Ion channels are well recognized as important therapeutic targets for treating a number of different pathophysiologies. Historically, however, development of drugs targeting this protein class has been difficult. Several challenges associated with molecular-based drug discovery include validation of new channel targets and identification of acceptable medicinal chemistry leads. Proof of concept approaches, focusing on combined molecular biological/pharmacological studies, have been successful. New, functional, high throughput screening (HTS) strategies developed to identify tractable lead structures, which typically are not abundant in small molecule libraries, have also yielded promising results. Automated cell-based HTS assays can be configured for many different types of ion channels using fluorescence methods to monitor either changes in membrane potential or intracellular calcium with high density format plate readers. New automated patch clamp technologies provide secondary screens to confirm the activity of hits at the channel level, to determine selectivity across ion channel superfamilies, and to provide insight into mechanism of action. The same primary and secondary assays effectively support medicinal chemistry lead development. Together, these methodologies, along with classical drug development practices, provide an opportunity to discover and optimize the activity of ion channel drug development candidates. A case study with voltage-gated sodium channels is presented to illustrate these principles.
Introduction
Ion channels are important drug targets because they play a crucial role in controlling a very wide spectrum of physiological processes (Hille, 2001), and because their dysfunction can lead to pathophysiology (Ashcroft, 2000). Given the strong historical precedent that exists for discovering and commercializing successful drugs that modulate the activity of voltage-gated sodium, calcium, or potassium channels, or ligand-gated ion channels, new generations of therapeutic agents are expected to result from targeting this protein family. Early ion channel drug discovery used classical pharmacological approaches, in which profiling in animal models, designed to simulate human disease states, was used to optimize compound activity, even if the nature of the molecular target was unclear. Serendipity, insight, and brute force effort drove these drug discovery efforts and resulted in a number of notable successes including successful therapies and discovery of research tools that have been invaluable in mapping out signaling pathways, purifying channel proteins, and characterizing gating mechanisms, all of which has sustained the present era of ion channel drug discovery (Garcia and Kaczorowski, 2005).
With the advent of a more complete understanding of cellular physiology and identification of the molecular components that constitute individual channel types and control their function, researchers are now focusing on a molecular-based strategy to identify drugs targeting this protein class. The molecular approach has been significantly strengthened by the advent of new technologies, including high throughput screening capabilities and automated electrophysiology. Despite these advances, the discovery and development of new ion channel drug candidates remains an arduous task. Significant challenges exist in the validation of new targets, which may be hindered by complex and potentially species-specific physiology (Yu and Catterall, 2004), difficulties in discovering acceptable small molecule leads, and the lack of biomarker and target engagement strategies to validate that drug exposure in patients is sufficient to differentiate negative from failed clinical trails. Each of these challenges is addressed in more detail in the remainder of this article, using examples from a drug discovery effort on voltage-gated sodium channels.
Identification and Validation of Ion Channel Targets
Drug discovery and development is a costly and time consuming process which, unfortunately, often meets with limited success. Issues that contribute to program failure include toxicity, due to the interaction of a development candidate with unrelated channels or other proteins (e.g., inhibition of Kv11.1, potentially leading to life threatening arrhythmias; Ashcroft, 2000), and selection of inappropriate targets due to uncertainties regarding the predictive nature of animal models used for preclinical testing when compared with human pathophysiology. An essential route to increased success in ion channel drug discovery is rigorous application of traditional and novel in vitro and in vivo target validation approaches, including genetic and pharmacological validation studies, expression profiling, and altering channel expression in model systems. Improvements in the target identification and validation stage can, arguably, have the greatest overall impact on ion channel drug discovery efforts.
Human genetics and gene ablation studies in rodents have identified a number of new ion channel targets (e.g., Nav1.7, Nav1.4, Cav2.2, KCNMA1, Kir1.1, Kir6.2-SUR2, KCNQ, etc.) (Ashcroft, 2000; Lifton et al., 2001; Yu and Catterall, 2004; Dib-Hajj et al., 2007). In addition to traditional knockout techniques, modulation of channel expression by regulation of promotor activity, siRNA technology, or the employment of dominant-negative interference strategies can be used to aid in the validation of novel targets.
As an example of human genetic validation, recent evidence has pointed to Nav1.7 as the most promising sodium channel target for new analgesics (Dib-Hajj et al., 2007). The syndrome of Congenital Indifference to Pain has been linked to nonsense mutations in Nav1.7 in individuals from 12 families representing 8 nationalities. These individuals have a complete inability to sense pain, and yet they appear normal in all other respects, including intelligence, physical development, motor and autonomic reflexes, and sensation with the exception of the sense of smell. Additionally, several human gain of function mutations have been identified in Nav1.7 channels and shown to be linked to inherited pain disorders such as Inherited Erythromelalgia or Paroxysmal Extreme Pain Disorder (Dib-Hajj et al., 2007). Interestingly, the human loss of Nav1.7 function was not replicated in mice, where a conventional knockout is lethal, and a nociceptor-specific knockout (Nassar et al., 2004) develops normally but displays a mild pain phenotype (resistance to inflammatory pain).
Given the possibility of compensatory changes in genetically derived disease models, the most convincing target validation is derived from pharmacological proof of concept in an animal model reflecting human physiology. Such validation can be obtained through use of existing small molecule channel modulators, peptide neurotoxins, or antibodies specifically developed to inhibit channel function. Specific examples of target validation using existing drugs (lidocaine, tricyclic antidepressants) or peptides (Ziconotide and GxTX) are discussed below.
Systemic administration of the local anesthetic lidocaine is approved for the treatment of neuropathic pain (Priest and Kaczorowski, 2007). At clinically used concentrations, block of Nav1 channels appears to be the only mode of action of this agent. Similarly, tricyclic antidepressants, such as amitriptyline, which are efficacious in treating neuropathic pain, possess a broad spectrum of pharmacological activities including inhibition of Nav1 channels. A comparison between therapeutic efficacy and ability to inhibit Nav1.7 channels for two classes of antidepressants, tricyclics and serotonin reuptake inhibitors, suggests a role of sodium channel inhibition in the efficacy of these compounds in treating neuropathic pain (Dick et al., 2007). Tricyclic antidepressants were potent sodium channel inhibitors and their potency in binding to the inactivated state of Nav1.7 was within the range of plasma concentrations required for the treatment of neuropathic pain. In contrast, antidepressant serotonin reuptake inhibitors that are not effective in treating post-herpetic neuralgia or diabetic neuropathy were weaker inhibitors of Nav1.7, with in vitro potencies mostly above their therapeutic concentration ranges. These data suggest that inhibition of voltage-gated sodium channels may contribute to the anti-hyperalgesic efficacy of tricyclic antidepressants and is further support for targeting sodium channels to treat chronic pain with more potent and selective inhibitors.
Another example of pharmacological validation comes from the use of peptides, such as Ziconotide, a synthetic analogue of a peptide contained in cone snail venom, and a potent blocker of the N-type voltage-gated calcium channel, Cav2.2 (Miljanich, 2004). Ziconotide was developed clinically as a treatment for intractable pain by intrathecal administration. In vivo pharmacological results with Ziconotide strongly support the hypothesis that a systemic small molecule inhibitor of Cav2.2 should be useful for treating pain. Similarly, in vitro studies with the spider toxin peptide GxTX demonstrate that the pancreatic β cell delayed rectifying potassium channel, Kv2.1 (KCNB1) is a potential target for enhancing glucose-dependent insulin secretion, and thus for the treatment of type II diabetes (Herrington et al., 2006). Since peptidyl modulators of ion channels are abundant in venoms, they are rich sources for reagents useful in proof of concept studies. In addition, small molecule natural product channel modulators, including indole diterpene blockers of high conductance, calcium-activated potassium channels (KCNMA1), have been used as probes in target validation studies (Garcia and Kaczorowski, 2001).
Lead Identification and Characterization
The most challenging aspect of ion channel drug discovery may be the identification of an appropriate, small molecule drug lead with desirable chemical properties that qualify it for exploration by medicinal chemistry (MacCoss and Baillie, 2004). This is a key element in successful ion channel preclinical drug development. Ion channels have traditionally been considered difficult targets to engage in high throughput functional screening formats, and large scale screening campaigns have often yielded a paucity of potent, selective hits. The scarceness of acceptable ion channel leads may derive from the long-standing emphasis within the pharmaceutical industry on programs directed at G protein–coupled receptors, kinases, and other enzymes, leading to biased sample collections. Successful targeting of ion channels critically requires robust and sensitive, mechanism-based, HTS technologies that can detect a vanishingly small number of actives in large compound libraries, or in other words, can “find a needle in a haystack” (Herrington et al., 2005; Garcia and Kaczorowski, 2006). Implementation of reliable and informative counter-screens for likely off-target activities is essential for meaningful hit assessment and lead prioritization to ensure that resources are not wasted in pursuing nondevelopable chemical structures. Recent introduction of fluorescent, cell-based screening technologies has enabled dependable HTS and ultra high throughput screening (UHTS) paradigms, allowing screening of libraries consisting of millions of compounds in a timely, cost effective manner, and thereby allow detection of true lead structures with adequate initial potency, selectivity across ion channel superfamilies, and defined mechanisms of action. Early mechanistic studies are also important, since a particular mechanism of action may lead to functional selectivity for the target channel through state dependence and/or use-dependent channel inhibition.
In one general configuration, HTS assays can be instituted for many different types of ion channels by establishing cell lines heterologously expressing the target in a context where changes in the activity of the channel of interest can affect the cellular plasma membrane potential. Potential sensitive fluorescent dyes can then be used to monitor changes in membrane potential of such cells grown in high density, multiwell format plates during screening procedures (Gonzalez and Maher, 2002). Detection of an active compound is achieved if addition of test compound causes a corresponding change in membrane potential. This strategy works well to identify sodium or potassium channel modulators, and such paradigms can be used to screen for ligand-gated ion channel modulators, as well. Identification of both channel inhibitors and channel openers can be accomplished using this general screening method (Garcia et al., 2007). For detecting voltage-gated calcium channel effectors, a similar approach can be adopted, except that fluorescent dye indicators are employed to monitor the concentration of intracellular calcium. Cotransfection with an inwardly rectifying potassium channel, together with the controlled variation in extracellular potassium concentration, has been used to control cellular membrane potential, in order to establish the most sensitive and mechanistically meaningful assay configuration (Xia et al., 2004). All of these screening formats are amenable to application of ultrahigh throughput automation strategies.
Until recently, the characterization of screening hits using manual voltage clamp techniques was slow and tedious, because of the low throughput inherent to this technique. However, secondary screening of initial hits has now been revolutionized with the use of new automated patch clamp technologies that can confirm a compound's direct functional effects on the channel, determine its selectivity across superfamilies of ion channels, and provide mechanistic insights, all accomplished in a very rapid fashion. Several different platforms are commercially available with specific features determining their optimal application, from specializing in high throughput analysis to more quantitative measurements of channel activity using complex voltage protocols (Priest et al., 2007). Hopefully, automated patch clamp technologies will soon be adapted to UHTS requirements, enabling a new set of UHTS approaches toward finding novel ion channel modulators. Presently, the combination of biochemical and biophysical approaches is needed to identify useful lead structures. Together, these strategies, along with more classical drug development techniques, provide a means for discovering and optimizing the activity of potential ion channel drug development candidates for almost any member of the various ion channel super families.
Case Study: Discovering Inhibitors of Voltage-gated Sodium Channels
As a way of illustrating the issues related to ion channel drug discovery outlined above, the remainder of this article will describe a case study focusing on the identification of voltage-gated sodium channel inhibitors to treat chronic neuropathic pain. Treatment of pain is a serious medical issue and there is a major effort in the pharmaceutical industry to develop new therapies for this condition. In particular, the treatment of neuropathic pain, defined as “chronic pain that results from a primary lesion or dysfunction of the peripheral nervous system” by the International Association for the Study of Pain (IASP), remains a major unmet medical need.
It is clear that voltage-gated sodium (Nav1) channels play a key role in the origination and propagation of sensory nerve action potentials necessary for pain signaling. Local applications of nonsubtype-selective sodium channel blockers, such as novocaine, provide complete pain relief through conduction block. However, this approach to pain relief is limited to very few applications, such as dental procedures, since sodium channels are also vital to conduction in the heart, CNS, skeletal muscle, and nonnociceptive sensory neurons. The Nav1 super family is comprised of 10 members (Yu and Catterall, 2004). Seven of these subtypes, Nav1.1, Nav1.3, Nav1.5, Nav1.6, Nav1.7, Nav1.8, and Nav1.9, are present in the peripheral nervous system (PNS). Of these, Nav1.7, Nav1.8, and Nav1.9 are expressed predominantly in nociceptive neurons, and Nav1.3 is predominantly embryonic, but is up-regulated in the adult PNS after injury. This limited expression pattern makes these subtypes attractive targets for the development of novel analgesic agents. However, their relative contribution to pain signaling, and specifically to neuropathic pain signaling, is unclear and may vary with different etiologies and sensory qualities of pain.
In the absence of molecular selectivity for one Nav1 subtype, it is possible to specifically target Nav1 channels in a given conformational state while preserving sodium channel–dependent impulse conduction. This type of state-dependent inhibition is the basis for the therapeutic window seen with sodium channel blocking anticonvulsants and antiarrhythmics, such as lamotrigine and lidocaine. These drugs have higher affinity for channels in the open and/or inactivated states than for resting, closed channels. This mechanism of inhibition favors binding in rapidly firing or partially depolarized tissues. Neuropathic pain should be sensitive to this inhibitory mechanism, since it is thought to arise from injury-induced areas of depolarizations, a hypothesis that is supported by the clinical efficacy of lidocaine administered systemically at subanesthetic doses. Moreover, nonsubtype-selective, state-dependent block may afford the greatest efficacy, since individual knockout of Nav1.3, Nav1.7, Nav1.8, or Nav1.9 did not provide convincing evidence for a dominant role of any of these channels in neuropathic pain signaling.
Based on this rationale, a decision was made to initially pursue nonsubtype selective, state-dependent Nav1 inhibitors, while monitoring molecular selectivity by testing compounds of interest on Nav1.7, Nav1.5 (the primary cardiac sodium channel) and Nav1.8 in parallel.
A membrane potential–based assay was used to screen ∼200,000 compounds on Nav1.8 stably expressed in a recombinant cell line. This HTS assay was based on fluorescence resonance energy transfer (FRET) between two members of a membrane potential–sensitive dye pair developed by Aurora Biosciences (Priest et al., 2004). Nav1.8 channels were preincubated with test compound and the chemical agonist deltamethrine in the absence of extracellular sodium. Subsequent addition of sodium resulted in membrane depolarization and Nav1 block was quantified as interference with that cellular depolarization process.
Although the initial screen on Nav1.8 yielded a variety of hits, only a single compound was considered a viable lead for medicinal chemistry efforts. Before committing resources to this lead, the compound, a disubstituted succinimide termed BPBTS (N-{[2'-(aminosulfonyl) biphenyl-4-yl] methyl }-N'-(2,2'-bithien-5-yl methyl)succinimide), was examined in detail by manual whole cell voltage clamp. BPBTS was found to inhibit all Nav1 subtypes with similar potency, and inhibition was dependent on membrane potential and stimulation frequency. This inhibitory mechanism was consistent with higher affinity of the compound for channels in the open and inactivated state, compared with channels in the resting state. In addition, BPBTS was two orders of magnitude more potent than the clinically used anticonvulsant and antiarrhythmic Nav1 blockers, inhibiting the inactivated state of Nav1.8, Nav1.7, Nav1.5, and Nav1.2, with Ki values of 0.09, 0.15, 0.08, and 0.14 μM and the resting state with Kr values of 1.5, 1.3, 0.3, and 1.2 μM, respectively (Priest et al., 2004).
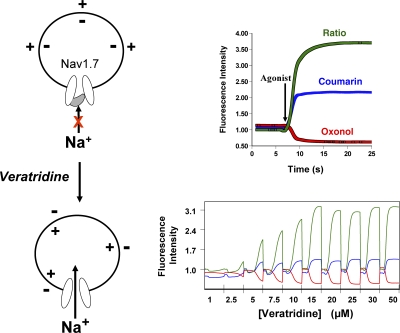
As such, BPBTS was an attractive lead for medicinal chemistry; its main liabilities being a poor pharmacokinetic profile. Over the course of profiling analogues of BPBTS, as well as published Nav1 inhibitors, using the membrane potential–based fluorescent screening assay, structure-based discrepancies between potencies determined in the fluorescent assay and by electrophysiology were noted for a few compounds. These discrepancies were traced to an interaction between these compounds and the agonist veratridine used to open Nav1.7 channels. Subsequently, the fluorescent assay was modified such that Nav1 channels were preincubated with test compound in physiological extracellular sodium concentrations and Nav1-dependent depolarization was initiated by agonist addition (Fig. 1). Channel inhibitory potencies measured in this modified assay correlated very well with the inactivated state inhibition determined by electrophysiology across many structural classes of Nav1 inhibitors (Felix et al., 2004; Liu et al., 2006).
Figure 1.
A functional, membrane potential FRET-based assay for Nav1.7 channels. In the absence of other ionic conductances that can hyperpolarize the cell, heterologous expression of Nav1.7 channels provides a system where at the cell resting membrane potential most channels will reside in the nonconducting inactivated state. Removal of fast inactivation by the addition of veratridine shifts the channel's equilibrium to the conductive, open state that allows sodium entry leading to cell depolarization. The changes in voltage can be monitored with a pair of FRET voltage-sensing dyes, coumarin and oxonol. Cell depolarization alters the distribution of oxonol across the membrane, causing a change in the FRET signal. In the presence of a Nav1.7 inhibitor, channel equilibrium shifts toward the inactivated, drug-bound conformation, reducing the number of channels that will be available for veratridine modification, and preventing the agonist-induced FRET signal. The dose–response curve for the veratridine-induced change in FRET signal is steep, suggesting that modification of a small number of Nav1.7 channels is sufficient to cause cell depolarization.
Although analogues of BPBTS failed to surpass the initial lead in potency, medicinal chemistry succeeded at improving the pharmacokinetic profile, eventually generating trans-N-{[2'-(aminosulfonyl)biphenyl-4-yl]methyl }-N-methyl-N'-[4-(trifluoromethoxy)benzyl]cyclopentane-1,2-dicarboxamide (CDA54) with 44% oral bioavailability, one hour half life, and a clearance rate of 14 ml/min/kg, which was profiled extensively in vivo (Brochu et al., 2006). In two rat models of neuropathic pain, CDA54 (10 mg/kg, given orally) significantly reduced nerve injury–induced behavioral hypersensitivity by 44–67%. The same dose/plasma concentration of CDA54 did not affect acute nociception (rat hot plate assay), motor coordination (rat rotorod assay), or cardiac conduction (electrophysiological parameters measured in the cardiovascular dog). These properties are in contrast to those of current sodium channel blockers used in the clinic, which cause impaired motor coordination in rats and CNS side effects in man at all efficacious doses. Interestingly, upon oral dosing, the brain to plasma ratio for CDA54 was 0.03. In contrast, clinically used Nav1 blockers accumulate in the CNS, with a brain to plasma ratio greater than 10 for mexiletine. These data obtained with CDA54 strongly suggested that inhibition of PNS sodium channels alone is efficacious in animal models of neuropathic pain, and that limiting CNS exposures of Nav1 inhibitors is a viable approach to developing Nav1 inhibitors with an improved therapeutic index.
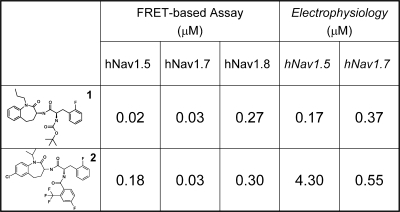
A UHTS campaign, using the membrane potential–based assay described to screen for inhibitors of Nav1.7, discovered the novel 1-benzazepin-2-one channel inhibitors (Hoyt et al., 2007; Williams et al., 2007). This class of inhibitors demonstrated a defined structure–activity relationship and, when evaluated in vivo, members of this series were orally efficacious in rodent neuropathic pain and epilepsy models. Importantly, some members of this class displayed molecular selectivity for Nav1.7 channels (Williams et al., 2007). For example, compound 2 of Fig. 2 was highly state dependent and ∼10-fold selective for Nav1.7 over Nav1.8 and Nav1.5. The most potent, albeit not subtype-selective, member of this class of Nav1.7 inhibitors (compound 1, BNZA; Fig. 2) was tritiated. [3H]BNZA binds with high affinity (Kd of 1.6 nM) to recombinant Nav1.7 channels. This is the first demonstration of high-affinity ligand binding to Nav1.7 and provides a valuable screening tool with which to search for Nav1.7-selective compounds. Data obtained with the 1-benzazepin-2-one structural series suggest that Nav1.7-selective analogues can be identified, and with the appropriate pharmacokinetic and drug metabolism properties, such compounds could be developed as analgesic agents, potentially displaying improved tolerability over existing drugs used to treat neuropathic pain. Support for the feasibility of developing subtype-selective sodium channel inhibitors as novel analgesics comes from the recent report of a high affinity Nav1.8 selective agent, which given intraperitoneally was efficacious in a wide range of rodent pain models (Jarvis et al., 2007).
Figure 2.
1-Benzazepin-2-one Nav1 inhibitors. The structures of two 1-benzazepin-2-one Nav1 inhibitors are illustrated together with their potencies for hNav1.5, hNav1.7, and hNav1.8 channels as determined in functional membrane potential, FRET-based assays. The estimated potencies of these compounds for the inactivated state of hNav1.5 and hNav1.7 channels, as determined from electrophysiological recordings, are also presented. Note that only compound 2 displays selectivity for the hNav1.7 channel. Both compounds are weaker inhibitors of the hNav1.8 channel.
A potential alternative approach to searching for subtype-selective sodium channel inhibitors would be to screen for compounds that target channel gating mechanisms. Several peptides have previously been shown to modify gating of sodium channels, but few small molecules, especially inhibitors, have been described to function in this fashion. One such agent is ProTx-II, a 30–amino acid peptide purified from tarantula venom; this peptide blocks sodium channels and shows selectivity for Nav1.7 (Smith et al., 2007). ProTx-II binds to the resting state of sodium channels and shifts the voltage dependence of channel activation to more depolarized potentials. Strong depolarizations overcome channel inhibition, which is a hallmark of this type of gating modifier peptide. One possible strategy to identify small molecule mimetics of a gating modifier peptide is to radiolabel ProTx-II in biologically active form, and to develop a binding assay with Nav1.7 channels heterologously expressed in a cell line. Screening for small molecules that modulate ProTx-II binding could reveal new classes of channel inhibitors that partition into the membrane and interfere with movement of the gating paddle, thereby preventing channel opening. An added advantage of this type of UHTS is that high concentrations of test compounds could be employed, a situation that is precluded in dye-based screening due to fluorescence interference that typically occurs with high concentrations of many small organic molecules. Given that some gating modifier peptides bind to regions that are unique to specific channels within a super family, subtype-selective inhibitors might be identified using such a strategy.
Conclusions
Although identification of novel sodium channel inhibitors was used to illustrate current molecular approaches to ion channel drug discovery, these principles can be generalized to any ion channel target. It would appear that establishment and orchestration of functional UHTS is no longer the rate-determining step in ion channel drug development. Rather, the synthesis of ion channel friendly small molecule libraries to facilitate lead discovery, and the establishment of meaningful clinical paradigms, including development of target engagement indices, to test rigorously an agent's therapeutic potential in man, are now the key elements to focus on in order to make development of new ion channel drugs a successful enterprise.
Acknowledgments
The authors would like to thank members of the Ion Channel, Medicinal Chemistry, and Pharmacology Departments at the Merck Research Laboratories in Rahway, NJ, who contributed to the work cited in this review, and Dr. Steve Hess for his editorial suggestions.
Abbreviations used in this paper: BPBTS, N-{[2'-(aminosulfonyl)biphenyl-4-yl]methyl }-N'-(2,2'-bithien-5-ylmethyl)succinimide; FRET, fluorescence resonance energy transfer; HTS, high throughput screening; Nav, voltage-gated sodium channel; PNS, peripheral nervous system; UHTS, ultra HTS.
References
- Ashcroft, F. 2000. Ion Channels and Disease. Academic Press, San Diego, CA. 481 pp..
- Brochu, R.M., I.E. Dick, J.W. Tarpley, E. McGowan, D. Gunner, J. Herrington, P.P. Shao, D. Ok, C. Li, W.H. Parsons, et al. 2006. Block of peripheral nerve sodium channels selectively inhibits features of neuropathic pain in rats. Mol. Pharmacol. 69:823–832. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj, S.D., T.R. Cummins, J.A. Black, and S.G. Waxman. 2007. From genes to pain: Na(v)1.7 and human pain disorders. Trends Neurosci. 30:555–563. [DOI] [PubMed] [Google Scholar]
- Dick, I.E., R.M. Brochu, Y. Purohit, G.J. Kaczorowski, W.J. Martin, and B.T. Priest. 2007. Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J. Pain. 8:315–324. [DOI] [PubMed] [Google Scholar]
- Felix, J.P., B.S. Williams, B.T. Priest, R.M. Brochu, I.E. Dick, V.A. Warren, L. Yan, R.S. Slaughter, G.J. Kaczorowski, M.M. Smith, and M.L. Garcia. 2004. Functional assay of voltage-gated sodium channels using membrane potential-sensitive dyes. Assay Drug Dev. Technol. 2:260–268. [DOI] [PubMed] [Google Scholar]
- Garcia, M.L., and G.J. Kaczorowski. 2001. Pharmacology of high-conductance, Ca2+-activated potassium channels. In Potassium Channels in Cardiovascular Biology. A.A. Rusch, editor. Academic/Plenum Publishers, New York. 219–234.
- Garcia, M.L., and G.J. Kaczorowski. 2005. Potassium channels as targets for therapeutic intervention. Sci. STKE. 2005:pe46. [DOI] [PubMed] [Google Scholar]
- Garcia, M.L., and G. Kaczorowski. 2006. Critical success. European Pharmaceutical Review. 11:42–45. [Google Scholar]
- Garcia, M.L., D.-M. Shen, and G.J. Kaczorowski. 2007. High-conductance calcium-activated potassium channels: validated targets for smooth muscle relaxants? Expert Opin. Ther. Patents. 17:832–842. [Google Scholar]
- Gonzalez, J.E., and M.P. Maher. 2002. Cellular fluorescent indicators and voltage/ion probe reader (VIPR) tools for ion channel and receptor drug discovery. Receptors Channels. 8:283–295. [PubMed] [Google Scholar]
- Herrington, J., O. McManus, and L. Kiss. 2005. The road ahead and how to get there. European Pharmaceutical Review. 10:79–84. [Google Scholar]
- Herrington, J., Y.P. Zhou, R.M. Bugianesi, P.M. Dulski, Y. Feng, V.A. Warren, M.M. Smith, M.G. Kohler, V.M. Garsky, M. Sanchez, et al. 2006. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes. 55:1034–1042. [DOI] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion Channels of Excitable Membranes. Third edition. Sinauer Associates, Inc., Sunderland, MA. 814 pp.
- Hoyt, S.B., C. London, D. Gorin, M.J. Wyvratt, M.H. Fisher, C. Abbadie, J.P. Felix, M.L. Garcia, X. Li, K.A. Lyons, et al. 2007. Discovery of a novel class of benzazepinone Na(v)1.7 blockers: potential treatments for neuropathic pain. Bioorg Med Chem Lett. 17:4630–4634. [DOI] [PubMed] [Google Scholar]
- Jarvis, M.F., P. Honore, C.-C. Shieh, M. Chapman, S. Joshi, X.-F. Zhang, M. Kort, W. Carroll, B. Marron, R. Atkinson, et al. 2007. From the cover: A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA. 104:8520–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifton, R.P., A.G. Gharavi, and D.S. Geller. 2001. Molecular mechanisms of human hypertension. Cell. 104:545–556. [DOI] [PubMed] [Google Scholar]
- Liu, C.J., B.T. Priest, R.M. Bugianesi, P.M. Dulski, J.P. Felix, I.E. Dick, R.M. Brochu, H.G. Knaus, R.E. Middleton, G.J. Kaczorowski, et al. 2006. A high-capacity membrane potential FRET-based assay for NaV1.8 channels. Assay Drug Dev. Technol. 4:37–48. [DOI] [PubMed] [Google Scholar]
- MacCoss, M., and T.A. Baillie. 2004. Organic chemistry in drug discovery. Science. 303:1810–1813. [DOI] [PubMed] [Google Scholar]
- Miljanich, G.P. 2004. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 11:3029–3040. [DOI] [PubMed] [Google Scholar]
- Nassar, M.A., L.C. Stirling, G. Forlani, M.D. Baker, E.A. Matthews, A.H. Dickenson, and J.N. Wood. 2004. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA. 101:12706–12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest, B.T., and G.J. Kaczorowski. 2007. Blocking sodium channels to treat neuropathic pain. Expert Opin. Ther. Targets. 11:291–306. [DOI] [PubMed] [Google Scholar]
- Priest, B.T., M.L. Garcia, R.E. Middleton, R.M. Brochu, S. Clark, G. Dai, I.E. Dick, J.P. Felix, C.J. Liu, B.S. Reiseter, et al. 2004. A disubstituted succinamide is a potent sodium channel blocker with efficacy in a rat pain model. Biochemistry. 43:9866–9876. [DOI] [PubMed] [Google Scholar]
- Priest, B.T., A.M. Swensen, and O.B. McManus. 2007. Automated electrophysiology in drug discovery. Curr. Pharm. Des. 13:2325–2337. [DOI] [PubMed] [Google Scholar]
- Smith, J.J., T.R. Cummins, S. Alphy, and K.M. Blumenthal. 2007. Molecular interactions of the gating modifier toxin ProTx-II with Nav 1.5: implied existence of a novel toxin binding site coupled to activation. J. Biol. Chem. 282:12687–12697. [DOI] [PubMed] [Google Scholar]
- Williams, B.S., J.P. Felix, B.T. Priest, R.M. Brochu, K. Dai, S.B. Hoyt, C. London, Y.S. Tang, J.L. Duffy, W.H. Parsons, et al. 2007. Characterization of a new class of potent inhibitors of the voltage-gated sodium channel Nav1.7. Biochemistry. 46:14693–14703. [DOI] [PubMed] [Google Scholar]
- Xia, M., J.P. Imredy, K.S. Koblan, P. Bennett, and T.M. Connolly. 2004. State-dependent inhibition of L-type calcium channels: cell-based assay in high-throughput format. Anal. Biochem. 327:74–81. [DOI] [PubMed] [Google Scholar]
- Yu, F.H., and W.A. Catterall. 2004. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE. 2004:re15. [DOI] [PubMed] [Google Scholar]