The purpose of the Perspectives in General Physiology is to provide a forum where scientific uncertainties or controversies can be discussed in an authoritative, yet open, manner.
The Perspectives are solicited by the editors—often based on recommendations by members of the editorial advisory board. To frame the issue, two or more experts are invited to present brief points of view on the problem, which are published consecutively in the Journal. The comments and opinions expressed in the Perspectives are those of the authors and not necessarily those of the Editors or the Editorial Advisory Board. The Perspectives are accompanied by a few editorial paragraphs that introduce the problem—and invite the submission of comments, in the form of letters to the editor, which are published in a single, predetermined issue (usually three months after publication of the Perspective).
In this issue of the Journal, Gregory Kaczorowski, Owen McManus, Birgit Priest and Maria Garcia (Merck Research Laboratories, Rahway, NJ), Kenneth Rhodes and James Trimmer (Biogen Idec, Cambridge, MA, and University of California, Davis, CA), Jon Sack, Oleg Shamotienko, and Oliver Dolly (Dublin City University, Dublin, Ireland), Jens Lundbæk (Technical University of Denmark, Lyngby, Denmark), and Stephen Tucker and Thomas Baukrowitz (University of Oxford, Oxford, UK, and Friedrich Schiller University, Jena, Germany) provide different perspectives on the challenges involved in the development of new drugs that target ion channels—or any other membrane protein for that matter.
The Perspectives were inspired by a workshop on How to Drug an Ion Channel, which was organized by Jon Sack and Oliver Dolly and held at Dublin City University in July 2007. The contributions to the Perspectives represent only a fraction of the presentations at the workshop; they were chosen because they focus on a set of recurring topics in the discussions at the workshop.
The history of drug development has rarely been punctuated by triumphs of rational design. Aside from a few notable exceptions (e.g., Van Epps, H.L. 2006. J. Exp. Med. 203:259), Pasteur's statement that “In the fields of observation chance favors only the prepared mind” characterizes most successful therapeutic ventures (e.g., Ban, T.A. 2006. Dialogues Clin. Neurosci. 8:335–344). The serendipitous routes taken by researchers are exemplified by the discoveries of antibiotics to treat bacterial infections (Moberg, C.L., and Z.A. Cohn. 1990. Launching the Antibiotic Era. The Rockefeller University Press, New York), diuretics (Eknoyan, G. 1997. Kidney Int. Suppl. 59:S118–S126) and calcium channel blockers (Fleckenstein, A. 1983. Circ. Res. 52:I3–I16) to treat cardiovascular diseases, valproate to treat seizures (Löscher 1999 #46890), the sulfonylureas to treat diabetes (Kleinsorge, H. 1998. Exp. Clin. Endocrinol. Diabetes. 106:149–151), and lithium and chlorpromazine to treat mood disorders and psychoses (Jacobsen 1986 #48080). Not surprisingly, therefore, drugs were introduced in clinical practice without clear understanding of their molecular target(s) and mechanism(s) of action. Indeed, the mechanism(s) underlying many drugs in current use, such as valproate (Rosenberg, R. 2007. Cell. Mol. Life Sci. 64:2090–2103) or lithium (Quiroz, J.A., T.D. Gould, and H.K. Manji. 2004. Mol. Interv. 4:259–272), have yet to be fully elucidated.
In the genomic era, the focus of pharmaceutical research is on specific molecular targets that are relevant for the disease of interest, with potential drug candidates being identified using appropriate high-throughput in vitro screens. Because ion channels play key roles in many different physiological processes, they become important potential drug targets; they also pose particular problems because of the existence of numerous channel isoforms with differential cellular and subcellular expression, which complicates both the identification of the relevant channel isoform(s) that should be targeted and the development of drugs with sufficient selectivity for the intended target(s).
A key consideration in drug development thus becomes target identification and validation and, once this has been accomplished, the identification of small molecule lead compounds that allow for exploration by medicinal chemistry methods. In this series, Kaczorowski et al. summarize the challenges, which include the development of appropriate high-throughput screens, using inhibitors of voltage-dependent sodium channels as a case study. A key conclusion is that it, even in the absence of molecular selectivity, is possible to target a desired channel population by exploiting state-dependent drug-channel interactions that favor the drug-induced stabilization of a specific state in channels with a particular profile, such as the inactivated state in voltage-dependent channels in rapidly firing or partially depolarized cells.
Target identification becomes a particular problem in cases where it may be a heterooligomeric combination of different subunits, as is the case for many potassium channels. Because different heterooligomers may have different pharmacological profiles and cellular expression, the challenge of target validation becomes acute. This challenge is discussed by Rhodes and Trimmer and by Sack et al. Rhodes and Trimmer address the issues in a critical analysis of the strengths and potential pitfalls associated with the use of antibody-based methods. Sack et al. present a complementary approach using concatenated potassium channel constructs in which the subunits are linked into a single open reading frame, such that the subunit stoichiometry and organization is well defined and suitable for defining the pharmacological profile of potassium channels with the equivalent subunit composition.
Once a target has been validated, and a suitable drug lead has been discovered, determining its mechanism of action is rarely trivial. In the case of ion channels, maybe the simplest mechanism is the classic channel or pore block, in which the drug in question binds in the channel's pore to block ion movement. In many cases, however, a drug modulates channel function by stabilizing either a nonconducting or a conducting channel state (to either inhibit or potentiate channel function), through allosteric mechanisms that are distinct from a channel, or pore, block. Generally, any molecule or experimental maneuver that alters the free energy difference between different channel states, or the kinetics of interconversion between different states, will be a modulator of channel function. The shift between different channel states is usually ascribed to different drug affinities to the different channel states, but channel function may be altered by mechanisms that do not involve direct binding to the target, as in the screening of interfacial charges, which alters the interfacial potential and thus the gating of voltage-dependent channels.
It is in this context important that many drugs are amphiphiles, meaning that they will adsorb at the lipid bilayer/solution interface to alter lipid bilayer properties, which in turn may alter the free energy difference associated with membrane protein conformational transitions (Andersen, O.S., and R.E. Koeppe II. 2007. Annu. Rev. Biophys. Biomol. Struct. 36:107–130). That is, amphiphilic drugs may alter the function of a channel (or any other membrane protein) without direct binding to the channel. The mechanistic basis for such bilayer-dependent changes in channel function is discussed by Jens Lundbæk, who presents a framework for how to approach possible bilayer-dependent drug effects due to amphiphile-induced changes in bilayer physical properties—by examining how the drug in question alters the function of well-characterized reporter channels, such as the gramicidin channels, or by testing how amphiphiles with well-characterized bilayer-modifying affects the target channel's function. In addition to the regulation caused by changes in bilayer physical properties, channel function may also be regulated by changes in surface density of specific lipids, such as the polyphosphoinositides, in which case the distinction between specific and nonspecific effects become particularly challenging. This regulation, and the challenges associated with dissecting out the underlying mechanism, is discussed by Tucker and Baukrowitz, who review the complex interplay between polyphosphoinositides and other negatively charged lipids and lipid metabolites in the regulation of inward rectifier potassium channels.
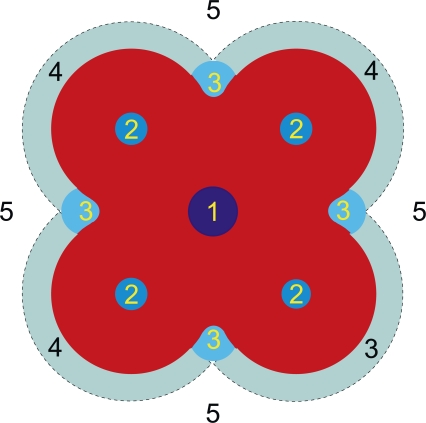
Overall, these Perspectives show that ion channel function can be altered by many different, nonexclusive mechanisms. A perusal of the contributions shows that one in general needs to consider, at least five different mechanisms of drug action—ranging from the classic pore block (1 in Fig. 1), over allosteric regulation due to (specific) drug binding to the target protein (2 and 3 in Fig. 1), to the allosteric regulation that arises from (more nonspecific) drug-induced changes in lipid packing adjacent to the protein (3 and 4 in Fig. 1) and in bilayer material properties (5 in Fig. 1).
Figure 1.
Schematic representation of different, nonexclusive mechanisms by which one can drug an ion channel: (1) a drug may bind in the pore to block ion movement; (2) a drug may bind to a site wholly formed by the protein, to either inhibit or potentiate the channel function by altering the free energy difference between different channel states; (3) an amphiphilic drug may bind specifically to a defined site composed of both the protein and the bilayer lipids in which case its effects could also involve changes in the bilayer deformation energy associated with channel conformational changes; (4) an amphiphilic drug may accumulate nonspecifically at the protein/bilayer interface to alter local lipid packing, and thereby alter the bilayer deformation energy contribution to channel's conformational changes; and (5) an amphiphilic drug may adsorb at the lipid bilayer/solution interface to alter the bilayer deformation energy associated with the channel conformational changes.
Because a given drug could exert its action by any combination of the above mechanisms, and because any amphiphilic molecule will adsorb at the bilayer/solution interface, one of the challenges in future drug development becomes to identify the relative contribution of each of these mechanisms to a potential drug's overall effects—for then to optimize for the desired drug effects. It is in context important that drugs with a high affinity for the lipid bilayer/solution interface, relative to sites 1–3, may exert a significant part of their effects through mechanisms 4 and 5, which would make them promiscuous modulators of membrane protein function.
Letters to the editor related to these Perspectives will be published in the August 2008 issue of the Journal of General Physiology. Letters to the editor should be received no later than Monday June 25, 2008, in order to allow for editorial review. The letters may be no longer than two printed pages (approximately six double-spaced pages) and will be subject to editorial review. They may contain no more than one figure, no more than 15 references, and no significant references to unpublished work. Letters should be prepared according to the Journal's instructions and can be submitted electronically at www.jgp.org, as an e-mail attachment to jgp@mail.rockefeller.edu.