Abstract
The currently circulating H3N2 and H1N1 subtypes of influenza A virus cause a transient, febrile upper respiratory illness in most adults and children (“seasonal influenza”), but infants, the elderly, immunodeficient and chronically ill persons may develop life-threatening primary viral pneumonia or complications such as bacterial pneumonia. By contrast, avian influenza viruses such as the H5N1 virus that recently emerged in Southeast Asia can cause severe disease when transferred from domestic poultry to previously healthy people (“avian influenza”). Most H5N1 patients present with fever, cough and shortness of breath that progress rapidly to adult respiratory distress syndrome. In seasonal influenza, viral replication remains confined to the respiratory tract, but limited studies indicate that H5N1 infections are characterized by systemic viral dissemination, high cytokine levels and multiorgan failure. Gastrointestinal infection and encephalitis also occur. The licensed anti-influenza drugs (the M2 ion channel blockers, amantadine and rimantadine, and the neuraminidase inhibitors, oseltamivir and zanamivir) are beneficial for uncomplicated seasonal influenza, but appropriate dosing regimens for severe seasonal or H5N1 viral infections have not been defined. Treatment options may be limited by the rapid emergence of drug-resistant viruses. Ribavirin has also been used to a limited extent to treat influenza. This article reviews licensed drugs and treatments under development, including high-dose oseltamivir; parenterally administered neuraminidase inhibitors, peramivir and zanamivir; dimeric forms of zanamivir; the RNA polymerase inhibitor T-705; a ribavirin prodrug, viramidine; polyvalent and monoclonal antibodies; and combination therapies.
Keywords: Influenza, Influenza virus, Avian influenza, H5N1 influenza virus, Adamantane, Neuraminidase, Peramivir, Oseltamivir, Zanamivir, Antiviral therapy
1. Introduction: influenza viruses
Influenza viruses have a multipartite, negative-sense, single-stranded RNA genome and a lipid envelope. They are divided into three genera, A, B and C within the family Orthomyxoviridae, based on the antigenic properties of the viral nucleoprotein. Influenza B and C viruses principally infect humans, usually causing mild illness in children, and undergo only gradual antigenic variation (Wright and Webster, 2001). By contrast, the influenza A viruses are maintained in a vast natural reservoir in wild waterfowl and shorebirds, from which they emerge to cause disease in domestic poultry, horses, pigs and humans. Their ability to infect the human respiratory tract and the periodic emergence of antigenically novel agents through genomic reassortment enable the influenza A viruses to cause worldwide epidemics with high morbidity and mortality (“pandemic influenza”).
Individual isolates of influenza A virus are classified by subtype, based on the antigenic identity of two glycoproteins embedded in the virion's lipid envelope, the hemagglutinin (HA) and the neuraminidase (NA) (Fig. 1 ). Studies of avian viruses have identified 16 antigenically distinct variants of HA and 9 of NA, leading to the existence of a large number of viral subtypes with different HA/NA pairs. Protective immunity resulting from previous infection or vaccination is based principally on neutralizing antibodies against HA, and to a lesser extent on antibodies to NA (Hayden and Palese, 2002).
Fig. 1.
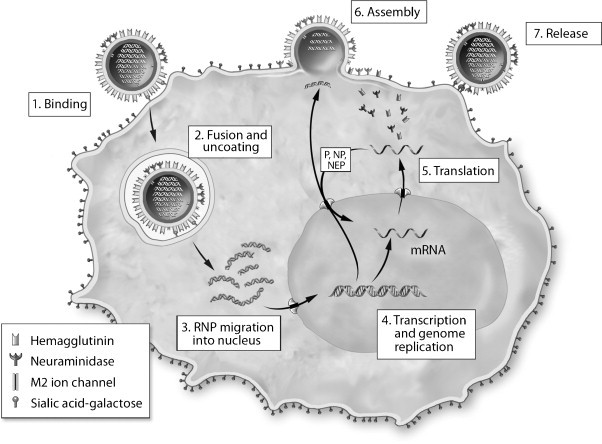
The influenza A virus replication cycle. The virion core contains eight RNA genome segments encapsidated by nucleoprotein (RNPs) and embedded together with associated polymerase (P) molecules in a matrix of M1 proteins. After binding to sialic acid–galactose linked to a cell-surface glycoprotein or glycolipid, the virion is taken up in an endocytic vesicle, where acidification triggers a conformational change that bring viral and endosomal membranes together. Acidification also produces a flow of protons through the M2 ion channel into the interior of the virion, causing the RNPs to dissociate from the M1 matrix and be released into the cytoplasm. They are then transported to the nucleus, where a viral polymerase complex performs transcription and genome replication. The resulting mRNAs move to the cytoplasm and are translated, producing new RNP protein components that are transported back to the nucleus to associate with nascent genome segments. The exit of new RNPs from the nucleus is aided by the viral NS2 (nuclear export protein, NEP). Meanwhile, nascent HA, NA and M2 molecules pass through the Golgi apparatus and undergo glycosylation before moving to the cell membrane. HA is also cleaved to form two chains linked by a disulfide bond. Virion assembly occurs as RNPs and M1 proteins associate with cytoplasmic tails of HA and NA. Successful release of new virus particles requires that NA cleave sialic acid from galactose on the cell surface or on adjacent virions to prevent HA binding.
The emergence of new pandemic subtypes of influenza A virus occurs through two pathways. The first is for an avian virus to infect and cause disease in a human (“avian influenza”) and evolve during the course of illness to become transmissible from person to person. The second is made possible by the multipartite genome, which permits a circulating seasonal agent to acquire new HA and NA genes through reassortment (“antigenic shift”), when a cell is co-infected by a seasonal virus and either an avian virus or one that has adapted to a mammalian host, such as pigs. Novel viruses emerged several times during the 20th century. In 1918, an avian H1N1 virus acquired the capacity for efficient respiratory transmission, resulting in a catastrophic pandemic. As the H1N1 virus continued to circulate, the progressive accumulation of mutations in the H1 and N1 genes permitted antigenically “drifted” variants to return and re-infect the same populations, causing recurrent outbreaks of illness (“seasonal influenza”). In 1957, a new reassortant virus appeared in which the two surface glycoproteins of H1N1 were replaced by avian H2 and N2. A second reassortment event produced H3N2 influenza in 1968, after which the H2N2 virus soon ceased to circulate.
As each new virus emerged and spread around the world, influenza morbidity and mortality increased markedly, then declined as the human population acquired some degree of immunity and the agent underwent progressive antigenic drift (Wright and Webster, 2001). A seasonal variant of the H1N1 virus reappeared in 1977, apparently through an accidental laboratory release, and it continues to circulate along with the H3N2 virus, both in humans and in pigs. Because an influenza A virus with a novel HA has not entered the human population since 1968, the emergence of a new pandemic strain is likely. Such a virus might obtain its HA from an agent such as the avian/swine reassortant H2N3 that has been detected in the USA (Ma et al., 2008), or from any of several purely avian strains that have infected humans during the past decade (see below).
At present, the most concerning scenario would be the pandemic spread of the highly virulent avian H5N1 virus that first appeared in Hong Kong in 1997, causing lethal primary viral pneumonia in 6 of 18 confirmed human infections (Chan, 2002, To et al., 2001, Yuen et al., 1998). Although cases ceased once all local poultry had been destroyed, the virus continued to spread and reassort among wild birds, and since 2003 it has re-emerged as the cause of poultry outbreaks and sporadic cases of severe human illness in several southeast Asian countries (Beigel et al., 2005, Li et al., 2004), and has been carried by migratory birds and the transport of domestic poultry to Indonesia and across Asia to Africa and Europe. As of December 17, 2007, a total of 340 confirmed human cases had been reported in 13 countries, with 209 deaths (Shinya et al., 2005, WHO, 2007). Fortunately, only a few instances of person-to-person transmission have been documented.
Vaccines are the principal defense against influenza, but because it takes time to produce an antigenically appropriate and immunogenic product and deliver it to entire populations, antiviral drugs will be a principal countermeasure to reduce the impact of a new pandemic (Monto, 2006, Moscona, 2005a). The current armamentarium of licensed anti-influenza medications consists of four drugs: two adamantanes, amantadine and rimantadine (Fig. 2 ), and two NA inhibitors, the oral drug oseltamivir (Tamiflu®) and the inhaled medication zanamivir (Relenza®) (Fig. 3 ) (De Clercq, 2006). Because their licensure was obtained based on studies in healthy adults with uncomplicated seasonal influenza, little is known about how these drugs should be used to treat severe disease. In particular, only anecdotal information is available on the treatment of fulminant H5N1 virus infections with oseltamivir. Therapy of severe influenza is made even more challenging by the rapid emergence of drug-resistant viruses, especially during treatment with the adamantanes, and by the lack of an approved parenteral medication that can be administered to patients incapable of swallowing or inhaling a drug. The only other licensed drug with anti-influenza activity, ribavirin (Virazole®), has been used to only a very limited extent to treat severe infections (see below) (Fig. 4 ).
Fig. 2.
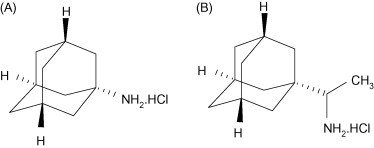
Licensed antivirals that block the influenza A M2 ion channel: amantadine (A); rimantadine (B) (Courtesy of Pieter Leyssen).
Fig. 3.
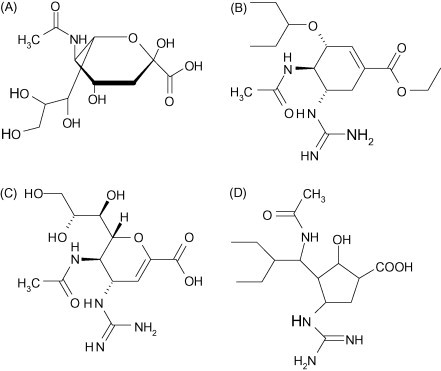
The structure of N-acetylneuraminic (sialic) acid (A) and antiviral drugs that compete with it for the active site of the influenza A or B neuraminidase: oseltamivir (B); zanamivir (C); peramivir (D) (Courtesy of Pieter Leyssen).
Fig. 4.
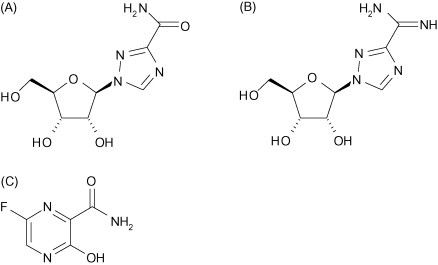
Nucleoside analogues that interfere with influenza virus RNA polymerase function: ribavirin (A); viramidine (B); T-705 (C) (Courtesy of Pieter Leyssen).
2. HA and NA
Influenza virions have two major surface glycoproteins: the HA, which binds to sialic acid covalently linked to the terminal galactose of an oligosaccharide on a glycoprotein or glycolipid, and the NA, which cleaves sialic acid from galactose (Figs. 1 and 3A). The presence of these two competing activities on the virion surface appears to have come about because sialic acid coupled to galactose is present not only on respiratory epithelial cells, but on a variety of molecules in respiratory mucus and even on oligosaccharide components of the HA itself. NA is therefore needed to minimize the binding of HA to “decoy” receptors, so as to enable virions to reach their target cells and to depart from them at the end of the replication cycle. If NA is genetically deleted from an influenza A virus, the initiation of infection is drastically inhibited, and nascent virions clump together and are unable to spread through a cell monolayer (Matrosovich et al., 2004, Wagner et al., 2002). Because HA and NA must work in tandem to achieve efficient infection, reassortant viruses with new combinations of these genes may not replicate efficiently.
Once an influenza A virus succeeds in crossing from its reservoir host to another species, the binding specificity of the virion HA appears to play a role in determining the outcome of infection. The HA of the currently circulating H3N2 and H1N1 viruses binds preferentially to sialic acid coupled with an α(2,6) linkage to galactose, which is present on cell-surface glycoproteins throughout the human respiratory tract, helping to explain why seasonal influenza occasionally develops into primary viral pneumonia (Ibricevic et al., 2006, Nicholls et al., 2007). By contrast, avian viruses make use of α(2,3)-linked sialic acids, which until the occurrence of viral pneumonia in patients in the 1997 Hong Kong H5N1 outbreak were thought to be absent from human respiratory epithelium. Recent studies have shown that, while α(2,6)-linked sialic acids predominate from the nasal cavities down to the bronchi, α(2,3)-linked sialic acids are present on bronchiolar lining cells, type II pneumocytes and alveolar macrophages, providing a possible explanation for the predominance of lower respiratory tract symptoms in H5N1 patients and the rapid development of ARDS (Ibricevic et al., 2006, Shinya et al., 2006, van Riel et al., 2006).
Although the nature and distribution of sialic acid–galactose linkages in the respiratory tract helps to explain some aspects of severe seasonal and avian influenza, it is clear that they are not the sole determinant of the course of infection. Thus, it has been shown that H5N1 viruses are able to replicate in explants of human upper respiratory tract tissues and that seasonal viruses can cause fatal infection in knockout mice lacking detectable α(2,6)-linked sialic acid and infect cells from which sialic acid has been enzymatically removed (Glaser et al., 2007, Nicholls et al., 2007, Stray et al., 2000).
3. Severe seasonal influenza
As pandemic viruses continue to circulate and undergo slow antigenic drift, they can return to infect the same populations, causing a generally mild illness in healthy children and adults characterized by fever, malaise, nasal congestion, sneezing and other upper respiratory symptoms that resolve within a few days without specific therapy. Young children experiencing their first bout of influenza tend to shed virus longer than persons who are reinfected by the same viral subtype, but they generally suffer no ill effects (Sato et al., 2005). However, seasonal viruses can also cause severe disease in very young infants, elderly people and those with immunodeficiency, cardiopulmonary disease or other chronic illnesses; in the United States, influenza A virus infections are responsible for nearly 40,000 deaths every year.
Severe seasonal influenza can occur when viral infection spreads from the upper to the lower respiratory tract to produce primary influenza pneumonia, characterized by respiratory distress, hypoxemia, hypotension and bilateral interstitial infiltrates (Wright and Webster, 2001). Bacterial pneumonia can also develop, either simultaneously with or following influenza virus infection. Limited studies have shown that while patients with uncomplicated influenza stop shedding virus in nasopharyngeal secretions by the third day of illness, virus can often be detected 5–7 days or longer after symptom onset in more severely ill hospitalized patients (Ison et al., 2003, Leekha et al., 2007, McGeer et al., 2007, Nicholson et al., 2000, Treanor et al., 2000). Prolonged viral shedding despite antiviral therapy is common in immunocompromised persons, especially transplant recipients (Ison et al., 2006). Drug-resistant viruses often emerge in this setting.
4. Avian influenza
Most avian influenza subtypes cause only mild infections, or no illness at all, in humans (Beare and Webster, 1991). When an H7N7 virus caused an outbreak among poultry workers in the Netherlands in 2003, 349 people developed conjunctivitis, 90 had an influenza-like illness, but one died from primary viral pneumonia (Fouchier et al., 2004, Koopmans et al., 2004). An H9N2 virus has caused an illness indistinguishable from seasonal influenza in a number of children in Hong Kong (Peiris et al., 1999, Butt et al., 2005) and an H7N3 virus caused conjunctivitis and mild respiratory tract illness in two confirmed cases in Canada (Tweed et al., 2004).
The H5N1 virus that has emerged in Southeast Asia is much more virulent than other avian influenza A viruses, and has an unusually broad host range, causing lethal disease in many species of wild and domestic birds and in small and large felines, rodents and primates. The virus recovered in Hong Kong in 1997 was more pathogenic for mice than other H5 subtype viruses (Dybing et al., 2000), and isolates recovered since 2003 have been even more virulent for mice and ferrets, capable without prior adaptation of causing severe pulmonary disease and spreading outside the respiratory tract to infect multiple organs, including the brain (Govorkova et al., 2007, Maines et al., 2005).
Although mild cases have been identified, most patients with H5N1 virus infections in Southeast Asia and Turkey have developed severe pulmonary disease. Patients typically present with fever, cough and difficulty breathing; primary viral pneumonia develops quickly, with pulmonary infiltrates on chest X-ray (Beigel et al., 2005, Buchy et al., 2007, De Jong et al., 2005a, De Jong et al., 2006, Oner et al., 2006, To et al., 2001, Yuen et al., 1998). Ventilatory support is usually required within 48 h of hospital admission. Limited patient studies indicate that, in contrast to seasonal influenza, signs of lower respiratory tract involvement are more prominent than upper respiratory tract symptoms, and viral titers are higher in pharyngeal than nasal secretions (De Jong et al., 2006). The illness progresses rapidly to acute respiratory distress syndrome (ARDS) and multiorgan failure, in a pattern reminiscent of severe acute respiratory syndrome (SARS) (Ng et al., 2006). Diarrhea is also common, and a few patients have presented with only severe diarrhea and/or neurologic changes that progressed rapidly to lethal encephalitis, with pulmonary disease either developing late or absent (Apisarnthanarak et al., 2004, De Jong et al., 2005a). Clinical laboratory and pathologic findings include leukopenia, marked lymphopenia with low T-cell counts, thrombocytopenia, hemophagocytosis, hepatic central lobular necrosis and acute renal tubular necrosis.
As noted, the rapid development of primary viral pneumonia in H5N1 infections may reflect the ability of the viral HA to bind to α(2,3)-linked sialic acid on human bronchioloalveolar cells, including type II pneumocytes. Because the latter cells give rise to new squamous type I pneumocytes following most types of lung injury, their loss through viral infection could lead to ARDS. Severe illness may also reflect more intense and widespread viral replication than occurs in seasonal influenza. A number of other factors may also contribute to the heightened virulence of the H5N1 virus, including greater suppression of host type I interferon responses by the NS1 protein (Garcia-Sastre, 2006), more efficient function of the RNA polymerase (Salomon et al., 2006) and altered activity of the PB1-F2 protein (Conenello et al., 2007). Enhanced viral replication is reflected in high titers of virus in nasopharyngeal or pharyngeal secretions and the presence of viral RNA in the spleen, small and large intestines and cerebrospinal fluid and in the blood of fatal cases, as detected by PCR (Buchy et al., 2007, De Jong et al., 2005a, De Jong et al., 2006, Uiprasertkul et al., 2005). In the series of patients studied by De Jong et al., extensive viral replication was accompanied by high levels of cytokines and chemokines (IP-10, MCP-1, IL-8, IL-6 and IL-10) in the plasma (2006). Titers were proportional to the pharyngeal viral load, and were highest in fatal cases.
The apparent ability of recent H5N1 isolates to induce more intense inflammatory responses than seasonal influenza viruses is mirrored by findings in the laboratory. Primary human macrophages infected with a 1997 Hong Kong virus produced significantly more TNF-α and interferon-β than cells infected with H3N2 or H1N1 seasonal viruses; the response profile was similar to that induced by lipopolysaccharide (Cheung et al., 2002, Seo et al., 2002). Recent H5N1 isolates also induced much stronger secretion of IP-10, IFN-β, IL-6 and RANTES than an H1N1 virus in primary human bronchial epithelial cells and type II pneumocytes (Chan et al., 2005). However, studies in knockout mice have given contradictory results, either showing that the absence of a TNF-α or IL-6 response does not reduce mortality (Salomon et al., 2007) or that animals lacking a functional TNF-α receptor suffer a milder illness (Szretter et al., 2007). In any case, there is no evidence that the intense inflammatory response seen in H5N1 cases should be regarded as the cause of illness, because glucocorticoid treatment did not improve the outcome of infection in mice and has not altered the course of fatal illness in humans (Arabi et al., 2007, Carter, 2007, Salomon et al., 2007). Although it has been suggested that the intense inflammatory response seen in H5N1 influenza constitutes a form of immune dysregulation or “cytokine storm” (Osterholm, 2005), it may simply be a consequence of severe and extensive viral infection.
5. Targets for antiviral therapy
5.1. Binding to target cells
5.1.1. Antibody therapies
Treatment with anti-influenza virus antibodies could potentially be of benefit in severe influenza, by preventing the binding of virions to target cells (Fig. 1, Step 1) and marking infected cells for destruction by complement or T cells, but no immunoglobulin product is in clinical use. The most extensive effort to assess such therapies took place during the 1918 pandemic, when severely ill patients were sometimes treated with whole blood, plasma or serum from convalescing survivors. In 8 published reports reviewed by Luke et al., the overall case fatality rate was 16% among 336 patients who received convalescent blood products, while 37% of 1219 untreated patients died (Luke et al., 2006). Distinct improvement in the patients’ condition was often noted following therapy. Along the same line, B. Zhou et al. (2007) and H. Zhou et al. (2007) recently reported the prompt recovery of a severely ill H5N1 patient treated with plasma from a convalescent survivor.
More definitive evidence of antibody efficacy has been obtained in laboratory animals. Ramisse et al. (1998) demonstrated that normal human immunoglobulin (IVIG) contains sufficient anti-influenza antibodies to protect mice from lethal pneumonia when inoculated intranasally up to 8 h after infection with an H3N2 virus (1998). F(ab′)2 fragments were equally active, indicating that protection was based on neutralization, rather than on Fc-receptor-bearing cells or complement. More recently, Lu et al. (2006) showed that a single injection of purified F(ab′)2 fragments derived from immunoglobulin of hyperimmunized horses protected mice against a lethal H5N1 virus challenge. A murine monoclonal antibody (mab) targeting a conserved site on the HA of H1 and H2 viruses protected mice against both agents (Okuno et al., 1994), and mabs to H1, given either intact or as Fab fragments, prevented lethal H1N1 infection in SCID mice (Mozdzanowska et al., 2003, Palladino et al., 1995). Humanized mabs also prevented death when given to mice up to 3 days after an otherwise lethal H5N1 virus challenge (Hanson et al., 2006). Similarly, fully human mabs developed using immortalized B cells from Vietnamese H5N1 survivors protected mice when administered up to 72 h after H5N1 virus challenge (Simmons et al., 2007). Although HA is the most important target of the antibody response, pooled serum from mice immunized against the NA of a seasonal H1N1 virus cross-protected mice against an H5N1 virus, suggesting that persons previously infected with an H1N1 virus will be partially resistant to H5N1 (Gillim-Ross and Subbarao, 2007, Sandbulte et al., 2007).
Although artificially generated antibodies appear to have therapeutic potential, pooled human immunoglobulin from convalescent patients or vaccinees may prove more efficacious in the setting of severe illness and may be more readily approved for human use. An effort has therefore been initiated to develop a polyclonal immunoglobulin against avian influenza for intravenous administration through plasmapheresis of vaccine recipients (www.clinicaltrials.gov: NCT00383071).
5.1.2. Other approaches
Other mechanisms now under development for blocking the binding of the virion HA to sialic acid on target cells include the topical delivery of a recombinant sialidase to remove sialic acid (Malakhov et al., 2006) and treatment with a naturally occurring cyanobacterial lectin, cyanovirin, that binds to high-mannose oligosaccharides on the virion HA (O’Keefe et al., 2003).
5.2. Uptake into cells
5.2.1. Blockade of the M2 ion channel
The adamantanes, amantadine and rimantadine, exert their antiviral activity by blocking the M2 ion channel, preventing virion uncoating and the release of genome segments into the cytoplasm (Fig. 1, Step 2). Historically, they have been used almost exclusively to prevent infection or to reduce the duration of uncomplicated seasonal influenza; their benefit in treating severe disease has not been defined. The adamantanes are inexpensive and highly stable in storage, but treatment is frequently complicated by gastrointestinal and central nervous system side-effects. Their use has also been significantly restricted by the rapid emergence of drug-resistant viruses that retain full virulence and transmissibility (Hayden, 2006).
Although most community isolates of H1N1 influenza virus are still sensitive to the adamantanes, resistance of H3N2 viruses has increased rapidly, so that 61% of isolates from Asia and 92% from the United States in 2006 were drug-resistant (Bright et al., 2005, Bright et al., 2006). This change has been attributed to the availability of over-the-counter amantadine (Hayden, 2006), the licensure of memantine, an amantadine derivative used to treat Alzheimer's disease (Shah, 2006), and the use of amantadine to prevent H9 viral infections on poultry farms in China (Cyranoski, 2005). Resistance is also widespread in the recently emerged Asian H5N1 viruses, with only 20% of poultry-derived isolates in China, and none of those in Thailand, sensitive to the drugs (Buranathai et al., 2007, Cheung et al., 2006, He et al., 2008). The WHO therefore recommends that amantadine or rimantadine not be used to prevent or treat H5N1 infections unless the infecting virus is known or likely to be sensitive (Schunemann et al., 2007).
5.3. Transcription and genome replication
5.3.1. Ribavirin
Ribavirin (Virazole®) is a guanosine analogue that is licensed for the treatment of hepatitis C in combination with interferon-α and for aerosol therapy of respiratory syncytial virus infections in infants (Fig. 4A). The compound acts both indirectly, by lowering intracellular GTP levels through the inhibition of inosine 5′-monophosphate dehydrogenase, and directly, by interfering with transcription and genome replication (Fig. 1, Step 4). Because of its multiple mechanisms of action, ribavirin is able to inhibit a broad range of RNA and DNA viruses, and resistant viruses are rarely observed (see Leyssen et al., 2008).
Ribavirin is less active against the influenza viruses than the adamantanes or NA inhibitors. In an early evaluation, a once-daily oral dose of 1 g of ribavirin begun after the onset of symptoms had no effect on the course of illness, but benefits were observed when the dosage was quadrupled (Smith et al., 1980, Stein et al., 1987). Aerosolized ribavirin also reduced the duration of fever in young children with influenza, but sometimes caused bronchospasm (Knight and Gilbert, 1987, Rodriguez et al., 1994). In contrast to the licensed anti-influenza drugs, ribavirin is available in intravenous form. When delivered in that manner, ribavirin eliminated virus from two patients and produced improvement in a third with severe influenza or parainfluenza virus infection (Hayden et al., 1996). However, the drug's clinical utility may be limited by the risk of hemolytic anemia, and its teratogenic properties proscribe its use in pregnant women.
5.3.2. Viramidine
Viramidine, a prodrug of ribavirin, has similar activity against seasonal and H5N1 influenza A viruses, but is less toxic (Fig. 4B) (Sidwell et al., 2005). Interestingly, efficacy against a lethal H1N1 virus was demonstrated in mice when the drug was administered in their drinking water. Perhaps because viramidine has less inhibitory effect on cellular DNA synthesis than ribavirin, it has produced a significantly lower incidence of hemolytic anemia (Gish, 2006). The drug is now in Phase 3 trials for the treatment of hepatitis C.
5.3.3. T-705
The pyrazine derivative T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is a broad-spectrum inhibitor of RNA viruses, including influenza A, B and C, that is now in Phase I testing (Fig. 4C) (Furuta et al., 2002, Furuta et al., 2005, Gowen et al., 2007). On entering the cell, the compound is initially converted to its monophosphate by phosphoribosyl transferase, then to its triphosphate by cellular kinase, in which form it appears to inhibit the RNA-dependent RNA polymerase (Furuta et al., 2005). In contrast to ribavirin, T-705 does not interfere with cellular DNA or RNA synthesis and has little effect on IMP dehydrogenase. It was highly active in vitro against a panel of seasonal and H5N1 influenza viruses, including amantadine- and oseltamivir-resistant agents (Sidwell et al., 2007). High doses caused no cytotoxicity, and repeated virus passage in the presence of the drug did not result in resistance.
Though somewhat less active than oseltamivir against influenza viruses in vitro, T-705 was more protective in vivo. In a lethal model of H1N1 virus infection in mice, 200 mg/kg/day given orally beginning 1 or 13 h after infection prevented death and reduced lung viral titers below the limit of detection, while treatment begun at 25 h gave 71% survival (Furuta et al., 2002, Takahashi et al., 2003). In a mouse model of lethal H5N1 infection, once-daily treatment prevented death even when initiated at 96 h postinfection, when the animals were already ill (Sidwell et al., 2007). Single large doses were nontoxic and highly protective, consistent with a long intracellular half-life.
5.3.4. Other approaches
Antisense DNA oligomers and short, interfering RNA (siRNA) molecules that target sequences in influenza virus mRNA have shown efficacy in murine and avian models of severe influenza (Ge et al., 2004, Ge et al., 2006, Tompkins et al., 2004, Wu et al., 2008, H. Zhou et al., 2007). Providing that methods can be developed to deliver them efficiently to infected cells, their ability to block the translation of viral mRNA (Fig. 1, Step 5) would potentially give such sequence-based therapies a valuable role in the treatment of human disease. These approaches are discussed in an accompanying article by Spurgers et al. (this issue).
5.4. Assembly and exit of nascent virions
Because NA activity is required for nascent influenza virions to exit the infected cell and prevent them from clumping to each other, analogues of sialic acid that block the enzyme are effective antiviral drugs (Fig. 1, Step 7; Fig. 3). The amino acids that make up the active site are highly conserved across the nine known NA subtypes. The structure of the H5N1 NA and potential approaches to designing new drugs are discussed in detail by Russell et al. (2006).
5.4.1. Oseltamivir
In multiple controlled trials in healthy adults, a 5-day course of twice-daily doses of 75 mg of oseltamivir shortened the course of laboratory-confirmed seasonal influenza to a modest extent, reduced nasal viral titers and lowered the incidence of lower respiratory tract complications requiring antibiotic treatment (Kaiser et al., 2003, Nicholson et al., 2000, Treanor et al., 2000, Cooper et al., 2003, Jefferson et al., 2006). Treatment was more effective the earlier it was begun in the disease course. A recent study found that the same regimen significantly reduced mortality in elderly or chronically ill patients hospitalized with laboratory-confirmed influenza, even if treatment was begun more than 48 h after symptom onset (McGeer et al., 2007).
Studies in laboratory animals indicate that the regimen of oseltamivir that was developed to treat uncomplicated seasonal influenza is inadequate for H5N1 infections. Although a dose of 1 mg/kg/day for 5 days was sufficient to prevent death in mice infected with a 1997 Hong Kong virus, 10 mg/kg/day for 8 days was needed against a 2004 virus from Viet Nam (Govorkova et al., 2001, Yen et al., 2005b). In ferrets infected with the same isolate, 5 mg/kg/day started 4 h after inoculation prevented death, but 25 mg/kg was required if therapy was delayed until 24 h postchallenge (Govorkova et al., 2007). Recent WHO guidelines recommended that the standard regimen of oseltamivir be used to treat patients with confirmed or strongly suspected H5N1 infections (Schunemann et al., 2007, World Health Organization, 2006). However, the report also noted that severely ill patients might benefit from higher doses (300 mg/day) and/or a 7–10-day course of therapy, but that prospective studies would be required to establish benefit. A multi-center study was initiated in Asia and the US in June 2007 to compare standard vs. high-dose regimens of oseltamivir (150 mg/day vs. 300 mg/day for adults with normal renal function) for 5 days or 10 days of therapy for severe seasonal and avian influenza (clinicaltrials.gov identifier: NCT00298233).
Resistance to NA inhibitors develops less commonly than to the adamantanes. Depending on the specific mutation, a virus may become resistant to oseltamivir, but remain sensitive to zanamivir (Gubareva et al., 2001, Moscona, 2005a, Moscona, 2005b, Moscona, 2008). The first step in the development of resistance is often a mutation in HA that reduces its affinity for sialic acid. Probably because a proper balance of HA/NA activity is required for the maximally efficient spread of virus among respiratory epithelial cells, resistant viruses that arise during the course of drug treatment usually replicate to lower levels in vitro and have a diminished ability to cause disease and be transmitted among ferrets (Carr et al., 2002, Herlocher et al., 2002, Herlocher et al., 2004, Zürcher et al., 2006). However, when reverse genetics methods were used to introduce certain resistance mutations into H5N1 viruses, the agents retained their replication capacity and virulence (Yen et al., 2005a, Yen et al., 2005b, Yen et al., 2006, Yen et al., 2007).
Reports of viruses resistant to oseltamivir were rare until recently, when two studies in Japan found that almost 20% of children treated with the drug shed resistant viruses (Kiso et al., 2004). Subtherapeutic dosing may have played a role, as similar resistance was not seen in US children treated with doses adjusted for weight (Moscona, 2005a). Oseltamivir-resistant H5N1 viruses have also been recovered from a few patients in Southeast Asia. Virus recovered from a girl who was treated first with a prophylactic, then with a therapeutic dose of oseltamivir and survived infection showed a resistant subpopulation, while viruses recovered from two other patients who died despite the early initiation of oseltamivir therapy showed a critical mutation in the NA active site (De Jong et al., 2005a, De Jong et al., 2005b, Le et al., 2005). H5N1 viruses with the H274Y substitution in NA that emerge during oseltamivir treatment retain full susceptibility to zanamivir (De Jong et al., 2005b, Gubareva et al., 2001).
5.4.2. Aerosolized zanamivir
Because NA acts outside of virus-infected cells, it can be inhibited by a topically administered drug. Aerosolized zanamivir (Relenza®) is effective in reducing the impact of seasonal influenza in previously healthy adults, when started before or soon after the onset of symptoms (Hayden et al., 1997). However, the drug is much less useful for severely ill patients who are unable to inhale it, or whose pulmonary infections are inaccessible to topical therapy (Medeiros et al., 2007). No experience has been reported in using zanamivir to prevent or treat H5N1 infections.
5.4.3. Intravenous zanamivir
Because it is active against a broad range of influenza A viruses and drug resistance is rare, intravenous zanamivir is being evaluated as a potential therapy for severe influenza. So far its efficacy has only been formally tested against uncomplicated seasonal influenza. Even though the drug's 2-h plasma half-life is shorter than that of oseltamivir or peramivir, twice-daily infusions beginning 4 h before intranasal H1N1 virus challenge produced significant reductions in fever, upper respiratory tract illness and viral shedding in volunteers (Calfee et al., 1999, Kaiser et al., 2003). A Phase I trial comparing the pharmacokinetics and interactions of oral oseltamivir and intravenous zanamivir is under development (www.clinicaltrials.gov: NCT00540501).
5.4.4. Multimeric forms of zanamivir
Efforts to develop “second generation” NA inhibitors have explored the activity of chemically modified or multimeric forms of the licensed compounds. Ether derivatives of zanamivir showed increased potency in vitro, improved oral availability and greater efficacy in mice (Honda et al., 2002, Masuda et al., 2003). Dimeric forms of zanamivir are particularly promising variants that can act in a manner similar to antibodies to cross-link influenza virions. Dimers in which the linking group was 14–18 atoms in length were 100–1000-fold more potent in vitro than the monomeric drug (Macdonald et al., 2004, Macdonald et al., 2005). The in vivo half-life of such constructs is also greatly increased. Administered intranasally, dimeric zanamivir had a residence time in rat lung exceeding 1 week, and a single dose prevented death in mice when given 7 days before virus challenge.
5.4.5. Peramivir
The synthesis of a new NA inhibitor, peramivir (RWJ-270201), through structure-based drug design was reported by Babu et al. (2000). The drug inhibits all nine NA subtypes and is at least as active as oseltamivir and zanamivir against H5N1 viruses in vitro (Govorkova et al., 2001) Peramivir offers the advantage of a markedly longer half-life of binding to the NA active site, permitting less frequent dosing (Bantia et al., 2006, Gubareva et al., 2001). Whereas the dissociation half-life of zanamivir and oseltamivir from an N9 enzyme was 1.25 h, that of peramivir exceeded 1 day. A single intramuscular injection 24 or 48 h postinfection was completely protective in mice challenged with a lethal dose of an H1N1 or H3N2 virus (Bantia et al., 2006).
Although oral and intranasal administrations were protective in mice and ferrets (Chand et al., 2005, Sweet et al., 2002), peramivir has low oral bioavailability in humans. Thus, the drug was well tolerated when given by mouth to human volunteers infected with influenza A or B virus, but reduction of symptoms failed to achieve statistical significance (Bantia et al., 2006). Subsequent development has therefore focused on parenteral administration. A Phase I trial of intravenous peramivir showed that the drug had a 22-h half-life in the plasma and was well tolerated (Beigel et al., 2007). A Phase II study is now under way to evaluate single-dose intramuscular peramivir for uncomplicated seasonal influenza (www.clinicaltrials.gov: NCT00419263), and a second randomized controlled trial will compare the efficacy of 5 days of intravenous peramivir to the current approved regimen of oral oseltamivir for severe influenza (www.clinicaltrials.gov: NCT00453999).
5.5. Combination therapy
Treatment with a combination of antiviral drugs with different mechanisms of action could potentially improve the outcome of severe influenza through additive (or possibly synergistic) suppression of viral replication, and might also reduce the required dose for each drug, limiting toxicity and the emergence of drug-resistant mutants (Tsiodras et al., 2007). Efforts to model this approach in vitro and in vivo have employed combinations of adamantanes and NA inhibitors. Thus, the passage of H1N1, H3N2 and H5N1 viruses in the presence of amantadine or oseltamivir resulted in the acquisition of drug resistance, but when the procedures were repeated in the presence of both drugs, no changes were detected (Ilyushina et al., 2006). When rimantadine was tested in combination with zanamivir, oseltamivir or peramivir, all three combinations showed synergistic activity against H3N2 and H1N1 isolates (Govorkova et al., 2004). In mice infected with an H5N1 virus sensitive to both oseltamivir and amantadine, combination therapy produced an additive benefit: oseltamivir alone gave 30% and amantadine alone gave 60% survival, but combination treatment increased the rate to 90% (Ilyushina et al., 2007). Combination therapy also significantly decreased viral titers in the lungs and prevented spread to the brain.
In the only reported study in humans, treatment of severe seasonal influenza with rimantadine plus zanamivir was suggestive of virologic benefit, compared to rimantadine alone (Ison et al., 2003). Given the range of new anti-influenza therapies that are now under development, a much greater variety of combinational approaches may soon be available for testing.
6. Conclusion
The existence of a vast reservoir of influenza A viruses in wild waterfowl and shorebirds and the ability of these agents to jump species barriers means that they cannot be eradicated and will always pose a threat to the health of humans and domestic animals. Currently available antiviral therapies were developed with the aim of reducing the impact of seasonal influenza, rather than to treat life-threatening disease, but the emergence of the virulent H5N1 virus has forced the world to take another look at influenza and begin to approach it with the seriousness it deserves. New and better antiviral therapies are needed, both to deal with the next pandemic and to reduce the toll of illness and death produced every year by seasonal influenza. Fortunately, current efforts show promise of achieving this goal.
Acknowledgments
The assistance of Johan Neyts and Kanta Subbarao in reviewing the final version of this paper is greatly appreciated.
References
- Apisarnthanarak A., Kitphati R., Thongphubeth K., Patoomanunt P., Anthanont P., Auwanit W., Thawatsupha P., Chittaganpitch M., Saeng-Aroon S., Waicharoen S., Apisarnthanarak P., Storch G.A., Mundy L.M., Fraser V.J. Atypical avian influenza (H5N1) Emerg. Infect. Dis. 2004;10:1321–1324. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y., Gommersall C., Ahmed Q., Boynton B., Memish Z. The critically ill avian influenza A (H5N1) patient. Crit. Care Med. 2007;35:1397–1403. doi: 10.1097/01.CCM.0000262940.34596.4B. [DOI] [PubMed] [Google Scholar]
- Babu Y.S., Chand P., Bantia S., Kotian P., Dehghani A., El-Kattan Y., Lin T.H., Hutchison T.L., Elliott A.J., Parker C.D., Ananth S.L., Horn L.L., Laver G.W., Montgomery J.A. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- Bantia S., Arnold C.S., Parker C.D., Upshaw R., Chand P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir. Res. 2006;69:39–45. doi: 10.1016/j.antiviral.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Beare A.S., Webster R.G. Replication of avian influenza viruses in humans. Arch. Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- Beigel, J., Harman, L.A., Collis, P.J., McCullough, A., Kilpatrick., J.M., Ruff, D., Mead, E., Alexander, W.J., 2007. Pharmacokinetic and safety evaluations of escalating doses of peramivir administered intravenously in healthy volunteers. ICAAC 2007, Poster A-1408.
- Beigel J.H., Farrar J., Han A.M., Hayden F.G., Hyer R., de Jong M.D., Lochindarat S., Nguyen T.K., Nguyen T.H., Tran T.H., Nicoll A., Touch S., Yuen K.Y. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Bright R.A., Medina M.J., Xu X., Perez-Oronoz G., Wallis T.R., Davis X.M., Povinelli L., Cox N.J., Klimov A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- Bright R.A., Shay D.K., Shu B., Cox N.J., Klimov A.I. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295:891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- Buchy P., Mardy S., Vong S., Toyoda T., Aubin J.T., Miller M., Touch S., Sovann L., Dufourcq J.B., Richner B., Tu P.V., Tien N.T., Lim W., Peiris J.S., Van der Werf S. Influenza A/H5N1 virus infection in humans in Cambodia. J. Clin. Virol. 2007;39:164–168. doi: 10.1016/j.jcv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Buranathai C., Amonsin A., Chaisigh A., Theamboonlers A., Pariyothorn N., Poovorawan Y. Surveillance activities and molecular analysis of H5N1 highly pathogenic avian influenza viruses from Thailand, 2004–2005. Avian Dis. 2007;51:194–200. doi: 10.1637/7594-040306R.1. [DOI] [PubMed] [Google Scholar]
- Butt K.M., Smith G.J., Chen H., Zhang L.J., Leung Y.H., Xu K.M., Lim W., Webster R.G., Yuen K.Y., Peiris J.S., Guan Y. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 2005;43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee D.P., Peng A.W., Cass L.M., Lobo M., Hayden F.G. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob. Agents Chemother. 1999;43:1616–1620. doi: 10.1128/aac.43.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J., Ives J., Kelly L., Lambkin R., Oxford J., Mendel D., Tai L., Roberts N. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antivir. Res. 2002;54:79–88. doi: 10.1016/s0166-3542(01)00215-7. [DOI] [PubMed] [Google Scholar]
- Carter M.J. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J. Med. Microbiol. 2007;56:875–883. doi: 10.1099/jmm.0.47124-0. [DOI] [PubMed] [Google Scholar]
- Chan P.K. Outbreak of avian influenza A(H5N1) virus infection in Hong Kong in 1997. Clin. Infect. Dis. 2002;34(Suppl 2):S58–S64. doi: 10.1086/338820. [DOI] [PubMed] [Google Scholar]
- Chan M.C., Cheung C.Y., Chui W.H., Tsao S.W., Nicholls J.M., Chan Y.O., Chan R.W., Long H.T., Poon L.L., Guan Y., Peiris J.S. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand P., Bantia S., Kotian P.L., El-Kattan Y., Lin T.H., Babu Y.S. Comparison of the anti-influenza virus activity of cyclopentane derivatives with oseltamivir and zanamivir in vivo. Bioorg. Med. Chem. 2005;13:4071–4077. doi: 10.1016/j.bmc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Cheung C.L., Rayner J.M., Smith G.J., Wang P., Naipospos T.S., Zhang J., Yuen K.Y., Webster R.G., Peiris J.S., Guan Y., Chen H. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 2006;193:1626–1629. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- Cheung C.Y., Poon L.L., Lau A.S., Luk W., Lau Y.L., Shortridge K.F., Gordon S., Guan Y., Peiris J.S. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Conenello G., Zamarin D., Perrone L., Palese P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PloS Pathogens. 2007;3:e141. doi: 10.1371/journal.ppat.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N.J., Sutton A.J., Abrams K.R., Wailoo A., Turner D., Nicholson K.G. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326:1235–1242. doi: 10.1136/bmj.326.7401.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski D. China's chicken farmers under fire for antiviral abuse. Nature. 2005;435:1009. doi: 10.1038/4351009a. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006;5:1015–1025. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong M.D., Bach V.C., Phan T.Q., Vo M.H., Tran T.T., Nguyen B.H., Beld M., Le T.P., Truong H.K., Nguyen V.V., Tran T.H., Do Q.H., Farrar J. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N. Engl. J. Med. 2005;352:686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- De Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N., Hoang D.M., Chau N.V., Khanh T.H., Dong V.C., Qui P.T., Cam B.V., Ha do Q., Guan Y., Peiris J.S., Chinh N.T., Hien T.T., Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong M.D., Tran T.T., Truong H.K., Vo M.H., Smith G.J., Nguyen V.C., Bach V.C., Phan T.Q., Do Q.H., Guan Y., Peiris J.S., Tran T.H., Farrar J. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- Dybing J.K., Schultz-Cherry S., Swayne D.E., Suarez D.L., Perdue M.L. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J. Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Watanabe Y., Narita H., Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier R.A., Schneeberger P.M., Rozendaal F.W., Broekman J.M., Kemink S.A., Munster V., Kuiken T., Rimmelzwaan G.F., Schutten M., Van Doornum G.J., Koch G., Bosman A., Koopmans M., Osterhaus A.D. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. Antiviral response in pandemic influenza viruses. Emerg. Infect. Dis. 2006;12:44–47. doi: 10.3201/eid1201.051186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad Sci. U.S.A. 2004;101(23):8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Pastey M., Kobasa D., Puthavathana P., Lupfer C., Bestwick R.K., Iversen P.L., Chen J., Stein D.A. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 2006;50(11):3724–3733. doi: 10.1128/AAC.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillim-Ross L., Subbarao K. Can immunity induced by the human influenza virus N1 neuraminidase provide some protection from avian influenza H5N1 viruses? PLoS Med. 2007;4:e91. doi: 10.1371/journal.pmed.0040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gish R. Treating HCV with ribavirin analogues and ribavirin-like molecules. J. Antimicrob. Chemother. 2006;57:8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- Glaser L., Conenello G., Paulson J., Palese P. Effective replication of human influenza viruses in mice lacking a major α2,6 sialyltransferase. Virus Res. 2007;126:9–18. doi: 10.1016/j.virusres.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Govorkova E.A., Fang H.B., Tan M., Webster R.G. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob. Agents Chemother. 2004;48:4855–4856. doi: 10.1128/AAC.48.12.4855-4863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Ilyushina N.A., Boltz D.A., Douglas A., Yilmaz N., Webster R.G. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob. Agents Chemother. 2007;51:1414–1424. doi: 10.1128/AAC.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova E.A., Leneva I.A., Goloubeva O.G., Bush K., Webster R.G. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob. Agents Chemother. 2001;45:2723–2732. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen B.B., Wong M.H., Jung K.H., Sanders A.B., Mendenhall M., Bailey K.W., Furuta Y., Sidwell R.W. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob. Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva L.V., Webster R.G., Hayden F.G. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob. Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B.J., Boon A.C.M., Lim A.P.C., Webb A., Ooi E.E., Webby R.J. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F.G. Antiviral resistance in influenza viruses—implications for management and pandemic response. N. Engl. J. Med. 2006;354:785–788. doi: 10.1056/NEJMp068030. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Osterhaus A.D., Treanor J.J., Fleming D.M., Aoki F.Y., Nicholson K.G., Bohnen A.M., Hirst H.M., Keene O., Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N. Engl. J. Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Hayden F.G., Palese P. Influenza virus. In: Richman D., Whitley R., Hayden F., editors. Clinical Virology. 2nd ed. ASM Press; Washington, DC: 2002. pp. 891–920. [Google Scholar]
- Hayden F.G., Sable C.A., Connor J.D., Lane J. Intravenous ribavirin by constant infusion for serious influenza and parainfluenza virus infection. Antivir. Ther. 1996;1:51–56. [PubMed] [Google Scholar]
- He G., Qiao J., Dong C., He C., Zhao L., Tian Y. Amantadine resistance among H5N1 avian influenza viruses isolated in Northern China. Antivir. Res. 2008;77:72–76. doi: 10.1016/j.antiviral.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Herlocher M.L., Carr J., Ives J., Elias S., Truscon R., Roberts N., Monto A.S. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antivir. Res. 2002;54:99–111. doi: 10.1016/s0166-3542(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Herlocher M.L., Truscon R., Elias S., Yen H.L., Roberts N.A., Ohmit S.E., Monto A.S. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J. Infect. Dis. 2004;190:1627–1630. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- Honda T., Masuda T., Yoshida S., Arai M., Kaneko S., Yamashita M. Synthesis and anti-influenza virus activity of 7-O-alkylated derivatives related to zanamivir. Bioorg. Med. Chem. Lett. 2002;12:1925–1928. doi: 10.1016/s0960-894x(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Ibricevic A., Pekosz A., Walter M.J., Newby C., Battaile J.T., Brown E.G., Holtzman M.J., Brody S.L. Influenza virus receptor specificity and cell tropism in mouse and human airway epithelial cells. J. Virol. 2006;80:7469–7480. doi: 10.1128/JVI.02677-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina N.A., Bovin N.V., Webster R.G., Govorkova E.A. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant variants. Antivir. Res. 2006;70:121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Ilyushina N.A., Hoffmann E., Solomon R., Webster R.G., Govorkova E.A. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir. Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- Ison M.G., Gnann J.W., Jr., Nagy-Agren S., Treannor J., Paya C., Steigbigel R., Elliott M., Weiss H.L., Hayden F.G. Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antivir. Ther. 2003;8:183–190. [PubMed] [Google Scholar]
- Ison M.G., Gubarova L.V., Atmar R.L., Treanor J., Hayden F.G. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J. Infect. Dis. 2006;193:760–764. doi: 10.1086/500465. [DOI] [PubMed] [Google Scholar]
- Jefferson T., Demicheli V., Rivetti D., Jones M., Di Pietrantonj C., Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006;367:303–313. doi: 10.1016/S0140-6736(06)67970-1. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Wat C., Mills T., Mahoney P., Ward P., Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- Kiso M., Mitamura K., Sakai-Tagawa Y., Shiraishi K., Kawakami C., Kimura K., Hayden F.G., Sugaya N., Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- Knight V., Gilbert B.E. Ribavirin aerosol treatment of influenza. Infect. Dis. Clin. North Am. 1987;1:441–457. [PubMed] [Google Scholar]
- Koopmans M., Wilbrink B., Conyn M., Natrop G., van der Nat H., Vennema H., Meijer A., van Steenbergen J., Fouchier R., Osterhaus A., Bosman A. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- Le Q.M., Kiso M., Someya K., Sakai Y.T., Nguyen T.H., Nguyen K.H., Pham N.D., Nguyen H.H., Yamada S., Muramoto Y., Horimoto T., Takada A., Goto H., Suzuki T., Suzuki Y., Kawaoka Y. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- Leekha S., Zitterkopf N.L., Espy M.J., Smith T.F., Thompson R.L., Sampathkumar P. Duration of influenza A virus shedding in hospitalized patients and implications for infection control. Infect. Control Hosp. Epidemiol. 2007;28:1071–1076. doi: 10.1086/520101. [DOI] [PubMed] [Google Scholar]
- Leyssen P., De Clercq E., Neyts J. Molecular strategies to inhibit the replication of RNA viruses. Antivir. Res. 2008;78:9–25. doi: 10.1016/j.antiviral.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.S., Guan Y., Wang J., Smith G.J., Xu K.M., Duan L., Rahardjo A.P., Puthavathana P., Buranathai C., Nguyen T.D., Estoepangestie A.T., Chaisingh A., Auewarakul P., Long H.T., Hanh N.T., Webby R.J., Poon L.L., Chen H., Shortridge K.F., Yuen K.Y., Webster R.G., Peiris J.S. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- Lu J., Guo Z., Pan X., Wang G., Zhang D., Li Y., Tan B., Ouyang L., Yu X., Lu J. Passive immunotherapy for influenza A H5N1 virus infection with equine hyperimmune globulin F(ab′)2 in mice. Respir. Res. 2006;7:43. doi: 10.1186/1465-9921-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- Ma W., Vincent A., Gramer M., Brockwell C., Lager K., Janke B., Gauger P., Patnayak D., Webby R., Richt J. Identification of H2N3 influenza A viruses from swine in the United States. Proc. Natl. Acad. Sci. U.S.A. 2008;104:20949–20954. doi: 10.1073/pnas.0710286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S.J., Cameron R., Demaine D.A., Fenton R.J., Foster G., Gower D., Hamblin J.N., Hamilton S., Hart G.J., Hill A.P., Inglis G.G., Jin B., Jones H.T., McConnell D.B., McKimm-Breschkin J., Mills G., Nguyen V., Owens I.J., Parry N., Shanahan S.E., Smith D., Watson K.G., Wu W.Y., Tucker S.P. Dimeric zanamivir conjugates with various linking groups are potent, long-lasting inhibitors of influenza neuraminidase including H5N1 avian influenza. J. Med. Chem. 2005;48:2964–2971. doi: 10.1021/jm040891b. [DOI] [PubMed] [Google Scholar]
- Macdonald S.J., Watson K.G., Cameron R., Chalmers D.K., Demaine D.A., Fenton R.J., Gower D., Hamblin J.N., Hamilton S., Hart G.J., Inglis G.G., Jin B., Jones H.T., McConnell D.B., Mason A.M., Nguyen V., Owens I.J., Parry N., Reece P.A., Shanahan S.E., Smith D., Wu W.Y., Tucker S.P. Potent and long-acting dimeric inhibitors of influenza virus neuraminidase are effective at a once-weekly dosing regimen. Antimicrob. Agents Chemother. 2004;48:4542–4549. doi: 10.1128/AAC.48.12.4542-4549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines T.R., Lu X.H., Erb S.M., Edwards L., Guarner J., Greer P.W., Nguyen D.C., Szretter K.J., Chen L.M., Thawatsupha P., Chittaganpitch M., Waicharoen S., Nguyen D.T., Nguyen T., Nguyen H.H., Kim J.H., Hoang L.T., Kang C., Phuong L.S., Lim W., Zaki S., Donis R.O., Cox N.J., Katz J.M., Tumpey T.M. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhov M.P., Aschenbrenner L.M., Smee D.F., Wandersee M.K., Sidwell R.W., Gubareva L.V., Mishin V.P., Hayden F.G., Kim D.H., Ing A., Campbell E.R., Yu M., Fang F. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob. Agents Chemother. 2006;50:1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T., Shibuya S., Arai M., Yoshida S., Tomozawa T., Ohno A., Yamashita M., Honda T. Synthesis and anti-influenza evaluation of orally active bicyclic ether derivatives related to zanamivir. Bioorg. Med. Chem. Lett. 2003;13:669–673. doi: 10.1016/s0960-894x(02)01039-9. [DOI] [PubMed] [Google Scholar]
- Matrosovich M.N., Matrosovich T.Y., Gray T., Roberts N.A., Klenk H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004;78:12665–12667. doi: 10.1128/JVI.78.22.12665-12667.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer A., Green K., Plevneshi A., Shigayeva A., Siddiqi N., Raboud J., Low D.E. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 2007;45 doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- Medeiros R., Rameix-Welti M.A., Lorin V., Ribaud P., Manuguerra J.C., Socie G., Scieux C., Naffakh N., van der Werf S. Failure of zanamivir therapy for pneumonia in a bone-marrow transplant recipient infected by a zanamivir-sensitive influenza A (H1N1) virus. Antivir. Ther. 2007;12:571–576. [PubMed] [Google Scholar]
- Monto A.S. Vaccines and antiviral drugs in pandemic preparedness. Emerg. Infect. Dis. 2006;12:55–60. doi: 10.3201/eid1201.051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- Moscona A. Oseltamivir resistance—disabling our influenza defenses. N. Engl. J. Med. 2005;353:2633–2636. doi: 10.1056/NEJMp058291. [DOI] [PubMed] [Google Scholar]
- Moscona A. Medical management of influenza infection. Annu. Rev. Med. 2008;59:397–413. doi: 10.1146/annurev.med.59.061506.213121. [DOI] [PubMed] [Google Scholar]
- Mozdzanowska K., Feng J., Gerhard W. Virus-neutralizing activity mediated by the Fab fragment of a hemagglutinin-specific antibody is sufficient for the resolution of influenza virus infection in SCID mice. J. Virol. 2003;77:8322–8328. doi: 10.1128/JVI.77.15.8322-8328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W.F., To K.F., Lam W.W., Ng T.K., Lee K.C. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum. Pathol. 2006;37:381–390. doi: 10.1016/j.humpath.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Chan M.C., Chan W.Y., Wong H.K., Cheung C.Y., Kwong D.L., Wong M.P., Chui W.H., Poon L.L., Tsao S.W., Guan Y., Peiris J.S. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat. Med. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- Nicholson K.G., Aoki F.Y., Osterhaus A.D., Trottier S., Carewicz O., Mercier C.H., Rode A., Kinnersley N., Ward P. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe B., Smee D., Turpin J., Saucedo C., Gustafson K., Mori T., Blakeslee D., Buckheit R., Boyd M. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y., Matsumoto K., Isegawa Y., Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J. Virol. 1994;68:517–520. doi: 10.1128/jvi.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oner A.F., Bay A., Arslan S., Akdeniz H., Sahin H.A., Cesur Y., Epcacan S., Yilmaz N., Deger I., Kizilyildiz B., Karsen H., Ceyhan M. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 2006;355:2179–2185. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- Osterholm M.T. Preparing for the next pandemic. N. Engl. J. Med. 2005;352:1839–1842. doi: 10.1056/NEJMp058068. [DOI] [PubMed] [Google Scholar]
- Palladino G., Mozdzanowska K., Washko G., Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris M., Yuen K.Y., Leung C.W., Chan K.H., Ip P.L., Lai R.W., Orr W.K., Shortridge K.F. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- Ramisse F., Deramoudt F.X., Szatanik M., Bianchi A., Binder P., Hannoun C., Alonso J.M. Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins or F(ab′)2 fragments. Clin. Exp. Immunol. 1998;111:583–587. doi: 10.1046/j.1365-2249.1998.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez W.J., Hall C.B., Welliver R., Simoes E.A., Ryan M.E., Stutman H., Johnson G., Van Dyke R., Groothuis J.R., Arrobio J. Efficacy and safety of aerosolized ribavirin in young children hospitalized with influenza: a double-blind, multicenter, placebo-controlled trial. J. Pediatr. 1994;125:129–135. doi: 10.1016/s0022-3476(94)70139-3. [DOI] [PubMed] [Google Scholar]
- Russell R.J., Haire L.F., Stevens D.J., Collins P.J., Lin Y.P., Blackburn G.M., Hay A.J., Gamblin A.J., Skehel J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006;443:45–49. doi: 10.1038/nature05114. [DOI] [PubMed] [Google Scholar]
- Salomon R., Franks J., Govorkova E.A., Ilyushina N.A., Yen H.L., Hulse-Post D.J., Humberd J., Trichet M., Rehg J.E., Webby R.J., Webster R.G., Hoffmann E. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 2006;203:689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Hoffmann E., Webster R.G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbulte M.R., Jimenez G.S., Boon A.C., Smith L.R., Treanor J.J., Webby R.J. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Hosoya M., Kato K., Suzuki H. Viral shedding in children with influenza virus infections treated with neuraminidase inhibitors. Pediatr. Infect. Dis. J. 2005;24:931–932. doi: 10.1097/01.inf.0000180976.81055.ce. [DOI] [PubMed] [Google Scholar]
- Schunemann H.J., Hill S.R., Kakad M., Bellamy R., Uyeki T.M., Hayden F.G., Yazdanpanah Y., Beigel J., Chotpitayasunondh T., Del Mar C., Farrar J., Tran T.H., Ozbay B., Sugaya N., Fukuda K., Shindo N., Stockman L., Vist G.E., Croisier A., Nagjdaliyev A., Roth C., Thomson G., Zucker H., Oxman A.D. WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect. Dis. 2007;7:21–31. doi: 10.1016/S1473-3099(06)70684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Hoffmann E., Webster R.G. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 2002;8:950–954. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- Shah R.C. Shift shown in influenza A adamantane resistance. JAMA. 2006;296:1585–1586. doi: 10.1001/jama.296.13.1585-b. [DOI] [PubMed] [Google Scholar]
- Shinya K., Ebina M., Yamada S., Ono M., Kasai N., Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Shinya K., Hatta M., Yamada S., Takada A., Watanabe S., Halfmann P., Horimoto T., Neumann G., Kim J.H., Lim W., Guan Y., Peiris M., Kiso M., Suzuki T., Suzuki Y., Kawaoka Y. Characterization of a human H5N1 influenza A virus isolated in 2003. J. Virol. 2005;79:9926–9932. doi: 10.1128/JVI.79.15.9926-9932.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R.W., Bailey K.W., Wong M.H., Barnard D.L., Smee D.F. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antivir. Res. 2005;68:10–17. doi: 10.1016/j.antiviral.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sidwell R.W., Barnard D.L., Day C.W., Smee D.F., Bailey K.W., Wong M.H., Morrey J.D., Furuta Y. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C.P., Bernasconi N.L., Suguitan A.L., Mills K., Ward J.M., Chau N.V., Hien T.T., Sallusto F., Ha do Q., Farrar J., de Jong M.D., Lanzavecchia A., Subbarao K. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.B., Charette R.P., Fox J.P., Cooney M.K., Hall C.E. Lack of effect of oral ribavirin in naturally occurring influenza A virus infection. J. Infect. Dis. 1980;141:548–554. doi: 10.1093/infdis/141.5.548. [DOI] [PubMed] [Google Scholar]
- Stein D.S., Creticos C.M., Jackson G.G., Bernstein J.M., Hayden F.G., Schiff G.M., Bernstein D.I. Oral ribavirin treatment of influenza A and B. Antimicrob. Agents Chemother. 1987;31:1285–1287. doi: 10.1128/aac.31.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray S.J., Cummings R.D., Air G.M. Influenza virus infection of desialylated cells. Glycobiology. 2000;10:649–658. doi: 10.1093/glycob/10.7.649. [DOI] [PubMed] [Google Scholar]
- Sweet C., Jakeman K.J., Bush K., Wagaman P.C., McKown L.A., Streeter A.J., Desai-Krieger D., Chand P., Babu Y.S. Oral administration of cyclopentane neuraminidase inhibitors protects ferrets against influenza virus infection. Antimicrob. Agents Chemother. 2002;46:996–1004. doi: 10.1128/AAC.46.4.996-1004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter K.J., Gangappa S., Lu X., Smith C., Shieh W.J., Zaki S.R., Sambhara S., Tumpey T.M., Katz J.M. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Furuta Y., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Shiraki K. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 2003;14:235–241. doi: 10.1177/095632020301400502. [DOI] [PubMed] [Google Scholar]
- To K.F., Chan P.K., Chan K.F., Lee W.K., Lam W.Y., Wong K.F., Tang N.L., Tsang D.N., Sung R.Y., Buckley T.A., Tam J.S., Cheng A.F. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. U.S.A. 2004;101(23):8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor J.J., Hayden F.G., Vrooman P.S., Barbarash R., Bettis R., Riff D., Singh S., Kinnersley N., Ward P., Mills R.G. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- Tsiodras S., Mooney J.D., Hatzakis A. Role of combination antiviral therapy in pandemic influenza. BMJ. 2007;334:293–294. doi: 10.1136/bmj.39105.428981.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweed S.A., Skowronski D.M., David S.T., Larder A., Petric M., Lees W., Li Y., Katz J., Krajden M., Tellier R., Halpert C., Hirst M., Astell C., Lawrence D., Mak A. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 2004;10:2196–2199. doi: 10.3201/eid1012.040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiprasertkul M., Puthavathana P., Sangsiriwut K., Pooruk P., Srisook K., Peiris M., Nicholls J.M., Chokephaibulkit K., Vanprapar N., Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Riel D., Munster V.J., de Wit E., Rimmelzwaan G.F., Fouchier R.A., Osterhaus A.D., Kuiken T. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Wagner R., Matrosovich M., Klenk H.D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2006. Advice on use of oseltamivir.
- World Health Organization, 2007. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO, vol. 2007, no. August 15.
- Wright P.F., Webster R.G. Orthomyxoviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1533–1579. [Google Scholar]
- Wu Y., Zhang G., Li Y., Jin Y., Dale R., Sun L.Q., Wang M. Inhibition of highly pathogenic avian H5N1 influenza virus replication by RNA oligonucleotides targeting NS1 gene. Biochem. Biophys. Res. Commun. 2008;365(2):369–374. doi: 10.1016/j.bbrc.2007.10.196. [DOI] [PubMed] [Google Scholar]
- Yen H.L., Herlocher L.M., Hoffmann E., Matrosovich M.N., Monto A.S., Webster R.G., Govorkova E.A. Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility. Antimicrob. Agents Chemother. 2005;49:4075–4084. doi: 10.1128/AAC.49.10.4075-4084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L., Hoffmann E., Taylor G., Scholtissek C., Monto A.S., Webster R.G., Govorkova E.A. Importance of neuraminidase active-site residues to the neuraminidase inhibitor resistance of influenza viruses. J. Virol. 2006;80:8787–8795. doi: 10.1128/JVI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L., Ilyushina N.A., Salomon R., Hoffmann E., Webster R.G., Govorkova E.A. Neuraminidase inhibitor-resistant recombinant A/Vietnam/1203/04 (H5N1) influenza viruses retain their replication efficiency and pathogenicity in vitro and in vivo. J. Virol. 2007;81:12418–12426. doi: 10.1128/JVI.01067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H.L., Monto A.S., Webster R.G., Govorkova E.A. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J. Infect. Dis. 2005;192:665–672. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
- Yuen K.Y., Chan P.K., Peiris M., Tsang D.N., Que T.L., Shortridge K.F., Cheung P.T., To W.K., Ho E.T., Sung R., Cheng A.F. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- Zhou B., Zhong N., Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zhou H., Jin M., Yu Z., Xu X., Peng Y., Wu H., Liu J., Liu H., Cao S., Chen H. Effective small interfering RNAs targeting matrix and nucleocapsid protein gene inhibit influenza A virus replication in cells and mice. Antivir. Res. 2007;76(2):186–193. doi: 10.1016/j.antiviral.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Zürcher T., Yates P.J., Daly J., Sahasrabudhe A., Walters M., Dash L., Tisdale M., McKimm-Breschkin J.L. Mutations conferring zanamivir resistance in human influenza virus N2 neuraminidases compromise virus fitness and are not stably maintained in vitro. J. Antimicrob. Chemother. 2006;58:723–732. doi: 10.1093/jac/dkl321. [DOI] [PubMed] [Google Scholar]


