Abstract
ATP-dependent proteases control diverse cellular processes by degrading specific regulatory proteins. Understanding how these regulatory proteins are targeted to ATP-dependent proteases is of central importance to understanding their biological role as regulators. Recent work has shown that protein substrates are specifically transferred to ATP-dependent proteases through different routes. These routes can function in parallel or independently. In all of these targeting mechanisms it can be useful to separate two steps: substrate binding to the protease and initiation of degradation.
To be active, newly synthesized protein chains must fold into three-dimensional structures, but regulated unfolding is also critically important in some biological processes, such as protein degradation by ATP-dependent proteases and protein translocation across membranes [1]. Unfolding is required during degradation because the proteolytic sites of the ATP-dependent proteases are sequestered deep inside the proteases’ structures and accessible only through narrow openings. Similarly, unfolding is required during several translocation processes because the protein import channels in some organelles are not wide enough for native proteins to fit through them. The mechanisms of unfolding in both types of processes are similar to each other but different from that of unfolding induced by heat or chemical denaturants. Here we discuss how the requirement for protein unfolding during degradation affects the way ATP-dependent proteases select their substrates.
ATP-dependent proteases
ATP-dependent proteases degrade short-lived regulatory proteins and thereby control cellular processes such as signal transduction, cell cycle, and gene transcription. The proteases also clear misfolded and aggregated proteins from the cell and produce some of the peptides to be displayed at cell surface as part of adaptive immune response. In eukaryotes, these functions are performed mainly by the proteasome. In prokaryotes and the organelles of eukaryotes, the functions are fulfill by analogues of the proteasome, such as the ClpAP, ClpXP, HslUV, FtsH, and Lon proteases. Although ATP-dependent proteases show only relatively little sequence identity, they share a common architecture [2].
The ATP-dependent proteases all form large multisubunit particles (Figure 1). In the simplest case, FtsH protease, the particle consists 6 copies of a 71 kDa subunit forming a complex of approximately 425 kDa, and in the most complex case, the proteasome, the particle consists of some 40 different subunits forming a complex of 2 MDa molecular weight [3,4]. The subunits are mostly arranged in six or seven subunit rings that stack on top of each other to form cylindrical structures [2]. The proteolytic sites in all of these proteases are buried deep inside the particles and are accessible only through channels that are too narrow to allow folded proteins to pass through them [2,5]. This arrangement prevents the unintentional degradation of proteins. The ATPase subunits sit at the entrance to the proteolytic channels where they gate the channels and select and unfold substrates for degradation [2,5] (Figure 1).
Figure 1.
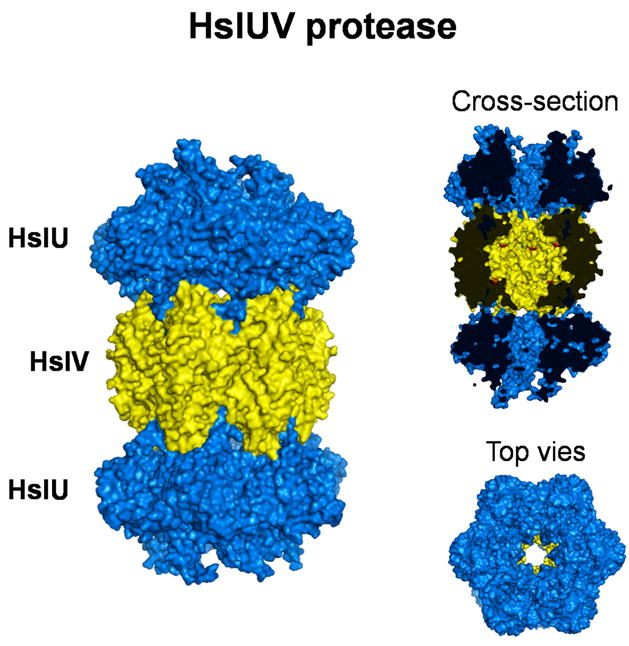
Structures of the bacterial ATP-dependent protease HslUV (PDB 1G3I). The protease subunits HlsV are shown in yellow, the ATPase subunits HslU are shown in blue. A side-on cross section reveals the active site of proteolysis (red dots) in the catalytic chamber and the degradation channel that connects the active site to the exterior of the protease. End-on view shows the sixfold axis of symmetry. Structures were produced by PyMOL.
Unfolding presumably occurs at the surface of the protease and the subsequent proteolysis proceeds sequentially along the substrate’s polypeptide chain [6] (Figure 2). Unfolding during degradation can be much faster than spontaneous global unfolding, and the susceptibility of a protein to unfolding by the proteases is largely determined by the stability of its local structure first encountered by the protease and not the stability of the overall structure against global unfolding [6]. Proteins are more easily unraveled from surface α-helices and loops than from buried β-strands [6]. In the simplest model, the proteases catalyze unfolding by pulling at the polypeptide chain, perhaps simply as a consequence of the translocation of the polypeptide chain into the degradation channel [1]. Once the protein reaches the proteolytic sites, it is hydrolysed into 3–30 amino acids-long peptides [7,8] (Figure 2).
Figure 2.
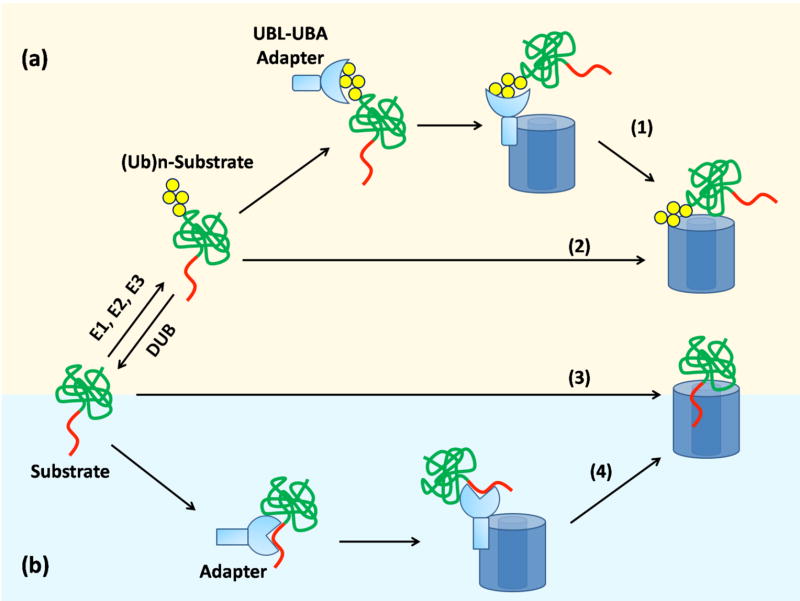
Pathways regulating the transfer of substrate to proteases in eukaryotes (a) and bacteria (b). Subunits of 26S proteasome bind to the polyubiquitin chain of modified substrate (2) or to exposed polypeptide sequence of the substrate (3). Alternatively, adaptor proteins that bind the polyubiquitin chain and the proteasome simultaneously can mediate targeting(1). Bacterial proteases recognize substrate via exposed sequence tags (3) or via adaptor proteins (4).
Besides their role in protein degradation, some ATP-dependent proteases are involved in nonproteolytic functions and most regulatory ATPase complexes show chaperone-like activity. Unfolding of a misfolded protein by ATP-dependent proteases can disrupt inappropriate intermolecular interactions and thus assist proper protein folding if it is uncoupled from degradation. The proteasome also functions as a regulator of a variety of cellular processes including gene transcription, DNA repair, and chromatin remodeling [9]. The chaperone-like activity of the proteasome ATPase ring may also induce conformational changes in the targeted factors involved in such cellular processes.
Substrate targeting to proteases
The proteases’ proteolytic sites show little intrinsic sequence preference [10] and instead substrate specificity is conferred by the regulatory complexes selecting the proteins to unfold and translocate to the degradation sites. There are three main pathways by which substrate proteins are targeted to the different proteases (Figure 3).
Figure 3.
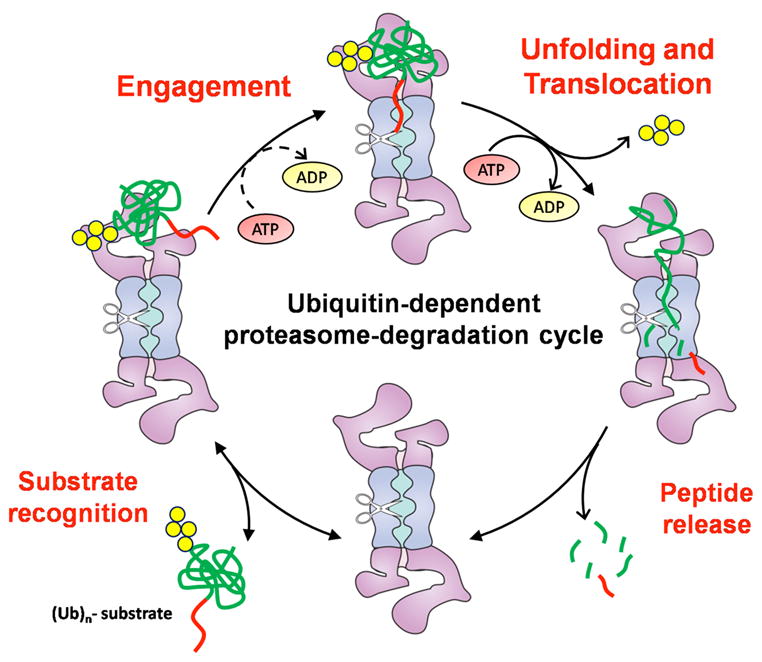
The degradation cycle of the proteasome. Polyubiquitinated (Ubn) proteins bind to the proteasome through the ubiquitin chain (bottom left). Unfolding and degradation (top right) occur only after the substrate has engaged the proteasome through an unstructured region (red strings) (top left). Once the substrate is engaged, it is degraded sequentially along the polypeptide chain from its unstructured initiation site (bottom right).
In eukaryotes, most substrate proteins are targeted to the proteasome by the covalent attachment of many copies of the small protein ubiquitin. Ubiquitination is carried out by a cascade of three enzymes, E1, E2, and E3, which act sequentially to attach the ubiquitin moieties to the acceptor protein. Typically, the C-terminus of ubiquitin forms an isopeptide bond with the ε-amino group of lysine residues in the substrate protein but in some rare cases ubiquitin may also be conjugated through the substrate’s N-terminus or a cysteine side chain [11–13]. Yeast encodes a single E1, a few dozen E2s, and hundreds of E3 enzymes. The enzymes pass the ubiquitin from the E1 to one of the E2s and on to the substrates, which are recognized by an E3 enzyme. Once the first ubiquitin is attached to substrate, the E3 can continue to function and attach more and more ubiquitins to lysines in the first ubiquitin. However, in some cases, further extension of the polyubiquitin chain is mediated by an additional conjugating factor (E4), which binds to preformed ubiquitin chain and catalyze multiubiquitin chain assembly in conjunction with E1, E2, and E3 [14]. The minimal proteasome targeting signal or degron consists of four ubiquitin moieties linked to each other by isopeptide bonds between carboxy termini and Lys48 [15]. This polyubiquitin degron is recognized by the 19S regulatory particle of the proteasome through two surfaces formed by the ATPase subunits Rpn10 and Rpt5 [16,17].
Once attached, a polyubiquitin chain keeps being modified and can grow and shrink [18]. The length of the polyubiquitin chains affects degradation [19]. For example, the E3 anaphase-promoting complex (APC) coordinates the order of substrate degradation during the cell cycle and the timing by which substrates are degraded depends on the processivity of their ubiquitination by APC [19]. Substrates that acquire long ubiquitination chains quickly are degraded earlier than substrates that are ubiquitinated slowly [19].
During degradation, the polyubiquitin chain must be removed from the substrate because the proteasome cannot translocate more than two or three polypeptide chains through the degradation channel at the same time. Cells contain a large number deubiquitinating enzymes (DUBs) [20] and at least two of them, Rpn11 and Ubp6, are located in the 19S regulatory particle and as such components of the proteasome [3,4,21–23]. Rpn11 removes entire ubiquitin chains from the substrate by cleaving the isopeptide bond between the substrate and the first ubiquitin to recycle ubiquitin and to allow substrate’s degradation [22,23]. Ubp6 trims the chain from the free end and may serve as a timer [24]: when the ubiquitinated substrate binds to the proteasome, the proteasome will try to engage its substrate while Ubp6 shortens the ubiquitin chains from their distal end. If the ubiquitin chain has been removed before the proteasome has begun to degrade the protein, it escapes until it is ubiquitinated again and rebinds the proteasome.
The length of the ubiquitin chain appears to be regulated further and it was found recently that the E3 ligase Hul5 associates with the DUB Ubp6 on the 19S regulatory particle [4,25]. The ubiquitin ligase activity of Hul5 promotes degradation by extending the number of ubiquitin moieties in the tag on substrates whereas the deubiquitinating activity of Ubp6 antagonizes degradation by trimming ubiquitin from the tag [25]. In other word, Hul5 activity adds back to the Ubp6 timer and thereby increases the chance of the degradation before it drop off the proteasome. The balance between these two opposing activities may fine tune the proteasome’s substrate specificity and thus regulate degradation [26].
The way the ubiquitin moieties are attached to each other also matters. Some polyubiquitin chains are linked through the Lys11 and Lys63 residues of ubiquitin but the extent to which they are involved in proteasome degradation is unclear [27]. Chains linked through Lys63 can serve as a nonproteolytic signaling, such as endocytosis, DNA repair, and protein sorting and trafficking. However, at least in vitro Lys63 linked ubiquitin chains also target proteins to the proteasome. Ubiquitin modifications linked through Lys6, 27, 29, 33 are rare [28]. In addition, many proteins are modified by single ubiquitin molecules and these modifications are involved in a wide range of processes unrelated to proteasomal degradation, such as endocytosis, virus budding, and chromatin structure [29].
A second step in ubiquitin-dependent proteasome targeting
Ubiquitination by itself does not always lead to rapid degradation [30,31] and effective proteolysis of a folded protein requires the presence of an unstructured region in the substrate [32,33], either at the ends of the polypeptide chain or internally [32,34]. The unstructured regions serves as the degradation initiation site and proteolysis continues from there along the polypeptide chain. Thus, ubiquitin tagging allows the protease to recognize its substrate proteins, and degradation then begins with proteolysis of initiation site [32]. The unstructured region functions to engage the unfolding machinery of the proteasome and is indispensable for the degradation of folded proteins. Thus, protein targeting to the proteasome appears to have two steps: recognition of the ubiquitin modification and initiation at the unstructured region; proteasome degrons have two components: a ubiquitination signal and an initiation site (Figure 2).
This initiation step in protein targeting could play an important role in substrate selection by the proteasome. Even relatively small differences in initiation may affect degradation efficiency if one takes into account the dynamic nature of the ubiquitin modification discussed above. If the ubiquitin modification is disassembled by the proteasome’s deubiquitinating enzymes before the substrate is fully engaged through its initiation site, the substrate will escape degradation until it is ubiquitinated again and the proteasome makes a new attempt at proteolysis.
Some proteins can bind to the proteasome yet escape proteolysis, presumably because the proteasome cannot initiate degradation on the substrate. In some cases the reason may simply be that the substrate lacks a suitable unstructured region. For example, the cyclin dependent kinase cdk2 folds into a compact structure devoid of disordered regions that could serve as initiation sites [35]. However, in other cases, proteins that are targeted to the proteasome contain long unstructured regions yet remain stable [36–39]. The E2 enzyme CDC34 autoubiquitinates on a long C-terminal unstructured region but does not get proteolysed [36,37]. Similarly, proteasome-targeting adapters such as Rad23 (see below) bind to the proteasome and contain unstructured regions yet remain stable [38,39]. These findings suggest that not all unstructured regions can serve as initiation sites. Some data indicate that an unstructured region has to be of a certain minimal length and be located close to the ubiquitin tag to allow the proteasome to engage its substrate effectively [32] but the relationship between the two components of the degradation signal needs to be analyzed further. Intriguingly, the proteasome may also have preferences for the amino acid sequence of the initiation site and it appears that sequences with a strongly biased amino acid composition do not serve as efficient degradation initiation sites [30,40]. Indeed, the unstructured regions in CDC34 and Rad23 consist of simple or low complexity amino acid sequences [41] and may therefore not function well as initiation site. What could the biochemical basis be for the effect of sequence composition on proteasome binding? Presumably, the proteasome recognizes certain, yet to be defined, sequence motifs in its substrates when it binds to them during initiation and degradation after removal of the ubiquitin modification. These motifs will be less well represented the simpler the amino acid composition of a peptide sequence and thus, regions with simpler amino acid composition may function less well as degradation initiation sites.
The selection of the initiation site will be of particular importance when a proteasome substrate is part of larger complex. The proteasome is able to remodel these complexes by degrading specific subunits without affecting other components [42,43]. For example, several steps in the cell cycle are controlled by the degradation of cyclins while they are bound to cyclin-dependent kinase subunit [44] or the degradation of cdk inhibitor while it is bound to the cyclin cdk complex [36]. The removal of the cyclin stops the kinase from functioning until a new cyclin binds whereas degradation of the inhibitor releases the kinase activity of the cyclin cdk complex. Similarly, the transcription factor NFκB is inhibited when bound to a IκB [45]. During activation, the IκB is degraded by the proteasome without affecting the other subunits of NFκB [46]. The explanation for these observations seemed to be that degradation begins specifically at the ubiquitination site but we now know that this mechanism may not always apply [32,33]. It will be interesting to determine whether the two components of the targeting signal could work together when separated onto two different polypeptides chains in a complex.
Targeting signals in the primary sequence of the substrates
Most proteins are targeted to prokaryotic ATP-dependent proteases by sequence motifs present in their primary structure from the moment that they are synthesized. However, at least one substrate tagging system also exists in prokaryotes in the form of the SsrA RNA quality control system in E. coli [47]. SsrA is a small RNA that enters the A-site of ribosomes stalled at the 3’ end of damaged mRNA. The ribosome switches template to the SsrA and becomes programmed to add an 11 residue tag to the C-terminus of the nascent polypeptide before encountering a stop codon. The ssrA tag targets the substrate for quick degradation by ATP-dependent proteases [47,48]. Several other consensus motifs have been defined for degradation signals [49,50]. Some of these motifs appear to be specific for particular proteases, others can target proteins to several different proteases at the same time. For example, the C-terminal ssrA tag is recognized by ClpAP, ClpXP and FtsH proteases [47,50,51] and the signal in UmuD’s N-terminus targets the protein to both ClpXP and Lon [52,53].
The consensus motifs in the degradation tags are relatively short (around 10 amino acids long) and they seem to be able to perform both functions of the two components of the proteasome targeting signal: they allow the protease to recognize its substrate and to initiate degradation. However, in some circumstances, initiation and binding sites can be separated. For example, an artificial substrate protein containing an internal ClpAP targeting tag requires an additional sequence tag at its C terminus for efficient degradation by ClpAP [54]. In this case, the internal targeting tag appears to tether the substrate to the protease and the C-terminal sequence serves as the initiation site [54].
The targeting signals are recognized by the proteases through loops in the central channel of the ATPase ring [55–58]. The best characterized of these is a loop containing the sequence GYVG, which is highly conserved in most proteolytic AAA+ ATPases and has been implicated directly in protein unfolding and translocation [55]. Other loops facing the central channel, such as an RKH loop in ClpX and two loops in ClpA D1 domain, also participate in the signal recognition [57,58]. The cooperation between the various loops probably allows ATP-dependent proteases to interact with the broad range of substrates.
In eukaryotes too some proteins are targeted to the proteasome directly by sequence motifs in their primary structure [59,60]. Degradation of thymidylate synthase (TS) and ornithine decarboxylase (ODC) by the proteasome is mediated by specific sequences, at the N terminus for TS and at the C terminus for ODC, and does not depend on ubiquitin [59,60]. These degradation signals also serve as both the protease binding site and the degradation initiation site. Finally, the proteasome can interact with misfolded or natively unfolded proteins lacking any known targeting signals in an ubiquitin-independent manner [61,62]. However, the relevance of this interaction to protein degradation is not clear.
Adapter proteins
The separation between protease binding and degradation initiation is clearest when proteolysis is mediated by adaptor proteins that bind both protease and substrate but escape degradation themselves. In eukaryotes, the DNA repair proteins Rad23 and Dsk2 can target ubiquitinated proteins for degradation [63,64]. They interact with the proteasome through a ubiquitin-like domain (UBL) and with the ubiquitin modification in substrates through two ubiquitin-association domains (UBAs) [37,38,63,65–67]. Thus, UBL-UBA proteins appear to deliver ubiquitinated proteins to the proteasome where they are subsequently degraded. Some E3 ubiquitin ligases have also been implicated in substrate delivery to the proteasome [68]. These E3 ligases interact with 26S proteasome directly or via other adapter proteins and the association could promote substrate degradation either directly by increasing the local concentration of substrate at the proteasome, or indirectly by increasing the length of polyubiquitin chain and thereby enhancing the affinity of the substrate for the proteasome. For some substrates, targeting can be even more complicated and lead through an additional ATPase ring complex called Cdc48 or p97 [69]. Cdc48/p97 can interact with both E3s and DUBs and may unfold proteins prior to proteasome degradation [68]. Some ubiquitinated proteins appear to be recruited to CDC48 by adapter proteins similar to Rad23 and Dsk2 but containing UBX domains instead of the UBL domain [68].
Prokaryotes also use adapter proteins for their ATP-dependent proteases [70,71]. SspB is one such adapter and it promotes degradation of several substrates, including that of ssrA-tagged proteins by ClpXP [72,73]. SspB interacts with residues in the ssrA tag as well as with ClpX, thereby increasing the effective local concentration of the substrate at the protease and facilitating its degradation [74,75]. Some bacterial adapter proteins alter substrate preferences. For example, the ClpS adapter protein specifically inhibits the degradation of ssrA-tagged substrates by ClpAP but stimulates ClpAP to degrade aggregated proteins and possibly N-end rule substrates [76–78]. The use of adaptors allows for an additional level of regulation of degradation. The alternative σ factor σS controls the expression of many stress response genes in E. coli and during exponential growth in the absence of stress its concentration is kept low by ClpXP. However, σS is not recognized by ClpXP and its degradation requires the adaptor protein RssB [79]. The antiadaptor IraP controls σS concentration by binding directly to RssB [80].
Additional layers of substrate targeting
The various routes to degradation described above overlap (Figure 3). For example, degradation of several proteasome substrates including p21/Cip1, c-Jun, c-Fos, p53, and RPN4, are mediated by both ubiquitin-dependent and ubiquitin-independent routes [81–83]. Although these proteins are usually ubiquitinated, they are degraded even when their ubiquitination is inhibited. The ubiquitin-dependent pathways themselves also show overlap. In the yeast, polyubiquitinated proteins are recognized by the proteasome subunit Rpn10 directly and by adapter proteins, such as Rad23 and Dsk2 [37,68,84,85]. Cells lacking one of these polyubiquitin receptors are viable, but double or triple deletions of these receptors have synthetic defect in the recognition and degradation of ubiquitinated substrates. This observation indicates that Rpn10, Rad23 and Dsk2 may represent distinct receptor pathways that link ubiquitinated substrates to the proteasome. Other ubiquitin receptor factors, such as an intrinsic Rpt5 subunit and Cdc48 adapter complex, may also participate in the transfer pathways [68].
Cooperative targeting with adapter proteins is also observed in bacterial proteases. Bacterial targeting signals, such as ssrA motif, are often recognized by the adapter protein. Thus, bacterial proteases can recognize their substrates either directly or via adapter proteins [78,86,87]. These multiple pathways work in parallel with and independently from one another and converge at the initiation step. The pathways are modulated depending on cellular condition and may help the cell fine tune the levels of individual proteins.
Conclusions
In summary, protein substrates are specifically targeted to the ATP-dependent proteases through many different routes. The pathway taken by any substrate may change in response to the cellular environment. For the proteasome, targeting appears to have two components: substrate binding, which for most substrates is mediated by the ubiquitin modification, and initiation of degradation at a separate site. In prokaryotic proteases, the distinction between binding and initiation sites is less clear cut but can be demonstrated in a few cases. In both proteasome degradation and degradation mediated by prokaryotic proteases, the binding step can be mediated by adaptor proteins. We propose that for folded proteins the availability of initiation sites contributes to substrate selection. Thus, studying the way in which degradation is initiated may provide useful insights into the specificity of degradation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prakash S, Matouschek A. Protein unfolding in the cell. Trends Biochem Sci. 2004;29:593–600. doi: 10.1016/j.tibs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister W, Walz J, Zühl F, Seemüller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 3.Verma R, Chen S, Feldman R, Schieltz D, Yates J, Dohmen J, Deshaies RJ. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol Biol Cell. 2000;11:3425–3439. doi: 10.1091/mbc.11.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leggett DS, Hanna J, Borodovsky A, Crosas B, Schmidt M, Baker RT, Walz T, Ploegh H, Finley D. Multiple associated proteins regulate proteasome structure and function. Mol Cell. 2002;10:495–507. doi: 10.1016/s1097-2765(02)00638-x. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CN, Finley D. Protein translocation channels in the proteasome and other proteases. Cell. 1997;91:431–434. doi: 10.1016/s0092-8674(00)80427-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001;7:627–637. doi: 10.1016/s1097-2765(01)00209-x. [DOI] [PubMed] [Google Scholar]
- 7.Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- 8.Shotland Y, Koby S, Teff D, Mansur N, Oren DA, Tatematsu K, Tomoyasu T, Kessel M, Bukau B, Ogura T, et al. Proteolysis of the phage lambda CII regulatory protein by FtsH (HflB) of Escherichia coli. Mol Microbiol. 1997;24:1303–1310. doi: 10.1046/j.1365-2958.1997.4231796.x. [DOI] [PubMed] [Google Scholar]
- 9.Collins GA, Tansey WP. The proteasome: a utility tool for transcription? Curr Opin Genet Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R. The proteasome. Annu Rev Biophys Biomol Struct. 1999;28:295–317. doi: 10.1146/annurev.biophys.28.1.295. [DOI] [PubMed] [Google Scholar]
- 11.Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A. A novel site for ubiquitination: the N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J. 1998;17:5964–5973. doi: 10.1093/emboj/17.20.5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Saadon R, Fajerman I, Ziv T, Hellman U, Schwartz AL, Ciechanover A. The tumor suppressor protein p16(INK4a) and the human papillomavirus oncoprotein-58 E7 are naturally occurring lysine-less proteins that are degraded by the ubiquitin system. Direct evidence for ubiquitination at the N-terminal residue. J Biol Chem. 2004;279:41414–41421. doi: 10.1074/jbc.M407201200. [DOI] [PubMed] [Google Scholar]
- 13.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 14**.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. The paper describes a novel enzymatic activity in the ubiquitination cascade that is responsible for multiubiquitination. [DOI] [PubMed] [Google Scholar]
- 15**.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. The paper describes a novel enzymatic activity in the ubiquitination cascade that is responsible for multiubiquitination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveraux Q, Ustrell V, Pickart C, Rechsteiner M. A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem. 1994;269:7059–7061. [PubMed] [Google Scholar]
- 17.Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature. 2002;416:763–767. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- 18**.Ellison MJ, Hochstrasser M. Epitope-tagged ubiquitin. A new probe for analyzing ubiquitin function. J Biol Chem. 1991;266:21150–21157. The paper shows that ubiquitin modification is dynamic. [PubMed] [Google Scholar]
- 19**.Rape M, Reddy SK, Kirschner MW. The processivity of multiubiquitination by the APC determines the order of substrate degradation. Cell. 2006;124:89–103. doi: 10.1016/j.cell.2005.10.032. This study shows how the extent of ubiquitination of a substrate affects degradation. [DOI] [PubMed] [Google Scholar]
- 20.Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Guterman A, Glickman MH. Complementary roles for Rpn11 and Ubp6 in deubiquitination and proteolysis by the proteasome. J Biol Chem. 2004;279:1729–1738. doi: 10.1074/jbc.M307050200. [DOI] [PubMed] [Google Scholar]
- 22**.Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 23**.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 24**.Lam YA, Xu W, DeMartino GN, Cohen RE. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature. 1997;385:737–740. doi: 10.1038/385737a0. The last three papers (22–24) characterize the deubiquitination activities on the proteasome. [DOI] [PubMed] [Google Scholar]
- 25**.Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, et al. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell. 2006;127:1401–1413. doi: 10.1016/j.cell.2006.09.051. The paper shows that the proteasome also contains a subunit that extends ubiquitin chains on a substrate and characterizes this activity. [DOI] [PubMed] [Google Scholar]
- 26.Kraut DA, Prakash S, Matouschek A. To degrade or release: ubiquitin-chain remodeling. Trends Cell Biol. 2007;17:419–421. doi: 10.1016/j.tcb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Rao H. What’s Ub chain linkage got to do with it? . Science STKE. 2006;2006:pe18. doi: 10.1126/stke.3302006pe18. [DOI] [PubMed] [Google Scholar]
- 28.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 29.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 30.Bachmair A, Varshavsky A. The degradation signal in a short-lived protein. Cell. 1989;56:1019–1032. doi: 10.1016/0092-8674(89)90635-1. [DOI] [PubMed] [Google Scholar]
- 31**.Johnson ES, Bartel B, Seufert W, Varshavsky A. Ubiquitin as a degradation signal. Embo J. 1992;11:497–505. doi: 10.1002/j.1460-2075.1992.tb05080.x. These two papers (30 and 31) are classics in the biochemical characterization of the ubiquitin pathway. They provide a detailed description of the degron (degradation signal) in a substrate protein and by doing so also suggest that degron may have other functions in addition to serving as ubiquitination sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Prakash S, Tian L, Ratliff KS, Lehotzky RE, Matouschek A. An unstructured initiation site is required for efficient proteasome-mediated degradation. Nat Struct Mol Biol. 2004;11:830–837. doi: 10.1038/nsmb814. The paper describes the requirement of an unstructured region in proteasome substrates as a second component to degrons in addition the ubiquitination site for effective degradation. [DOI] [PubMed] [Google Scholar]
- 33**.Takeuchi J, Chen H, Coffino P. Proteasome substrate degradation requires association plus extended peptide. Embo J. 2007;26:123–131. doi: 10.1038/sj.emboj.7601476. The paper extends the observation for the requirement for unstructured initiation sites to substrates targeted to the proteasome in an ubiquitin independent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massague J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 36.Verma R, McDonald H, Yates JR, Deshaies RJ. Selective degradation of ubiquitinated sic1 by purified 26s proteasome yields active s phase cyclin-cdk. Mol Cell. 2001;8:439–448. doi: 10.1016/s1097-2765(01)00308-2. [DOI] [PubMed] [Google Scholar]
- 37.Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Muller B, Feng MT, Tubing F, Dittmar GA, Finley D. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol. 2002;4:725–730. doi: 10.1038/ncb845. [DOI] [PubMed] [Google Scholar]
- 38.Schauber C, Chen L, Tongaonkar P, Vega I, Lambertson D, Potts W, Madura K. Rad23 links DNA repair to the ubiquitin/proteasome pathway. Nature. 1998;391:715–718. doi: 10.1038/35661. [DOI] [PubMed] [Google Scholar]
- 39.Heessen S, Masucci MG, Dantuma NP. The UBA2 domain functions as an intrinsic stabilization signal that protects Rad23 from proteasomal degradation. Mol Cell. 2005;18:225–235. doi: 10.1016/j.molcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Tian L, Holmgren RA, Matouschek A. A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-κB. Nat Struct Mol Biol. 2005;12:1045–1053. doi: 10.1038/nsmb1018. [DOI] [PubMed] [Google Scholar]
- 41.Wootton JC, Federhen S. Analysis of compositionally biased regions in sequence databases. Methods Enzymol. 1996;266:554–571. doi: 10.1016/s0076-6879(96)66035-2. [DOI] [PubMed] [Google Scholar]
- 42**.Johnson ES, Gonda DK, Varshavsky A. cis-trans recognition and subunit-specific degradation of short-lived proteins. Nature. 1990;346:287–291. doi: 10.1038/346287a0. [DOI] [PubMed] [Google Scholar]
- 43**.Hochstrasser M, Varshavsky A. In vivo degradation of a transcriptional regulator: the yeast alpha 2 repressor. Cell. 1990;61:697–708. doi: 10.1016/0092-8674(90)90481-s. The two papers (42 and 43) demonstrated that the proteasome can remodel protein complexes by specifically degrading some subunits and leaving others unaffected. [DOI] [PubMed] [Google Scholar]
- 44.Stewart E, Kobayashi H, Harrison D, Hunt T. Destruction of Xenopus cyclins A and B2, but not B1, requires binding to p34cdc2. EMBO J. 1994;13:584–594. doi: 10.1002/j.1460-2075.1994.tb06296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 47**.Keiler KC, Waller PR, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. The paper describes a protein tagging system that targets proteins to degradation in bacteria. [DOI] [PubMed] [Google Scholar]
- 48.Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flynn JM, Neher SB, Kim YI, Sauer RT, Baker TA. Proteomic discovery of cellular substrates of the ClpXP protease reveals five classes of ClpX-recognition signals. Mol Cell. 2003;11:671–683. doi: 10.1016/s1097-2765(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 50.Herman C, Thevenet D, Bouloc P, Walker GC, D’Ari R. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB. Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith CK, Baker TA, Sauer RT. Lon and Clp family proteases and chaperones share homologous substrate- recognition domains. Proc Natl Acad Sci USA. 1999;96:6678–6682. doi: 10.1073/pnas.96.12.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez M, Frank EG, Levine AS, Woodgate R. Lon-mediated proteolysis of Escherichia coli UmuD mutagenesis protein: in vitro degradation and identification of residues required for proteolysis. Genes Dev. 1998;12:3889–3899. doi: 10.1101/gad.12.24.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez M, Rasulova F, Maurizi MR, Woodgate R. Subunit-specific degradation of UmuD/D’ heterodimer by ClpXP protease: the role of trans recognition in UmuD’ stability. EMBO J. 2000;19:5251–5258. doi: 10.1093/emboj/19.19.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoskins JR, Wickner S. Two peptide sequences can function cooperatively to facilitate binding and unfolding by ClpA and degradation by ClpAP. Proc Natl Acad Sci U S A. 2006;103:909–914. doi: 10.1073/pnas.0509154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Song JJ, Franklin MC, Kamtekar S, Im YJ, Rho SH, Seong IS, Lee CS, Chung CH, Eom SH. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure (Camb) 2001;9:177–184. doi: 10.1016/s0969-2126(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 56**.Yamada-Inagawa T, Okuno T, Karata K, Yamanaka K, Ogura T. Conserved pore residues in the AAA protease FtsH are important for proteolysis and its coupling to ATP hydrolysis. J Biol Chem. 2003;278:50182–50187. doi: 10.1074/jbc.M308327200. [DOI] [PubMed] [Google Scholar]
- 57**.Hinnerwisch J, Fenton WA, Furtak KJ, Farr GW, Horwich AL. Loops in the Central Channel of ClpA Chaperone Mediate Protein Binding, Unfolding, and Translocation. Cell. 2005;121:1029–1041. doi: 10.1016/j.cell.2005.04.012. These papers (56 and 57) begin to identify substrate binding sites on AAA+ proteases. [DOI] [PubMed] [Google Scholar]
- 58**.Farrell CM, Baker TA, Sauer RT. Altered specificity of a AAA+ protease. Mol Cell. 2007;25:161–166. doi: 10.1016/j.molcel.2006.11.018. The authors show that loops, which surround the entrance to the central pore of the ClpX hexamer, participate in degron recognition. Mutations in this loop change substrate specificity of ClpX P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59**.Zhang M, Pickart CM, Coffino P. Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate. EMBO J. 2003;22:1488–1496. doi: 10.1093/emboj/cdg158. The paper provides a detailed biochemical characterization of ubiquitin independent targeting to the proteasome for a protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forsthoefel AM, Pena MM, Xing YY, Rafique Z, Berger FG. Structural determinants for the intracellular degradation of human thymidylate synthase. Biochemistry. 2004;43:1972–1979. doi: 10.1021/bi035894p. [DOI] [PubMed] [Google Scholar]
- 61.Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, Finley D, Schmidt M. The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol. 1999;1:221–226. doi: 10.1038/12043. [DOI] [PubMed] [Google Scholar]
- 62.Strickland E, Hakala K, Thomas PJ, DeMartino GN. Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J Biol Chem. 2000;275:5565–5572. doi: 10.1074/jbc.275.8.5565. [DOI] [PubMed] [Google Scholar]
- 63.Rao H, Sastry A. Recognition of specific ubiquitin conjugates is important for the proteolytic functions of the ubiquitin-associated domain proteins Dsk2 and Rad23. J Biol Chem. 2002;277:11691–11695. doi: 10.1074/jbc.M200245200. [DOI] [PubMed] [Google Scholar]
- 64.Madura K. Rad23 and Rpn10: perennial wallflowers join the melee. Trends Biochem Sci. 2004;29:637–640. doi: 10.1016/j.tibs.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Hiyama H, Yokoi M, Masutani C, Sugasawa K, Maekawa T, Tanaka K, Hoeijmakers JH, Hanaoka F. Interaction of hHR23 with S5a. The ubiquitin-like domain of hHR23 mediates interaction with S5a subunit of 26 S proteasome. J Biol Chem. 1999;274:28019–28025. doi: 10.1074/jbc.274.39.28019. [DOI] [PubMed] [Google Scholar]
- 66.Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol. 2001;3:939–943. doi: 10.1038/ncb1001-939. [DOI] [PubMed] [Google Scholar]
- 67.Elsasser S, Chandler-Militello D, Muller B, Hanna J, Finley D. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J Biol Chem. 2004;279:26817–26822. doi: 10.1074/jbc.M404020200. [DOI] [PubMed] [Google Scholar]
- 68.Elsasser S, Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat Cell Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- 69.Woodman PG. p97, a protein coping with multiple identities. J Cell Sci. 2003;116:4283–4290. doi: 10.1242/jcs.00817. [DOI] [PubMed] [Google Scholar]
- 70.Baker TA, Sauer RT. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem Sci. 2006;31:647–653. doi: 10.1016/j.tibs.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoskins JR, Sharma S, Sathyanarayana BK, Wickner S. Clp ATPases and their role in protein unfolding and degradation. Adv Protein Chem. 2001;59:413–429. doi: 10.1016/s0065-3233(01)59013-0. [DOI] [PubMed] [Google Scholar]
- 72**.Levchenko I, Seidel M, Sauer RT, Baker TA. A specificity-enhancing factor for the ClpXP degradation machine. Science. 2000;289:2354–2356. doi: 10.1126/science.289.5488.2354. This paper describes the discovery and functional characterization of the first adaptor protein for ClpX P, SspB. [DOI] [PubMed] [Google Scholar]
- 73.Flynn JM, Levchenko I, Sauer RT, Baker TA. Modulating substrate choice: the SspB adaptor delivers a regulator of the extracytoplasmic-stress response to the AAA+ protease ClpXP for degradation. Genes Dev. 2004;18:2292–2301. doi: 10.1101/gad.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levchenko I, Grant RA, Wah DA, Sauer RT, Baker TA. Structure of a delivery protein for an AAA+ protease in complex with a peptide degradation tag. Mol Cell. 2003;12:365–372. doi: 10.1016/j.molcel.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 75.Wah DA, Levchenko I, Rieckhof GE, Bolon DN, Baker TA, Sauer RT. Flexible linkers leash the substrate binding domain of SspB to a peptide module that stabilizes delivery complexes with the AAA+ ClpXP protease. Mol Cell. 2003;12:355–363. doi: 10.1016/s1097-2765(03)00272-7. [DOI] [PubMed] [Google Scholar]
- 76.Dougan DA, Reid BG, Horwich AL, Bukau B. ClpS, a substrate modulator of the ClpAP machine. Mol Cell. 2002;9:673–683. doi: 10.1016/s1097-2765(02)00485-9. [DOI] [PubMed] [Google Scholar]
- 77.Erbse A, Schmidt R, Bornemann T, Schneider-Mergener J, Mogk A, Zahn R, Dougan DA, Bukau B. ClpS is an essential component of the N-end rule pathway in Escherichia coli. Nature. 2006;439:753–756. doi: 10.1038/nature04412. [DOI] [PubMed] [Google Scholar]
- 78.Wang KH, Sauer RT, Baker TA. ClpS modulates but is not essential for bacterial N-end rule degradation. Genes Dev. 2007;21:403–408. doi: 10.1101/gad.1511907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Gottesman S, Hoskins JR, Maurizi MR, Wickner S. The RssB response regulator directly targets sigma(S) for degradation by ClpXP. Genes Dev. 2001;15:627–637. doi: 10.1101/gad.864401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80**.Bougdour A, Wickner S, Gottesman S. Modulating RssB activity: Ira P, a novel regulator of sigma(S) stability in Escherichia coli. Genes Dev. 2006;20:884–897. doi: 10.1101/gad.1400306. The paper describes a novel mechanism of regulation of protein degradation by ClpXP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jariel-Encontre I, Pariat M, Martin F, Carillo S, Salvat C, Piechaczyk M. Ubiquitinylation is not an absolute requirement for degradation of c-Jun protein by the 26 S proteasome. J Biol Chem. 1995;270:11623–11627. doi: 10.1074/jbc.270.19.11623. [DOI] [PubMed] [Google Scholar]
- 82.Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell. 2000;5:403–410. doi: 10.1016/s1097-2765(00)80435-9. [DOI] [PubMed] [Google Scholar]
- 83.Ju D, Xie Y. Proteasomal degradation of RPN4 via two distinct mechanisms, ubiquitin-dependent and -independent. J Biol Chem. 2004;279:23851–23854. doi: 10.1074/jbc.C400111200. [DOI] [PubMed] [Google Scholar]
- 84.Verma R, Oania R, Graumann J, Deshaies RJ. Multiubiquitin chain receptors define a layer of substrate selectivity in the ubiquitin-proteasome system. Cell. 2004;118:99–110. doi: 10.1016/j.cell.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 85.Lambertson D, Chen L, Madura K. Pleiotropic defects caused by loss of the proteasome-interacting factors Rad23 and Rpn10 of Saccharomyces cerevisiae. Genetics. 1999;153:69–79. doi: 10.1093/genetics/153.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86**.McGinness KE, Baker TA, Sauer RT. Engineering controllable protein degradation. Mol Cell. 2006;22:701–707. doi: 10.1016/j.molcel.2006.04.027. This paper and the next (86 and 87) demonstrate a complete understanding of the protein targeting to ClpXP by artificially modulating the flow of substrates to degradation through different pathways. Changes by site directed mutagenesis in degron, protease and adaptor lead to the predicted changes in protease - substrate interactions. [DOI] [PubMed] [Google Scholar]
- 87.McGinness KE, Bolon DN, Kaganovich M, Baker TA, Sauer RT. Altered tethering of the SspB adaptor to the ClpXP protease causes changes in substrate delivery. J Biol Chem. 2007;282:11465–11473. doi: 10.1074/jbc.M610671200. [DOI] [PubMed] [Google Scholar]