Abstract
The purpose of this study was to determine the role of nitric oxide (NO) in the down-regulation of human CYP enzymes and mRNAs by an inflammatory stimulus in cultured human hepatocytes. We focused on CYP2B6, because previous studies showed that rat CYP2B proteins undergo an NO-dependent degradation in response to inflammatory stimuli. To ensure high level expression of CYP2B6, the inducer phenytoin was present at all times. Stimulation of cells with a mixture of TNFα, IL-1β and IFNγ (ILmix) down-regulated CYP2B6 mRNA and protein to 9% and 19% of control levels. The NO donor NOC-18 down-regulated CYP2B6 protein to 30% of control, with only a small effect on CYP2B6 mRNA. NOS inhibitors attenuated the down-regulation of CYP2B6 protein, but not mRNA, by ILmix. These findings demonstrate that the post-transcriptional NO dependent down-regulation of CYP2B enzymes, observed previously in rat hepatocytes, is conserved in human CYP2B6. This mechanism is specific for CYP2B6 among the enzymes tested. No evidence was found for regulation of CYP2E1 mRNA or protein by NO. NOC-18 treatment down-regulated CYP3A4 mRNA to 50% of control. However, NOS inhibitors failed to block the effects of ILmix on CYP3A4 expression.
Keywords: nitric oxide, cytochrome P450, CYP2B6, inflammation, cytokines, protein degradation
Introduction
Cytochromes P450 (CYPs) are heme-thiolate monooxygenases that serve diverse physiological roles including the synthesis and catabolism of hormones, eicosanoids, bile acids and retinoids [1]. The mammalian CYPs expressed in the liver, which are the focus of this paper, have a primary role in the metabolism clearance of most therapeutic drugs, and metabolism by CYPs is also necessary for the formation of toxic and carcinogenic metabolites of environmental chemicals [1].
Infectious and inflammatory diseases in humans, or diseases with an inflammatory component, are associated with decreased clearances of several CYP substrates [2–5], and increased incidence of adverse effects of drugs with a low therapeutic index like theophylline [2] CYP-selective effects have been inferred from the clearance of diagnostic substrates [6]. Moreover, many of these effects can be recapitulated in human hepatocyte cultures exposed to inflammatory cytokines [7–9]. In both humans and animals, transcriptional suppression of CYPs is an early event that is a major contributor to many of the subsequent effects observed on CYP activities [10]. On the other hand, there is clear evidence that inflammatory stimuli can regulate CYP proteins levels independently of transcriptional effects [11], and that enzyme activities can also be inhibited in the absence of changes in CYP protein levels [12].
Systemic inflammation causes a dramatic increase in hepatic NO production due to induction of inducible nitric oxide synthase (NOS2) in hepatocytes [13]. NO can bind to P450s and cause inhibition by reversible or irreversible pathways [14, 15], and therefore NO may be one cause of the enzyme inhibition observed in inflammatory states [16, 17]. Stimulation of NO synthesis may also be involved in the down-regulation of hepatic CYP mRNAs or proteins by inflammation, because these effects could be blocked or attenuated by NOS inhibitors in either in vivo or cell culture models [18–20]. However, the role of NO in down-regulation of P450 expression is still not fully understood. We found no effects of NOS inhibition on the suppression of hepatic P450 2C11, 3A2 and 2E1 mRNAs or proteins in rats injected with LPS or IL-1 [21]. We also demonstrated that NO is not required for down-regulation of CYP3A and 2E1 using wild type and NOS2-null mice [22]. On the other hand we recently found evidence for a role of NO in the post-translational suppression of phenobarbital-induced CYP2B1 in rat hepatocytes by bacterial endotoxin (lipopolysaccharide, LPS) [11, 23, 24]. Exposure of rat hepatocytes to LPS resulted in a rapid decrease in microsomal CYP2B1 protein, which was blocked by NOS inhibitors and mimicked by NO donors [11]. We developed a model in which CYP2B1 can be regulated by two independent mechanisms [23]: an NO-independent pre-translational mechanism which occurs at low LPS concentrations; and a rapid NO-dependent suppression of CYP2B protein.
Evidence for the participation of NO in inflammatory down-regulation of human CYP enzymes is sparse. NO donors can down-regulate CYP mRNAs and proteins in colon carcinoma [25, 26] and hepatocarcinoma [27] cell lines, and it was reported that in HT-29 cells, NOS2 inhibitors or antisense oligonucleotides could block the down-regulation of CYP3A4 protein by ceramide [28], a putative mediator of the effects of pro-inflammatory cytokines on CYP expression [29]. We are aware of one published study on the regulation of CYP expression by physiological or pharmacological NO in primary human hepatocytes, which is the optimal system to study regulation of the human drug-metabolizing CYPs. In that work, limited evidence was provided for the attenuation by an inhibitor of NOS of the down-regulation of CYP1A2 and 3A4 mRNAs and protein by interferon-γ [30].
Due to the strong evidence that NO synthesized by NOS2 caused a posttranscriptional down-regulation of CYP2B proteins in rat hepatocytes (see above), we set out to test the hypothesis that NO would have a similar role in the regulation of human CYP2B6. CYP2B6 may contribute to the metabolism of up to 25% of all pharmaceutical agents and is responsible for 3–5% of the total hepatic P450 [31, 32] although there is large interindividual variation in CYP2B6 expression [33, 34]. Due to the paucity of information about human hepatic CYP regulation by NO, we also studied two other important CYP enzymes. CYP3A4 is the predominant P450 in the human liver, comprising about 25% of the total hepatic P450 protein, and metabolizes 40% of all currently employed drugs [10]‥ CYP2E1 has broad substrate specificity and has an important role in the activation of many small molecules to toxic or carcinogenic metabolites [35]. In contrast to most CYPs, it is relatively refractory to inflammatory regulation in animal models.
In this paper, we tested the abilities of the NO donor (Z)-1-[2-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (NOC-18) to down-regulate CYP 2B6, 2E1 and 3A4 mRNAs and proteins in human hepatocytes, and of NOS inhibitors to block the inflammatory down-regulation of these gene products. Our findings document that the NO-dependent mechanism for down-regulation of CYP2B proteins observed in the rat is preserved in human CYP2B6. CYP3A4 is down-regulated at the mRNA level by pharmacological but not NOS-derived NO, whereas human CYP2E1 is refractory to cytokine down-regulation like its rodent counterparts.
Materials and Methods
Reagents
All chemicals were purchased from Sigma-Aldrich (St Louis, MO) unless otherwise stated. Tumor necrosis factor α (TNF α) was from Invitrogen (Carlsbad, CA), interferon-γ (IFNγ) was from BD Pharmagen (San Diego, CA) and NOC-18 was from Alexis Biochemicals (San Diego, CA). Specific antibodies were purchased to CYP3A4 (Xenotech, Lenexa, KA), CYP2B6 (Gentest, BD biosciences, Discovery Labwear, MA) and CYP2E1 (Daiichi Pure Chemical Co., Japan). NOS inhibitors tested included 1400W, LY83583 and l-N6-(1-Iminoethyl) lysine (NIL) from Calbiochem (EMD Biosciences, San Diego, CA).
Human hepatocyte cultures and treatments
These studies were carried out in accordance with the Declaration of Helsinki, and were designated exempt from review by the Emory University Institutional Review Board. Human hepatocytes were obtained from Dr Steven Strom at the University of Pittsburgh via the NIH-sponsored Liver Tissue Procurement and Distribution System program. They were prepared from livers not suitable for transplantation within 24 h of procurement. Details on liver donors are provided in Table 1. Hepatocytes were isolated as described in Strom et al [36] by a three-step collagenase perfusion technique and cultured on collagen coated 12 well plates (10 × 106 cells per plate, for RNA measurements) or T25 flasks (3 × 106 cells per flask, for protein measurements). Cells were cultured for 48 h prior to delivery in hepatocyte maintenance medium(HMM) (Cambrex Bioscience Walkersville Inc.). Upon receipt, cells were placed at 37°C in 5% CO2 and medium was changed 1–2 h later to Williams E cell culture media supplemented with 10 nM insulin, 25 nM dexamethazone, 10mg/ml penicillin/streptomycin and 100µM phenytoin [37]. Phenytoin was present throughout the experiments. Media were changed daily.
Table 1.
Donor Information
| Cells | Sex | Age | Race | Cause of Death |
|---|---|---|---|---|
| 1234 | M | 56 | Caucasian | Anoxia/Cardiac Arrest |
| 1261 | M | 21 months | Caucasian | Cerebrovascular Disease |
| 1274 | F | 55 | Black | Anoxia/Cardiac Arrest |
| 1280 | F | 36 | Caucasian | Anoxia/Cardiac Arrest |
| 1281 | M | 16 | Caucasian | Head Trauma |
| 1286 | M | 50 | Caucasian | Intracranial Hemorrhage |
| 1293 | M | 50 | Black | Cerebrovascular Disease |
| 1307 | F | 12 | Caucasian | Anoxia |
| 1318 | M | 15 Months | Caucasian | Unknown |
After a 48 h induction period, cells were exposed to a mixture (ILmix) of IL-1 (5 ng/ml), TNFα (10 ng/ml) and IFNγ (10 ng/ml) for 24 h to simulate an inflammatory event. Cells were also treated for 24 h with a range of NOS inhibitors in the presence or absence of the cytokine combination described above (ILmix). In each experiment some cells were treated for 24 h with NOC-18, which was prepared as a stock in high pH water just prior to use and added to the media at a final concentration of 500µM. All treatment groups were in triplicate or quadruplicate for each human hepatocyte preparation. At the end of the 24 h incubation period, media samples were removed and reserved for assay of the stable end products of NO production, nitrate + nitrite (NOx) using the Griess reaction [38]. Cells were harvested for preparation of microsomes or cell lysates, which were used to quantitate CYP protein expression. When enough cells were available, RNA was also collected from a number of the human cell batches.
Preparation of microsomes, cell lysates and RNA
All cells were washed twice in ice cold PBS. T25 flasks were scraped in ice cold PBS to remove cells. Cells were then pelleted and the supernatant discarded. For cell lysates, cells were resuspended in 200µl of cell lysis buffer (50 mM Tris pH 7.4, 0.1% SDS, 0.5% NP40, 1% EDTA), and sonicated for 10–12 sec with a ultrasonic cell disruptor Microsan (Misonix Inc, Farmingdale NY) on power setting 15. Protease inhibitor cocktail containing 4-(2-aminoethyl) benzenesulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin was added and lysates were stored at −80°C. Microsomes were prepared following the methods of Tippin et al [39] with some adaptations. Cells were harvested as described above, then resuspended in 1 ml of homogenization buffer (50 mM Tris – HCl pH 7.0, 150 mM KCl, 2 mM EDTA. Cells were then sonicated three times for 10 seconds at power setting 15, with icing in between. Cell lysates were centrifuged at 9000g for 20 minutes at 4°C in a bench top centrifuge. The supernatant was then centrifuged at 400,000g for 15mins at 4°C in a Beckman Optima TLX ultracentrifuge (Beckman Coulter Inc, Fullerton, CA). The microsomal pellet was resuspended in 200 µl of 0.25 M sucrose and stored at −80°C. Protein levels were measured using the BSA assay from Pierce (Thermo Fisher Scientific, Waltham, MA). Total RNA was prepared from 12-well plates using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturers directions. RNA concentrations and quality were determined spectrophotometrically by measuring absorbance at 260nm and 260/280nm ratio.
Reverse transcriptase-real time PCR
Purified total RNA was reverse transcribed using the Superscript first strand synthesis system for RT-PCR kit (Invitrogen) according to the manufacturer’s instructions. Primers for the human CYPs studied are listed in Table 2. When available, published primer pairs were utilized. Primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed using the Primer Select software program (DNASTAR, Inc., Madison, WI). Real time RT-PCR analysis was carried out on an ABI 7300 sequence detection system (Applied Biosystems, Foster City, CA) to quantify relative P450 mRNA expression in human hepatocytes. Reactions were carried out in a total volume of 25µl using Sybr green master mix reagent (Applied Biosystems), 2 µl of cDNA for template with 10 µM forward and reverse primers. All reactions were carried out in duplicate. Assay plates were incubated at 50 °C for 2 minutes, 95 °C for 10 minutes followed by 40 cycles of 95°C for 15 seconds plus 1 minute at the relevant annealing temperature (see Table 2 for details). Levels of the housekeeping gene GAPDH were monitored in parallel in order to normalize the total amount of cDNA in each sample. Livak and Schmittgen [40] described the Ct method that was used to calculate the amount of target P450 in a treated sample normalized to GAPDH and relative to the control P450 samples. Results are expressed as relative levels of P450 mRNA with expression in control samples arbitrarily defined as one.
Table 2.
RT-PCR Primer List
| CYP | RT-PCR Primer | Annealing Temp | Product Size | Reference |
|---|---|---|---|---|
| 3A4 | Fwd cct tac aca tac aca ccc ttt gga agt | 58 | 382 | [47] |
| Rvd agc tca atg cat gta cag aat ccc cgg tta | ||||
| 2B6 | Fwd atg ggg cac tga aaa aga ctg a | 58 | 283 | [47] |
| Rvd aga ggc ggg gac act gaa tga c | ||||
| 2E1 | Fwd gac tgt ggc cga cct gtt | 58 | 297 | [48] |
| Rvd act acg act gtg ccc ttg g | ||||
| GAPDH | Fwd gga cca cca gcc cca gca aga g | 58 | 103 | |
| Rvd gag gag ggg aga ttc agt gtg gtg | ||||
SDS-PAGE and Western blotting
SDS-PAGE and Western blotting were carried out as described by Cheng et al. [41] with modifications. Equal amounts of sample (10–25 µg of protein) were loaded into each lane of a 5–15% gradient polyacrylamide gel (Criterion Tris-HCl gel, BioRad, Hercules, CA) for analysis. Blots were blocked by incubating in PBS containing 0.05% Tween-20 (PBS-Tween) and milk for approximately 1 hour and then incubated overnight at 4 °C with the respective primary antibodies. Antibodies for CYP2B6, 3A4 and 2E1 diluted according to manufacturers specifications in PBS-Tween. The blots were washed 3 times for 10 min in PBS-Tween prior to incubation with the horseradish peroxidase coupled secondary antibody (Jackson Immunoresearch Laborites, West Grove, PA). The membrane was incubated for a further hour at room temperature and washed again prior to development. The intensity of stained bands was measured by densitometry using a Kodak EDAS 290 imaging station and Imaging Software 4.0.1, (Eastman Kodak, California).
Statistical analysis
For data from a single human hepatocyte preparation, ANOVA and Tukey’s test were used for statistical comparisons between groups. Nonparametric statistics were used to analyze data combined from several human hepatocyte preparations, in order to account for interindividual variability in the magnitudes of the responses. For these analyses, a Kruskal-Wallis test followed by Mann-Whitney U-tests with Holm-Bonferroni correction were employed. Significance was set at p<0.05 for all tests.
Results
Optimization of experimental conditions
Preliminary experiments were conducted to optimize conditions for CYP2B6 expression. In order to be able to consistently detect this enzyme in our hepatocyte preparations, it was necessary to induce CYP2B6 expression in the cells. Phenytoin was selected as the CYP inducer based on the work of LeCluyse et al [37] in which CYP2B6 displayed a 5-fold induction and CYP3A4 and 8-fold induction in terms of enzyme activity. Early experiments (data not shown) in which cells were stimulated with IL-1 only resulted in about a 2-fold induction in NOx production and limited CYP2B6 protein down regulation. Therefore, based on work by Nussler et al [42]we tested a mixture (ILmix) of IL-1 (5 ng/ml), TNFα (10 ng/ml) and IFNγ (10 ng/ml) to simulate an inflammatory event. This combination resulted in a 10-fold induction of NO production and robust down-regulation of CYP2B6 protein, and was therefore used for all further experiments. NOx levels, NOS mRNA and CYP2B6 protein levels were monitored in response to ILmix exposure in 2 individuals, HH1234 and HH1261, (data not shown). Elevation of NO levels in media were detectable at 16–18 h, and by 24 h a 20–50 fold increase over control was recorded. NOS mRNA levels rose as early as 6 h and were still elevated at 24 h. CYP2B6 protein was clearly down regulated by 16 h. Allowing for variability, all subsequent human subject data was collected 24 h post-treatment when NOS and NO levels were significantly increased and CYP2B6 levels were clearly down regulated in response to the inflammatory stimulus.
Evaluation of NOS inhibitors
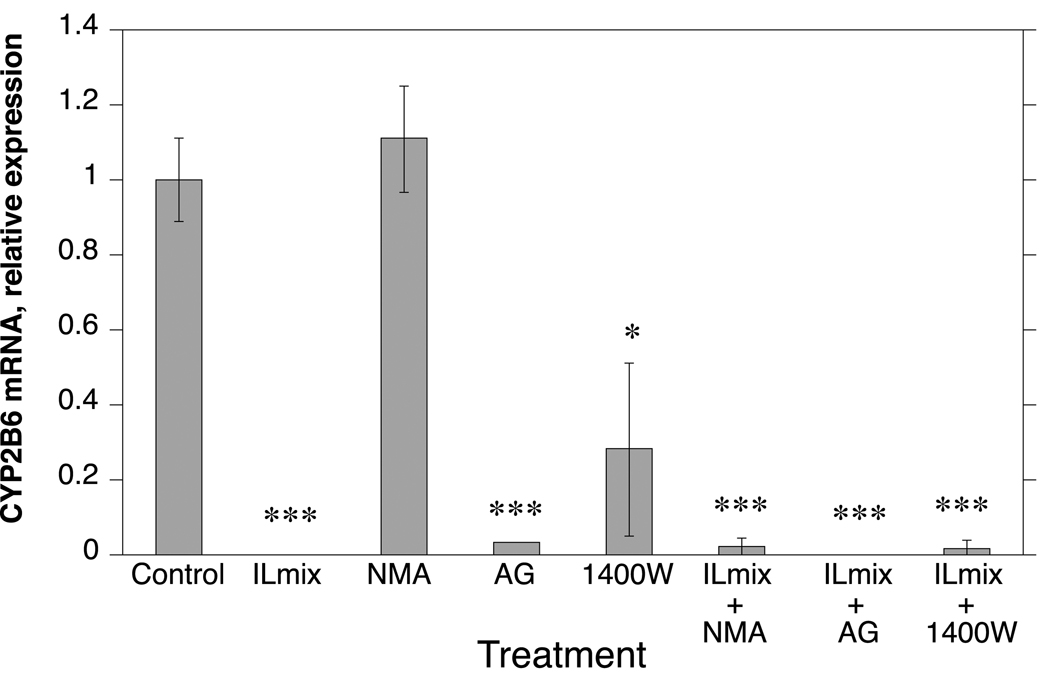
In order to study the role of NO in CYP protein regulation, it was necessary to identify NOS inhibitors that did not by themselves affect CYP expression. Therefore, we evaluated a number of NOS inhibitors for their effects on CYP2B6 expression (Fig. 1). Aminoguanidine (300µM), an irreversible inhibitor of multiple NOS isoforms, and 1400W (8µM) an irreversible and specific NOS 2 inhibitor both had clear effects on CYP2B6 RNA (Fig.1) and were not used in further experiments for this reason. We also tested LY83583 (4µM), a guanylyl cyclase inhibitor which has been shown to block induction of NOS2 in rat hepatocytes [11], but it did not block NOx production in human hepatocytes (not shown) and was removed from analysis.
Fig. 1.
Effect of inflammation and NOS inhibitors on expression of CYP2B6 mRNA in human hepatocytes. Cells from human hepatocyte preparation HH 1261 were treated for 24 h as shown, and then harvested for analysis of CYP2B6 mRNA expression by RT-real time PCR as described above. All treatments were carried out in triplicate. AG, aminoguanidine. Values represent means ± S.E.M. a, significantly different from control, p < 0.05.
N-methyl-l-arginine (NMA) (300µM), a competitive inhibitor of NOS enzymes, did not affect CYP2B6 mRNA expression in the presence or absence of ILmix (Fig. 1), and was therefore selected for further study. We also examined the effects of NIL (300 µM), a more selective inhibitor of NOS2. Both NMA and NIL effectively inhibited NO production, although the extent of inhibition varied somewhat among individuals (Fig. 2). Neither NMA nor NIL themselves affected CYP2B6 RNA or protein levels (Fig. 3, Fig. 4, Fig. 5).
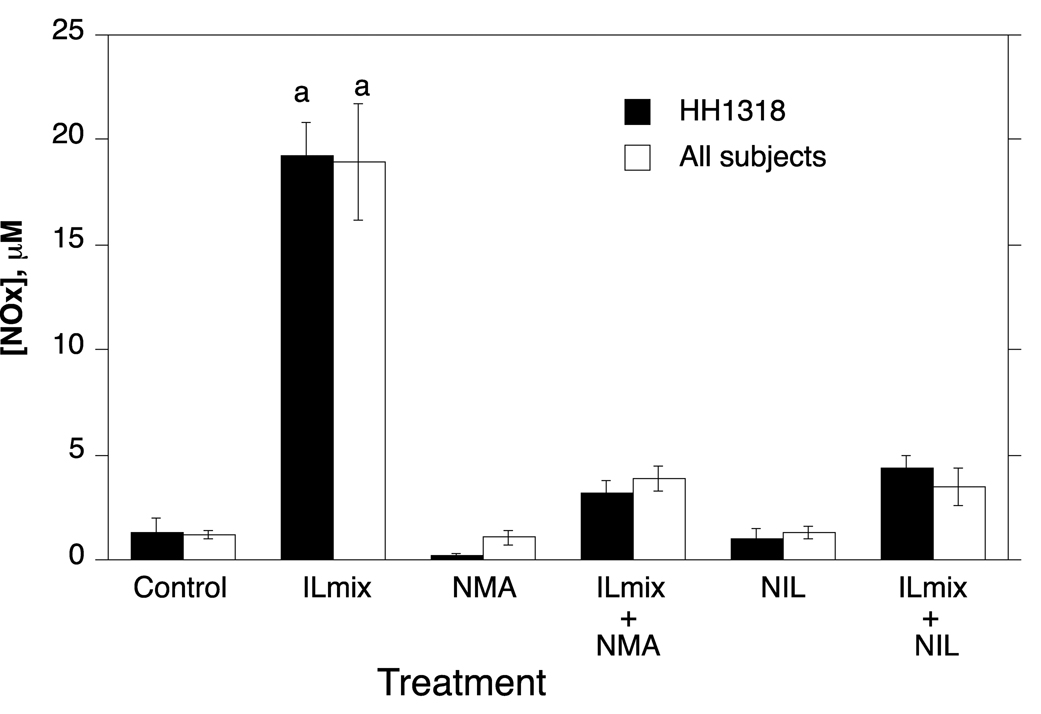
Fig. 2.
NOx concentrations in human hepatocyte cultures. Cells from human hepatocyte preparations were treated for 24 h with NIL or NMA (300 µM) inhibitors in the presence or absence of ILmix as shown. Media were harvested for analysis of the concentration of nitrate = nitrite (NOx) as a measure of NO production. Values represent means ± S.E.M. for each group. a, significantly different from control, p < 0.05. Values for HH1318 are the means of three culture flasks. n values for the combined data are given in the text.
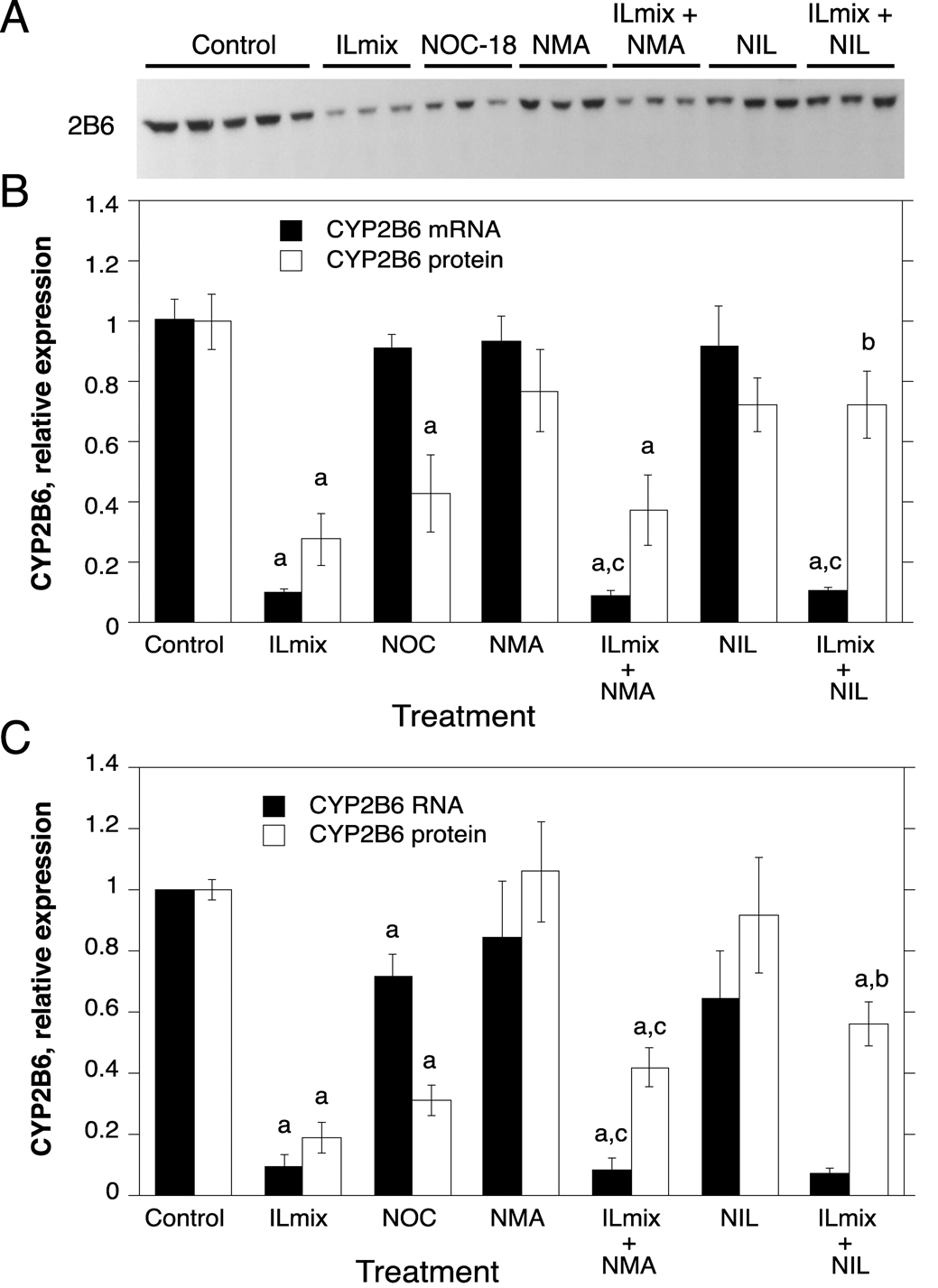
Fig. 3.
Regulation of CYP2B6 protein and mRNA expression by NO donors, ILmix and NOS inhibitors. Cells from human hepatocyte preparations were treated for 24 h with NOC-18 (NOC), or with NOS inhibitors in the presence or absence of ILmix as shown. Cells were harvested for analysis of CYP2B6 mRNA and/or protein expression. A, Western blot of samples from HH1318. B, Quantitative analysis of CYP2B6 protein and RNA expression in HH1318 (n=3). C. Combined analyses of results from multiple human hepatocyte preparations. Not all experimental conditions were analyzed in each preparation, and the n value for each group is stated in the text. Values represent means ± S.E.M. for each group. a, significantly different from control; b, significantly different from ILmix group; c, significantly different from group treated with NOS inhibitor alone, p<0.05. In panel C, no statistical comparisons were made between mRNA levels in groups treated with NIL because the n value in these groups was only 2.
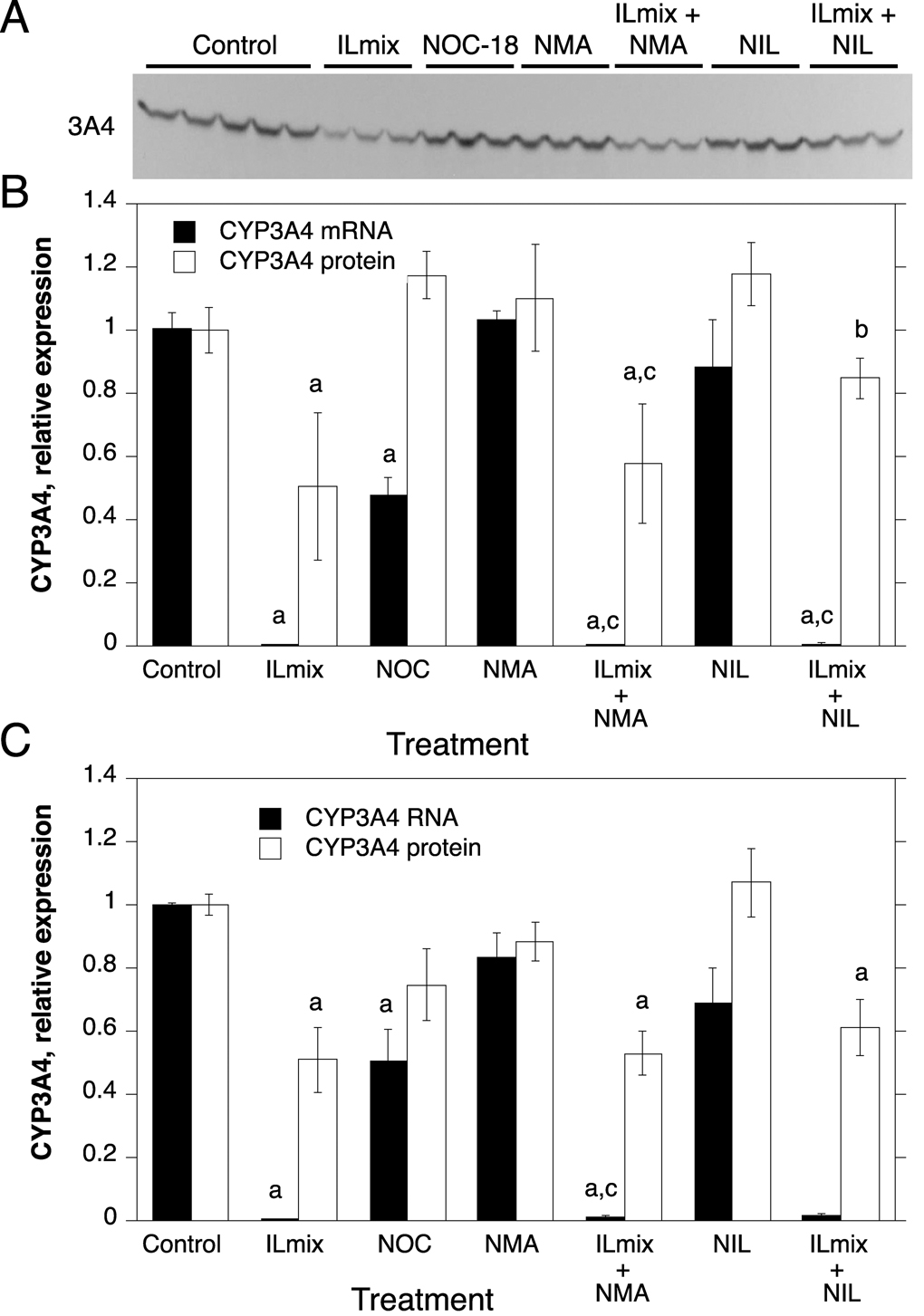
Fig. 4.
Regulation of CYP3A4 protein and mRNA expression by NO donors, ILmix and NOS inhibitors. Cells from human hepatocyte preparations were treated for 24 h with NOC-18 (NOC), or with NOS inhibitors in the presence or absence of ILmix as shown. Cells were harvested for analysis of CYP2B6 mRNA and/or protein expression. A, Western blot of samples from HH1318. B, Quantitative analysis of CYP3A4 protein and RNA expression in HH1318 (n=3). C. Combined analyses of results from multiple human hepatocyte preparations. Not all experimental conditions were analyzed in each preparation, and the n value for each group is stated in the text. Values represent means ± S.E.M. for each group. a, significantly different from control; b, significantly different from ILmix group; c, significantly different from group treated with NOS inhibitor alone, p<0.05. In panel C, no statistical comparisons were made between mRNA levels in groups treated with NIL because the n value in these groups was only 2.
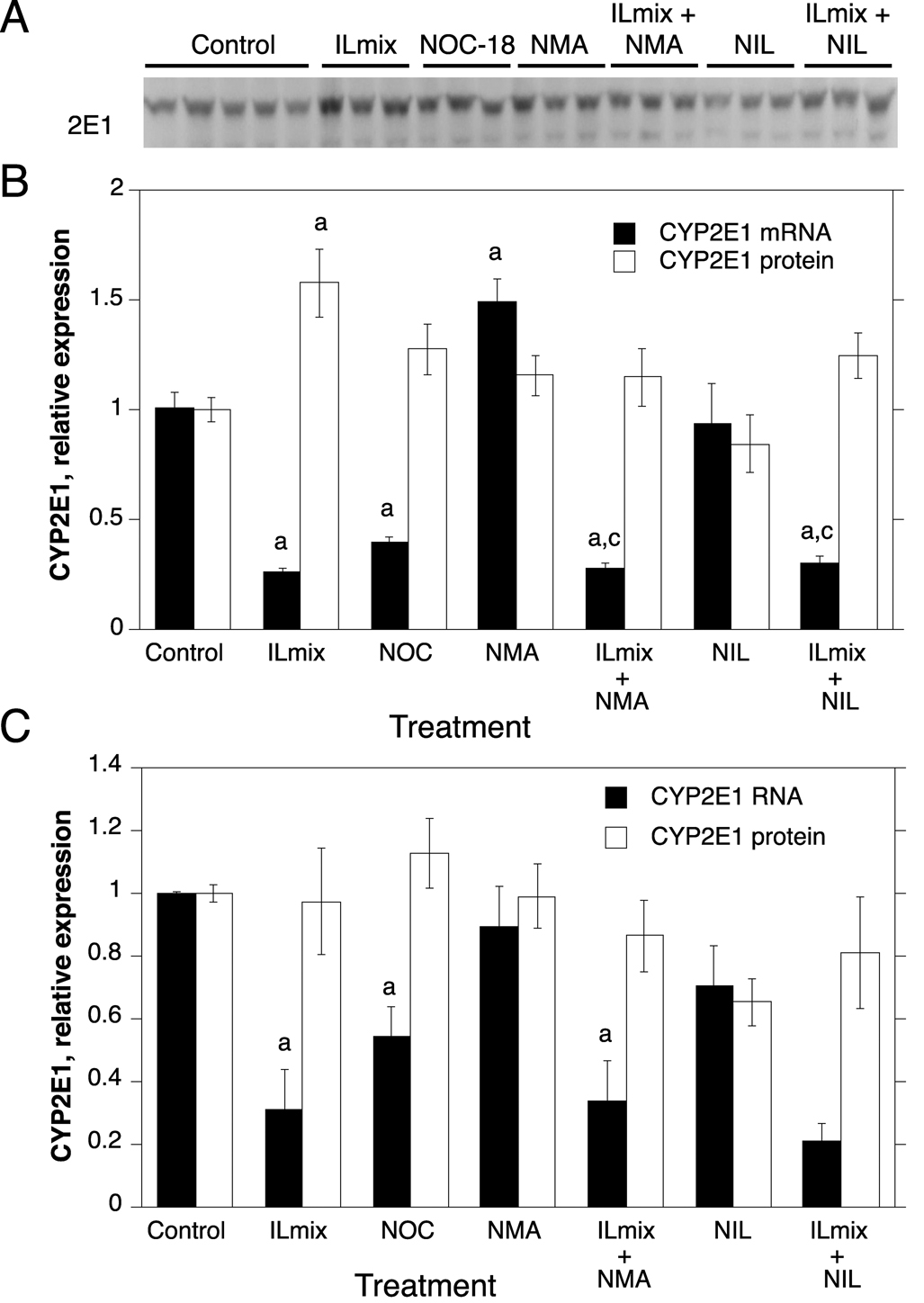
Fig. 5.
Regulation of CYP2E1 protein and mRNA expression by NO donors, ILmix and NOS inhibitors. Cells from human hepatocyte preparations were treated for 24 h with NOC-18 (NOC), or with NOS inhibitors in the presence or absence of ILmix as shown. Cells were harvested for analysis of CYP2E1 mRNA and/or protein expression. A, Western blot of samples from HH1318. B, Quantitative analysis of CYP2E1 protein and RNA expression in HH1318 (n=3). C. Combined analyses of results from multiple human hepatocyte preparations. Not all experimental conditions were analyzed in each preparation, and the n value for each group is stated in the text. Values represent means ± S.E.M. for each group. a, significantly different from control; b, significantly different from ILmix group; c, significantly different from group treated with NOS inhibitor alone, p<0.05. In panel C, no statistical comparisons were made between mRNA levels in groups treated with NIL because the n value in these groups was only 2.
Due to the limited supply of human hepatocytes, not all treatments or analyses could be performed in every human hepatocyte preparation. CYP mRNA and protein expression were assessed together in six hepatocyte preparations (HH 1274, 1280, 1281, 1286 and 1318). Only mRNA expression was measured in HH1261, and only protein levels were measured in HH 1293 and 1307. In the ensuing Figures, data are presented for a single representative hepatocyte preparation (HH1318) in which all of the treatments were performed (panels A and B), together with the averaged data for all of the hepatocyte preparations tested for each condition or parameter (panels C).
Regulation of CYP expression by the NO donor NOC 18
Treatment with the NO donor caused a clear down-regulation of CYP2B6 protein to 43% of control in HH1318 (Fig. 3A, B), and a similar decrease (31% of control) in the data for the averaged preparations (Fig. 3C, n=6). However, NOC treatment did not affect CYP2B6 mRNA in HH1318, and had only a small effect on the mRNA (71% of control) in the averaged data (Fig. 3C, n=5).
In sharp contrast to CYP2B6, exposure of the cells to NOC-18 had no effect on CYP3A4 protein levels (n=7), but down-regulated CYP3A4 mRNA to 48% and 51% of control in HH1318 and the combined data (n=5), respectively (Fig. 4). Similarly to CYP3A4, NOC-18 down-regulated CYP2E1 mRNA to 40% of control in HH1318 (Fig. 5B), and to 55% of control in the panel of hepatocytes tested (Fig 5C, n=5). NOC-18 treatment had no significant effect on CYP2E1 protein levels (Fig 5C, n=5).
Effect of NOS inhibition on NOx production and regulation of CYP mRNAs by cytokines
The cytokine mixture, ILmix, caused a profound down-regulation of the mRNAs for CYPs 2B6, 3A4 and 2E1 in both HH1318 and in the data from the combined experiments (Fig. 3, Fig. 4 and Fig. 5; n = 6). This was accompanied by a robust functional induction of NOS2 as indicated by elevated NOx levels in the media (Fig. 2). CYP3A4 mRNA expression was essentially ablated, whereas CYP2B6 and 2E1 mRNAs were suppressed to 10% and 30% of control, respectively. The NOS inhibitors NMA and NIL each inhibited NOx production in response to ILmix stimulation, although NIL was slightly more effective than NMA (Fig. 2). NMA (n=6) did not affect the mRNA expression of CYPs 2B6, 3A4 or 2E1 in the presence or absence of ILmix stimulation (Fig. 3, Fig. 4, Fig. 5). However, NIL also had no significant effect on CYP mRNA suppression in HH1318 (Fig. 3, Fig. 4, Fig. 5), but because CYP mRNAs could only be measured in two of the preparations that were treated with NIL, no statistical comparisons were made in the combined data concerning these groups.
Effect of NOS inhibition on regulation of CYP proteins by cytokines
Of the three CYPs tested, CYP2B6 protein was the most sensitive to down-regulation by ILmix, being suppressed to 19% of control levels (Fig. 3C, n=7). CYP3A4 protein was significantly down-regulated to 51% of control (Fig. 4C, n=7), whereas CYP2E1 protein levels were not significantly affected (Fig 5C, n=5).
Neither NMA (n=5) or NIL (n=4) affected the expression of CYP2B6 protein in the absence of ILmix (Fig. 3). NIL blocked the down-regulation of CYP2B6 protein by ILmix, because CYP2B6 levels in hepatocytes treated with ILmix plus NIL were significantly higher than in hepatocytes that received ILmix alone, and not significantly different from hepatocytes treated with NIL alone (Fig. 3B,C, n=4). NMA tended to have the same effect (19% of control in cells stimulated with ILmix, compared to 42% of control in cells treated with ILmix and NMA), although this effect narrowly failed to achieve statistical significance (p=0.056, Fig. 3C, n=5).
In HH1318, NIL, but not NMA, attenuated the down-regulation of CYP3A4 protein by ILmix. However, this observation was not reproduced in the combined data from multiple preparations (Fig. 4C, n=4–5). CYP2E1 protein was unaffected by any of the treatments examined in the combined data (Fig. 5, n=3–5), although a significant increase in CYP2E1 protein was noted in HH1318 exposed to ILmix (Fig 5B).
To address the possibility that modification of CYP2B6 by NO blocks the reactivity of the protein with the CYP2B6 antibody, we treated insect cell microsomes expressing CYP2B6 or pooled human liver microsomes, (Gentest, Woburn, MA) with 500 µM NOC-18, 150 µM S-nitrosoglutathione (a nitrosylating agent) or 300 µM peroxynitrite for 1 h, then analyzed the reactivity of the CYP2B6 with the antibody by Western blotting. None of the treatments affected the CYP2B6 band intensity (data not shown), indicating that any CYP2B6 modification by these agents did not affect its reactivity with the antibody. This is consistent with the fact that the immunogenic peptide for these antibodies, residues 247–266, contains no Tyr or Cys residues that are the main amino acid targets of reactive nitrogen species.
Discussion
Two lines of evidence from this work argue for a significant role of NO in the down-regulation of CYP2B6 protein by pro-inflammatory mediators. Firstly, blockade of NO production with NOS inhibitors attenuated the down-regulation of CYP2B6 protein by an inflammatory stimulus. Secondly, an NO donor mimicked the down-regulation of CYP2B6 protein by inflammation. This NO-dependent down-regulation of CYP2B6 is achieved by a post-transcriptional mechanism, because neither NOS inhibitors failed to block the decline in CYP2B6 mRNA expression due to ILmix, and the NO donor affected CYP2B6 mRNA expression to a much lesser degree. This regulatory mechanism is unique to CYP2B6 among the three CYPs tested in this study, and is further illustrated by the fact that CYP2B6 protein is much more sensitive to inflammatory stimulation than is CYP3A4 or CYP2E1, despite that CYP3A4 mRNA is more profoundly affected than is CYP2B6 mRNA.
Thus, the NO-dependent regulation of CYP2B proteins previously identified in rat hepatocytes [11] is preserved in human hepatocytes. Interestingly, this mechanism appears to be absent in mouse liver [24] but the reason for this species difference remains to be determined. Regardless of the reason, our results suggest that CYP2B6-dependent drug clearance or bioactivation may be more susceptible to impairment during disease conditions that favor hepatic NO production (e.g. bacterial sepsis). Therapeutic agents that release nitric oxide (e.g sodium nitroprusside, nitroglycerin) are also likely to inhibit CYP2B6-dependent metabolism via stimulation of enzyme degradation. The consequences of this mechanism will be longer lasting than transient inhibition due to formation of nitrosoheme complexes. Caution should always be exercised when extrapolating from hepatocytes to humans. Our experiments were done in the presence of the inducer phenytoin, to obtain sufficient CYP2B6 expression for measurement. Since the induction mechanism is transcriptional, we assume that uninduced CYP2B6 protein will be similarly affected. However, this remains to be determined.
We speculated earlier that the NO-dependent down-regulation of CYP2B proteins in rat hepatocytes involves the modification of CYP2B amino acids by one ore more reactive nitrogen species, and that this modification targets the protein for ubiquitin-dependent proteasomal degradation [23]. There is precedent for such a mechanism of regulation of another hepatic protein, iron-regulatory protein-2. Nitrosylation of specific cysteine residues on this protein causes it to be ubiquitinated and degraded by the proteasome [43]. Recent studies in our laboratory have demonstrated that indeed rat CYP2B1 is ubiquitinated in an NO-dependent manner, and that the rapid disappearance of the protein is blocked by proteasome inhibitors [44]. Studies are under way to determine whether this mechanism also pertains to CYP2B6.
Interestingly, the NO-dependent down-regulation observed with CYP2B6 does not extend to CYP2E1 or CYP3A4, despite the fact that each of these proteins has been shown to undergo proteasomal degradation when modified by suicide substrates [45]. No evidence was obtained in this study for any involvement of NO in the post-transcriptional regulation of human CYP2E1. In contrast, the NO donor NOC-18 down-regulated CYP2E1 mRNA. Zamora et al reported that overexpression of NOS2 via an adenoviral vector or treatment with the NO donor S-nitroso-N-acetylpennicillamine caused an induction of CYP2E1 mRNA and protein in mouse hepatocytes [46]. The differential effects of NO observed in these two studies may reflect a species difference. Regardless of this, the failure of NMA or NIL to block the down-regulation of CYP2E1 mRNA by ILmix suggests that NO does not play a prominent role in the physiological regulation of human CYP2E1.
CYP3A4 expression was suppressed at the RNA level in cells exposed to the NO donor, which is consistent with previous work in Caco-2 cells, a human colon carcinoma cell line, in which NO donors inhibited the induction of CYP3A4 mRNA by 1,25-dihydroxyvitamin D3 [25, 26]. The mechanism of the effect in Caco-2 cells apparently involves the induction of c-myc, which represses CYP3A4 transcription [26]. It would be interesting to determine if this mechanism is involved in the suppression of CYP3A4 by NO donors in hepatocytes. However, NO formed in the hepatocytes does not play a critical role in the profound down-regulation of CYP3A4 that occurs in response to an inflammatory stimulus, because we found that NOS inhibition failed to even partially reverse this effect. This conclusion is in contrast to the findings of Donato et al [30], who reported that down-regulation of CYP1A2 and 3A4 protein and mRNAs caused by treatment of the cells with IFNγ were reversed by NO inhibition. However, the data provided to support this conclusion were limited, and not supported by statistical analyses. Furthermore, the induction of NO production by IFNγ was minimal in that study.
In conclusion, this work provides clear evidence for the existence of a physiological or pathophysiological regulation of CYP2B6 protein by nitric oxide formed during an inflammatory response in human hepatocytes. This effect is independent of the transcriptional regulation of CYP2B6 mRNA, and does not exist for the human CYP enzymes CYP3A4 and 2E1. Future studies will attempt to define the mechanism by which NO production causes the selective degradation of CYP2B6.
Acknowledgements
This work was supported by grant GM069971 from the National Institutes of Health. Hepatocytes were provided by Dr. Steven Strom via the Liver Tissue Procurement and Distribution System, NIH Contract N01-DK-9-2310. C-M Lee was supported by a Training Grant from the National Institutes of Health (T32ES01287).
We thank Kimberley Pierce and Malik Raynor for expert technical assistance.
List of Abbreviations
- CYP
cytochrome P450
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- IFNγ
interferon-γ
- NIL
l-N6-(1-Iminoethyl) lysine
- NMA
N-methyl-l-arginine
- NOC-18
(Z)-1-[2-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate
- NOS
nitric oxide synthase
- NOx
nitrate + nitrite
- TNFα
tumor necrosis factor–α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nature Rev. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer MJ, Furukawa CT, Koup JR, Shapiro GG, Pierson WE, Bierman CW. Altered theophylline clearance during an influenza B outbreak. Pediatrics. 1982;69:476–480. [PubMed] [Google Scholar]
- 3.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J. Clin. investigation. 1994;94:2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carcillo JA, Doughty L, Kofos D, Frye RF, Kaplan SS, Sasser H, Burckart GJ. Cytochrome P450 mediated-drug metabolism is reduced in children with sepsis-induced multiple organ failure. Intensive Care Med. 2003;29:980–984. doi: 10.1007/s00134-003-1758-3. [DOI] [PubMed] [Google Scholar]
- 5.Rivory LP, Slaviero KA, Clarke SJ. Hepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase response. Br. J. Cancer. 2002;87:277–280. doi: 10.1038/sj.bjc.6600448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frye RF, Zgheib NK, Matzke GR, Chaves-Gnecco D, Rabinovitz M, Shaikh OS, Branch RA. Liver disease selectively modulates cytochrome P450--mediated metabolism. Clin. Pharmacol. Ther. 2006;80:235–245. doi: 10.1016/j.clpt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, Beaune P, Guillouzo A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol. Pharmacol. 1993;44:707–715. [PubMed] [Google Scholar]
- 8.Muntane-Relat J, Ourlin JC, Domergue J, Maurel P. Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology. 1995;22:1143–1153. [PubMed] [Google Scholar]
- 9.Sunman JA, Hawke RL, LeCluyse EL, Kashuba AD. Kupffer cell-mediated IL-2 suppression of CYP3A activity in human hepatocytes. Drug Metab. Dispos. 2004;32:359–363. doi: 10.1124/dmd.32.3.359. [DOI] [PubMed] [Google Scholar]
- 10.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu. Rev. Pharmacol. Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari L, Peng N, Halpert JR, Morgan ET. Role of nitric oxide in down-regulation of CYP2B1 protein, but not RNA, in primary cultures of rat hepatocytes. Mol. Pharmacol. 2001;60:209–216. doi: 10.1124/mol.60.1.209. [DOI] [PubMed] [Google Scholar]
- 12.Bleau AM, Levitchi MC, Maurice H, Du Souich P. Cytochrome P450 inactivation by serum from humans with a viral infection and serum from rabbits with a turpentine-induced inflammation: the role of cytokines. Br. J. Pharmacol. 2000;130:1777–1784. doi: 10.1038/sj.bjp.0703486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit. Care Med. 2000;28:N37–N52. doi: 10.1097/00003246-200004001-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem. Biophys. 1993;300:115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 15.Vuppugalla R, Mehvar R. Hepatic disposition and effects of nitric oxide donors: Rapid and concentration-dependent reduction in the cytochrome P450-mediated drug metabolism in isolated perfused rat livers. J. Pharmacol. Exp. Ther. 2004;310:718–727. doi: 10.1124/jpet.104.065557. [DOI] [PubMed] [Google Scholar]
- 16.Veihelmann A, Brill T, Blobner M, Scheller I, Mayer B, Prolls M, Himpel S, Stadler J. Inhibition of nitric oxide synthesis improves detoxication in inflammatory liver dysfunction in vivo. Am. J. Physiol. 1997;273:G530–G536. doi: 10.1152/ajpgi.1997.273.2.G530. [DOI] [PubMed] [Google Scholar]
- 17.Kourylko O, Fradette C, Arcand M, du Souich P. Modulation of CYP1A2 and CYP3A6 catalytic activities by serum from rabbits with a turpentine-induced inflammatory reaction and interleukin 6. Drug Metab. Dispos. 2006;34:27–35. doi: 10.1124/dmd.105.006528. [DOI] [PubMed] [Google Scholar]
- 18.Carlson TJ, Billings RE. Role of nitric oxide in the cytokine-mediated regulation of cytochrome P-450. Mol. Pharmacol. 1996;49:796–801. [PubMed] [Google Scholar]
- 19.Stadler J, Trockfeld J, Schmalix WA, Brill T, Siewert JR, Greim H, Doehmer J. Inhibition of cytochromes P4501A by nitric oxide. Proc. Natl. Acad. Sci. USA. 1994;91:3559–3563. doi: 10.1073/pnas.91.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khatsenko OG, Gross SS, Rifkind AB, Vane JR. Nitric oxide is a mediator of the decrease in cytochrome P450-dependent metabolism caused by immunostimulants. Proc. Natl. Acad. Sci.USA. 1993;90:11147–11151. doi: 10.1073/pnas.90.23.11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan ET. Regulation of Cytochrome P450 by Inflammatory Mediators: Why and How? Drug Metab. Dispos. 2001;29:207–212. [PubMed] [Google Scholar]
- 22.Sewer MB, Barclay TB, Morgan ET. Down-regulation of cytochrome P450 mRNAs and proteins in mice lacking a functional NOS2 gene. Mol. Pharmacol. 1998;54:273–279. doi: 10.1124/mol.54.2.273. [DOI] [PubMed] [Google Scholar]
- 23.Morgan ET, Li-Masters T, Cheng PY. Mechanisms of cytochrome P450 regulation by inflammatory mediators. Toxicology. 2002;181–182:207–210. doi: 10.1016/s0300-483x(02)00283-4. [DOI] [PubMed] [Google Scholar]
- 24.Li-Masters T, Morgan ET. Down-regulation of phenobarbital-induced cytochrome P4502B mRNAs and proteins by endotoxin in mice: independence from nitric oxide production by inducible nitric oxide synthase. Biochem. Pharmacol. 2002;64:1703–1711. doi: 10.1016/s0006-2952(02)01423-5. [DOI] [PubMed] [Google Scholar]
- 25.Hara H, Mitani N, Adachi T. Inhibitory effect of nitric oxide on the induction of cytochrome P450 3A4 mRNA by 1,25-dihydroxyvitamin D3 in Caco-2 cells. Free Radic. Res. 2000;33:279–285. doi: 10.1080/10715760000301441. [DOI] [PubMed] [Google Scholar]
- 26.Watabe M, Isogai Y, Numazawa S, Yoshida T. Role of c-Myc in nitric oxide-mediated suppression of cytochrome P450 3A4. Life Sci. 2003;74:99–108. doi: 10.1016/j.lfs.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Hara H, Adachi T. Contribution of hepatocyte nuclear factor-4 to down-regulation of CYP2D6 gene expression by nitric oxide. Mol. Pharmacol. 2002;61:194–200. doi: 10.1124/mol.61.1.194. [DOI] [PubMed] [Google Scholar]
- 28.Chun YJ, Lee S, Yang SA, Park S, Kim MY. Modulation of CYP3A4 expression by ceramide in human colon carcinoma HT-29 cells. Biochem. Biophys. Res. Commun. 2002;298:687–692. doi: 10.1016/s0006-291x(02)02541-x. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Nikolova-Karakashian M, Merrill AH, Jr, Morgan ET. Regulation of cytochrome P450 2C11 (CYP2C11) gene expression by interleukin-1, sphingomyelin hydrolysis, and ceramides in rat hepatocytes. J. Biol. Chem. 1995;270:25233–25238. doi: 10.1074/jbc.270.42.25233. [DOI] [PubMed] [Google Scholar]
- 30.Donato MT, Guillen MI, Jover R, Castell JV, Gomez-Lechon MJ. Nitric oxide-mediated inhibition of cytochrome P450 by interferon-gamma in human hepatocytes. J. Pharmacol. Exp. Ther. 1997;281:484–490. [PubMed] [Google Scholar]
- 31.Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, Martin H, Beaune P, de Waziers I. Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics. 1999;9:295–306. [PubMed] [Google Scholar]
- 32.Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, Yan B, Negishi M, LeCluyse EL. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab. Dispos. 2004;32:348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez FJ. The 2006 Bernard B. Brodie Award Lecture. Cyp2e1. Drug Metab. Dispos. 2007;35:1–8. doi: 10.1124/dmd.106.012492. [DOI] [PubMed] [Google Scholar]
- 36.Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- 37.LeCluyse E, Madan A, Hamilton G, Carroll K, DeHaan R, Parkinson A. Expression and regulation of cytochrome P450 enzymes in primary cultures of human hepatocytes. J. Biochem. Mol. Toxicol. 2000;14:177–188. doi: 10.1002/(sici)1099-0461(2000)14:4<177::aid-jbt1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 39.Tippin TK, Hamilton G, Moore L, Beaudet EJ, Jolley S, Brodie TA, Andrews RC, Becherer JD, McDougald DL, Gaul MD, Hoivik DJ, Mellon-Kusibab K, Lehmann J, Kliewer S, Novick S, Laethem R, Zhao Z, LeCluyse EL. CYP3A induction by N-hydroxyformamide tumor necrosis factor-alpha converting enzyme/matrix metalloproteinase inhibitors use of a pregname X receptor activation assay and primary hepatocyte culture for assessing induction potential in humans. Drug Metab. Dispos. 2003;31:870–877. doi: 10.1124/dmd.31.7.870. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Cheng PY, Wang M, Morgan ET. Rapid transcriptional suppression of rat cytochrome P450 genes by endotoxin treatment and its inhibition by curcumin. J. Pharmacol. Exp. Ther. 2003;307:1205–1212. doi: 10.1124/jpet.103.057174. [DOI] [PubMed] [Google Scholar]
- 42.Nussler AK, Geller DA, Sweetland MA, Di Silvio M, Billiar TR, Madariaga JB, Simmons RL, Lancaster JR., Jr Induction of nitric oxide synthesis and its reactions in cultured human and rat hepatocytes stimulated with cytokines plus LPS. Biochem. Biophys. Res. Commun. 1993;194:826–835. doi: 10.1006/bbrc.1993.1896. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Wing SS, Ponka PS. S-nitrosylation of IRP2 regulates its stability via the ubiquitin-proteaso me pathway. Mol. Cell. Biol. 2004;24:330–337. doi: 10.1128/MCB.24.1.330-337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CM, Kim BY, Li L, Morgan ET. Nitric oxide-dependent proteasomal degradation of cytochrome P450 2B proteins. J. Biol. Chem. 2007 doi: 10.1074/jbc.M708821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correia MA, Sadeghi S, Mundo-Paredes E. Cytochrome P450 ubiquitination: branding for the proteolytic slaughter? Annu. Rev. Pharmacol. Toxicol. 2005;45:439–464. doi: 10.1146/annurev.pharmtox.45.120403.100127. [DOI] [PubMed] [Google Scholar]
- 46.Zamora R, Vodovotz Y, Alarcon L, Betten B, Loughran PA, Aulak KS, Stuehr DJ, Gibson KF, Billiar TR. Nitric oxide from the inducible nitric oxide synthase (iNOS) increases the expression of cytochrome P450 2E1 in iNOS-null hepatocytes in the absence of inflammatory stimuli. Arch. Biochem. Biophys. 2001;390:287–294. doi: 10.1006/abbi.2001.2391. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Antona C, Jover R, Gomez-Lechon MJ, Castell JV. Quantitative RT-PCR measurement of human cytochrome P-450s: application to drug induction studies. Arch. Biochem. Biophys. 2000;376:109–116. doi: 10.1006/abbi.2000.1697. [DOI] [PubMed] [Google Scholar]
- 48.Wilkening S, Stahl F, Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]