followed by 2 s trains of 0.2-ms pulses of 60 Hz at the indicated intervals. The muscles were either stimulated in K.R. buffer (filled symbols) or in air, after the buffer had been aspirated (open symbols). Each point represents the mean of observations on four muscles with bars denoting SEM. None of the differences between force recorded in buffer and in air reached statistical significance.
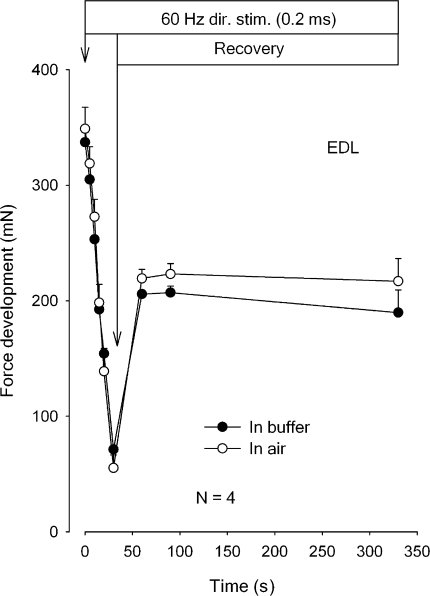
To explore the possible effects of much more pronounced excitation-induced K+ efflux, experiments were performed using isolated rat EDL muscles. In this preparation previous measurements of excitation-induced K+ efflux showed values 6.6-fold higher than those seen in soleus muscles from the same animals (Clausen et al., 2004). From Fig. 3 it can be seen that in rat EDL, there is no significant difference between the absolute force development and force recovery in air or in buffer. It should be noted that in buffer the rate of force decline in EDL was 3.9-fold faster than in soleus and in air 4.7-fold faster. After 300 s of subsequent rest, tetanic force of EDL had recovered to ∼60% of the initial force, which is clearly lower than in soleus. These observations indicate that the rate of force decline is the same without or with access to diffusional clearance of K+ into the buffer, no matter whether the rate of force decline or the efflux of K+ is large or small.
Figure 3.
Time course of force decline and recovery in rat EDL during 60 Hz direct stimulation in buffer or in air. Experimental conditions as described in the legend to Fig. 2, but muscles were stimulated continuously for 30 s, followed by 0.5-s trains of 0.2-ms pulses of 60 Hz at the indicated intervals. The muscles were either stimulated in K.R. buffer (filled symbols) or in air, after the buffer had been aspirated (open symbols). Each point represents the mean of observations on four muscles with bars denoting SEM. None of the differences between force development in buffer or in air reached statistical significance.
Effects of Muscle Dimensions, Ouabain, and Salbutamol on Contractile Performance
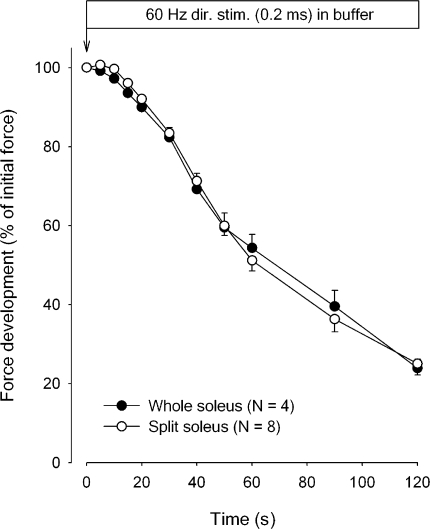
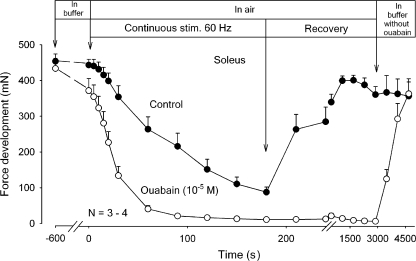
The influence of altering the diffusional distance was explored by comparing the time course of force decline in whole soleus muscles with that of muscle strips weighing 8–15 mg, prepared from the contralateral soleus muscle. These experiments were performed using buffer so as to allow diffusional clearance of K+. The thinner muscle strips with cross-sectional areas of 29–58% of that of the intact muscles should give shorter diffusional delay for the K+ leaving the muscle cells than the intact muscle. Therefore, it seems reasonable to assume that the clearance of K+ by diffusion was more efficient in the strips than in the intact muscles, allowing better maintenance of force and a slower rate of force decline. As shown in Fig. 4, however, there was no significant difference between the time course of the decline of average force in whole muscles and in strips prepared from the contralateral muscles. This together with the abovementioned observations indicate that only a minor part of the intracellular K+ lost during excitation is cleared from the extracellular space by diffusion into the surrounding buffer. Therefore, most of the K+ would have to be cleared by reaccumulation into the cells. This possibility was explored by inhibiting or stimulating the rate of K+ uptake via the Na+,K+ pump and following the contractile performance in air. As shown in Fig. 5, preincubation for 10 min in K.R. with 10−5 M ouabain caused a decrease of 14% in the force recorded at the onset of continuous stimulation at 60 Hz. During the first 20 s of continuous stimulation, the rate of force decline was accelerated by 565% in comparison to the controls without ouabain. It seems reasonable to assume that these changes reflect ouabain-induced inhibition of K+ reaccumulation in the muscle cells and ensuing accumulation of K+ in the interstitial water space. It should be noted that the inhibitory effect of ouabain on contractile performance was not restored even after a recovery period of 2,820 s in air. In contrast, subsequently returning the muscles to K.R. buffer without ouabain induced almost complete force recovery in 1,500 s, demonstrating that following washout of the glycoside in buffer, the inhibitory effect of ouabain on contractility was completely reversible. Control muscles not exposed to ouabain showed almost the same time course of force decline during continuous 60-Hz stimulation as shown in Fig. 2 and ∼90% recovery of tetanic force in air. Henceforth, they maintained contractile performance for around 1,800 s in air, but showed no further force recovery following return to K.R. buffer.
Figure 4.
Effect of splitting soleus muscles on the time course of force decline during continuous direct stimulation in buffer for 120 s at 60 Hz. Each point represents the mean of observations on four whole muscles or eight splits obtained from the contralateral muscles of the same rats. Bars indicate SEM. None of the differences between force development in intact muscles or in splits reached statistical significance.
Figure 5.
Effects of ouabain on tetanic force development in rat soleus exposed to direct stimulation at 60 Hz in K.R. buffer or in air. Experimental conditions as described in the legend to Fig. 2, but muscles were first tested using 2-s trains of 60 Hz stimulation in buffer without (filled symbols) or with (open symbols) ouabain (10−5 M). After 10 min of incubation in buffer without or with ouabain, the buffer was aspirated and all muscles exposed to continuous stimulation at 60 Hz for 180 s, followed by 2-s trains of 60-Hz stimulation at the indicated intervals. Finally all muscles were washed three times in K.R. without ouabain, left in buffer, and tetanic force tested at the indicated intervals. Each point represents the mean of observations on three to four muscles with bars denoting SEM.
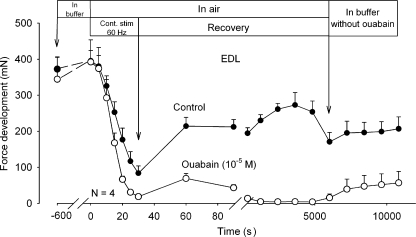
As shown in Fig. 6, the same experiment was repeated using EDL muscles. Again, the untreated control muscles showed a much faster force decline than soleus and incomplete force recovery. Return to buffer caused no further force recovery. The addition of ouabain increased the rate of force decline by 51% and reduced total force to 0. Return to buffer without ouabain caused only slow and modest force recovery.
Figure 6.
Effects of ouabain on tetanic force development in rat soleus exposed to direct stimulation at 60 Hz in buffer or in air. Experimental conditions as described in the legend to Fig. 5, but contractility was tested using 0.5-s pulses of 60 Hz. Each point represents the mean of observations on four muscles with bars denoting SEM.
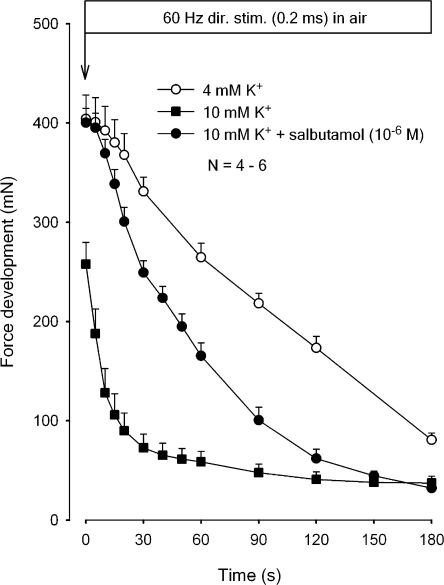
In the next series of experiments, the effects of elevated [K+]o and Na+,K+ pump stimulation with salbutamol were explored in soleus muscles with measurements of force development in air. As shown in Fig. 7, preincubation for 20 min with buffer containing 10 mM K+ caused a significant reduction in the initial force compared with muscles preincubated in buffer containing 4 mM K+ (−38%, P < 0.001). In the same muscles, there was a considerable increase (625%) in the rate of force decline during the first 20 s of the subsequent continuous stimulation. The addition of salbutamol (10−6 M) to the preincubation buffer containing 10 mM K+ induced complete restoration of initial force and partial restoration of the rate of force decline (to 180% of the controls incubated at 4 mM K+). It should be noted that parallel experiments with stimulation in K.R. buffer gave closely similar values for the time course of force decline in buffer containing 4 mM K+, 10 mM K+, without or with salbutamol (n = 6–12, unpublished data).
Figure 7.
Effects of high [K+]o without or with salbutamol on force decline in rat soleus. Experimental conditions as described in the legend to Fig. 2. All muscles were preincubated for 20 min in K.R. containing 4 mM K+ or 10 mM K+ without or with 10−6 M salbutamol. Then buffer was aspirated and all muscles exposed to continuous stimulation at 60 Hz for 180 s. Each point represents the mean of observations on four to six muscles with bars denoting SEM.
Excitation-induced Loss of K+ and Gain of Na+
The effects of stimulation in air on the loss of intracellular K+ and the gain of intracellular Na+ were estimated in soleus and EDL muscles. It can be assumed that during stimulation, the K+ released from the muscle cells is primarily accumulated in the extracellular space. Concomitantly, Na+ is taken up into the intracellular space. To quantify these changes, the muscles were washed in ice-cold Na+-free buffer for 4 × 15 min. We have previously shown that during this wash, extracellular K+ and Na+ are removed, allowing quantification of the excitation-induced changes in the intracellular contents of these ions. During 60 min of washout in the ice-cold Na+-free buffer, there is no loss of intracellular K+, whereas intracellular Na+ undergoes a reduction, which in soleus can be corrected for by multiplying the final Na+ content by a factor of 1.49 (Buchanan et al., 2002) and in EDL by a factor of 1.46 (Hansen et al., 2005).
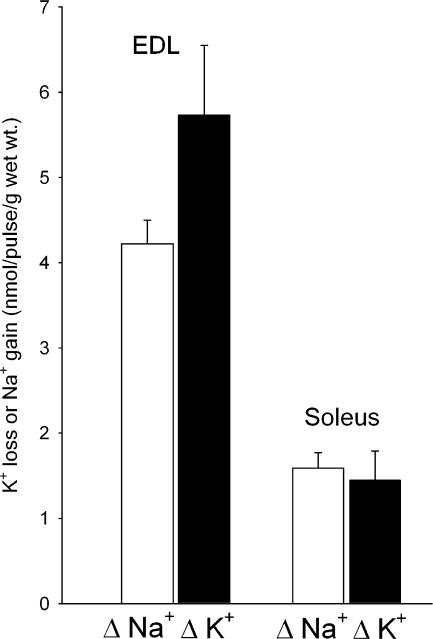
Earlier studies have shown that during shorter-lasting excitation in buffer (5–30 s), the release of K+ per pulse is ∼6.6-fold faster in EDL than in soleus (Clausen et al., 2004). It was of interest, therefore, to determine whether the marked difference in K+ release between soleus and EDL could be detected by measuring the difference between the net release of K+ during stimulation in air. The excitation-induced loss of K+ and gain of Na+ were estimated after 60 Hz of continuous stimulation of soleus (60 s or 3,600 pulses) and EDL (30 s or 1,800 pulses) muscles in air, followed by a washout in ice-cold buffer. As shown in Fig. 8, the net loss of K+ measured as the difference between K+ contents of resting and stimulated muscles amounted to 5.73 ± 0.82 nmol/pulse/g wet wt in EDL and 1.45 ± 0.34 nmol/pulse/g wet wt in soleus (P = 0.011). This indicates that the net loss of K+ per pulse is fourfold larger in EDL than in soleus. As can be seen from Fig. 8, the gain of Na+ amounted to 4.22 ± 0.28 nanomol/pulse/g wet wt in EDL and 1.59 ± 0.18 nanomol/pulse/g wet wt in soleus (P = 0.014), corresponding to a 2.7-fold difference in the net gain of Na+ per pulse.
Figure 8.
The excitation-induced net loss of K+ and gain of Na+ in rat soleus and EDL. Experimental conditions as described in the legend to Fig. 2. Following equilibration for 30 min in K.R. buffer, contralateral muscles from the same animal were either allowed to rest or stimulated in air at 60 Hz for 60 s (soleus) or 30 s (EDL). For each pair of contralateral muscles, the excitation-induced loss of K+ and gain of Na+ were calculated from the differences in K+ and Na+ contents, respectively, and expressed as nmol/pulse/g wet wt (Δ Na+ and Δ K+). Each column represents the mean of observations on 12 muscles with bars denoting SEM, The difference between the excitation-induced Na+ uptake in soleus and EDL muscles was significant (P = 0.0l4). Likewise, the difference between excitation-induced K+ release of soleus and EDL muscles was significant (P = 0.011).
As shown in Table I, measurements of the 14C-sucrose space of the two muscles showed no significant difference between resting and stimulated muscles. The mean values for 14C-sucrose space of resting and stimulated muscles were 23.6 and 21.3% for soleus and EDL, respectively, which is in close agreement with those obtained in our previous studies (Clausen and Gissel, 2005). I assume that these values represent the size of the extracellular space and that before the onset of stimulation this contains 4 mM K+. By dividing the values for the total amount of K+ released into the extracellular space (in soleus 5.200 nmol/g wet wt and in EDL 10.300 nmol/g wet wt) by the mean values of 14C-sucrose space it could be calculated that at the end of the continuous stimulation in air, the average [K+]o in the extracellular space of soleus and EDL had increased from 4 mM to 26.0 and 52.4 mM, respectively.
TABLE I.
14C-sucrose Space in Resting and Stimulated Soleus and EDL Muscles
| 14C-sucrose space (% of wet wt.) | |
|---|---|
| Resting soleus | 22.9 ± 3.4 |
| Soleus stimulated for 60 s | 24.2 ± 2.8 |
| Resting EDL | 21.7 ± 1.9 |
| EDL stimulated for 30 s | 20.9 ± 1.2 |
Muscles were mounted on electrodes for isometric contractions and incubated for 90 min in K.R. containing 1 mM sucrose and 3.7 kBq/ml of 14C-sucrose. Then all muscles were moved into empty tubes and carefully blotted to remove the last drips of buffer and stimulated at 60 Hz or allowed to rest. Immediately after the stimulation, the muscles were blotted, weighed, and soaked overnight in 0.3 M TCA. The TCA extract was sampled for counting of 14C-sucrose. Each value is the mean of measurements on 3 muscles ± SEM.
In other experiments it was examined whether the net loss of K+ from EDL muscles was higher when the muscles were stimulated in buffer than in air. Following 30 s of continuous stimulation of muscles at 60 Hz in K.R. buffer or in air, the muscles were washed for 4 × 15 min in ice-cold Na+-free Tris-sucrose buffer, blotted, weighed, and taken for flame photometric determination of total K+ contents. The difference between K+ contents of resting and contralateral stimulated muscles was 9.1 ± 1.7 μmol/g wet wt in the muscle pairs kept in air and 9.5 ± 1.4 μmol/g wet wt in those kept in buffer (n = 9 vs. 7, P = 0.87). This indicates that during the 30 s of stimulation in K.R. buffer, the average concentration of K+ in the extracellular space of EDL muscles reaches the same level (48.6 mM) as in the muscles stimulated in air (46.7 mM), in keeping with the close similarity in loss of force (Fig. 3).
DISCUSSION
The major new information gained from the present study is that when isolated rat skeletal muscles are allowed to work in air, their contractile performance over 30–180 s of continuous 60-Hz stimulation is closely similar to that shown in the standard K.R. incubation medium. It is generally assumed that the K+ released from muscles working in vitro is primarily cleared by diffusion into the surrounding buffer. When the muscles are working in air, however, this clearance pathway is closed. Since this causes no impairment of contractile performance, clearance of K+ by diffusion into the incubation medium seems to be of minor importance during the early phase of our in vitro experiments. Comparison of the net loss of K+ following stimulation in air and in buffer shows no difference, indicating that during 30 s of 60 Hz stimulation of EDL muscles, the relatively large loss of K+ from the muscle cells into the extracellular water space is not diffusing into the incubation medium. Moreover, marked reduction in the size of the soleus muscle preparation, which would facilitate diffusional clearance of K+, causes no reduction in the rate of force decline. Hence, it seems reasonable to assume that the clearance of K+ depends on reaccumulation into the muscle cells. In keeping with this, inhibition of K+ reaccumulation via the Na+,K+ pumps markedly accelerates the force decline (by 565%) taking place during continuous stimulation of soleus muscles. A similar increase in the force decline (by 625%) is obtained by augmenting extracellular K+ by preincubation in buffer containing 10 mM K+. Conversely, the increase in the rate of force decline induced by high [K+]o is counterbalanced by stimulation of the Na+,K+ pumps with the β2 agonist salbutamol. Taken together, these results indicate that clearance of extracellular K+ via the Na+,K+ pumps plays a major role in the maintenance of muscle contractility. Further evidence for the crucial role of extracellular K+ in determining contractile performance emerges from the observation that during continuous stimulation, the rate of K+ release per pulse is fourfold faster in EDL than in soleus. As shown in Figs. 2 and Figs.3, the rate of force decline in EDL is 3.9-fold (in buffer) and 4.7-fold (in air) faster than in soleus, which is very similar to the difference observed between isolated mouse soleus and EDL muscles (Pagala et al., 1998). The correlation between the excitation-induced K+ release and force decline indicate that the difference in fatigability of soleus and EDL is due to differences in the rate of excitation-induced accumulation of K+ in the extracellular space. The much lower contractile endurance in muscles containing predominantly fast-twitch type II fibers can be related to a faster reduction in the amplitude of the compound action potentials (Pagala et al., 1984, 1998; Clausen et al., 2004).
In the presence of ouabain (10−5 M), the initial rate of force decline is 74% faster in EDL than in soleus. Since this difference is appreciably smaller than in the absence of ouabain (290–370%), it is possible that only part of it reflects a difference in the rate of excitation-induced K+ efflux. Part of the difference in force decline may be due to a lower rate of Na+,K+ pump–mediated K+ reaccumulation in EDL. Thus, we found that during 2-Hz stimulation, the Na+,K+ pump–mediated 86Rb uptake in soleus is 57% larger than in EDL, possibly because in soleus, the Na+,K+ pumps are more sensitive to increases in intracellular Na+ than in EDL (Everts and Clausen, 1992).
Measurements of the difference between the K+ content of the resting and the stimulated muscles indicate that during continuous stimulation at 60 Hz, the average extracellular concentration of K+ reaches values of 26.0 mM in soleus (after 60 s) and 52.4 mM in EDL (after 30 s), respectively. These values represent the net result of the excitation-induced release of K+ from the working muscle cells minus the K+ that has been cleared into the muscle cells during the entire stimulation period. It should be noted, however, that during the continuous stimulation, soleus (60 s) and EDL (30 s) lose respectively 35 and 83% of their initial force (Fig. 2 and 3), probably due to loss of excitability and ensuing dropout of action potentials (Clausen et al., 2004). This implies that, on average, the total number of action potentials in soleus (in 60 s) and in EDL (in 30 s) is reduced by ∼17 and 41%, respectively. It could be calculated that this accounts for the fact that the excitation-induced losses of K+ per pulse in soleus and EDL obtained in the present study are smaller than those measured in a previous study, where shorter intervals of stimulation were used, allowing better maintenance of the generation of action potentials (Clausen et al., 2004). It should be noted that the excitation-induced loss of muscle K+ per action potential observed in our laboratory is in the same range as those found in other intact muscle fibers (for references see Clausen, 2003). It is interesting that the present as well as a previous study (Clausen et al., 2004) showed that the excitation-induced loss of K+ was not significantly different from the gain of Na+, both in soleus (P = 0.38) and in EDL (P = 0.07). This is in keeping with the usual one-for-one exchange of Na+ and K+ taking place during the action potentials.
The stimulation frequency of 60 Hz used in the present study was chosen for technical reasons, allowing more accurate measurements of Na+,K+ exchange and characterization of the rates of fatigue. The initial peak tetanic force reached in soleus and EDL is similar to those obtained by stimulating the same muscles from 35-d-old rats with intact blood supply (Close, 1964). In rat soleus with intact circulation, peak tetanic force at 30°C was 20 N/cm2 of cross-sectional area (Swoap et al., 1997). In soleus of the size used in the present study, the cross-sectional area is 0.016 cm2, and the peak tetanic force measured in air is 38 g, corresponding to 23.3 N/cm2, which is in good agreement with the value obtained by Swoap et al. (1997). 60 Hz is higher than the frequencies measured in skeletal muscles of spontaneously active rats in vivo (Hennig and Lømo, 1985), raising the question of whether low frequency muscle work also in vivo gives rise to loss of K+ from the muscles and a gain of Na+. Sréter (1963) found that in the red fibers of rat gastrocnemius in vivo, 4 min of stimulation at 5 Hz caused a 14% decrease in [K+]i and an 81% increase in [Na+]i. In rat EDL muscle in vivo, 15 min of indirect 5-Hz stimulation increased [Na+]i by 100% and decreased [K+]i by 30% (Nagaoka et al., 1994). In man, voluntary biking, which is assumed to be associated with an average stimulation frequency of around 6 Hz, leads to a net loss of K+ from the working muscles (Sjøgaard, 1990; Hallen et al., 1994). Moreover, in the venous blood leaving intensely working human leg muscles, plasma K+ may increase to around 8 mM within 1 min (Medbø and Sejersted, 1990). Studies with microdialysis probes indicate that during plantar flexion exercise, extracellular K+ in the gastrocnemius muscles increases to 11.5 mM, and there is a considerable net release of K+ from the working muscles to the venous blood (Green et al., 2000). In keeping with this, it was shown that during work, skeletal muscle cells undergo depolarization, both in vitro (Hanson, 1974; Balog et al., 1994) and in vivo (Locke and Solomon, 1967), which may contribute to the loss of excitability and contractility shown in several studies (for review see Clausen, 2003).
The present results indicate that for the short-term maintenance of contractile performance, access to diffusional clearance of K+ to a surrounding incubation medium plays only a minor role. The clearance of K+ may take place by reaccumulation into the muscle cells via the Na+,K+ pumps. During static work with isometric contractions, the blood vessels may be compressed (Barcroft and Millen, 1939; Humphreys and Lind, 1963), causing cessation of circulation. Under these conditions, K+ has to be cleared by transport into the cells. The muscles seem to have adapted to maintain force during such critical situations by generating the large number of Na+,K+ pumps, which they have been shown to contain. Following 10 s of intense excitation of isolated rat soleus muscles, the net efflux of intracellular Na+ as measured over the following 30 s has been shown to increase up to 22-fold, reaching the theoretical maximum capacity of the Na+,K+ pumps present in the same muscle (Clausen, 2003). Due to the electrogenic action of the Na+,K+ pump, this contributes to the maintenance of membrane potential and excitability. It is surprising, however, that even when the average extracellular concentration of K+ in soleus and EDL reaches 26 and 52 mM, respectively, the muscles are still able to produce, albeit weakened, contractions. This may be attributed to the recently discovered improvement of excitability brought about by intracellular accumulation of lactic acid with ensuing acidification and inactivation of Cl− channels (Pedersen et al., 2005). It is important that in K+-inhibited muscles, the effects of Na+,K+ pump stimulation by catecholamines and lactic acid on force recovery are additive (De Paoli et al., 2007). Both effects are rapid in onset, and in working muscles, metabolic acidosis and Na+,K+ pump stimulation act concomitantly to maintain excitability.
Acknowledgments
We thank Ann-Charlotte Andersen, Tove Lindahl Andersen, Marianne Stürup-Johansen, and Vibeke Uhre for skilled technical assistance.
This study was supported by grants from The Danish Medical Research Council (j. nr. 22-04-0241), The Danish Biomembrane Research Center, Aarhus Universitets Forskningsfond, and the Karen Elise Jensen Foundation.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: EDL, extensor digitorum longus; TCA, trichloroacetic acid.
References
- Balog, E.M., L.V. Thompson, and R.H. Fitts. 1994. Role of sarcolemma action potentials and excitability in muscle fatigue. J. Appl. Physiol. 76:2157–2162. [DOI] [PubMed] [Google Scholar]
- Barcroft, H., and J. Millen. 1939. The blood flow through muscle during sustained contractions. J. Physiol. 97:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie, B., D.N. Jones, and J.J. Woods. 1979. Excitation frequency and muscle fatigue: electrical responses during human voluntary and stimulated contractions. Exp. Neurol. 64:414–427. [DOI] [PubMed] [Google Scholar]
- Buchanan, R., O.B. Nielsen, and T. Clausen. 2002. Excitation- and β2-agonist-induced activation of the Na+-K+ pump in rat soleus muscle. J. Physiol. 545:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, T. 2003. Na+-K+ pump regulation and skeletal muscle contractility. Physiol. Rev. 83:1269–1324. [DOI] [PubMed] [Google Scholar]
- Clausen, T. 2008. Role of Na+,K+-pumps and transmembrane Na+,K+-distribution in muscle function. Invited review. Acta Physiol. 192:339–349. [DOI] [PubMed] [Google Scholar]
- Clausen, T., and H. Gissel. 2005. Role of Na+,K+ pumps in restoring contractility following loss of cell membrane integrity in rat skeletal muscle. Acta Physiol. Scand. 183:263–271. [DOI] [PubMed] [Google Scholar]
- Clausen, T., and O.B. Nielsen. 2007. Potassium, Na+,K+-pumps and fatigue in rat muscle. J. Physiol. 584:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, T., K. Overgaard, and O.B. Nielsen. 2004. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta Physiol. Scand. 180:209–216. [DOI] [PubMed] [Google Scholar]
- Close, R. 1964. Dynamic properties of fast and slow skeletal muscles in the rat during development. J. Physiol. 173:74–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paoli, F.V., K. Overgaard, T.H. Pedersen, and O.B. Nielsen. 2007. Additive protective effects of the addition of lactic acid and adrenaline on excitability and force in isolated rat skeletal muscle depressed by elevated extracellular K+. J. Physiol. 581:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts, M.E., and T. Clausen. 1992. Activation of the Na-K pump by intracellular Na in rat slow- and fast-twitch muscle. Acta Physiol. Scand. 145:353–362. [DOI] [PubMed] [Google Scholar]
- Everts, M.E., and T. Clausen. 1994. Excitation-induced activation of the Na+-K+-pump in rat skeletal muscle. Am. J. Physiol. 266:C925–C934. [DOI] [PubMed] [Google Scholar]
- Fenn, W.O. 1940. The role of potassium in physiological processes. Physiol. Rev. 20:377–415. [Google Scholar]
- Green, S., H. Langberg, D. Skovgaard, J. Bülow, and M. Kjær. 2000. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J. Physiol. 529:849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen, J., L. Gullestad, and O.M. Sejersted. 1994. K+ shifts of skeletal muscle during bicycle exercise with and without β-adrenoceptor blockade. J. Physiol. 477:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, A.K., T. Clausen, and O.B. Nielsen. 2005. Effects of lactic acid and catecholamines on contractility in fast-twitch muscles exposed to hyperkalemia. Am. J. Physiol. Cell Physiol. 289:C104–C112. [DOI] [PubMed] [Google Scholar]
- Hanson, J. 1974. The effects of repetitive stimulation on the action potential and the twitch of rat muscle. Acta Physiol. Scand. 90:387–400. [DOI] [PubMed] [Google Scholar]
- Hennig, R., and T. Lømo. 1985. Firing patterns of motor units in normal rats. Nature. 314:164–166. [DOI] [PubMed] [Google Scholar]
- Humphreys, P.W., and A.R. Lind. 1963. The blood flow through active and inactive muscles of the forearm during sustained hand-grip contractions. J. Physiol. 166:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D.A. 1981. Muscle fatigue due to changes beyond the neuromuscular junction. Ciba Found. Symp. 82:178–196. [DOI] [PubMed] [Google Scholar]
- Locke, S., and H.C. Solomon. 1967. Relation of resting potential of rat gastrocnemius and soleus muscles to innervation, activity, and the Na-K pump. J. Exp. Zool. 166:377–386. [DOI] [PubMed] [Google Scholar]
- McKenna, M.J., J. Bangsbo, and J.M. Renaud. 2007. Muscle K+, Na+, Cl− disturbances and Na+,K+-pump inactivation: implications for fatigue. J. Appl. Physiol. 104:288–295. [DOI] [PubMed] [Google Scholar]
- Medbø, J.I., and O.M. Sejersted. 1990. Plasma potassium changes with high intensity exercise. J. Physiol. 421:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K.T., and T. Clausen. 2007. The importance of limitations in aerobic metabolism, glycolysis, and membrane excitability for the development of high-frequency fatigue in isolated rat soleus muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R2001–R2011. [DOI] [PubMed] [Google Scholar]
- Nagaoka, R., S. Yamashita, M. Mizuno, and N. Akaike. 1994. Intracellular Na+ and K+ shifts induced by contractile activities of rat skeletal muscles. Comp. Biochem. Physiol. A Physiol. 109:957–965. [DOI] [PubMed] [Google Scholar]
- Overgaard, K., O.B. Nielsen, J.A. Flatman, and T. Clausen. 1999. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J. Physiol. 518:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagala, M.K., T. Namba, and D. Grob. 1984. Failure of neuromuscular transmission and contractility during muscle fatigue. Muscle Nerve. 7:454–464. [DOI] [PubMed] [Google Scholar]
- Pagala, M.K., K. Ravindran, T. Namba, and D. Grob. 1998. Skeletal muscle fatigue and physical endurance of young and old mice. Muscle Nerve. 21:1729–1739. [DOI] [PubMed] [Google Scholar]
- Pedersen, T.H., F. de Pauli, and O.B. Nielsen. 2005. Increased excitability of acidified skeletal muscle: role of chloride conductance. J. Gen. Physiol. 125:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff, R.L., L. Simoncini, and W. Stühmer. 1988. Slow sodium channel inactivation in mammalian muscle: a possible role in regulating excitability. Muscle Nerve. 11:502–510. [DOI] [PubMed] [Google Scholar]
- Sejersted, O.M., and G. Sjøgaard. 2000. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 80:1411–1481. [DOI] [PubMed] [Google Scholar]
- Sjøgaard, G. 1990. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol. Scand. Suppl. 593:1–60. [PubMed] [Google Scholar]
- Sjøgaard, G. 1996. Potassium and fatigue, the pros and cons. Acta Physiol. Scand. 156:257–264 (invited review.). [DOI] [PubMed] [Google Scholar]
- Sréter, F.A. 1963. Cell water, sodium, and potassium in stimulated red and white mammalian muscles. Am. J. Physiol. 205:1295–1298. [DOI] [PubMed] [Google Scholar]
- Swoap, S.J., V.J. Caiozzo, and K.M. Baldwin. 1997. Optimal shortening velocities for in situ power production of rat soleus and plantaris muscles. Am. J. Physiol. 273:C1057–C1063. [DOI] [PubMed] [Google Scholar]