Summary
Neurexins and neuroligins play an essential role in synapse function, and their alterations are linked to autistic spectrum disorder. Interactions between neurexins and neuroligins regulate inhibitory and excitatory synaptogenesis in vitro through a “splice insert signaling code”. In particular, neurexin 1β carrying an alternative splice insert at site SS#4 interacts with neuroligin 2 (found predominantly at inhibitory synapses) but much less so with other neuroligins (those carrying an insert at site B and prevalent at excitatory synapses). The structure of neurexin 1β+SS#4 reveals dramatic rearrangements to the “hyper-variable surface”, the binding site for neuroligins. The splice insert protrudes as a long helix into space, triggers conversion of loop β10-β11 into a helix rearranging the binding site for neuroligins, and 3) rearranges the Ca2+-binding site required for ligand binding increasing its affinity. Our structures reveal the mechanism by which neurexin 1β isoforms acquire neuroligin splice isoform selectivity.
Introduction
Neurexins are cell surface adhesion molecules found at synapses, located predominantly on pre-synaptic membranes (reviewed Craig & Kang, 2007; Ushkaryov et al., 1992; Graf et al., 2004; Missler et al., 2003; Dean et al., 2003; Taniguchi et al., 2007). The extracellular domains of α-neurexins consist of 6 LNS/LG (Laminin, Neurexin, Sex hormone binding globulin/Laminin G) domains interspersed by 3 EGF-like repeats. The ectodomains of β-neurexins are made up of only a short β-neurexin specific sequence and a single LNS/LG domain that is identical in amino acid sequence to its α-neurexin counterpart, the sixth LNS/LG domain. The repertoire of neurexins is dramatically increased by alternative splicing of mRNA transcripts at 5 positions in α-neurexins and 2 positions in β-neurexins modifying regions that encode the extracellular domain to produce more than 2000 possible neurexin isoforms (Rowen et al., 2002; Tabuchi et al., 2002). As a consequence of splicing, highly conserved polypeptide splice inserts reaching 30 amino acids in length incorporate at highly conserved positions in the LNS/LG domains (splice site SS#2 in n1α_L2, splice site SS#3 in n1α_L4 and splice site SS#4 in n1β_L/n1α_L6 (Fig 1a) (Rowen et al., 2002; Tabuchi et al., 2002). SS#2, SS#3, and SS#4 map to loops that surround a Ca2+-binding site and form the “hyper-variable surface” (Fig 1b). Single neurexin LNS/LG domains suffice to bind protein ligands; binding is typically Ca2+-dependent and regulated through the presence (or absence) of splice inserts. Protein partners have been identified for different neurexin LNS/LG domains including: the post-synaptic adhesion molecules neuroligins (mediated by n1β_L/n1α_L6), the spider venom toxin α-latrotoxin (mediated by n1β_L/n1α_L6), and the extracellular matrix protein α-dystroglycan (mediated by n1α_L2 as well as n1β_L/n1α_L6) (Ichtchenko et al., 1995; 1996; Sugita et al., 1999; 2001; Bouchard et al., 2005; Chih et al., 2006; Graf et al., 2006). The protein partner interaction sites likely coincide with the hyper-variable surfaces on neurexin LNS/LG domains as has indeed been established for neuroligin 1 binding to neurexin 1β (Fig 1b) (Rudenko et al., 2001; Graf et al., 2006; Araç et al., 2007; Chen et al., 2007; Fabrichny et al., 2007). Given the diversity, modular domain structure, and multiplicity of protein partners, neurexins are well positioned to fine-tune synaptic protein networks through their protein:protein interactions.
Figure 1.
a) Extracellular domain of neurexin 1α (n1α) and neurexin 1β (n1β). LNS/LG domains (abbreviated from Laminin, Neurexin, Sex hormone binding globulin/Laminin G domain) are indicated as grey squares labeled ‘L1’ through ‘L6’, or in the case of neurexin 1β, ‘L’. EGF-like domains (abbreviated from Epidermal Growth Factor) are shown as black ovals labeled ‘A’, ‘B’ and ‘C’. A short β-neurexin specific sequence is indicated as ‘β-specific’. In addition to the extracellular domain, neurexins also contain a signal peptide, single transmembrane segment and a cytoplasmic tail (not shown). The five positions in the extracellular domain of α-neurexins that accommodate splice insert sites are indicated (SS#1, SS#2, SS#3, SS#4 and SS#5) as well as the two splice insert sites found in β-neurexins (SS#4 and SS#5). In the rat and bovine species n1α_L4 accommodates a glycine or a 10 a.a. insert at SS#3 (DCIRINCNSS) and n1β_L (with identical sequence to n1α_L6) accommodates either no insert or a 30 a.a. sequence at SS#4 (GNNDNERLAIARQRIPYRLGRVVDEWLLDK) (Ushkaryov et al., 1994; Ullrich et al., 1995). b) Structure of n1α_L2 (Sheckler et al., 2006). The splice insert sites SS#2, SS#3 and SS#4 are shown in yellow, green and magenta respectively, forming a region called the “hyper-variable surface” shaded in grey. A Ca2+-ion is observed experimentally in n1α_L2 at the center of the hyper-variable surface, shown as a blue sphere. Loops β2-β3, β6-β7 and β10-β11 identified to be crucial for synaptogenic activity and neuroligin binding are indicated (Graf et al., 2006).
The best studied neurexin:protein interaction, the neurexin 1β-neuroligin trans-synaptic bridge, spans the synaptic cleft and plays a key role in synapse maintenance and function (Craig & Kang, 2007). Neuroligins, like neurexins, undergo alternative splicing in their extracellular domain, but only at two sites: A (adding up to 40 a.a.) and B (adding a 9 a.a. insert) (Comoletti et al., 2006). Interactions between members of the neurexin and neuroligin family appear highly regulated by a “splice insert signaling code” (Boucard et al., 2005; Chih et al., 2006, Graf et al., 2006, Kang et al., 2007). Key features of this code involve: 1) a pronounced binding preference between specific neurexin alternative splice isoforms and specific neuroligin alternative splice isoforms (Boucard et al., 2005; Chih et al., 2006; Graf et al., 2006, Kang et al., 2007), and 2) a distinct distribution of neuroligin isoforms between excitatory (NL1/3/4) and inhibitory synapses (NL2) (Song et al., 1999; Graf et al., 2004; Varoqueaux et al., 2004; Prange et al., 2004; Chih et al., 2005; Levinson & El-Husseini 2005; Chubykin et al., 2007). In particular, the presence of a 30 amino acid splice insert at site SS#4 in n1β (n1β+SS#4) favors binding to neuroligin 2 (which is not spliced at site B and is found at GABAergic synapses) promoting GABAergic over glutamatergic synaptogenesis. N1β+SS#4 also supports binding to other neuroligins when they do not carry a splice insert at their site B. In contrast, n1β with no splice insert at SS#4 interacts with neuroligin isoforms regardless of whether the latter are spliced at their site B and consequently n1β is found at both glutamatergic as well as GABAergic synapses. The neurexin-neuroligin interaction is likely of fundamental importance because an imbalance of excitatory versus inhibitory synaptic activity is thought to be at the basis of a number of neurological disorders, and indeed mutations in neuroligins as well as neurexins have been linked to autistic spectrum disorder (Jamain et al., 2003; Laumonnier et al., 2004; Cline et al., 2005; Garber, 2007; Szatmari et al., 2007, Tabuchi et al., 2007).
To understand the mechanism by which alternative splicing of n1β generates selective preference for neuroligin isoforms, we solved the structure of n1β_L/n1α_L6 carrying its natural thirty amino acid alternative splice insert at splice site SS#4 (abbreviated here to n1β_L(30)) at 1.72 Å resolution in a Ca2+-containing form. To directly assess the structural impact of the splice insert at SS#4 we also solved the structure of the splice insert-free n1β in the presence of Ca2+ because only the metal-free form of n1β was known (Rudenko et al., 1999). To ascertain if n1β_L/n1α_L6 forms with and without a splice insert have Ca2+-binding sites at the hyper-variable surface with characteristic properties, we additionally solved the structure of n1α_L4 in the splice insert-free form. Our studies reveal major changes to the shape of n1β_L/n1α_L6 upon incorporation of the splice insert at SS#4, not only adding secondary structure but also inducing major rearrangements to a key portion of the hyper-variable surface. The rearrangements affect key residues in loop β10-β11 lining the hyper-variable surface and important for neuroligin binding. In addition, the presence of SS#4 increases the Ca2+-binding affinity of n1β, though overall the Ca2+-binding affinity appears low for the neurexin LNS/LG domains tested (n1β +/- SS#4 and n1α_L4 +/- SS#3). Our results indicate that incorporation of alternative splice inserts into neurexin LNS/LG domains can introduce profound changes to their structural and biochemical properties.
Results
Structure of n1β_L(30)
The structure of n1β_L(30) was solved in presence of Ca2+ and refined to 1.72 Å (Table 1, Experimental Procedures). Two independent monomers are found in the asymmetric unit with 198 and 196 a.a. respectively (residues 80 – 294 and 80-292), each comprising 14 β-strands and 2 α-helices (Fig 2). Electron density is not observed for the residues 199 - 215 in either monomer.
Table 1.
Data and Refinement Statistics Summary for n1β_L(30), n1β + Ca2+ and n1α_L4
| Data Set£ | Resolution(Å)§ | Reflections (total/unique) | Multiplicity | Completeness (%) | Rmerge¶(%) | I/σ |
|---|---|---|---|---|---|---|
| n1β_L(30) | 19.9-1.72(1.76 - 1.72) | 406535/49688 | 8.2 | 92.1 (73.1) | 5.4 (35.5) | 25.9 (3.1) |
| n1β+Ca2+ | 40.6-2.60(2.64 - 2.60) | 362517/73949 | 4.9 | 99.9 (99.8) | 6.5 (42.3) | 24.8 (4.3) |
| n1α_L4 | 23.7-1.04(1.08-1.04) | 1039051/82197 | 12.6 | 92.9/(60.5*) | 7.0 (29.6) | 37.9 (6.2) |
| Structure | n1β_L(30) | n1β+Ca2+ | n1α_L4 | |||
|
| ||||||
| Resolution (all reflections ∣F∣/σ ≥ 0.0) | 19.9 – 1.72 Å | 20.0 – 2.6 Å | 23.7 – 1.04 Å | |||
| Protein atoms (as alternative conformation) | 3023 atoms (95 atoms) | 12111 | 1477 (214) | |||
| Protein residues | 394 | 1600 | 175 | |||
| Solvent atoms | 353 | 113 | 130 | |||
| Molecules in asymmetric unit | 2 | 9 | 1 | |||
| Ligands | 2 Ca2+ | 3 Ca2+ | 1 Ca2+ | |||
| Unique reflections | 49679 | 73564 | 80871 | |||
| Working set/ Test set | 45909/3770 | 69823/3741 | 72763/8108 | |||
| Rwork | 19.75 % | 20.27 % | 14.65 % | |||
| Rfree | 22.47 % | 24.4 % | 16.52 % | |||
| Rmsd bond lengths | 0.023 Å | 0.017 Å | 0.016 Å | |||
| Rmsd bond angles | 1.7° | 1.6° | 1.9° | |||
| Rmsd NCS-related molecules (nr Cα atoms) | 0.26 Å (196) | 0.50 Å (173#) | n.a. | |||
| Bave of main chain atoms (nr atoms)& | 25.2 Å2 (1576) | 39.8 (6400) | 12.9 Å2 (700) | |||
| Bave of side chain atoms (nr atoms)& | 27.3 Å2 (1447) | 40.6 Å2 (5711) | 15.3 Å2 (777) | |||
| Bave of solvent atoms (nr atoms)& | 31.1 Å2 (353) | 33.0 Å2 (113) | 20.5 Å2 (133) | |||
| Bave of Ca2+-ions (nr ions)& | 20.9 Å2 (2) | 43.7 Å2 (3) | 6.7 Å2 (1) | |||
Outer shell statistics in parentheses;
Rmerge = Σ (∣ (I − <Imean>) ∣ / Σ (I);
Bave=average residual B-factor after TLS refinement by REFMAC;
1.08 – 1.12 Å 84.6% complete, rest of shells ≥ 95.7%;
except mol I with 151 Cα
Figure 2.
Ribbon diagram of n1β_L(30). The β-sandwich is shown in a face view (left) and side view (right). β-strands are depicted as light blue arrows. Helical residues are shown in pink with the exception of the residues belonging to the thirty amino acid splice insert at SS#4 which are shown in magenta (part of helix Sα1). Residues Pro199 - Ile215 (part of loop β10-β11) are not visible in the structure, and are depicted here as a dotted line. A Ca2+-ion is observed at the hyper-variable surface, shown here as a blue sphere, the N-terminus and C-terminus of the polypeptide chain are indicated with “N-term” and “C-term” respectively. Dimensions for the β-sandwich (cyan) and the protruding helix Sα1 (pink/magenta) are indicated.
Accommodating a splice insert at splice site SS#4
The thirty amino acid splice insert Gly201 – Lys230 interrupts loop β10 –β11 at the hyper-variable surface with residues Pro216 – Lys230 forming part of helix (Sα1) that protrudes perpendicularly into space (Fig 2). The helix Sα1 extends out more than 28 Å, almost doubling the height of the monomer (Fig 2), and adopts the same orientation in both molecules with respect to the β-sandwich (Fig 3a). Superimposing the molecules (using all 196 common Cα-atoms, rmsd of 0.26 Å) places the two Sα1 helices < 2° apart (rmsd of 0.39 Å for 18 Cα-atoms Arg218-Thr235). However, the Sα1 helices make a number of contacts with symmetry related molecules in the crystal lattice which could influence their orientation. The high quality of the electron density shows that the Sα1 helices are well-ordered, unlike residues Gly201 – Ile215 of the splice insert that are too flexible to be observed (Fig 3b, Fig 3c).
Figure 3.
Helix Sα1 adopts a well-defined orientation in the crystal structure of n1β_L(30). a) superposition of the crystallographically independent n1β_L(30) molecules A (yellow) and B (magenta) found in the asymmetric unit. b) omit map density for Sα1 in molecule A. c) omit map density for Sα1 in molecule B. The figures b) and c) display electron density contoured at 1σ from simulated annealed composite omit maps calculated by CNS (Brunger et al., 1998). The Ca2+-ion is shown as a blue sphere.
Structural rearrangements take place to accommodate Sα1
We compared the structures of n1β_L(30) and n1β (Table 1) in their Ca2+-containing forms to identify the structural rearrangements that accompany the integration of the splice insert at SS#4. In n1β, loop β10–β11 traverses the edge of the sandwich forming an integral part of the hyper-variable surface (Fig 4a, refer also to Fig 1b). In n1β_L(30), the extra residues added by the splice insert do not interact with the rest of the molecule (Fig 4a). However, in order to accommodate Sα1, residues in loop β10-β11 undergo drastic rearrangement altering their conformation compared to n1β (Fig 4a). Residues Gly231 – Thr235 that in n1β contribute to the long and extended loop β10-β11 traversing the rim of the β-sandwich, rearrange in n1β_L(30) into a helical conformation to form part of Sα1 (Fig 4b). In particular, Gly231- Arg232 adopt an α-helical conformation and Gln233-Leu234-Thr235 convert to a 310 helix extending Sα1 in n1β_L(30). The three residues N-terminal to SS#4 (Tyr198, Pro199 and Ala200) are disordered in n1β_L(30) unlike in n1β (Fig 4a and 4b).
Figure 4.

Structural comparison of n1β_L(30) and n1β. a) superposition of n1β_L(30) (grey Cα-trace) and n1β (cyan trace). The Sα1-helix seen only in n1β_L(30) is shown in maroon (the splice insert residues Pro216-Lys230) and in yellow (residues Gly231-Thr235). The residues Gly231-Thr235 in n1β are displayed in orange. b) Close-up of the structural rearrangements that occur to loop β10-β11 upon incorporation of a splice insert at SS#4 in n1β. N1β_L(30) in grey, yellow and maroon, and n1β in cyan. Relevant side chains are depicted in ball-and-stick (carbon, yellow or cyan, nitrogen, blue and oxygen, red respectively). The Ca2+-ion is shown as a blue sphere. A cyan arrow indicates where the splice insert site SS#4 maps in n1β.
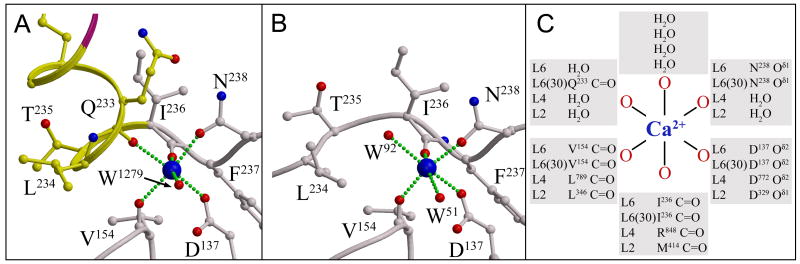
Structural characterization of Ca2+-binding sites of n1β_L(30), n1β, and n1α_L4
To investigate if the Ca2+-binding sites at the hyper-variable surfaces are coupled to alternative splicing of neurexin LNS/LG domains, we first identified the Ca2+-binding sites in the structures of n1β_L(30), n1β and n1α_L4 in presence 5mM Ca2+. A Ca2+-ion was identified in n1β_L(30) by difference Fourier techniques (see Experimental Procedures). The Ca2+ is bound at the hyper-variable surface with approximate octahedral geometry displaying Ca2+ to oxygen distances of 2.34 - 2.50 Å (as typical for proteins (Harding, 2006)) chelated by side chains Asp137 and Asn238, main chain carbonyls from Val154, Ile236 and Q233; the coordination (n=6) is completed by a water molecule (Wat1279 in mol A and Wat1211 in mol B) (Fig 5a). Difference Fourier techniques revealed a Ca2+-ion bound at the hyper-variable surface of n1β as well (see Experimental Procedures). The Ca2+-ion is bound with approximate octahedral geometry by side chain oxygens from Asp137 and Asn238, main chain carbonyls from Val154 and Ile236 at Ca2+ to oxygen distances in the range 2.26 – 2.66 Å while coordination n=6 is completed by two water molecules (shown for monomer D, Fig 5b). Only three out of nine n1β molecules in the asymmetric unit contain an ion bound at the Ca2+-binding site, because an arginine residue from a neighboring molecule (Arg109 or Arg286) forms a salt bridge with Asp137 blocking access in the rest of the molecules. While amino acid sequence conservation predicts a Ca2+-binding site at the hyper-variable surface of n1α_L(4), formed by the side chain of Asp772 (Oδ2), and main chain carbonyl oxygens from Leu789 and Arg848 (Fig 5c), in the crystal structure determined to 1.04 Å (Table 1) we find electron density that we interpret as a mixture between a Ca2+-ion (with three water molecules completing its coordination) and a Tris molecule with its NH3+/NH2-group occupying roughly the same position as the Ca2+-ion (an interpretation that is warranted by the very high resolution of the diffraction data)(Fig S1a, S1b, S1c). N1α_L(4) crystals were exposed to Tris-buffer less than one half hour prior to data collection, suggesting that n1α_L(4) has a surprisingly weak Ca2+-binding site.
Figure 5.
Ca2+-binding sites in neurexin LNS/LG domains. a) Ca2+-binding site observed in n1β_L(30), b) Ca2+-binding site observed in n1β, and c) schematic comparison of experimentally determined Ca2+-binding sites in neurexin LNS/LG domains n1α_L2, n1α_L4, n1β_L/n1α_L6 and n1β_L(30)/n1α_L6(30), with information for n1α_L2 obtained from Sheckler et al., 2006. Relevant side chains are depicted in ball-and-stick (carbon, yellow or grey, nitrogen, blue and oxygen, red respectively). The Ca2+-ion is shown as a blue sphere.
Comparing the Ca2+-binding site for n1β_L(30) and n1β reveals that incorporation of the splice insert at SS#4 alters the Ca2+-binding site of n1β_L/n1α_L6 structurally (Fig 5a and 5b). The rearrangements incurred to incorporate Sα1 in n1β_L(30), altering Gly231-Thr235 from an extended loop into an helical conformation, work to reposition Gln233 so that it ligands the Ca2+-ion with its backbone carbonyl, thereby displacing a water molecule (Wat92) that occupies the same position in n1β more fully burying the Ca2+-ion (Fig 5a and 5b). So, while no residue of the splice insert directly interacts with Ca2+- binding site, its incorporation results in an additional protein ligand being provided to the Ca2+-ion contributed by loop β10—β11. Our structures reveal that the minimal Ca2+-binding site in neurexin LNS/LG domains is formed by a side chain of an conserved aspartic acid residue and two main chain carbonyl residues, but that Ca2+-coordination is completed in very different ways leaving the Ca2+-ion solvent exposed (such as in n1α_L2 and n1α_L4) or almost completely buried, such as seen in n1β_L(30), where it is coordinated by 5 protein ligands (Fig 5c).
Alternative splicing of neurexin LNS/LG domains regulates Ca2+-binding affinity
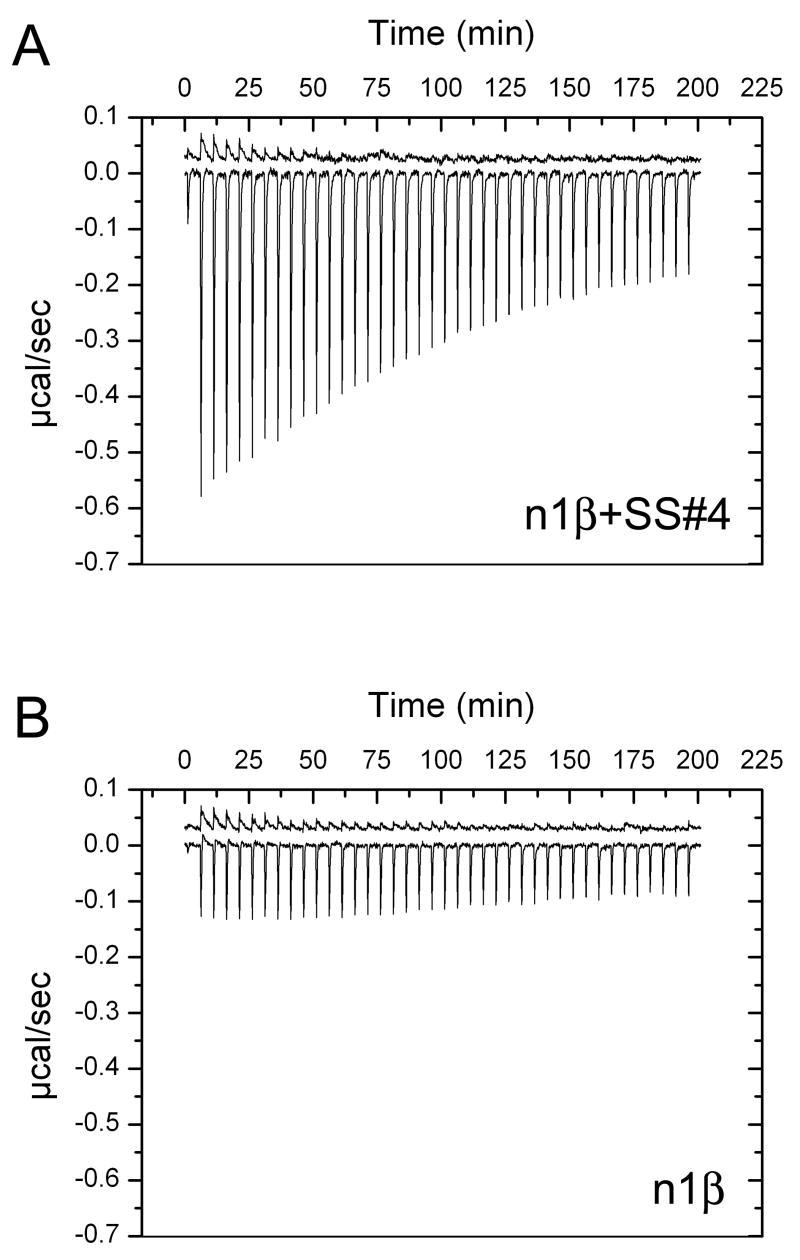
To understand if the presence of alternative splice inserts changes the affinity of the Ca2+-binding sites we used isothermal titration calorimetry (ITC) to characterize Ca2+-binding to n1β and n1α_L4, and their respective splice isoforms. Titration of a Ca2+-containing solution into n1β carrying the 30 a.a. splice insert at SS#4 (0.35 mM n1β+SS#4, 8.1 mM CaCl2) generated a clear but weak exothermic reaction (largest peak < 0.6 μcal/sec) that slowly saturated, indicating a metal-binding site increasingly occupied by Ca2+ (Fig. 6a). Titration of n1β lacking the splice insert at SS#4 with Ca2+ (under the identical experimental conditions) generated a clear but much weaker exothermic reaction that did not saturate significantly indicating lower affinity (Fig 6b). The heat evolved through titration of Ca2+ into n1α_L4 or its splice isoform n1α_L4(10) with an insert at SS#3 (0.35 mM protein in a GST-fusion form to increase solubility, 6 mM CaCl2) generated a very weak endothermic reaction, only slightly larger than the heat evolved upon dilution of the Ca2+ into buffer, indicating that Ca2+-binding was poorly/not measurable under the experimental conditions (Fig. S2). We conclude that alternative splicing of n1β alters both its three-dimensional structure, as well as its Ca2+-binding site, impacting the affinity for Ca2+ in a domain specific way.
Figure 6.
Isothermal titration calorimetric studies of n1β+SS#4 and n1β. Titrations were carried out using a Microcal VP-ITC as described in the Experimental Procedures. Each spike (with the exception of the first) represents the heat evolved in μcal/sec as a result of a 5 μl injection of CaCl2. a) 0.35 mM n1β+SS#4 in CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) titrated with 8.1 mM CaCl2 at 5°C. b) 0.35 mM n1β in CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) titrated with 8.1 mM CaCl2 at 5°C. For each run the heat of dilution of Ca2+ into buffer obtained by carrying out an identical titration into buffer with no macromolecule is also shown (top trace in each figure, vertically offset by an arbitrary amount for visualization purposes). In both cases, the heat evolved per injection was small and binding did not reach saturation precluding the fitting of a binding isotherm and derivation of a binding constant; titrations using higher protein concentrations were not possible due to limited protein solubility.
Discussion
Neurexins form a large family of synaptic adhesion molecules diversified through alternative splicing. The trans-synaptic bridge formed between specific presynaptic neurexin alternative splice isoforms and specific post-synaptic neuroligin alternative splice isoforms promotes excitatory and inhibitory synaptogenesis in vitro, controlling their balance. A “splice insert signaling code” is exploited regulating mutual binding affinities. In particular, studies using recombinant proteins as well as cell surface binding assays have revealed that n1β binds all four neuroligin 1 splice isoforms (i.e. +A+B, +A-B, -A+B and -A-B) and both neuroligin 2 isoforms (+A and -A), while n1β+SS#4 binds only isoforms of neuroligin 1 lacking the B-insert (i.e. +A-B and -A-B) and both neuroligin 2 isoforms (i.e. +A and -A, as neuroligin 2 is not spliced at the B-site)(Boucard et al., 2005; Chih et al., 2006). Incorporation of a 30 a.a. splice insert site at SS#4 in n1β therefore promotes inhibitory synapse formation in vitro, because on the one hand binding to neuroligin 2 is maintained which is specific to inhibitory synapses, and on the other hand binding is reduced to neuroligin isoforms that carry splice inserts at their site B and are located predominately at glutamatergic synapses (Boucard et al., 2005; Chih et al., 2006; Graf et al., 2006).
We have solved the structure of n1β_L(30) carrying its 30 amino acid alternative splice insert at splice site SS#4, the first structure of a neurexin LNS/LG domain accommodating a splice insert. The structure reveals that: 1) the 30 a.a. splice insert does not make contact with the rest of the molecule but forms a long α-helix protruding from the hyper-variable surface, 2) incorporation of the splice insert at SS#4 results in dramatic structural rearrangements to residues outside the splice insert, changing the molecular shape and properties of the hyper-variable surface, and 3) the Ca2+-binding site is rearranged to more fully obscure the Ca2+ from solvent indeed increasing its Ca2+-binding affinity (as observed by ITC). The conformation of helix Sα1 observed in the crystal structure may represent its solution structure as well (as opposed to being the result of lattice contacts) because 1) secondary structure prediction programs indicate that the splice insert contains helical content, 2) the Sα1 helices, though stabilized by the crystal packing, are identically orientated with respect to their β-sandwich cores in the two crystallographically independent molecules, and finally, 3) the Ca2+-binding site at the hyper-variable surface of n1β_L(30) anchors one end of the Sα1 helix in place. However, in the context of a complex with neuroligin helix Sα1 may undergo structural changes to adopt quite another conformation (see further on in Discussion). In stark contrast, structures of the Ca2+-containing forms of n1β and n1α_L4 reveal a large flat and open hyper-variable surface with metal binding sites that appear by ITC to be weakly occupied (if at all) and are largely solvent accessible.
Impact of an alternative splice insert at SS#4
The rearrangements observed in n1β_L(30) reveal a structural mechanism for the regulatory effect imparted by the splice insert at SS#4 and a mechanism for generating neuroligin splice isoform specificity.
Studies probing neuroligin 1 binding through mutagenesis of neurexin 1β pinpointed the hyper-variable surface as being an essential protein:protein interaction site (Graf et al., 2006). In particular, poly-alanine replacement of loop β2-β3 (Ser107Thr108Arg109), loop β6-β7 (Val154Gly155Thr156Asp157) or loop β10-β11 (Arg232Gln233Leu234Thr235), loops surrounding the Ca2+-binding site central to the hyper-variable surface (Fig 1), completely abolished both inhibitory as well as excitatory synaptogenic activity in neuron:transfected fibroblast co-culture assays, while other loops tested had no effect. These studies also showed that synaptogenic activity is abolished by mutation Arg109Ala on β2-β3 and diminished by mutation Asp158Ala in loop β6-β7, residues on either side of the Ca2+-binding site. Importantly, the individual mutations Arg232Ala, Gln233Ala and Thr235Ala displayed a regulatory effect because they only somewhat affected synaptogenic activity at inhibitory synapses (involving neuroligin 2) but significantly depressed synaptogenic activity at excitatory synapses (presumably involving neuroligin isoforms with a splice insert at site B). Direct cell surface binding studies showed that these same point mutations disrupted both neuroligin 1 (+B) and neuroligin 2 binding, but impacted the former more than the latter, indicating that residues Arg232, Gln233 and Thr235 appear to discriminate between neuroligin (splice) isoforms.
Our structures reveal a molecular mechanism for the generation of splice isoform specific neuroligin interactions whereby the shape of the hyper-variable surface is grossly altered by the presence of helix Sα1, and whereby “switch” residues Gly231Arg232Gln233Leu234Thr235 (loop β10-β11) occupy drastically different conformations in n1β_L(30) compared to the splice insert-free n1β (for example counterparts for Arg232 and Gln233 locate roughly 15.5 Å apart in the two structures) (Fig 4). Only certain neuroligin binding epitopes however are targeted for rearrangement because residues Arg109 in loop β2-β3 and Asp158 in loop β6-β7 (crucial for binding neuroligins regardless of splicing at their B-site and essential for both inhibitory and excitatory synaptogenic activity) are in the same conformation in n1β and n1β+SS#4. Though helix Sα1 may undergo conformational changes upon neuroligin binding its presence nonetheless must drastically influence the binding surfaces that can be generated. Our results indicate that β-neurexins employ a ‘splice insert-blocked’ or ‘splice insert-open’ hyper-variable surface together with switch residues Gly231Arg232Gln233Leu234Thr235 to generate distinctly different binding surfaces that discriminate between different neuroligins splice isoforms.
While this work was under peer-review three structures of neurexin 1β in complex with either neuroligin 1 or neuroligin 4 were published (Araç et al., 2007; Chen et al., 2007; Fabrichny et al., 2007). Using the highest resolution coordinates available (Araç et al., 2007) we docked n1β_L(30) onto n1β found in the complex with neuroligin 1B (-A -B) (rmsd of 0.5 Å using 168 Cα-atoms). It is immediately clear that the interface between neuroligin 1 and n1β is disrupted by the presence of the splice insert at SS#4 (Fig 7a). In particular, the mechanism by which neuroligin 1 interacts with the Ca2+-binding site of n1β using side chain E397 and backbone carbonyl Q395 to interact with the Ca2+-ion in a water-mediated manner (Chen et al. 2007, Araç et al. 2007) is not possible in n1β+SS#4 because one of the “switch” residues Q233 located in loop β10-β11 blocks access while its backbone carbonyl ligands the Ca2+-ion (Fig 7b). If helix Sα1 is anchored by its interaction to the Ca2+-ion then an n1β+SS#4:neuroligin complex would require neuroligin to pivot to an alternate position and binding mode to accommodate splice insert SS#4, or neuroligin to either bend helix Sα1 out of the way or displace Sα1 from the Ca2+-binding site by inducing conformational rearrangements. The presence of an insert at site B in neuroligin could hinder rearrangements required to form an n1β+SS#4:neuroligin complex explaining why inserts at site B and SS#4 appear less compatible together.
Figure 7.
Splice insert SS#4 disrupts the protein:protein interface seen in the n1β:neuroligin 1 complex. a) superposition of n1β_L(30) (grey Cα-trace) onto n1β (light cyan Cα-trace) of the n1β:neuroligin 1 complex (PDB Id: 3BIW), with neuroligin 1 shown as a cyan Cα-trace. The splice insert residues are in maroon, “switch” residues in yellow (n1β_L(30)) or orange (n1β) respectively. b) Close-up of the Ca2+-binding site in n1β_L(30) as docked on n1β of the n1β:neuroligin 1 complex. N1β_L(30) shown in grey and yellow, neuroligin 1 in cyan. Relevant side chains are depicted in ball-and-stick (carbon, grey, yellow or cyan; nitrogen, blue; oxygen, red). The Ca2+-ion is shown as a blue sphere. For the sake of clarity the side chain lle236 of n1β_L(30) and the trace of n1β are not shown. Orange arrows indicate the side chain of Glu397 and main chain carbonyl of Gln395 from neuroligin 1 that undergo (water-mediated) interaction with the Ca2+-binding site of n1β (Araç et al., 2007; Chen et al., 2007).
Ca2+-binding in neurexin LNS/LG domains
Our structures suggest that Ca2+ plays different roles in mediating neuroligin binding to n1β in presence or absence of a splice insert at SS#4. The Ca2+-binding site at the hyper-variable surface is absolutely essential for binding neuroligins, because mutating the n1β ligands chelating the Ca2+-ion abolishes synaptogenic activity (Graf et al., 2006). The structural mechanism of Ca2+-dependent ligand binding in the splice insert-free form of n1β (which is very similar in the Ca2+-free and Ca2+-bound form, results not shown) entails ligand completing the Ca2+-coordination mediated by a water molecule (Araç et al., 2007; Chen et al., 2007; Fabrichny et al., 2007). However, in the case of n1β+SS#4 where the Ca2+-ion is largely buried the mechanism of Ca2+-dependent ligand binding likely involves Ca2+ playing a key structural role, keeping the helix Sα1 helix fixed with respect to the rest of the molecule in a binding compatible or incompatible mode as required by the “splice insert signaling code”. In the physiological context of the synapse it remains to be determined if the increase in Ca2+-affinity observed for n1β+SS#4 is important for neuroligin binding, and whether the affinity of the Ca2+-binding site is further altered upon association with neuroligin.
Overall, the different neurexin LNS/LG domains in their isolated form appear to have surprisingly weak affinity Ca2+-binding sites, even though the domains n1α_L2, n1α_L2(8), n1α_L2(15), n1β, n1β+SS#4, n1α_L4 and n1α_L4(10) have required different experimental conditions for our ITC experiments complicating direct comparison (results presented here; Sheckler et al., 2006; Chen et al., 2007). Of course in the context of the intact neurexin ectodomains or as complexes with their respective protein partners the affinities of these Ca2+-binding sites likely change. Our findings nevertheless suggest that if Ca2+-levels do indeed fluctuate at the synaptic cleft, as has been suggested (Stanley et al., 2000; Smith et al., 2004; Hardingham et al., 2006), that neurexin protein:protein interactions might potentially be influenced. Alternative splicing as a mechanism to control Ca2+-binding sites has been shown in the case of the cytomatrix protein piccolo, however this protein is located at the pre-synaptic terminals, intracellularly at the active zone, where huge Ca2+ fluctuations are known to occur (Garcia et al., 2004). Our studies indicate that neurexin splice isoforms also possess distinct molecular, structural and Ca2+-binding properties and as such could be exploited to fine-tune neural network function and formation.
The recent finding that alterations in neurexins are linked with autism spectrum disorder (Szatmari et al., 2007), as indeed previously has also been shown for neuroligins, and the lethality observed in α-neurexin knock-out mice (Missler et al., 2003) suggests that neurexins are fundamentally important for proper brain function and human mental health. Study of isolated neurexin LNS/LG domains already provides important clues how alternative splicing of neurexin isoforms can subtly control synaptic protein interactions.
Experimental Procedures
Protein Over-expression and Purification
Rat neurexin 1β in the splice insert-containing (n1β+SS#4), the insert-free form (n1β), as well as the isolated LNS/LG domain (residues Ser77-Ser294) in the insert-containing form (n1β_L(30)) were expressed as thrombin-cleavable glutathione-S-transferase (GST) fusion proteins in E. coli BL21 (DE3) cells, while the fourth LNS/LG domain (residues Arg721– Ile909) from bovine neurexin 1α in the splice insert-containing (n1α_L4(10)) and the insert-free form (n1α_L4 were expressed as GST-fusions in Origami B cells. Proteins were purified as described by Rudenko et al., 1999 and Sheckler et al., 2006 as well were the intact GST-fusion proteins GST-n1α_L4 and GST-n1α_L4(10). As a final step, proteins were subject to size exclusion chromatography (Superdex 200 16/60) in 20 mM HEPES pH 7.5, 150 mM NaCl, 1 mM EDTA and concentrated to 8.5 - 10 mg/ml for n1β_L(30), n1β and n1β+SS4. N1α_L4 and n1α_L4(10) were typically gel-filtrated in 20 mM MES pH 6, 150 mM NaCl, 1 mM EDTA and concentrated to 3 and 5 mg/ml respectively for structural studies. Protein concentrations were determined using the method of Pace et al., 1995 and using the BIORAD protein assay with BSA as a standard. The residue numbering scheme for the domains assumes the presence of a splice insert at SS#3 (10 residues) and SS#4 (30 residues).
Crystallization and X-ray Data Collection
N1β_L(30) was crystallized with the symmetry of spacegroup P21 (cell dimensions a=84.1 Å, b=39.3 Å, c=85.4 Å, β= 115.5°) using the hanging drop method with a reservoir solution (1000 μl) containing 17% Peg 8000, 0.1 M Tris pH 8.5, 0.2 M MgCl2, 5 mM CaCl2, 0.5% β-octyl-glucoside at 4°C. Crystals of at least 0.3 mm in size were obtained within 1-2 weeks by micro-seeding hanging drops containing 1 μl of protein at 10 mg/ml and 1 μl reservoir solution; crystals were cryo-protected in paratone N oil: mineral oil (1:1) and flash frozen in liquid propane. N1β was crystallized with the symmetry of spacegroup P21212 (a=116.7 Å, b=195.7 Å, c=103.9 Å) as described in Rudenko et al., 1999. Crystals were soaked 21 hrs in Ca2+-containing mother liquor (24% Peg 5000 MME, 50 mM NaCacodylate pH 6.5, 5 mM AmSO4, 5 mM CaCl2), cryo-protected with additional presence of 30% glycerol and flash frozen in liquid propane. Crystals of n1α_L4 in the splice insert free form (spacegroup P63 and cell dimensions a=90.3 Å, b=90.3 Å, c=39.6 Å) were grown using the hanging drop method with a reservoir solution (1000 μl) containing 12.5% isopropanol, 0.1 M CHES pH 9.0, 5 mM CaCl2 at 20°C and hanging drops containing 1 μl of protein at 3 mg/ml and 1 μl reservoir solution. Crystals of the size 0.2 -0.3 mm were obtained within 1 week, cryo-protected in 10% isopropanol, 0.1 M Tris pH 8.5, 5 mM CaCl2, 40% Peg 400 for 20 minutes and flash frozen in liquid propane. N1α_L4 crystals were also soaked for 14 hrs in 10% isopropanol, 0.1 M Tris pH 8.5, containing 1 mM ErCl3 or 5 mM SrCl2 to replace Ca2+, then cryo-protected and frozen in the same manner. Crystals of n1α_L4(10) were not obtained, in spite of extensive screening for crystallization conditions.
Diffraction data for n1β_L(30) crystals were collected at APS COMCAT beamline (32-ID) on a MAR CCD 165 mm in unbinned mode at 1.0000 Å wavelength using a single crystal. The data was processed with MOSFLM (Leslie, 1992) and programs from the CCP4 suite (CCP4, 1994). Diffraction data for n1β crystals soaked in Ca2+ were collected at APS 19-BM on a custom built CCD at 0.9795 Å wavelength using a single crystal. The data was processed with HKL2000 (Otwinowski & Minor, 1997) and programs from the CCP4 suite (CCP4, 1994). Diffraction data for n1α_L4 crystals were collected at APS GM/CA-CAT beamline (23-ID) on a MAR CCD 300 mm at 0.9611 Å wavelength on a single crystal. The data was processed with HKL2000 (Otwinowski & Minor, 1997) and programs from the CCP4 suite (CCP4, 1994). Additional data was collect at LS-CAT 21-ID at APS on a MAR CCD 165 mm at 1.2795 Å and 0.7689 Å for crystals soaked in Er3+ and Sr2+ respectively. Data statistics for the data sets (all collected under cryogenic conditions) are given in Table 1.
Structure Determination, Model Building and Refinement
The structures of n1β_L(30) and n1α_L4 were determined by molecular replacement with CNS (Brunger et al., 1998) using n1β with no splice insert in the Ca2+-free form (PDB:1C4R) as a search model. N1β_L(30) was refined to 1.72 Å and contains two monomers in the asymmetric unit. N1α_L4 was refined to 1.04 Å and contains one monomer per asymmetric unit (residues 723 – 906). The structure of n1β in the Ca2+-bound form was determined by rigid body refinement of the 8 molecule packing detemined for n1β in the Ca2+-free form (pdb: 1C4R) (Rudenko et al., 1999). Amazingly, upon soaking the n1β crystals in a Ca2+-containing mother liquor, a ninth molecule becomes ordered in the crystals with interpretable electron density for all but 5 stretches of polypeptide, the largest being 11 residues long. Structures were built with O (Jones et al., 1991) and refined using REFMAC (Murshudov et al., 1997) (Table 1). The structures for n1α_L4, n1β, and n1β_L(30) have been deposited in the Protein Data Bank (ID: 2R16, 2R1D and 2R1B). The structures were validated using PROCHECK (CCP4, 1994). The Ramachandran plot reveals for n1β_L(30) that 88.6% of the residues are in the most favored regions, 9.8% in the additionally allowed region, and no residues are in the disallowed region; for n1α_L4 that 91.6 % of the residues are in the most favored regions, 8.4 % in the additionally allowed region, and no residues are in the disallowed region; and finally for n1β+Ca2+ that 85.1 % of the residues are in the most favored regions, 13.7 % in the additionally allowed region, and three residues out of a total of 1600 residues (0.2%, 9 molecules) are in the disallowed region. Figures were made using Molscript (Kraulis, 1991) and Raster3D (Merritt & Bacon, 1997).
Identification of Ca2+-binding Sites
Ca2+ binding sites in n1β_L(30) were identified as two strong positive electron densities (22.6 and 19.6 σ) corresponding to a single Ca2+-ion bound at the hyper-variable surface in each monomer in SigmaA-weighted difference Fourier maps (with coefficients m∣FoCa2+∣-D∣FcCa2+-free∣ and phases calculated with a protein/solvent model but no calcium atoms). A Ca2+-binding site at the hyper-variable surface of n1β was identified in three molecules (mol B, D, I) using two separate difference Fourier maps: 1) diffraction data from a crystal in presence of Ca2+ versus a crystal in presence of EDTA was used to generate coefficients (FoCa2+-FoCa2+-free) and combined with phases from a model with solvent but no Ca2+-ions revealing three strong density peaks (5.9, 6.9 and 7.5 σ); and 2) A sigmaA-weighted omit map (coefficients m∣FoCa2+∣-D∣Fcmodel – Ca2+∣ and phases calculated from a protein/solvent model minus calcium atoms also revealed three strong positive electron densities (10, 9.5 and 7.2 σ). A second possible site (Site II) was observed in n1β and n1β_L(30) in the sharp loop β4-β5 at the hyper-variable surface formed by side chain oxygen from Ser131, main chain carbonyls from Ser131, Ser132, Leu135 and completed by two water molecules (not shown). However, given the long distances to ligands (up to 2.5 – 2.7 Å) and inconsistent presence in difference Fourier maps the existence of Site II is unclear. The Ca2+-binding site in n1α_L4 was identified in SigmaA-weighted difference Fourier maps (with coefficients m∣FoCa2+∣-D∣Fcmodel -Ca2+∣ and phases calculated using a protein/solvent model with no calcium atoms) as a very strong positive electron density peak (35.1 σ) at the hyper-variable surface consistent with a Ca2+-ion bound with octahedral geometry (2.43 Å from Asp772 (Oδ2), 2.31 Å and 2.30 Å from the backbone carbonyl oxygens of residues Leu789 and Arg848, surrounded by three positive solvent peaks at 2.40 Å, 2.34 Å and 2.42 Å distance respectively). However, further inspection of the electron density indicated that the Ca2+-binding site is partially occupied by the NH3 +/NH2-group of a Tris molecule. Refinement with a Tris molecule alone at the Ca2+-binding site yielded a residual positive peak in the m∣Fo∣-D∣Fc∣ difference Fourier map of 17 σ, while refinement of solely a Ca2+-ion at this site yielded a negative peak of -12.5 σ, both results indicating that the Ca2+-ion occupancy is less than 1.0 (Supplemental Fig S1a and S1b). High resolution structures of n1α_L4 at 1.4 Å derived from crystals soaked in Er3+ or Sr2+ (Rmerge 4.5%, 96.6 % complete, 7.3x multiple, and Rmerge 5.9 %, 97.2% complete and 8.6x multiple respectively, data further not presented), revealed exceptionally high quality density for a Tris molecule bound at the Ca2+-binding site and no evidence of any metal ion bound (shown for Er3+-soaked crystals in Supplemental Fig S1c).
Isothermal Titration Calorimetry (ITC)
In preparation for ITC, protein was rendered metal-free by size exclusion chromatography in 10 mM PIPES pH 8, 100 mM NaCl, 5 mM EDTA and subsequently exhaustively buffer-exchanged into CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) prior to concentration to 8.5 or 9.7 mg/ml (0.35 mM) for n1β and n1β+SS#4 respectively. The proteins n1α_L4 and n1α_L4(10) required acidic conditions (pH ≤ 6.5) for solubility at 0.3 mM, conditions that could potentially lower Ca2+-binding affinity. To improve solubility therefore and perform titrations under the same conditions as n1β and n1β+SS#4 we produced metal-free GST-n1α_L4 and GST-n1α_L4(10) fusions proteins with the method above, buffer-exchanging the proteins into CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) and concentrating them to 16.3 and 16.7 mg/ml respectively (0.35 mM) in preparation for ITC. To verify our decalcification procedure, we decalcified bovine α-lactalbumin (Sigma) which has a single Ca2+-binding site with approximately 20 nM affinity, and demonstrated Ca2+-binding using similar titration conditions as described in Griko et al., 1999.
ITC was carried out using the procedures described in Sheckler et al., 2006. N1β and n1β+SS#4 at a protein concentration of 0.35 mM in CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) were titrated with 8.1 mM CaCl2 at 5°C using a Microcal VP-ITC in a series of 29 injections or 40 injections of 5 μl, including a single 1 μl injection at the start of each titration. GST-n1α_L4 and GST-n1α_L4(10) at 0.35 mM protein concentration in CHELEX-treated buffer (10 mM HEPES pH 8, 100 mM NaCl) were titrated with 6.0 mM CaCl2 at 5°C. For each protein the heat of dilution was obtained by carrying out a titration of Ca2+ directly into corresponding buffer (obtained during the final concentration step). While the calorimetric data for n1β+SS#4 could be integrated in a semi-automatic fashion and fit to a single site, insufficient heat evolved per injection for an accurate binding isotherm to be derived from which estimates of K could be calculated. After each titration, sample was extracted from the cell and centrifuged at 13 000 rpm and the appearance of a pellet investigated as a measure of protein precipitation. Very little precipitation was observed for the different proteins after ITC runs, with the exception of n1β+SS#4 where a small amount of precipitation were routinely observed, further complicating attempts to extract an accurate binding constant from the data. To check machine performance, before each series of titrations a control ITC titration was performed with lysozyme (Sigma) and a ligand N,N’,N”-Triacetylchitotriose (Sigma) which binds with a KD of approximately 4 μM (Cooper et al., 2001).
Supplementary Material
Acknowledgments
Funding was provided by the NIHM (RO1 MH077303). GR is a recipient of a scientist development award from the American Heart Association. Beamlines at Argonne National Laboratory, the Advanced Photon Source are gratefully acknowledged: COMCAT (32-ID), SBC (19-BM), GM/CA (23-ID) and LS-CAT (21-ID). We thank Todd Geders for collecting the diffraction data on native n1α_L4 crystals, Dr. Clay Brown and Jim Delproposto for expertise regarding over-expression of n1α_L4, and Dr. Demet Araç for communicating unpublished results. Full length n1α_bovine, rat neurexin 1β-SS#4 and rat neurexin 1β+SS#4 were the kind gift of Professor Thomas Südhof. Professor Johann Deisenhofer is most gratefully acknowledged for initial support of these studies and encouragement over the years. Lisa Henry provided assistance in initial protein purification and crystallization attempts and Sha Huang helpful technical discussions. We also thank Dr. Verna Fresca (Microcal) for extensive expert advice on processing ITC data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araç D, Boucard AA, Őzkan E, Strop P, Newell E, Südhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1β complex reveal specific protein:protein and protein-Ca2+ interactions. Neuron. 2007;56:1–12. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Brunger AT, A P, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Chen X, Liu H, Shim AHR, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2007;15:50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr Biol. 2005;15:R203–205. doi: 10.1016/j.cub.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, N. The CCP4 Suite: Programs for Protein Crystallography. Acta Cryst. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Sudhof TC, Taylor P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry. 2006;45:12816–12827. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- Cooper A, Johnson CM, Lakey JH, Nollmann M. Heat does not come in different colours: entropy-enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys Chem. 2001;93:215–230. doi: 10.1016/s0301-4622(01)00222-8. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its β-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Neuroscience. Autism’s cause may reside in abnormalities at the synapse. Science. 2007;317:190–191. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- Garcia J, Gerber SH, Sugita S, Sudhof TC, Rizo J. A conformational switch in the Piccolo C2A domain regulated by alternative splicing. Nat Struct Mol Biol. 2004;11:45–53. doi: 10.1038/nsmb707. [DOI] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griko YV, Remeta DP. Energetics of solvent and ligand-induced conformational changes in alpha-lactalbumin. Protein Sci. 1999;8:554–561. doi: 10.1110/ps.8.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding MM. Small revisions to predicted distances around metal sites in proteins. Acta Crystallogr D Biol Crystallogr. 2006;62:678–682. doi: 10.1107/S0907444906014594. [DOI] [PubMed] [Google Scholar]
- Hardingham NR, Bannister NJ, Read JC, Fox KD, Hardingham GE, Jack JJ. Extracellular calcium regulates postsynaptic efficacy through group 1 metabotropic glutamate receptors. J Neurosci. 2006;26:6337–6345. doi: 10.1523/JNEUROSCI.5128-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhang X, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by α-neurexins. J Biol Chem. 2007 doi: 10.1074/jbc.M703957200. In press, Epub on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraulis P. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structures. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AGW. Recent changes to the MOSFLM package for processing film and image plate data. Joint CCP4 + ESF-EAMCB Newsletter on Protein Crystallography. 1992;26 [Google Scholar]
- Levinson JN, El-Husseini A. Building excitatory and inhibitory synapses: balancing neuroligin partnerships. Neuron. 2005;48:171–174. doi: 10.1016/j.neuron.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Merritt EA, B DJ. Raster3D-photorealistic molecular graphics. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+-channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In: Carter JRMSCW, editor. Methods Enzymol. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps DL, Qin S, Minx P, Wilson RK, Hood L, Graveley BR. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002;79:587–597. doi: 10.1006/geno.2002.6734. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Hohenester E, Muller YA. LG/LNS domains: multiple functions -- one business end? Trends Biochem Sci. 2001;26:363–368. doi: 10.1016/s0968-0004(01)01832-1. [DOI] [PubMed] [Google Scholar]
- Rudenko G, Nguyen T, Chelliah Y, Sudhof TC, Deisenhofer J. The structure of the ligand-binding domain of neurexin Ibeta: regulation of LNS domain function by alternative splicing. Cell. 1999;99:93–101. doi: 10.1016/s0092-8674(00)80065-3. [DOI] [PubMed] [Google Scholar]
- Sheckler LR, Henry L, Sugita S, Sudhof TC, Rudenko G. Crystal structure of the second LNS/LG domain from neurexin 1alpha: Ca2+ binding and the effects of alternative splicing. J Biol Chem. 2006;281:22896–22905. doi: 10.1074/jbc.M603464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Bergsman JB, Harata NC, Scheller RH, Tsien RW. Recordings from single neocortical nerve terminals reveal a nonselective cation channel activated by decreases in extracellular calcium. Neuron. 2004;41:243–256. doi: 10.1016/s0896-6273(03)00837-7. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. Presynaptic calcium channels and the depletion of synaptic cleft calcium ions. J Neurophysiol. 2000;83:477–482. doi: 10.1152/jn.2000.83.1.477. [DOI] [PubMed] [Google Scholar]
- Sugita S, Khvochtev M, Sudhof TC. Neurexins are functional alpha-latrotoxin receptors. Neuron. 1999;22:489–496. doi: 10.1016/s0896-6273(00)80704-7. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, Liu XQ, Vincent JB, Skaug JL, Thompson AP, Senman L, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Südhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.