Abstract
Multiple mechanisms exist for the endocytosis of receptors from the cell surface. While the M1, M3, and M4 subtypes of muscarinic acetylcholine receptors internalize through the well-characterized mechanism of clathrin coated vesicles, the mechanism of M2 endocytosis is not well defined. Because the M2 and M4 receptors transduce their signals through the same second messengers but internalize though different pathways, we tested the ability of several small G-proteins to regulate the agonist-induced endocytosis of M2 and M4 in JEG-3 human choriocarcinoma cells. Dominant negative Rab5 as well as both wild type and dominant negative Rab11 inhibited M4 but not M2 endocytosis. In contrast, a dominant negative Arf6 as well as wild-type Rab22 increased M2 but not M4 endocytosis. We used immunocytochemistry to show that in unstimulated cells, the M2 and M4 receptors co-localize on the cell surface, whereas after stimulation M2 and M4 are in distinct vesicular compartments. In this study, we demonstrate that agonist-induced internalization of the M2 receptor utilizes an Arf6, Rab22 dependent pathway, while the M4 receptor undergoes agonist-induced internalization through a Rab5, Rab11 dependent pathway. Additionally, we show that Rab15 and RhoA are not involved in either pathway in JEG-3 cells.
Keywords: muscarinic, endocytosis, small G-proteins, GPCR, trafficking, vesicle
Introduction
Muscarinic acetylcholine receptors (mAChRs) are members of the G-protein coupled receptor (GPCR) superfamily whose members couple with heterotrimeric G-proteins to transduce extracellular signals into intracellular signal transduction cascades (Lanzafame et al., 2003; van Koppen and Kaiser, 2003). The mAChR family consists of five subtypes (M1–M5) which can be divided into two groups based on the signal transduction pathway to which they couple most efficiently; M1, M3, and M5 couple to the Gq family of G-proteins, while M2 and M4 couple to the Gi family (van Koppen and Kaiser, 2003).
Several mechanisms are responsible for regulating cell signal transduction following stimulation of a GPCR. In seconds to minutes after agonist exposure, desensitization, involving G-protein coupled receptor kinase (GRK) and β-arrestin, uncouples the receptor from its G-protein (Ferguson, 2001). Agonist-induced receptor endocytosis also occurs rapidly to remove receptors from the cell surface. Receptor endocytosis has several functions that depend on the specific receptor and cell type, including coupling receptors to different signal transduction cascades (Pierce, 2001), dephosphorylation and resensitization of receptor function (Claing et al., 2002), and initiating downregulation (von Zastrow, 2003) of the receptor. Additionally, internalization of one receptor can influence the signaling of an unrelated receptor, as endocytosis of the epidermal growth factor receptor contributes to the signal transduction of the adrenergic receptors (Pierce et al., 2000). Several mechanisms of receptor endocytosis have been characterized. The most well understood mechanisms are mediated by clathrin coated pits (Le Roy and Wrana, 2005), where a clathrin matrix assembles causing an invagination which is then pinched from the membrane in a dynamin-dependent fashion, and caveolae, which utilize caveolin and are sometimes associated with lipid rafts (Nichols 2003; Parton and Richards 2003). Perplexingly, several receptors have been shown to not internalize through either of these pathways, though the pathways that they utilize are not yet well-characterized. M1, M3, and M4 mAChRs undergo agonist-induced endocytosis in a dynamin-dependent manner through clathrin coated pits, while M2 displays a unique sensitivity to dynamin in HEK cells and does not utilize clathrin coated pits (Tolbert and Lameh, 1996; Schlador and Nathanson, 1997; Shockley et al., 1999; van Koppen, 2001; Roseberry and Hosey, 2001; Roseberry et al., 2001; Delaney et al., 2002). Some studies have shown an interaction between M2 and caveolin in cardiomyocytes (Feron et al., 1997), although the internalization of the M2 receptor in HEK293 cells has been reported to be independent of caveolae (Roseberry and Hosey, 2001).
We have previously shown that JEG-3 choriocarcinoma cells are an attractive system for the analysis of agonist-induced internalization of the M2 receptor. Initial work showed that the M2 but not the M1 receptor undergoes agonist-induced internalization in these cells (Goldman et al., 1996). These observations provided the first evidence that multiple pathways exist for agonist-induced internalization of the mAChRs. In addition, while M2 internalization can be increased by overexpression of GRK2 and β-arrestin-1 (Schlador and Nathanson, 1997), this is not the preferred endocytic pathway for M2 as demonstrated by the observation that M2/M1(6), an internalization deficient mutant M2, is able to undergo endocytosis only when coexpressed with these proteins (Schlador et al., 2000). The differential internalization of M1 and M2 has allowed identification of five specific amino acid residues residing in the third intracellular loop as well as the sixth and seventh transmembrane domains required for this M2-specific internalization pathway (Schlador et al., 2000). Like M1, the M3 receptor is also relatively resistant to agonist-induced internalization in JEG-3 cells (Goin and Nathanson, unpublished data), while the M4 receptor exhibits internalization that is comparable in magnitude to M2 (Schlador, 2000).
The Rab and Arf families of small G-proteins were originally discovered as GTPases involved in transport within the trans-Golgi network, but it has recently become clear that they are also involved in internalization of cell surface receptors. In fact, some members of these families are found on the plasma membrane and endosomes where their main function is in regulating vesicular transport (Takai et al., 2001). Arf6, Rab5, Rab11, and Rab4 have all been implicated in the endocytosis or recycling of the beta-adrenergic receptor following ligand stimulation (Premont et al., 1998; Seachrist et al., 2000; Moore et al., 2004). Additionally, Arf6 has been implicated in M2 endocytosis (Delaney et al., 2002; Houndolo et al., 2005) while Rab5 and Rab11 have been implicated in M4 endocytosis and recycling, respectively (Volpicelli et al., 2001; Volpicelli et al., 2002). To date, there has not been a systematic comparison of the regulation of different muscarinic receptor subtypes by small G-proteins. Therefore, we sought to determine whether the endocytic pathways of M2 and M4, which are highly homologous receptors with similar signal transduction properties, are completely distinct or if their pathways involve some shared modulators. We demonstrate here that the M2 endocytic pathway utilizes Arf6 and Rab22 while M4 uses Rab5 and Rab11 for its endocytic pathway. We further demonstrate that M2 and M4 do not colocalize in vesicles during their initial stages of endocytosis.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and penicillin-streptomycin (P/S) were obtained from Life Technologies. Lipofectamine2000 was from Invitrogen. N-[3H]methylscopolamine ([3H]NMS, 80-82 Ci/mmol) was purchased from Amersham. The chamber slides were from Nalge Nunc International. The anti-HA rabbit polyclonal antibody was from Pierce. The Alexa 568 conjugated anti-mouse antibody and the Alexa 488 conjugated anti-rabbit antibody were from Molecular Probes. The anti-Flag mouse monoclonal M2 antibody, carbamylcholine chloride (carbachol), atropine and all other reagents were purchased from Sigma.
Plasmids
The Flag-M2 pcDNA3.1 construct was generated by digesting pCDPS-Flag-M2 (Schlador and Nathanson, 1997) with Kpn1 and EcoR1 to remove the Flag-M2 coding region, which was then ligated into the pCDNA3.1 vector (Invitrogen). The HA-M4, HA-RhoA, HA-RhoAS19N, and HA-RhoAG14V constructs were purchased from the Guthrie (now the UMR) cDNA Resource Center. Rab5-GFP and Rab5S34N-GFP were the generous gift of Dr. Nigel Bunnett (UCSF) (Schmidlin et al., 2001). Dr. Aimee Powelka kindly provided the Arf6, Arf6T27N, and Arf6Q67L constructs (Powelka and Buckley, 2001). Dr. Ann Richmond (Vanderbilt University School of Medicine, Nashville, TN) kindly provided the Rab11 and Rab11S25N constructs (Fan et al., 2003). Rab15, Rab15N121I, Rab15T22N, and Rab5Q67L constructs were the generous gift of Dr. Lisa Elferink (University of Texas Medical Branch, Galveston, TX) (Zuk and Elferink, 1999). The Rab22, Rab22S19N, and Rab22Q64L constructs were the generous gift of Dr. Luis Mayorga (Universidad Nacional de Cuyo, Argentina) (Mesa et al., 2001).
Cell Culture and transfection
JEG-3 choriocarcinoma cells (American Type Culture Collection, Rockville, MD) were grown in DMEM supplemented with 10% FBS and 1% P/S in a 10% CO2 environment at 37° C. For assays, a 10 cm plate was transected using Lipofectamine2000 with 6–10 μg DNA each of both receptor and G-protein expression vectors.
Binding Assays
Cell surface expression of mAChRs was measured using the binding of the membrane impermeable radioligand N-[3H]methylscopolamine to intact cells using a previously described method (Schlador et al., 2000). Briefly, 24 hours post-transfection each 10 cm plate was split into two 6-well plates. 48 hours post-transfection, triplicate wells were stimulated with 1 mM carbachol for 15 or 30 min. All cells were placed on ice and washed three times with ice-cold phosphate-buffered saline (PBS: 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4). All wells were then incubated for four hours at 4°C with 1 nM [3H]NMS in 2 ml PBS. Three control wells also received 1 μM atropine to measure non-specific binding. Following the incubation, all wells were washed three times with ice-cold PBS, solublized with 0.5 ml 1% Triton X-100 and transferred to scintillation vials containing 3.5 ml scintillation fluid for counting. The percent of receptor remaining on the cell surface was determined by normalizing the 15 and 30 minute time points to the 0 min time point for each transfection type.
Recycling Assays
24 hours post-transfection each 10 cm plate was split into two 6-well plates. 48 hours post-transfection, triplicate wells were stimulated with 1 mM carbachol for 10 minutes. Following stimulation, one set of cells was washed 3 times and placed in the 37°C incubator for 30 minutes. Cells were then placed on ice and washed three times with ice-cold PBS. All wells were then incubated for four hours at 4°C with 1 nM [3H]NMS in 2 ml PBS. Three control wells also received 1 μM atropine to measure non-specific binding. Following the incubation, all wells were washed three times with ice-cold PBS, solublized with 0.5 ml 1% Triton X-100, and transferred to scintillation vials containing 3.5 ml scintillation fluid for counting. The percent of receptor remaining on the cell surface was determined by normalizing the carbachol treated cells to unstimulated cells for each transfection type.
Statistical Analysis
ANOVA was performed comparing all data from each individual experiment using Stat View (SAS, Cary, NC) statistical analysis software. For ANOVA with significant p values (p < 0.05), the Fisher’s PLSD post-hoc test was performed to obtain p values between each mock transfected and G-protein transfected culture as well as p values between wildtype and mutant G-protein transfected cultures for each set of conditions at each time point. Figures show conditions in which the p≤ 0.05.
Immunocytochemistry and confocal microscopy
Receptor cellular localization was visualized using a modified version of a previously described protocol (Beattie et al., 2000). 24 hours following cotransfection of N-terminal tagged receptors, cells from each 10 cm plate were split onto chamber slides at a density of 3.5 × 105 cells/chamber. 48 hours following transfection, live cells were labeled with antibodies directed at either the Flag or HA epitope of the receptor (4 μg/ml or 1:150, respectively, in 0.5 ml PBS) for 30 min at room temperature. After excess antibody was removed (2 washes in DMEM), cells were incubated for up to 30 minutes in 1 mM carbachol at 37°C in 2 ml DMEM. Endocytosis was terminated by placing the cells on ice and washing twice with ice-cold PBS. An acid wash (0.5 M NaCl, 0.2 N acetic acid) was then applied to the cells for 3 minutes to remove any remaining surface antibodies; control cells did not receive this treatment. Cells were fixed (4% paraformaldehyde) then permeabilized (0.25% Triton X-100 in PBS). After several washes in PBST (0.1% Tween-20 in PBS), cells were incubated with secondary antibodies in blocking buffer (3% bovine serum albumin, 0.25% Triton, 0.1% Tween-20 in PBS) for 45–60 min in the dark. Receptors were labeled with Alexa 568 goat anti-mouse antibody (1:500, to label Flag-tagged receptors) or FITC goat anti-rabbit antibody (1:300, to label HA tagged receptors). Images were collected using a Leica TCS SP1/NT confocal microscope (Leica Microsystems, Inc., Exton, PA.) with a 100X, 1.4 N.A. oil immersion lens.
Results
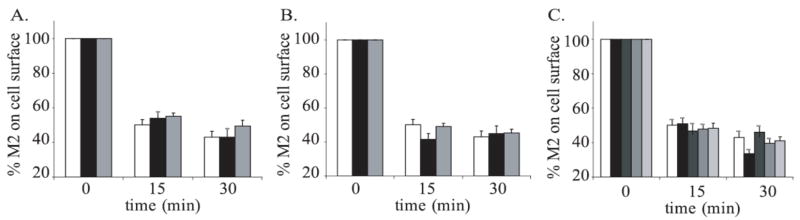
Previous work in our laboratory and others has shown that the M2 and M4 mAChRs internalize to similar extents with similar kinetics. We have focused on the endocytosis of these receptors in JEG-3 cells because our lab has previously characterized the mechanism of both M2 and M4 internalization in them, showing that while both M2 and M4 receptor internalization can be regulated by overexpression of GRK and β-arrestin, M2 preferentially internalizes through a mechanism independent of these proteins (Schlador et al., 2000; Schlador, 2000). In JEG-3 cells transiently transfected with either M2 or M4, we observe a 40–50% loss of cell surface binding sites for the membrane-impermeable radioligand [3H]NMS following 15 minutes of stimulation with 1 mM carbachol and further loss of cell surface binding with 30 minute stimulation (Fig. 1A). These results indicate that M4 internalizes to a similar extent as M2 following agonist activation. The level of receptor expression usually ranged from 4–25 fmol per well over the course of the experiments reported here. We have previously shown that the extent of agonist-induced mAChR internalization in transiently transfected JEG-3 cells is independent of receptor density over a greater than 13-fold range of expression levels (Goldman et al., 1996).
Figure 1. Time course of M2 and M4 internalization in JEG-3 cells.

A. JEG-3 cells were transfected with Flag-M2 (white bars) or HA-M4 (black bars) and stimulated for 0, 15 or 30 minutes with 1mM carbachol. B. JEG-3 cells were transfected with Flag-M2 (white bars) or HA-M4 (black bars) and either not stimulated (NS), stimulated for 10 minutes with 1mM carbachol (carb) or stimulated then washed and allowed to recover for 30 minutes (wash). C. HEK293 cells were treated as in B. Cell surface receptors were measured using the binding of [3H]NMS to intact cells as described in ‘Experimental Procedures.’ Data represent the means ± S.E. of two to six independent experiments, each performed in triplicate.
The interpretation of results from internalization experiments can be dependent on whether or not the receptor is recycled following internalization. Previous reports have shown that M4 recycles back to the cell surface in CHO, HEK293 and PC12 cells with the time required for recycling dependent on cell type (Bogatkewitsch et al., 1996; Krudewig et al., 2000: Volpicelli et al., 2002) whereas in HEK293 and JEG-3 cells M2 does not recycle (Vogler et al., 1998; Schlador et al., 2000). In order to determine if the M2 or M4 receptors could exhibit significant recycling in JEG-3 cells during the 30 minute agonist incubation period used in most of the experiments in this study, we examined the recycling of both M2 and M4 in JEG-3 cells following 10 minutes of carbachol stimulation and 30 minutes of recovery after the agonist was washed from the cells. We found that neither receptor recycled (Fig. 1B). As a control, we also examined the recycling of the receptors in HEK293 cells to ensure our experimental conditions are sufficient to detect recycling (Fig. 1C). Our results show that M4, but not M2, undergoes partial recycling to the surface of HEK293 cells following agonist-stimulation. This is in agreement with previous studies in HEK293 cells (Vogler et al., 1998; Krudewig et al., 2000).
M4 has previously been shown by immunocytochemistry to internalize through a clathrin coated pit mechanism dependent on dynamin, Rab5 and Rab11 in PC12 cells (Volpicelli et al., 2001; Volpicelli et al., 2002). To determine whether M4 internalizes through a similar mechanism in our cell culture system, we first cotransfected JEG-3 cells with M4 and either wild-type Rab5 (Rab5WT) or a dominant negative Rab5 (Rab5S34N) which cannot exchange GDP for GTP. The cells were then stimulated with 1 mM carbachol for 15 or 30 minutes and the loss of M4 from the cells’ surface was measured by [3H]NMS binding (Fig. 2A). At both the 15 and 30 minute time points there was a significant decrease in the extent of M4 internalization in cells cotransfected with Rab5S34N compared to cells cotransfected with Rab5WT, confirming the role of Rab5 in the endocytosis of M4 in JEG-3 cells. Endocytosis of M4 in cells transfected with Rab5WT was not statistically different from cells transfected with M4 alone.
Figure 2. The effects of Rab5, Rab 11 and Rab 15 on M4 internalization in JEG-3 cells.
A. JEG-3 cells were transfected with Flag-M4 alone (white bars) or with either Rab5WT (black bars) or Rab5S34N (dominant-negative; grey bars). B. JEG-3 cells were transfected with either Flag-M4 alone (white bars) or with either Rab11aWT (black bars) or Rab11aS25N (dominant-negative; grey bars). C. JEG-3 cells were transfected with Flag-M4 alone (white bars) and either Rab15WT (black bars), Rab15N121I (dominant-negative; dark grey bars), Rab15T22N (dominant-negative; medium grey bars), or Rab15Q67L (constitutively active; light grey bars). Following transfection, cells were stimulated for 0, 15, or 30 minutes with 1mM carbachol. Internalization of M4 receptors was measured using the binding of [3H]NMS to intact cells as described in ‘Experimental Procedures.’ Data represent the means ± S.E. of four to ten independent experiments, each performed in triplicate.
We next cotransfected M4 and either wild-type Rab11a (Rab11aWT) or a dominant negative Rab11a (Rab11aS35N), which cannot exchange GDP for GTP (Fig. 2B). In the cells cotransfected with either Rab11aWT or Rab11aS35N, there was a significant inhibition in the endocytosis of M4 compared to M4 alone at both the 15 and 30 minute time points. These results show that in JEG-3 cells, M4 internalizes through a mechanism involving Rab5 and Rab11.
We next tested the role of Rab15, another small G-protein that is associated with Rab5 and Rab11a mediated endocytosis (Zuk and Elferink, 1999), on M4 internalization. JEG-3 cells were cotransfected with M4 and either wild-type Rab15 (Rab15WT), a dominant negative Rab15 (Rab15T22N), which cannot exchange GDP for GTP, a constitutively active Rab15 (Rab15Q67L), which lacks GTPase activity, or another dominant negative Rab15 (Rab15N121I), which is unable to bind guanine nucleotides, following carbachol stimulation (Fig. 2C). ANOVA analysis shows no significant differences in M4 internalization between these conditions, indicating that Rab15 is not involved in M4 endocytosis. There is also no significant difference between cells transfected with M4 and Rab15WT and those transfected with M4 alone.
The M2 mAChR is thought to internalize through a clathrin independent mechanism (van Koppen, 2001; Roseberry and Hosey, 2001; Roseberry et al., 2001). Thus, we hypothesized that Rab5, Rab11a and Rab15 would not have an effect on M2 endocytosis. To determine whether Rab5 is involved in M2 internalization, JEG-3 cells were cotransfected with M2 and either Rab5WT or Rab5S34N and stimulated with 1 mM carbachol for 15 or 30 minutes. Cell surface receptors were measured by [3H]NMS binding (Fig. 3A). The results of this experiment show no difference between conditions. In addition, cells transfected with M2 alone showed no significant difference compared with cells transfected with M2 and Rab5WT. In JEG-3 cells cotransfected with M2 and either Rab11aWT or Rab11aS35N, ~55% of the M2 receptors internalized in an agonist dependent manner (Fig. 3B), indicating that Rab11a does not affect M2 endocytosis. There was also no difference between cells cotransfected with Rab11aWT and M2 and those transfected with M2 alone. To determine whether Rab15 is involved in agonist-induced M2 internalization, internalization assays were performed following stimulation of cells cotransfected with M2 and either Rab15WT, Rab15T22N, Rab15Q67L or Rab15N121I (Fig. 3C). ANOVA analysis of the data shows no difference in internalization between the conditions. There are also no differences between cotransfected cells and those transfected with M2 alone.
Figure 3. The effects of Rab5, Rab11 and Rab15 on M2 internalization in JEG-3 cells.
A. JEG-3 cells were transfected with Flag-M2 alone (white bars) or with either Rab5WT (black bars) or Rab5S34N (dominant-negative; grey bars). B. JEG-3 cells were transfected with Flag-M2 alone (white bars) and either Rab11aWT (black bars) or Rab11aS25N (dominant-negative; grey bars). C. JEG-3 cells were transfected with Flag-M2 alone (white bars) or with either Rab15WT (black bars), Rab15N121I (dominant-negative; dark grey bars), Rab15T22N (dominant-negative; medium grey bars), or Rab15Q67L (constitutively active; light grey bars). Following transfection, cells were stimulated for 0, 15 or 30 minutes with 1mM carbachol. Internalization of M2 receptors was measured using the binding of [3H]NMS to intact cells as described in ‘Experimental Procedures.’ Data represent the means ± S.E. of three to five independent experiments, each performed in triplicate.
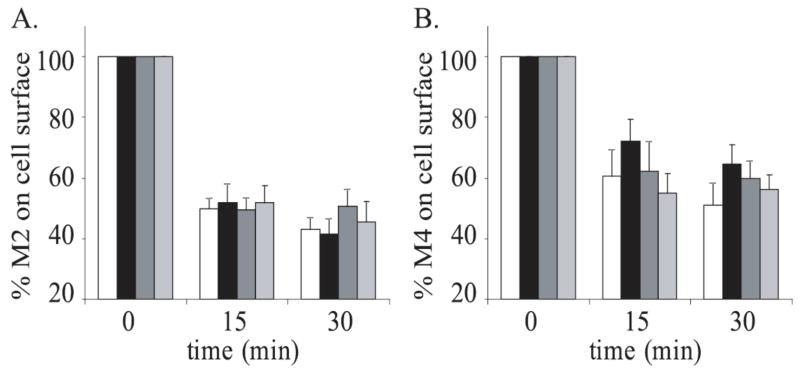
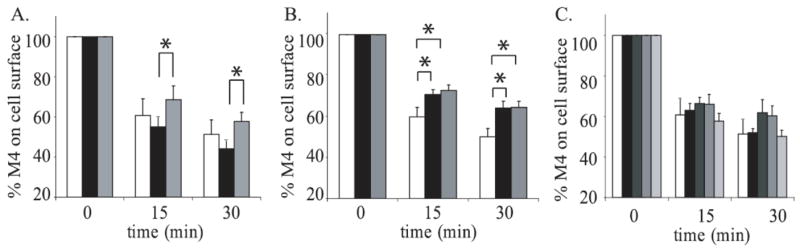
Several small G-proteins have been implicated in clathrin-independent endocytosis. Arf6 is associated with early endosome formation as well as the recycling of internalized proteins back to the cell surface. Previous studies using NMS binding and immunocytochemistry have shown Arf6 to regulate the initial steps of M2 endocytosis in HeLa cells (Delaney et al., 2002). To test the role of Arf6 in M2 agonist induced endocytosis in JEG-3 cells, cells were cotransfected with M2 and either wild-type Arf6 (Arf6WT), a dominant negative Arf6 (Arf6T22N), which cannot exchange GDP for GTP, or a constitutively active Arf6 (Arf6Q67L), which lacks GTPase activity. We found that Arf6T22N caused a dramatic increase in internalization of the receptor, while there was no difference between M2 internalization in the Arf6Q67L transfected cells when compared to cells transfected with Arf6WT nor between M2 internalization in cells transfected with receptor alone when compared to cells transfected with Arf6WT (Fig. 4A). Recently, Rab22 has been shown to regulate the recycling phase of the Arf6 endocytic pathway (Weigert et al., 2004); therefore, we tested its ability to influence M2 endocytosis in our system. 15 minutes following stimulation, we observed a significant increase in internalization of the receptor from the cell surface when M2 was co-expressed with Rab22WT compared to cells expressing M2 alone or M2 and a constitutively active Rab22 (Rab22Q69L), which lacks GTPase activity (Fig. 4B). This increase in internalization was not seen at the later time point. Co-expression of a dominant negative Rab22 (Rab22S19N), which cannot exchange GDP for GTP, showed no significant difference in M2 endocytosis when compared to all other conditions. We carried out similar experiments to determine whether Arf6 and Rab22 have a role in M4 endocytosis by cotransfecting M4 with the various small G-protein constructs (Fig. 4C & D). None of these constructs influenced the agonist-induced endocytosis of M4 when compared to their wild-type counterparts. Taken together, these results indicate that M2 endocytosis is dependent on both Arf6 and Rab22 and M4 endocytosis is independent of these small G-proteins.
Figure 4. The effects of Arf6 and Rab22 on M2 and M4 internalization in JEG-3 cells.
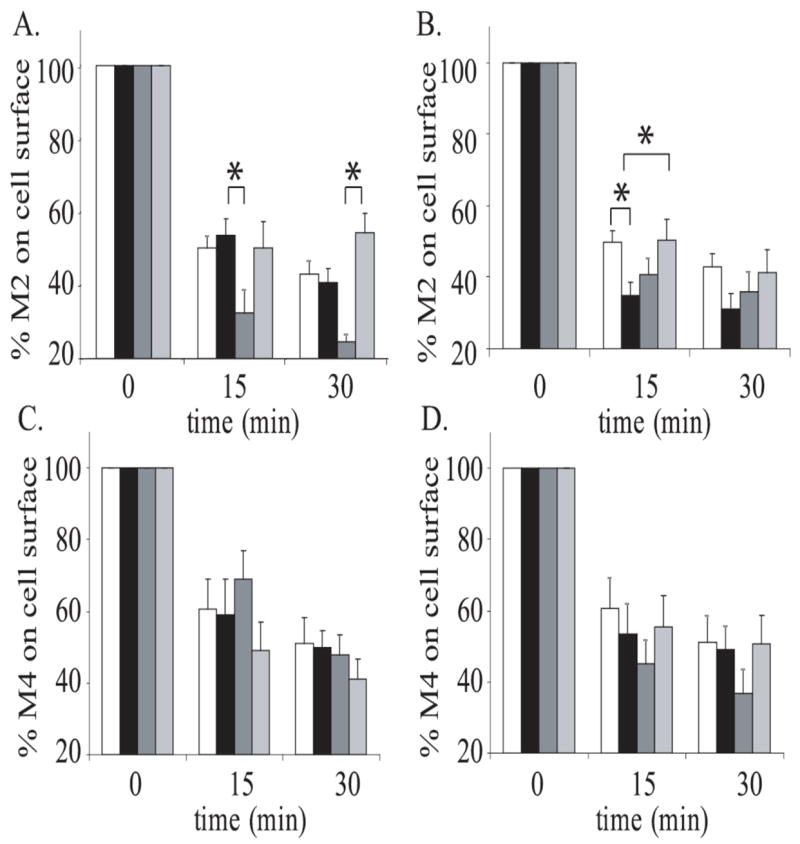
A. JEG-3 cells were transfected with Flag-M2 alone (white bars) or with either Arf6WT (black bars), Arf6T27N (dominant-negative; dark grey bars) or Arf6Q67L (constitutively active; light grey bars). B. JEG-3 cells were transfected with Flag-M2 alone (white bars) or with either Rab22WT (black bars), Rab22S19N (dominant-negative; dark grey bars), or Rab22Q69L (constitutively active; dark grey bars). C. JEG-3 cells were transfected with Flag-M4 alone (white bars) or with either Arf6WT (black bars), Arf6T27N (dominant-negative; dark grey bars), or Arf6Q67L (constitutively active; light grey bars). D. JEG-3 cells were transfected with Flag-M4 alone (white bars) or with either Rab22WT (black bars), Rab22S19N (dominant-negative; dark grey bars), or Rab22Q69L (constitutively active; light grey bars). Following transfection, cells were stimulated for 0, 15, or 30 minutes with 1mM carbachol. Internalization of M2 and M4 receptors was measured using the binding of [3H]NMS to intact cells as described in ‘Experimental Procedures.’ Data represent the means ± S.E. of three to eight independent experiments, each performed in triplicate.
Sequestration of M1 and M2 mAChRs has been demonstrated to be inhibited by overexpression of RhoA in HEK293 cells (Vogler et al., 1999). To determine the role of RhoA in M2 and M4 endocytosis in our system, we cotransfected JEG-3 cells with M2 or M4 and either wild-type RhoA (RhoAWT), a dominant negative RhoA (RhoAS19N) which is unable to exchange GDP for GTP, or a constitutively active RhoA (RhoAG14V), then stimulated with 1 mM carbachol for 15 or 30 minutes. Cell surface receptors were measured by [3H]NMS binding (Fig. 5A & B). No significant changes in M2 or M4 endocytosis occurred with coexpression of any RhoA constructs, indicating RhoA does not influence the endocytosis of either receptor subtype in our system.
Figure 5. The effects RhoA on M2 and M4 internalization in JEG-3 cells.
A. JEG-3 cells were transfected with Flag-M2 alone (white bars) or with either RhoAWT (black bars), RhoAG14V (constitutively active; dark grey bars), or RhoAS19N (dominant-negative; light grey bars). B. JEG-3 cells were transfected with Flag-M4 alone (white bars) or with either RhoAWT (black bars), RhoAG14V ((constitutively active; dark grey bars), or RhoAS19N (dominant-negative; light grey bars). Following transfection, cells were stimulated for 0, 15, or 30 minutes with 1mM carbachol. Internalization of M2 and M4 receptors was measured using the binding of [3H]NMS to intact cells as described in ‘Experimental Procedures.’ Data represent the means ± S.E. of five to six independent experiments, each performed in triplicate.
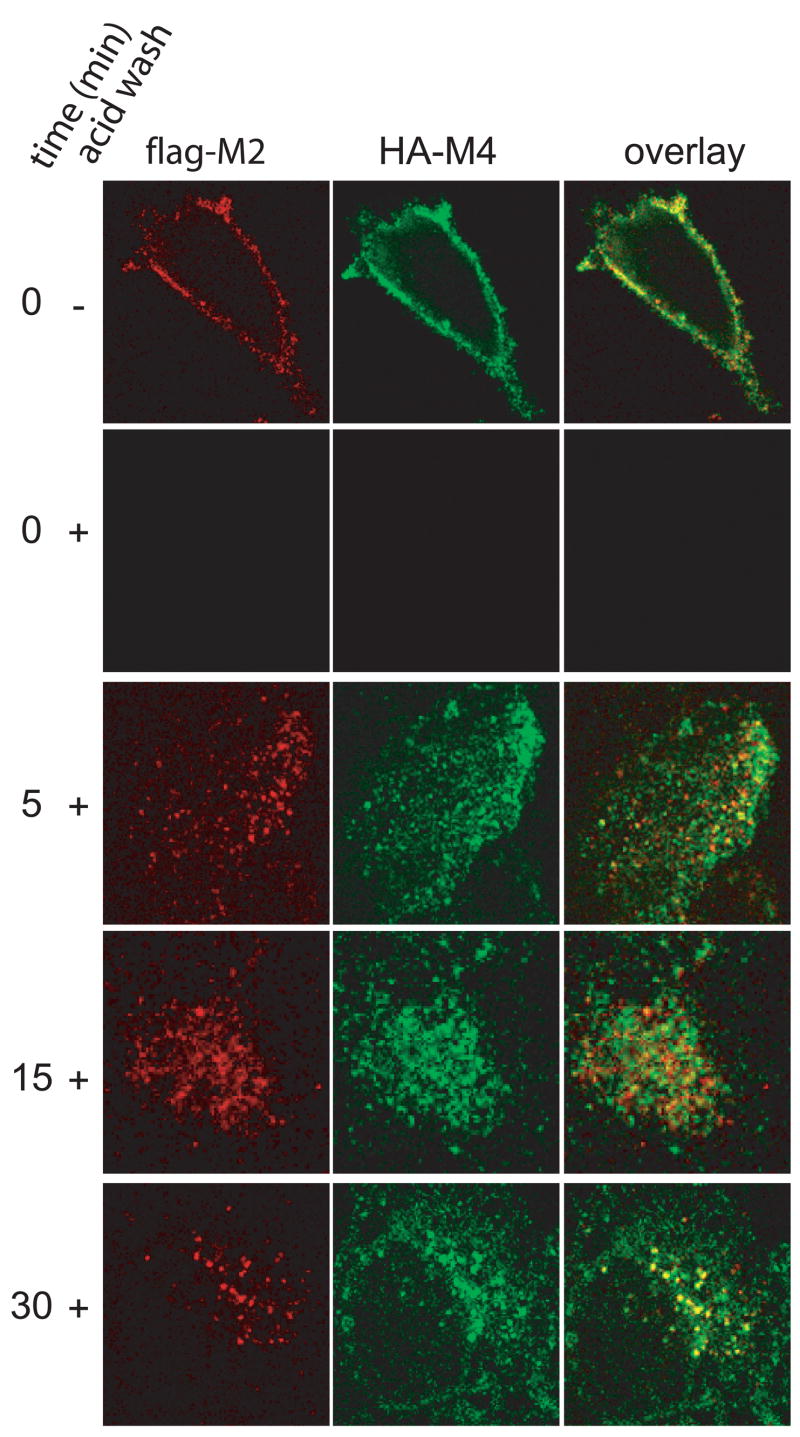
Our results thus far have placed M2 and M4 in separate endocytic pathways, which do not share any small G-protein regulators. To further confirm the differences between M2 and M4 endocytic pathways, we used immunocytochemistry to determine if the receptors would colocalize following stimulation. We tested whether the receptors are in distinct areas of the cell at all time points following stimulation by cotransfecting JEG-3 cells with Flag-tagged M2 and HA-tagged M4. In order to decrease background staining and to ensure that all receptor observed following stimulation were receptors internalized from the cell surface; we labeled live JEG-3 cells with a primary antibody directed at either a Flag- or HA- tagged receptor. Cells were stimulated with 1 mM carbachol for 5, 15 or 30 minutes, then placed on ice and any antibody remaining on the cell surface was removed using an acid wash. After being fixed and permeabilized, the cells were labeled with fluorescent secondary antibodies and visualized by confocal microscopy. In non-stimulated, non-acid washed cells, some, but not all of the M2 and M4 co-localized on the membrane (Fig. 6). The acid wash successfully stripped the antibody off of the cell surface of non-stimulated cells, assuring that all labeling in the stimulated, acid washed cells consisted of internalized receptors. At the 5 and 15 minute time points, the M2 and M4 receptors were localized to distinct vesicles, as evidenced by the complete lack of yellow in the merged confocal images. At 30 minutes approximately half of the cells showed significant co-localization, as seen by the presence of yellow signal due to the overlap of the M2 and M4 signals in the merged confocal images (Fig. 6), and the other half showed the receptors to be in distinct compartments as at the earlier time points (data not shown). Thus the M2 and M4 receptors initially undergo agonist-induced internalization using distinct vesicular pathways, consistent with the biochemical studies shown here, while the pathways appear to begin to merge at 30 minutes after agonist stimulation.
Figure 6. Co-localization of M4 with M2.
JEG-3 cells were transfected with HA-M4 and Flag-M2. The cells were stimulated for various times as indicated in the first column, acid washed as indicated in the second column and stained and analyzed by confocal microscopy as described in ‘Experimental Procedures.’ The first column of images represents staining for Flag-M2 only. The second column of images represents staining for HA-M4 only. The third column of images is an overlay of the first two columns. Images were taken at 100x magnification and are representative examples of images taken from five independent experiments.
Discussion
In this study we used overexpression of wildtype and mutant G-proteins to show that M2 and M4 internalize through distinct pathways using different subsets of small G-proteins. It has been shown previously that M1, M3, and M4 require β-arrestin and dynamin for endocytosis (Vogler et al., 1998; Vogler et al., 1999). Although M2 internalization is able to utilize this pathway, it preferentially uses a pathway independent of these proteins in JEG-3 cells (Schlador and Nathanson, 1997; van Koppen, 2001). Here we have compared the M2 and M4 endocytic pathways for their reliance on small G-proteins.
We first investigated the reliance of M2 and M4 endocytosis on a variety of small G-proteins that have previously been shown to associate with endosomes. We hypothesized that M4 internalization would be affected by small G-proteins known to associate with clathrin coated pits, Rab5, Rab11, and Rab15, while M2 internalization would be affected by those known to be involved with clathrin-independent endocytosis, Arf6, Rab22, and RhoA.
Recent studies have shown that Rab5, Rab11, and Rab15 are involved in clathrin coated pit mediated endocytosis (Stenmark et al., 1994; Moore et al., 1995; Zuk and Elferink, 1999; Wang et al., 2000). Additionally, Rab5 and Rab11 have been implicated in M4 internalization and recycling, respectively, in PC12 cells (Volpicelli et al., 2001; Volpicelli et al., 2002). We first tested the role of these G-proteins on agonist-induced internalization of M4 in JEG-3 cells (Fig. 2A, B). Our studies indicate that Rab5 plays a role in M4 internalization in JEG-3 cells. This finding correlates with earlier studies in PC12 cells though our results differ slightly. Volpicelli et al. used immunocytochemistry to demonstrate that the dominant negative Rab5S34N caused a decrease in M4 endocytosis at up to five minutes following agonist stimulation, but not at later time points. They suggest that this may be because the receptor is quickly transferred out of the early endosomes and into recycling endosomes which allows for more receptor to internalize. Our studies found a decrease in M4 endocytosis up to 30 minutes following agonist stimulation. The difference in the time points of our results could be due to increased sensitivity of the binding assay versus immunocytochemistry or differences in cell types: PC12 cells recycle M4 (Volpicelli et al., 2002) whereas JEG-3 cells do not (Fig. 1B).
We tested if the inability to detect recycling of the M4 receptor in JEG-3 cells reflected a lack of sensitivity of the assay or an intrinsic property of the JEG-3 cells; As shown in Fig. 1C, the M4 receptor (but not the M2 receptor) underwent partial recycling in HEK293 cells after agonist removal. This difference in regulatory properties is not surprising. Previous studies have shown that both the specificity of functional coupling and the regulatory properties and trafficking of a given receptor can be dependent on the celltype in which the receptor is expressed (Tietje and Nathanson, 1991; Goldman et al., 1996; Schlador et al., 2000). Indeed, even different stocks of the same cell line obtained from different laboratories in the same institution can show significant differences in the regulation of a specific receptor (Lefkowitz et al., 2002). Thus, the use of more than a single cell line can detect important aspects of receptor biology that would be missed by the use of a single celltype.
We found that coexpression of M4 with either wild-type Rab11 or dominant negative Rab11S35N resulted in an inhibition of endocytosis when compared to cells expressing only M4 (Fig. 2B). It is somewhat surprising that both the wild type and dominant negative Rab11 had similar effects on M4 internalization. There are several possible explanations for this observation. Volpicelli et al. (2002) showed that Rab11a was involved in endosomal recycling of M4 in PC12 cells. It is possible that the overexpression of wild-type Rab11 increases the rate of recycling, thus leading to more receptors on the cell surface. Because the M4 receptor does not recycle in JEG-3 cells, it is more likely that the wild-type Rab11 is increasing delivery of newly synthesized M4 to the plasma membrane (Urbe et al., 1993). The effects of the dominant negative Rab11S35N suggest that Rab11 may be involved in the early stages of M4 endocytosis. It would not be unusual for a single small G-protein to have multiple roles in endocytosis, as Arf6 was initially implicated in the recycling phase of endocytosis (D’Souza-Schorey et al., 1995) and it has recently been found to also be involved in the early stages of endocytosis of the M2 receptor (Delaney et al., 2002; Houndolo et al., 2005). In fact, Rab11 has recently been shown to regulate both the endocytosis and insulin-induced translocation to the plasma membrane of GLUT4 (Uhlig et al., 2005). Finally, it is possible that Rab11 can act as a protein scaffold independently of its activity as a GTPase. It would be interesting to determine if both mutant and wildtype Rab11 interact with one or more other proteins which regulate the trafficking of the M4 receptor but not the M2 receptor. Since Rab15 is a regulator of early endocytosis that co-localizes with Rab5 on early endosomes and with Rab11 on recycling endosomes (Zuk and Elferink, 1999), we wanted to know if it was also involved in M4 endocytosis. In JEG-3 cells cotransfected with M4 and either wild-type Rab15WT, dominant negatives Rab15T22N, Rab15N121I, or constitutive active Rab15Q67L, there were no significant differences in either rate or extent of internalization between any of the conditions (Fig. 2C) indicating that Rab15 does not play a role in M4 agonist-stimulated endocytosis.
We found that neither Rab5, Rab11 nor Rab15 are involved in M2 endocytosis. Because these proteins have been implicated in clathrin-mediated internalization, these results are consistent with the idea that M2 does not internalize through clathrin coated pits.
Several small G-proteins have also been shown to associate with a clathrin-independent endocytic pathway. Arf6 is distinct amongst the small G-proteins in that it is membrane associated in unstimulated cells (Cavenagh et al., 1996; Yang et al., 1998). It is also the only small G-protein previously reported to be involved in M2 endocytosis (Delaney et al., 2002; Houndolo et al., 2005). The first implication of Arf6 in the endocytosis of M2 showed that the constitutively active Arf6Q67L inhibited endocytosis while the dominant negative and wild-type Arf6 constructs had no effect (Delaney et al., 2002). We showed that Arf6 is involved in M2 agonist-induced endocytosis in JEG-3 cells; we found that the dominant negative Arf6T22N will stimulate internalization while Delaney et al. (Delaney et al., 2002) found that the constitutively active Arf6Q67L inhibits internalization. The lack of effect of the dominant-negative Arf6 reported by Delaney et al. (200) and the lack of effect of the constitutively active Arf6 found in the current study suggest that endogenous Arf6 plays a more important role in regulating M2 internalization in JEG-3 cells than in the HeLa cells used by Delaney et al. The decrease in M2 at the cell surface when coexpressed with dominant negative Arf6 is not due to a blockade of receptor recycling following internalization, because we are unable to observe M2 recycling in these cells. The decreased internalization by constitutively active Arf6 found by Delaney et al. (2002) and the increased internalization by dominant negative Arf6 found here suggest an inhibitory role for Arf6 in M2 endocytosis.
Rab22, has high sequence homology to Rab5 and associates with endosomes (Olkkonen et al., 1993), but has not yet been implicated in the internalization of GPCRs. More recently, Rab22 has been implicated in the recycling phase of the clathrin-independent endocytosis of MHCI (Weigert et al., 2004). Our results show that co-expression with Rab22WT increases M2 internalization 15 minutes following stimulation with carbachol. Studies to date have shown Rab22 to be associated with early endosomes and to interact specifically with EEA1 (Mesa et al., 2001; Kauppi et al., 2002), suggesting a role for Rab22 in vesicle fusion in a manner similar to Rab5. While the lack of effect of the constitutively active Rab22 in the current study is surprising in light of the inhibition caused by Rab22WT, these results together suggest that Rab22 may be involved in the fusion of M2 containing early endosomes to late endosomes. This is also consistent with the increased internalization at only the 15 minute time point as the increased fusion would speed up the endocytosis but not necessarily increase the extent of endocytosis. As expected, neither Arf6 nor Rab22 are important for M4 endocytosis.
The last small G-protein that we analyzed in this study is RhoA, which has also been implicated in clathrin-independent endocytosis and more specifically has been shown to affect the non-preferred pathway for M2 internalization in HEK293 cells (Vogler et al., 1999). Our results here show that RhoA is not involved in the endocytosis of either M2 or M4 in JEG-3 cells.
Transfection of the various G-proteins, when effective on mAChR internalization, attenuated but did not completely inhibit receptor internalization. This is consistent with previous observations that overexpression of small G-proteins could partially but not completely inhibit the internalization of a variety of G-protein coupled receptors (Seachrist et al., 2000; Volpicelli et al., 2001; Delaney et al., 2002; Moore et al., 2004). While the effects of overexpression can be dependent on the level of endogenous G-protein expression in the cell, a differential effect of a given G-protein construct on the regulation of the M2 and M4 receptors provides strong evidence for a differential involvement in receptor internalization. The specificity of the effects on mAChR internalization reported here is supported by the fact that G-protein constructs which affect M2 internalization did not affect M4 internalization, and constructs affecting M4 internalization did not affect M2 internalization.
Our immunocytochemical studies confirm that M2 and M4 internalize through distinct pathways,. After stimulation M2 and M4 are largely contained in separate vesicles. Even at the 30 minute time point, only ~50% of the cells observed had significant colocalization of the receptors. These results, which are the first description of simultaneous localization of the M2 and M4 receptors during agonist induced internalization, are striking because even though the receptors can be found in the same areas on the cell surface, they are able to separate into distinct endocytotic vesicles and employ internalization pathways which do not overlap. These results suggest that the M2 and M4 receptors do not interact or form heterodimers during the early stages of agonist-induced internalization. Interestingly, approximately half of the cells exhibited significant colocalization of the two internalized receptors at 30 minutes. These results indicate that while the M2 and M4 receptors initially utilize different endocytotic pathways, the receptors are beginning to share the same trafficking pathway at 30 minutes. This convergence of initially distinct trafficking pathways is consistent with the results of Delaney et al. (2002), who reported that the M2 receptor and transferrin receptor initially were internalized in distinct endocytotic vesicles and subsequently showed partial overlap in the same intracellular compartments. These studies have identified an additional small G-protein that appears to be involved in the M2 endocytic pathway and demonstrate that the M2 and M4 mAChR internalize through distinct pathways regulated by separate subsets of small G-proteins.
Acknowledgments
We thank Dr. Nigel Bunnett, Dr. Aimee Powelka, Dr. Ann Richmond, Dr. Lisa Elferink, and Dr. Luis Mayorga for supplying the DNA constructs for this work. We also thank Dr. Juan Goin and Dr. Matthew Cunningham for critical reading of the manuscript and Greg Martin for technical assistance with confocal microscopy. This research was supported by NIH grants HL44948 and GM07750.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neuroscience. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bogatkewitsch GS, Lenz W, Jakobs KH, Van Koppen CJ. Receptor internalization delays m4 muscarinic acetylcholine receptor resensitization at the plasma membrane. Molecular Pharmacology. 1996;50:424–429. [PubMed] [Google Scholar]
- Cavenagh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. Journal of Biological Chemistry. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Progress in Neurobiology. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Delaney KA, Murph MM, Brown LM, Radhakrishna H. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. Journal of Biological Chemistry. 2002;277:33439–33446. doi: 10.1074/jbc.M205293200. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Li G, Colombo MI, Stahl PD. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101:2115–2124. doi: 10.1182/blood-2002-07-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacological Review. 2001;53:1–24. [PubMed] [Google Scholar]
- Feron O, Smith TW, Michel T, Kelly RA. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. Journal of Biological Chemistry. 1997;272:17744–17748. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Schlador ML, Shapiro RA, Nathanson NM. Identification of a region required for subtype-specific agonist-induced sequestration of the m2 muscarinic acetylcholine receptor. Journal of Biological Chemistry. 1996;271:4215–4222. doi: 10.1074/jbc.271.8.4215. [DOI] [PubMed] [Google Scholar]
- Houndolo T, Boulay PL, Claing A. G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. Journal of Biological Chemistry. 2005;280:598–604. doi: 10.1074/jbc.M411456200. [DOI] [PubMed] [Google Scholar]
- Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. Journal of Cell Science. 2002;115:899–911. doi: 10.1242/jcs.115.5.899. [DOI] [PubMed] [Google Scholar]
- Krudewig R, Langer B, Vogler O, Markschies N, Erl M, Jakobs KH, van Koppen CJ. Distinct internalization of M2 muscarinic acetylcholine receptors confers selective and long-lasting desensitization of signaling to phospholipase C. Journal of Neurochemistry. 2000;74:1721–1730. doi: 10.1046/j.1471-4159.2000.0741721.x. [DOI] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors and Channels. 2003;9:241–60. [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nature Reviews Molecular and Cellular Biology. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Pierce KL, Luttrell LM. Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Molecular Pharmacology. 2002;62:971–974. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- Mesa R, Salomon C, Roggero M, Stahl PD, Mayorga LS. Rab22a affects the morphology and function of the endocytic pathway. Journal of Cell Science. 2001;114:4041–4049. doi: 10.1242/jcs.114.22.4041. [DOI] [PubMed] [Google Scholar]
- Moore I, Schell J, Palme K. Subclass-specific sequence motifs identified in Rab GTPases. Trends in Biochemical Sciences. 1995;20:10–12. doi: 10.1016/s0968-0004(00)88939-2. [DOI] [PubMed] [Google Scholar]
- Moore RH, Millman EE, Alpizar-Foster E, Dai W, Knoll BJ. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. Journal of Cell Science. 2004;117:3107–3117. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- Nichols B. Caveosomes and endocytosis of lipid rafts. Journal of Cell Science. 2003;116:4707–14. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Dupree P, Killisch I, Lutcke A, Zerial M, Simons K. Molecular cloning and subcellular localization of three GTP-binding proteins of the rab subfamily. Journal of Cell Science. 1993;106:1249–1261. doi: 10.1242/jcs.106.4.1249. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–1738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Maudsley S, Daaka Y, Luttrell LM, Lefkowitz RJ. Role of endocytosis in the activation of the extracellular signal-regulated kinase cascade by sequestering and nonsequestering G protein-coupled receptors. Proceedings of the National Academy of Sciences USA. 2000;97:1489–1494. doi: 10.1073/pnas.97.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powelka AM, Buckley KM. Expression of ARF6 mutants in neuroendocrine cells suggests a role for ARF6 in synaptic vesicle biogenesis. FEBS Letterrs. 2001;501:47–50. doi: 10.1016/s0014-5793(01)02624-2. [DOI] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proceedings of the National Academy of Sciences USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseberry AG, Bunemann M, Elavunkal J, Hosey MM. Agonist-dependent delivery of M(2) muscarinic acetylcholine receptors to the cell surface after pertussis toxin treatment. Molecular Pharmacology. 2001;59:1256–1268. doi: 10.1124/mol.59.5.1256. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Hosey MM. Internalization of the M2 muscarinic acetylcholine receptor proceeds through an atypical pathway in HEK293 cells that is independent of clathrin and caveolae. Journal of Cell Science. 2001;114:739–746. doi: 10.1242/jcs.114.4.739. [DOI] [PubMed] [Google Scholar]
- Schlador ML. Doctoral Dissertation, Department of Pharmacology. University of Washington; Seattle: 2000. Agonist-Dependent Regulation of Muscarinic Acetylcholine Receptor Expression and Function. [Google Scholar]
- Schlador ML, Grubbs RD, Nathanson NM. Multiple topological domains mediate subtype-specific internalization of the M2 muscarinic acetylcholine receptor. Journal of Biological Chemistry. 2000;275:23295–2302. doi: 10.1074/jbc.M002380200. [DOI] [PubMed] [Google Scholar]
- Schlador ML, Nathanson NM. Synergistic regulation of m2 muscarinic acetylcholine receptor desensitization and sequestration by G protein-coupled receptor kinase-2 and beta-arrestin-1. Journal of Biological Chemistry. 1997;272:18882–18890. doi: 10.1074/jbc.272.30.18882. [DOI] [PubMed] [Google Scholar]
- Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. Journal of Biological Chemistry. 2001;276:25427–25437. doi: 10.1074/jbc.M101688200. [DOI] [PubMed] [Google Scholar]
- Shockley MS, Tolbert LM, Tobin AB, Nahorski SR, Sadee W, Lameh J. Differential regulation of muscarinic M1 and M3 receptors by a putative phosphorylation domain. European Journal of Pharmacology. 1999;377:137–146. doi: 10.1016/s0014-2999(99)00303-9. [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. Journal of Biological Chemistry. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. Embo J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiological Reviews. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tietje KM, Nathanson NM. Embryonic chick heart expresses multiple muscarinic acetylcholine receptor subtypes. Isolation and characterization of a gene encoding a novel m2 muscarinic acetylcholine receptor with high affinity for pirenzepine. Journal of Biological Chemistry. 1991;266:17382–17387. [PubMed] [Google Scholar]
- Tolbert LM, Lameh J. Human muscarinic cholinergic receptor Hm1 internalizes via clathrin coated vesicles. Journal of Biological Chemistry. 1996;271:17335–17342. doi: 10.1074/jbc.271.29.17335. [DOI] [PubMed] [Google Scholar]
- Uhlig M, Passlack W, Eckel J. Functional role of Rab11 in GLUT4 trafficking in cardiomyocytes. Moecular and Cellular Endocrinology. 2005;235:1–9. doi: 10.1016/j.mce.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Letterrs. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacology and Therapeutics. 2003;98:197–220. doi: 10.1016/s0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- van Koppen CJ. Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors. Biochemical Society Transactions. 2001;29:505–508. doi: 10.1042/bst0290505. [DOI] [PubMed] [Google Scholar]
- Vogler O, Bogatkewitsch GS, Wriske C, Krummenerl P, Jakobs KH, van Koppen CJ. Receptor subtype-specific regulation of muscarinic acetylcholine receptor sequestration by dynamin. Distinct sequestration of m2 receptors. Journal of Biological Chemistry. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- Vogler O, Krummenerl P, Schmidt M, Jakobs KH, Van Koppen CJ. RhoA-sensitive trafficking of muscarinic acetylcholine receptors. Journal of Pharmacology and Experimental Therapeutics. 1999;288:36–42. [PubMed] [Google Scholar]
- Vogler O, Nolte B, Voss M, Schmidt M, Jakobs KH, van Koppen CJ. Regulation of muscarinic acetylcholine receptor sequestration and function by beta-arrestin. Journal of Biological Chemistry. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Lah JJ, Fang G, Goldenring JR, Levey AI. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. Journal of Neurosciences. 2002;22:9776–9784. doi: 10.1523/JNEUROSCI.22-22-09776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli LA, Lah JJ, Levey AI. Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. Journal of Biological Chemistry. 2001;276:47590–47598. doi: 10.1074/jbc.M106535200. [DOI] [PubMed] [Google Scholar]
- von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sciences. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR. Regulation of vesicle trafficking in madin-darby canine kidney cells by Rab11a and Rab25. Journal of Biological Chemistry. 2000;275:29138–29146. doi: 10.1074/jbc.M004410200. [DOI] [PubMed] [Google Scholar]
- Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Molecular Biology of the Cell. 2004;15:3758–3770. doi: 10.1091/mbc.E04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CZ, Heimberg H, D’Souza-Schorey C, Mueckler MM, Stahl PD. Subcellular distribution and differential expression of endogenous ADP-ribosylation factor 6 in mammalian cells. Journal of Biological Chemistry. 1998;273:4006–4011. doi: 10.1074/jbc.273.7.4006. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Elferink LA. Rab15 mediates an early endocytic event in Chinese hamster ovary cells. Journal of Biological Chemistry. 1999;274:22303–22312. doi: 10.1074/jbc.274.32.22303. [DOI] [PubMed] [Google Scholar]