Abstract
Lipoatrophy is a prevalent side effect of antiretroviral treatment of human immunodeficiency virus (HIV) infection. Its mechanisms are still disputed but include mitochondrial toxicity and, in particular, mitochondrial DNA (mtDNA) depletion induced by nucleoside reverse transcriptase inhibitors. To obtain an integrated evaluation of the mitochondrial alteration in lipoatrophy, we investigated the DNA, RNA, and protein levels in 15 samples of abdominal subcutaneous adipose tissue from HIV-infected patients with peripheral lipoatrophy and compared the results with those for 15 samples from age- and body mass index-matched controls. The DNA and RNA analyses used PCR-based techniques, while proteins were quantified with enzyme-linked immunosorbent assay and measurement of activities with spectrophotometric assays. Depletion of mtDNA and mtDNA-encoded MT-CO2 mRNA was present, but normal levels of mtDNA-dependent activity (cytochrome c oxidase) and protein (MT-CO2p) showed that it was compensated for. An increase in nuclear-DNA-dependent mitochondrial activities (citrate synthase and malate dehydrogenase) and protein (COX4I1p), as well as transcriptional up-regulation of nuclear-DNA-encoded mitochondrial genes (COX4I1 and UCP2), demonstrated increased mitochondrial biogenesis. However, the expression of the known transcription factors of mitochondrial biogenesis (TFAM, NRF1, GABPA, PPARGC1A, PPARGC1B, and PPRC1) was normal or decreased. Increased amounts of activated caspase 3 and of DDIT3 mRNA showed the induction of apoptosis and oxidative stress, respectively. The mtDNA content did not correlate with any other mitochondrial parameter. In conclusion, mtDNA content does not appear to be an accurate biomarker of mitochondrial alteration in lipoatrophic adipose tissue. The preservation of mtDNA-dependent mitochondrial functions occurred despite severe mtDNA depletion. The presence of significant oxidative stress and apoptosis did not correlate with the mtDNA content.
The lipodystrophy syndrome represents a major complication of antiretroviral therapy (ART) (5). Even if its occurrence seems markedly decreased with recently marketed antiretroviral drugs, its reversibility in lipodystrophic patients seems slow and possibly incomplete. The precise pathogenesis of lipodystrophy remains unclear to date (14, 30, 47). Its proposed deleterious mechanisms have included mitochondrial dysfunction, induction of apoptosis, altered cytokine secretion, and adipose tissue differentiation (4, 14, 18, 29, 36). Mitochondrial dysfunction could have a central role in the different mechanisms underlying lipoatrophy. It is a well-recognized inducer of apoptosis and oxidative stress (21). It also may directly impact adipogenesis, which has been shown to be accompanied by striking mitochondrial proliferation in murine adipocyte cell lines (50). Peripheral lipoatrophy is one component of the lipodystrophy syndrome that has been particularly associated with exposure to nucleoside reverse transcriptase inhibitors (NRTI), especially stavudine and zidovudine, which have been shown to be particularly toxic for mitochondrial function (19, 35, 45).
The inhibition by NRTI of mitochondrial DNA (mtDNA) polymerase γ (POLG), the sole DNA polymerase for mtDNA, has been proposed to have a central role in ART-related mitochondrial toxicity. It has been documented in vivo and in vitro (24). Inherited alterations of POLG provoke decrease of the mtDNA content (depletion) and the accumulation of mtDNA qualitative alterations, such as multiple deletions or point mutations (16, 41), which in turn induce the depletion of mtDNA-encoded proteins and respiratory defects. Such a sequence of events has been proposed for human immunodeficiency virus (HIV)-related lipoatrophy (24). mtDNA depletion has indeed been reproducibly shown in subcutaneous adipose tissue of ART-treated HIV-infected patients (7, 33, 34, 40, 49). Focal defects of an mtDNA-encoded subunit (MT-CO1p) (33) and of cytochrome c oxidase (COX) activity were shown in lipodystrophic adipose tissue by using histological approaches (15). The mtDNA content of adipose tissue could therefore be considered an accurate biomarker of mitochondrial alteration in adipose tissue.
The direct responsibility of mtDNA depletion has, however, been challenged by its correlation with NRTI exposure, but not with lipoatrophy (7, 34, 45, 49). Furthermore, the mtDNA content under NRTI has been shown to possibly diverge from its transcription (12, 28, 32) or from respiratory chain activities (17, 42, 46). Another confounding parameter is the mitochondrial proliferation that was observed in association with mtDNA depletion in HIV-infected patients' lipodystrophic adipose tissue (25, 33, 34, 37, 49). Intriguingly, the mitochondrial proliferation in HIV-related lipodystrophic adipose tissue did not appear to be regulated by the transcription factors involved in mitochondrial biogenesis (13), as several of them, including PPARγ coactivator 1 (PPARGC1A), nuclear respiratory factor 1 (NRF1), and mitochondrial transcription factor A (TFAM), were found to be expressed at normal or even decreased levels (20, 37).
In the present study, we performed an integrated evaluation of the mitochondrial alteration of lipoatrophic patients' adipose tissue at its diverse molecular levels (DNA, RNA, and protein). We demonstrated efficient preservation of mtDNA-dependent mitochondrial functions despite severe mtDNA depletion. This did not, however, prevent the induction of oxidative stress and apoptosis, two common consequences of mitochondrial alteration. The mtDNA content did not correlate with any other mitochondrial characteristics.
MATERIALS AND METHODS
Patients.
Pathological samples were obtained from 15 patients (13 were men) with marked facial lipoatrophy. Abdominal subcutaneous adipose tissue was removed by lipoaspiration. After centrifugation, a portion was injected into the cheeks (Coleman protocol) and the rest was kept for research purposes. All the patients gave their written informed approval to this protocol. The study was approved by the ANRS (National Agency for AIDS Research). The patients' immunological and viral statuses and their clinical histories were recorded at the time of the surgery and are summarized in Table 1. All the patients received at least two NRTI, and 10 of them were also treated with at least one protease inhibitor (PI). The NRTI most often used was stavudine (12 patients). It was given in association with lamivudine in 11 patients.
TABLE 1.
Main clinical and virologic characteristics of the HIV-infected patientsa
| Clinical or virologic characteristic | No./ratio or value [median (range)] |
|---|---|
| Age (yr) | 50.5 (37-67) |
| Sex (M/F) | 13/2 |
| BMI | 23.7 (21.3-28.3) |
| Viral load (copies of viral genomes/ml) | 465 (50-133,560) |
| CD4 (cells/ml) | 352 (100-868) |
| Triglyceridemia (mmol/liter) | 2.3 (1.0-8.0) |
| Cholesterolemia (mmol/liter) | 5.6 (3.2-9.9) |
| ApoB/ApoA1 | 1.3 (0.6-1.5) |
| HOMA | 4.1 (1.6-14.3) |
| Lactatemia (mmol/liter) | 1.9 (0.8-2.8) |
| Duration since onset of HIV infection (yr) | 11 (5-20) |
| Treatment duration (yr) | 3.5 (1.0-4.0) |
| Duration since onset of lipodystrophy (yr) | 2.0 (0.3-3.0) |
| Patients receiving 2 or 3 NRTI ± 1 NNRTIb, no PI | 5/15 |
| Patients receiving PI and 2 NRTI | 10/15 |
| Patients receiving stavudine | 12/15 |
Normal values: triglyceridemia, <1.6 mmol/liter; cholesterolemia, <6.5 mmol/liter; ApoB/ApoA1 ratio, 0.44 to 0.86 for men and 0.35 to 0.69 for women; HOMA [homeostasis model assessment = fasting insulin (mU/liter) × fasting glucose (mmol/liter)/22.5], <2.5; lactatemia, <2.5 mmol/liter.
NNRTI, nonnucleoside reverse transcriptase inhibitors.
DNA and RNA analyses were performed on all samples. Protein analyses could be obtained for a subset of eight samples due to the very limited amount of tissue.
Control subcutaneous fat samples were provided by the Association Française centre les Myopathies tissue repository bank. They were obtained from 15 men undergoing abdominal surgery in the absence of infection. They were matched to the patients' group with respect to age (median, 56.2, and range, 21 to 76 years) and body mass index (BMI) (median, 24.4, and range, 19.5 to 28.0 kg/m2).
Molecular analyses. (i) DNA analyses.
Total DNA from adipose tissue was prepared by using a standard protocol based on sodium dodecyl sulfate, proteinase K, and isopropanol precipitation. Multiple deletions of the mtDNA were searched for with an Expand Long Template PCR system long-range PCR kit (Boehringer) as described previously (48). The presence of mtDNA heteroplasmic point mutations was screened for by using denaturing gradient gel electrophoresis of tRNALeu(UUR) and tRNALys, as previously reported (43). Quantification of the mtDNA was performed by real-time PCR amplification on a Light Cycler (Roche Diagnostics) using Light Cycler FastStart DNA master Sybr green I mix (Roche Diagnostics), as reported previously (22). Briefly, the mtDNA copy number was evaluated by amplifying a fragment of the mtDNA 12S rRNA gene (MT-RNR1), while the amount of nuclear DNA in the same sample was evaluated by amplifying a fragment of the nuclear 28S rRNA gene (RNR1). The official gene symbols, primers, and conditions of the amplifications are shown in Table 2. The results are expressed as copy number per cell, considering that 10 pg of total DNA roughly represents the DNA content of one cell.
TABLE 2.
Real-time PCR conditions utilized for mtDNA and cDNA quantificationa
| Gene | Sense primer | Antisense primer | MgCl2 | Temp |
|---|---|---|---|---|
| MT-RNR1 | TAGCCCTAAACCTCAACAGT | TGCGCTTACTTTGTAGCCTTCAT | 5 | 62 |
| RNR1 | ATCCTTCGATGTCGGC | AGCACATACACCAAATGTCT | 6 | 60 |
| ACTB | CGACATGGAGAAAATCTGGC | CCATCCTGCGTCTGGACCT | 2.5 | 60 |
| DDIT3 | GCCAAAATCAGAGCTGGAAC | TCTTGCAGGTCCTCATACCA | 3.5 | 60 |
| MT-CO2 | TACGGCGGACTAATCTTCAA | AAAACAGATGCAATTCCCGG | 2.5 | 60 |
| COX4I1 | TGGATGAGAAAGTCGAGTTG | TCGTTATCATGTGGCAGAAG | 2.5 | 60 |
| CYBB | AATGCCCAATCCCTCAGTTT | TCCCACTTCCATTTTGAACC | 3.5 | 58 |
| NOX4 | TCCGTTGGTTTGCAGATTTAC | CAAAAGTTTCCACCGAGGAC | 3 | 58 |
| NRF1 | GCAACAGGAAAGAAACGGAA | ATTGTCCTCTGTATCTCACC | 2.5 | 60 |
| GABPA | TGCTGCACTGGAAGGCTATA | ACCGATATAGACCTCACCACA | 2.5 | 60 |
| TFAM | TGTGTATTTACCGAGGTGGT | TTAGAAGAATTGCCCAGCGT | 2.5 | 60 |
| PPARGC1B | CTGAAGATGACGTGGGT | TGATGTTGGGTAGGATGTG | 3.5 | 60 |
| PPARGC1A | AAGAGCGCCGTGTGATTTAT | ACCTACCGTTATACCTGTGA | 2.5 | 60 |
| PPRC1 | CCACGTACTCAGGGTT | GGTCTAGGGGCCTCTT | 3 | 60 |
| UCP2 | CAGGATCGGCCTCTACGAC | GTCAACGGTTCGGTTGTTGC | 3 | 60 |
For each amplification, serial dilutions of linearized plasmid vector pGEM-T Easy (Promega) containing the target gene as its insert were used as standards. All standard plasmids were purified with Nucleobond AX (Macherey-Nagel), linearized, and quantified by fluorometry using Sybr green I and λ phage DNA cut with HindIII as the standard (Invitrogen). Magnesium concentration is expressed as mmol/liter, and annealing temperature as °C.
(ii) RNA analyses.
Total RNA from adipose tissue biopsies was obtained by using an RNeasy lipid tissue mini kit from Qiagen (Courtaboeuf, France) (4). It was reverse transcribed with random hexamers and a SuperScript first-strand synthesis system for RT-PCR (Invitrogen) according to the manufacturer's instructions. The expression levels of the ACTB, MT-CO2, COX4I1, TFAM, NRF1, GABPA, PPARGC1A, PPARGC1B, PPRC1, DDIT3, CYBB, and NOX4 genes were evaluated by real-time PCR amplification of the cDNAs by using qPCR Mastermix plus for Sybr green I (Eurogentec). All results are expressed as copy number and normalized to the level of β-actin gene expression. The official gene symbols, primers, standards, and conditions of the amplifications are summarized in Table 2.
(iii) Protein analyses.
Protein analyses were performed on the infranatant layer (below the fat layer and above the pellet) of adipose tissue homogenized in 9 volumes of 50 mM Tris-HCl, pH 7.5, 150 mM KCl, 5 mM EDTA, 2 mM phenymethylsulfonyl fluoride and centrifuged at 750 × g and 4°C for 10 min. Aliquot samples were stored at −80°C before use. The semiquantitative evaluation of two mitochondrial proteins (MT-CO2p, encoded by mtDNA, and COX4p, encoded by nuclear DNA); of the cleaved (activated) form of caspase 3, a major apoptosis player; and of α-tubulin, a cytoskeleton protein used as the internal control of the amount of tissue, was performed by enzyme-linked immunosorbent assay (ELISA), as reported previously (26). Antibodies were either monoclonal antibodies (anti-active caspase 3 from BD Pharmingen, Tokyo, Japan; antitubulin 1A2 from TK/GE; and anti-COX4p graciously provided by Salvatore DiMauro, Columbia University, New York, NY) (44) or polyclonal antibodies against MT-CO2p, the specificities of which had been previously demonstrated (26). All antibodies were used at a 1/1,000 dilution. The results were expressed as the percentages of the results for one of the control samples arbitrarily chosen. Standard spectrophotometric methods were used to assess the citrate synthase (CS), COX, malate dehydrogenase (MDH), and phosphoglycerate kinase (PGK) activities (48).
Statistical analyses.
Only nonparametric tests were used. Comparison between groups was performed with the Mann and Whitney test, and correlation between quantitative parameters with the Spearman test using the Sigma Stat 3.1 application. The threshold for significance was set at a P value of 0.05.
RESULTS
Significant mtDNA depletion was present but compensated for at the posttranscriptional level.
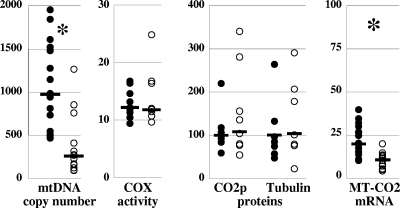
The mtDNA content of the adipose tissue was measured by real-time PCR of pathological and control adipose tissue samples. Despite large variations in the results for the pathological, as well as the control, samples (Fig. 1), the median mtDNA content was significantly decreased (P < 0.01) in the patients' adipose tissue (median, 270, and range, 95 to 1,269 mtDNA copies/cell) compared to the mtDNA content of the controls (median, 986, and range, 470 to 1,852 mtDNA copies/cell). Three patients' samples, however, had an mtDNA content that was above the lower limit of the control values. These patients did not differ from the others in terms of clinical presentation, disease and treatment duration, or composition of ART.
FIG. 1.
Significant mtDNA depletion was present but compensated for at the posttranscriptional level. The amount of mtDNA (mtDNA copy number) is expressed as mtDNA copy number/cell; the COX activity, a mitochondrial respiratory chain activity strictly dependent on normal function of the mtDNA, is expressed as nmol·min−1·mg−1 of protein in adipose tissue homogenate; the amounts of subunit 2 of COX (MT-CO2p), an mtDNA-encoded mitochondrial protein, and α-tubulin (Tubulin), a cytoskeleton component, were expressed as the percentages of the level in one of the controls, arbitrarily chosen. The steady-state level of mRNA from the MT-CO2 gene located on the mtDNA (MT-CO2 mRNA) was expressed as cDNA copies, normalized to the β-actin level. The open circles are values from samples of HIV-infected patients' adipose tissue, and the closed circles are values from control samples. The median value of each group of data is shown as a bar. The asterisks mark statistically significant differences between results for patients' and control samples.
Inherited alterations of the DNA polymerase γ gene in humans have been found to be responsible for multiple deletions and point mutations of mtDNA (41). We searched for these two types of genetic alterations in the DNA samples from control and patients' samples. The presence of large-sized rearrangements in mtDNA was excluded by long-range PCR amplification of the mtDNA, showing the expected 13-kb and 15-kb fragments without additional fragments of smaller size (data not shown). In parallel, significant sequence variation—heteroplasmic and/or potentially deleterious mutation—was excluded by denaturing gradient gel electrophoresis of tRNALeu(UUR) and tRNALys genes that have been reported as mtDNA mutation hotspots (43; data not shown).
To evaluate the functional impact of mtDNA depletion, we assayed the mtDNA-dependent COX activity, whose three catalytic subunits (MT-CO1p, MT-CO2p, and MT-CO3p) are encoded on the mtDNA. Efficient compensation of the mtDNA depletion was shown by the normal COX activity in the eight patients' samples that were available for that analysis (median, 12.2 nmol·min−1·mg−1 protein versus 11.8 nmol·min−1·mg−1 protein in control samples; P = 0.46) (Fig. 1). Only one of the eight samples had a normal mtDNA content; its COX activity was 10 nmol·min−1·mg−1 protein.
To assess if the compensation was associated with preservation of the amount of protein, we quantified MT-CO2p, one of the three mtDNA-encoded subunits of COX, using ELISA. The amounts of MT-CO2p appeared similar in the patients' and control samples (108% versus 100%, respectively; P = 0.87) (Fig. 1). It was 80% in the sole patient's sample with normal mtDNA content. The amounts of α-tubulin, a cytoskeleton component used as the internal control, were similar in patients' and control samples, thus showing equivalent loading (104% of the control value in patients' samples versus 100% in control samples; P = 0.99) (Fig. 1).
The preservation of mtDNA-dependent mitochondrial proteins and activities despite severe inherited mtDNA depletion has been previously related to increased transcription of the mtDNA (3). However, in lipodystrophic adipose tissue, the steady-state levels of the mtDNA-encoded CO2 subunit mRNA (MT-CO2) was significantly decreased in the patients' samples (median, 10.7 copies, normalized to the β-actin mRNA level, versus 19.9 in controls; P < 0.001), thus showing the absence of compensatory up-regulation of mtDNA transcription and implying that the preservation of CO2p was related to posttranscriptional mechanisms (Fig. 1). The amount of MT-COX2 mRNA did not correlate with the amount of mtDNA in the controls as well as it did in the patients' samples. In particular, two of the three patients' samples with normal mtDNA content had a small amount of MT-CO2 mRNA (7.9 and 13.0 copies, normalized to the β-actin level).
Increased mitochondrial population accompanied mtDNA depletion.
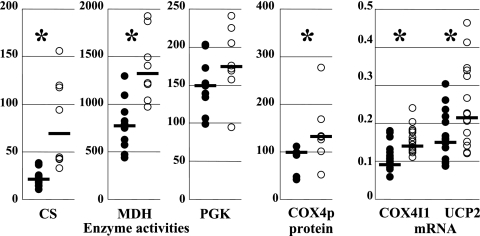
Although mitochondrial proliferation has been repeatedly reported in patients' lipodystrophic adipose tissue (25, 33, 34, 37, 49), it has been quantified only once (37). To evaluate the mitochondrial mass, we assayed two mitochondrial enzymatic activities that depend only on nuclear-DNA-encoded proteins. CS, a Krebs cycle enzyme, has a purely mitochondrial location, while MDH has both mitochondrial and cytosolic locations. PGK, a glycolysis enzyme with a purely cytosolic location, was used as the internal control (Fig. 2). A threefold increase in the mitochondrial mass was shown by the CS activity (median CS activity in patients' samples, 71 nmol·min−1·mg−1 protein, versus 23 in controls; P < 0.001), while the amount of MDH was increased 1.7 times (median MDH activity in patients' samples, 1,331 nmol·min−1·mg−1 protein, versus 784 in controls; P < 0.001). In contrast, the amounts of PGK were similar in patients and controls (median PGK activity in patients' samples, 176 nmol·min−1·mg−1 protein, versus 151 in controls; P = 0.17) (Fig. 2). The increase in mitochondrial proteins was also shown with the quantification by ELISA of COX4p, a nuclear-DNA-encoded subunit of COX, whose median amount was 132% in patients' samples, versus 100% in controls (P = 0.01) (Fig. 2). A significant increase in the transcription steady-state level of nuclear genes encoding structural mitochondrial proteins accompanied the increase in the mitochondrial population. The median number of copies, normalized to the β-actin level of COX4I1 mRNA, was 13.9 × 10−2 in patients' samples, versus 9.1 × 10−2 in controls (P < 0.01), while that of UCP2 mRNA was 21.5 × 10−2 in patients' samples, versus 15.1 × 10−2 in controls (P = 0.04) (Fig. 2).
FIG. 2.
Increased mitochondrial mass accompanied mtDNA depletion. The activities of CS, a mitochondrial enzyme of the tricarboxylic acid cycle, MDH, an enzyme with both mitochondrial and cytosolic locations, and PGK, a cytosolic enzyme of the glycolysis pathway, are expressed as nmol·min−1·mg−1 of protein in adipose tissue homogenate; the amounts of subunit 4 of COX (COX4p), a nuclear-DNA-encoded mitochondrial protein, were expressed as the percentages of the amount in one of the control samples; and the steady-state levels of mRNA from the nuclear DNA genes COX4I1 and UCP2 were expressed as cDNA copies, normalized to the β-actin cDNA level. The open circles show values from samples of HIV-infected patients' adipose tissue, and the closed circles show values from control samples. The median value of each group of data is shown as a bar. The asterisks mark statistically significant differences between results for patients' and control samples.
Increased mitochondrial population was not explained by transcriptional regulation of mitochondrial biogenesis.
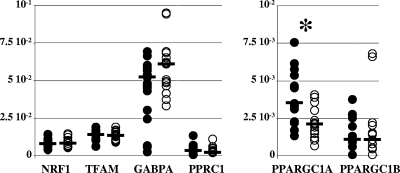
The increased levels of the mRNA of nuclear-DNA-dependent mitochondrial proteins suggested coordinated transcriptional up-regulation of mitochondrial biogenesis. In the control samples, transcriptional regulation of mitochondrial biogenesis was shown by the tight correlation between the levels of COX4I1 mRNA and NRF1 (P < 0.01) or TFAM (P = 0.01) mRNA or between the levels of COX4I1 mRNA and the CS (P < 0.01) or COX (P < 0.001) activities. In the patients' samples, however, in accordance with previously reported results (20, 37), we found that all known genes encoding transcription factors involved in mitochondrial biogenesis (10, 39) either had decreased (PPARGC1A, whose product is also known as PGC1α) or normal expression (NRF1; TFAM; GABPA, whose product is also known as NRF2; PPRC1, whose product is also known as PGC1-related coactivator [PRC]; and PPARGC1B, whose product is also known as PERC or PGC1β) (Fig. 3). Furthermore, the correlations between the levels of COX4I1 mRNA and of NRF1, CS, and COX were lost (P = 0.27, 0.88, and 0.57, respectively). The mitochondrial proliferation observed in the pathological adipose tissues was therefore not explained by a coordinated up-regulation of transcription factors involved in mitochondrial biogenesis.
FIG. 3.
The increased mitochondrial population was not explained by the increase of transcription factors involved in mitochondrial biogenesis. The steady-state levels of mRNA from all known genes encoding transcription factors involved in mitochondrial biogenesis are expressed as cDNA copies, normalized to the β-actin cDNA level. The closed circles show values for control samples, and the open circles show values for patients' samples. The median value of each group of data is shown as a bar. The asterisks mark statistically significant differences between results for pathological and control samples. The patients' and control samples had similar levels of NRF1 (8.35 × 10−3 in patients' samples versus 8.23 × 10−3 in controls; P = 0.74), of TFAM (1.35 × 10−2 in patients' samples versus 1.44 × 10−2 in controls; P = 0.71), of GABPA (also known as NRF2) (6.12 × 10−2 in patients' samples versus 5.25 × 10−2 in controls; P = 0.21), of PPRC1 (also known as PGC1-related coactivator [PRC]) (3.81 × 10−3 in patients' samples versus 5.07 × 10−3 in controls; P = 0.61), and of PPARGC1B (also known as PERC or PGC1β) (1.50 × 10−3 in patients' samples versus 1.34 × 10−3 in controls; P = 0.89). The amount of PPARGC1A (also known as PGC1α) was significantly decreased in patients' samples (2.13 × 10−3 versus 3.55 × 10−3 in controls; P < 0.01).
Increased apoptosis and oxidative stress were observed in patients' adipose tissue.
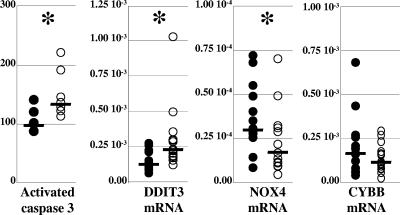
Increased apoptosis and oxidative stress are commonly associated with respiratory chain defects. Despite the preservation of the levels of COX activity, the altered ratio between MT-CO2p and COX4p implied that some COX complexes had an abnormal composition, thus provoking focal respiratory defects. In addition, mitochondrial proliferation has been demonstrated to be an essential factor for the induction of apoptosis in mitochondrial myopathy (1). The amount of the activated form of caspase 3, a hallmark of the cellular commitment to apoptosis (8), was therefore assessed with ELISA and found to be significantly increased in the patients' samples (median, 156% of control value, versus 110% in control samples; P = 0.02) (Fig. 4). Due to the very small amounts of the available adipose tissues preventing the direct evaluation of oxidative lesions of proteins, lipids, or DNA, we assessed the presence of oxidative stress by the steady-state expression level of DDIT3 (DNA damage-inducible transcript 3, also known as CHOP [C/EBP homologous protein]), a member of the CAAT/enhancer binding protein (C/EBP) family involved in the mitochondrial stress response (51) which has been previously shown to be regulated by mitochondrial reactive oxygen species (6). The steady-state level of DDIT3 mRNA was significantly increased in the patients' samples (median, 21.92 × 10−3 copies, normalized to the β-actin mRNA level, versus 11.58 × 10−3 in controls; P < 0.01), suggesting the presence of oxidative stress (Fig. 4). A cytosolic origin of the oxidative stress emanating from NAD(P)H oxidase activity (11) appeared unlikely, as the expression of the NAD(P)H oxidase catalytic subunit of adipocytes (NOX4) (27) was significantly decreased (median, 1.74 × 10−3 copies, normalized to the β-actin mRNA level, in patients' samples versus 3.01 × 10−3 in controls; P = 0.01), while that of the NAD(P)H oxidase catalytic subunit of phagocytes (CYBB) (2) was normal (median, 1.18 × 10−2 copies, normalized to the β-actin mRNA level, in patients' samples versus 1.66 × 10−2 in controls; P = 0.21) (Fig. 4). The content of mtDNA did not correlate with the markers of apoptosis or oxidative stress.
FIG. 4.
Increased apoptosis and oxidative stress were observed in pathological adipose tissues. The amount of the cleaved form of caspase 3 (Activated caspase 3), a hallmark of cell commitment to apoptosis, is expressed as the percentage of the amount in one control, arbitrarily chosen, and the steady-state levels of the mRNAs of DDIT3, a gene involved in the mitochondrial stress response; of NOX4, the gene encoding the adipocyte's NAD(P)H oxidase catalytic subunit; and of CYBB, the gene encoding the phagocytic cell's NAD(P)H oxidase catalytic subunit, were expressed as cDNA copies, normalized to the β-actin cDNA level. The median value of each group of data is shown as a bar on the graph. The asterisks mark statistically significant differences between results for pathological and control samples. Results for patients' samples are shown with open circles, and those for control samples with closed circles.
DISCUSSION
To evaluate the relevance of mtDNA content in mitochondrial alteration in HIV-related lipoatrophy, we performed an integrated evaluation of the mitochondrial function at its DNA, RNA, and protein levels, which had not been previously done. Our main findings were (i) the preservation of mtDNA-dependent activities and proteins despite severe mtDNA depletion, (ii) striking mitochondrial proliferation without up-regulation of the transcription factors involved in mitochondrial biogenesis, (iii) the occurrence of apoptosis and oxidative stress despite compensation of respiratory function at the global tissue level, and (iv) a striking absence of correlation between the mtDNA content of adipose tissue and any other mitochondrial characteristics. The cause of the observed alterations was not addressed in our study, whose design would not allow such interrogation (transversal analysis and the nature of the control samples).
Despite slight technical differences, the mtDNA copy numbers found in our study fell within the range published by others (33, 34). Our data showing normal levels of mtDNA-dependent enzyme activities and proteins unambiguously demonstrated efficient compensation of mtDNA depletion at the tissue level. They confirmed recent in vitro and animal data that have shown divergence between mtDNA content and respiratory chain activity (42, 46). They do not contradict the previously reported focal histological defects of mtDNA-dependent proteins or activities (33) which indicated the presence of scattered mitochondria with altered composition, a finding also inferred in our study from the abnormal ratios of mitochondrial proteins (MT-CO2p and COX4p) and of enzymatic activities (COX and CS). The decrease in the quantity/quality of mtDNA might be the primary feature in these scattered defective mitochondria.
Despite global compensation at the tissue level, altered mitochondrial composition and increased mitochondrial mass were associated with mitochondrion-borne deleterious mechanisms, such as oxidative stress and apoptosis (1). Indeed, the expression of DDIT3, a transcription factor induced by mitochondrial reactive oxygen species (6), was increased in patients' samples. Our data are in agreement with the recently reported induction of oxidative stress and DDIT3 expression in adipocytes under ART (23). Oxidative stress is considered a major triggering factor of apoptosis. It might, therefore, be responsible for the increased apoptosis observed in our study and in other studies (9, 18, 31).
The lack of correlation between the mtDNA level and the signs of apoptosis or oxidative stress argues against an essential primary responsibility of the mtDNA content in mitochondrial toxicity. That conclusion was reinforced by the presence of a significant defect of transcription of the mtDNA-encoded MT-CO2 in two patients with normal amounts of mtDNA, which showed mitochondrial toxicity independent of the mtDNA amount.
Mitochondrial proliferation has been previously reported (25, 33, 34, 49). Our quantification of CS activity, a method commonly used for the evaluation of mitochondrial population (3, 38), showed a 300% increase that was very close to the 289% increase previously reported (34). The molecular mechanisms underlying that striking mitochondrial proliferation remained elusive. Although the increased expression of the nuclear genes encoding mitochondrial proteins (COX4I1 and UCP2) suggested transcriptional regulation of mitochondrial biogenesis, we found that the expression of all the known transcription factors involved in mitochondrial biogenesis was normal or decreased in accordance with previously reported results for some of them (20, 37). The triggering factors of mitochondrial proliferation therefore remain to be identified. Whether these factors are involved in the normal function of adipose tissue or are specifically induced in response to ART toxicity will have to be evaluated.
In conclusion, the proposed sequence of events linking the decrease of the mtDNA content to defects of mtDNA-encoded proteins and respiratory activities is not confirmed at the level of lipoatrophic adipose tissue, whose mtDNA content is dissociated from the level of mtDNA transcription, the amount of mtDNA-encoded protein, and the levels of mtDNA-dependent activities. The absence of correlation between mtDNA content and apoptosis or oxidative stress further showed that the mtDNA content of adipose tissue cannot be considered an accurate biomarker of mitochondrial alteration in that tissue.
Acknowledgments
This work was supported by grants from ANRS (Agence Nationale de Recherche sur le SIDA), Sidaction, and INSERM (Institut National de la Santé et de la Recherche Médicale). M. J. Kim received a fellowship from Sidaction.
We thank Corinne Vigouroux, Philippe Levan, Pierre Marie Girard, and Willy Rozenbaum (Rothschild Hospital, AP-HP, Paris, France) for providing clinical and biological data, as well as samples from the patients analyzed in the paper. We also are grateful to the AFM tissue repository for providing control adipose tissue samples.
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Auré, K., G. Fayet, J. P. Leroy, E. Lacène, N. B. Romero, and A. Lombès. 2006. Apoptosis in mitochondrial myopathies is linked to mitochondrial proliferation. Brain 129:1249-1259. [DOI] [PubMed] [Google Scholar]
- 2.Babior, B. M., J. D. Lambeth, and W. Nauseef. 2002. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397:342-344. [DOI] [PubMed] [Google Scholar]
- 3.Barthélémy, C., H. Ogier de Baulny, J. Diaz, M. A. Cheval, P. Frachon, N. Romero, F. Goutieres, M. Fardeau, and A. Lombès. 2001. Late-onset mitochondrial DNA depletion: DNA copy number, multiple deletions, and compensation. Ann. Neurol. 49:607-617. [PubMed] [Google Scholar]
- 4.Bastard, J. P., M. Caron, H. Vidal, V. Jan, M. Auclair, C. Vigouroux, J. Luboinski, M. Laville, M. Maachi, P. M. Girard, W. Rozenbaum, P. Levan, and J. Capeau. 2002. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 359:1026-1031. [DOI] [PubMed] [Google Scholar]
- 5.Carr, A., and D. A. Cooper. 2000. Adverse effects of antiretroviral therapy. Lancet 356:1423-1430. [DOI] [PubMed] [Google Scholar]
- 6.Carriere, A., M. C. Carmona, Y. Fernandez, M. Rigoulet, R. H. Wenger, L. Penicaud, and L. Casteilla. 2004. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J. Biol. Chem. 279:40462-40469. [DOI] [PubMed] [Google Scholar]
- 7.Cherry, C. L., M. E. Gahan, J. C. McArthur, S. R. Lewin, J. F. Hoy, and S. L. Wesselingh. 2002. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J. Acquir. Immune Defic. Syndr. 30:271-277. [DOI] [PubMed] [Google Scholar]
- 8.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 9.Domingo, P., X. Matias-Guiu, R. M. Pujol, E. Francia, E. Lagarda, M. A. Sambeat, and G. Vazquez. 1999. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS 13:2261-2267. [DOI] [PubMed] [Google Scholar]
- 10.Finck, B. N., and D. P. Kelly. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 116:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa, S., T. Fujita, M. Shimabukuro, M. Iwaki, Y. Yamada, Y. Nakajima, O. Nakayama, M. Makishima, M. Matsuda, and I. Shimomura. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 114:1752-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galluzzi, L., M. Pinti, G. Guaraldi, C. Mussini, L. Troiano, E. Roat, C. Giovenzana, E. Nemes, M. Nasi, G. Orlando, P. Salomoni, and A. Cossarizza. 2005. Altered mitochondrial RNA production in adipocytes from HIV-infected individuals with lipodystrophy. Antivir. Ther. 10(Suppl. 2):M91-M99. [PubMed] [Google Scholar]
- 13.Garesse, R., and C. G. Vallejo. 2001. Animal mitochondrial biogenesis and function: a regulatory cross-talk between two genomes. Gene 263:1-16. [DOI] [PubMed] [Google Scholar]
- 14.Gougeon, M. L., L. Penicaud, B. Fromenty, P. Leclercq, J. P. Viard, and J. Capeau. 2004. Adipocytes targets and actors in the pathogenesis of HIV-associated lipodystrophy and metabolic alterations. Antivir. Ther. 9:161-177. [PubMed] [Google Scholar]
- 15.Hammond, E., D. Nolan, I. James, C. Metcalf, and S. Mallal. 2004. Reduction of mitochondrial DNA content and respiratory chain activity occurs in adipocytes within 6-12 months of commencing nucleoside reverse transcriptase inhibitor therapy. AIDS 18:815-817. [DOI] [PubMed] [Google Scholar]
- 16.Hudson, G., and P. F. Chinnery. 2006. Mitochondrial DNA polymerase-gamma and human disease. Hum. Mol. Genet. 15(Spec. No. 2):R244-R252. [DOI] [PubMed] [Google Scholar]
- 17.Igoudjil, A., A. Abbey-Toby, K. Begriche, A. Grodet, K. Chataigner, G. Peytavin, M. Maachi, M. Colin, M. A. Robin, P. Letteron, G. Feldmann, D. Pessayre, and B. Fromenty. 2007. High doses of stavudine induce fat wasting and mild liver damage without impairing mitochondrial respiration in mice. Antivir. Ther. 12:389-400. [PubMed] [Google Scholar]
- 18.Jan, V., P. Cervera, M. Maachi, M. Baudrimont, M. Kim, H. Vidal, P. M. Girard, P. Levan, W. Rozenbaum, A. Lombes, J. Capeau, and J. P. Bastard. 2004. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir. Ther. 9:555-564. [PubMed] [Google Scholar]
- 19.Joly, V., P. Flandre, V. Meiffredy, N. Leturque, M. Harel, J. P. Aboulker, and P. Yeni. 2002. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS 16:2447-2454. [DOI] [PubMed] [Google Scholar]
- 20.Kannisto, K., J. Sutinen, E. Korsheninnikova, R. M. Fisher, E. Ehrenborg, K. Gertow, A. Virkamaki, T. Nyman, H. Vidal, A. Hamsten, and H. Yki-Jarvinen. 2003. Expression of adipogenic transcription factors, peroxisome proliferator-activated receptor gamma coactivator 1, IL-6 and CD45 in subcutaneous adipose tissue in lipodystrophy associated with highly active antiretroviral therapy. AIDS 17:1753-1762. [DOI] [PubMed] [Google Scholar]
- 21.Kujoth, G. C., A. Hiona, T. D. Pugh, S. Someya, K. Panzer, S. E. Wohlgemuth, T. Hofer, A. Y. Seo, R. Sullivan, W. A. Jobling, J. D. Morrow, H. Van Remmen, J. M. Sedivy, T. Yamasoba, M. Tanokura, R. Weindruch, C. Leeuwenburgh, and T. A. Prolla. 2005. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309:481-484. [DOI] [PubMed] [Google Scholar]
- 22.Labarthe, F., D. Dobbelaere, L. Devisme, A. De Muret, C. Jardel, J. W. Taanman, F. Gottrand, and A. Lombès. 2005. Clinical, biochemical and morphological features of hepatocerebral syndrome with mitochondrial DNA depletion due to deoxyguanosine kinase deficiency. J. Hepatol. 43:333-341. [DOI] [PubMed] [Google Scholar]
- 23.Lagathu, C., B. Eustace, M. Prot, D. Frantz, Y. Gu, J. P. Bastard, M. Maachi, S. Azoulay, M. Briggs, M. Caron, and J. Capeau. 2007. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. 12:489-500. [PubMed] [Google Scholar]
- 24.Lewis, W., B. J. Day, and W. C. Copeland. 2003. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2:812-822. [DOI] [PubMed] [Google Scholar]
- 25.Lloreta, J., P. Domingo, R. M. Pujol, J. A. Arroyo, N. Baixeras, X. Matias-Guiu, M. Gilaberte, M. A. Sambeat, and S. Serrano. 2002. Ultrastructural features of highly active antiretroviral therapy-associated partial lipodystrophy. Virchows Arch. 441:599-604. [DOI] [PubMed] [Google Scholar]
- 26.Lombès, A., J. R. Mendell, H. Nakase, R. J. Barohn, E. Bonilla, M. Zeviani, A. J. Yates, J. Omerza, T. L. Gales, K. Nakahara, et al. 1989. Myoclonic epilepsy and ragged-red fibers with cytochrome oxidase deficiency: neuropathology, biochemistry, and molecular genetics. Ann. Neurol. 26:20-33. [DOI] [PubMed] [Google Scholar]
- 27.Mahadev, K., H. Motoshima, X. Wu, J. M. Ruddy, R. S. Arnold, G. Cheng, J. D. Lambeth, and B. J. Goldstein. 2004. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24:1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallon, P. W., P. Unemori, R. Sedwell, A. Morey, M. Rafferty, K. Williams, D. Chisholm, K. Samaras, S. Emery, A. Kelleher, D. A. Cooper, and A. Carr. 2005. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J. Infect. Dis. 191:1686-1696. [DOI] [PubMed] [Google Scholar]
- 29.McComsey, G. 2002. Update on mitochondrial toxicity of antiretrovirals and its link to lipodystrophy. AIDS Rev. 4:140-147. [PubMed] [Google Scholar]
- 30.McComsey, G., and J. F. Maa. 2003. Host factors may be more important than choice of antiretrovirals in the development of lipoatrophy. AIDS Read. 13:539-542. [PubMed] [Google Scholar]
- 31.McComsey, G. A., D. M. Paulsen, J. T. Lonergan, S. M. Hessenthaler, C. L. Hoppel, V. C. Williams, R. L. Fisher, C. L. Cherry, C. White-Owen, K. A. Thompson, S. T. Ross, J. E. Hernandez, and L. L. Ross. 2005. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS 19:15-23. [DOI] [PubMed] [Google Scholar]
- 32.Miro, O., S. Lopez, M. Rodriguez de la Concepcion, E. Martinez, E. Pedrol, G. Garrabou, M. Giralt, F. Cardellach, J. M. Gatell, F. Vilarroya, and J. Casademont. 2004. Upregulatory mechanisms compensate for mitochondrial DNA depletion in asymptomatic individuals receiving stavudine plus didanosine. J. Acquir. Immune Defic. Syndr. 37:1550-1555. [DOI] [PubMed] [Google Scholar]
- 33.Nolan, D., E. Hammond, I. James, E. McKinnon, and S. Mallal. 2003. Contribution of nucleoside-analogue reverse transcriptase inhibitor therapy to lipoatrophy from the population to the cellular level. Antivir. Ther. 8:617-626. [PubMed] [Google Scholar]
- 34.Nolan, D., E. Hammond, A. Martin, L. Taylor, S. Herrmann, E. McKinnon, C. Metcalf, B. Latham, and S. Mallal. 2003. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17:1329-1338. [DOI] [PubMed] [Google Scholar]
- 35.Nolan, D., and S. Mallal. 2004. Complications associated with NRTI therapy: update on clinical features and possible pathogenic mechanisms. Antivir. Ther. 9:849-863. [PubMed] [Google Scholar]
- 36.Nolan, D., and S. Mallal. 2004. The role of nucleoside reverse transcriptase inhibitors in the fat redistribution syndrome. J. HIV Ther. 9:34-40. [PubMed] [Google Scholar]
- 37.Pace, C. S., A. M. Martin, E. L. Hammond, C. D. Mamotte, D. A. Nolan, and S. A. Mallal. 2003. Mitochondrial proliferation, DNA depletion and adipocyte differentiation in subcutaneous adipose tissue of HIV-positive HAART recipients. Antivir. Ther. 8:323-331. [PubMed] [Google Scholar]
- 38.Rustin, P., D. Chretien, T. Bourgeron, B. Gerard, A. Rotig, J. M. Saudubray, and A. Munnich. 1994. Biochemical and molecular investigations in respiratory chain deficiencies. Clin. Chim. Acta 228:35-51. [DOI] [PubMed] [Google Scholar]
- 39.Scarpulla, R. C. 2002. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim. Biophys. Acta 1576:1-14. [DOI] [PubMed] [Google Scholar]
- 40.Shikuma, C. M., N. Hu, C. Milne, F. Yost, C. Waslien, S. Shimizu, and B. Shiramizu. 2001. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS 15:1801-1809. [DOI] [PubMed] [Google Scholar]
- 41.Spelbrink, J. N., J. M. Toivonen, G. A. Hakkaart, J. M. Kurkela, H. M. Cooper, S. K. Lehtinen, N. Lecrenier, J. W. Back, D. Speijer, F. Foury, and H. T. Jacobs. 2000. In vivo functional analysis of the human mitochondrial DNA polymerase POLG expressed in cultured human cells. J. Biol. Chem. 275:24818-24828. [DOI] [PubMed] [Google Scholar]
- 42.Stankov, M. V., T. Lucke, A. M. Das, R. E. Schmidt, and G. M. Behrens. 2007. Relationship of mitochondrial DNA depletion and respiratory chain activity in preadipocytes treated with nucleoside reverse transcriptase inhibitors. Antivir. Ther. 12:205-216. [PubMed] [Google Scholar]
- 43.Sternberg, D., E. Chatzoglou, P. Laforet, G. Fayet, C. Jardel, P. Blondy, M. Fardeau, S. Amselem, B. Eymard, and A. Lombes. 2001. Mitochondrial DNA transfer RNA gene sequence variations in patients with mitochondrial disorders. Brain 124:984-994. [DOI] [PubMed] [Google Scholar]
- 44.Tritschler, H. J., E. Bonilla, A. Lombès, F. Andreetta, S. Servidei, B. Schneyder, A. F. Miranda, E. A. Schon, B. Kadenbach, and S. DiMauro. 1991. Differential diagnosis of fatal and benign cytochrome c oxidase-deficient myopathies of infancy: an immunohistochemical approach. Neurology 41:300-305. [DOI] [PubMed] [Google Scholar]
- 45.van der Valk, M., M. Casula, G. J. Weverlingz, K. van Kuijk, B. van Eck-Smit, H. J. Hulsebosch, P. Nieuwkerk, A. van Eeden, K. Brinkman, J. Lange, A. de Ronde, and P. Reiss. 2004. Prevalence of lipoatrophy and mitochondrial DNA content of blood and subcutaneous fat in HIV-1-infected patients randomly allocated to zidovudine- or stavudine-based therapy. Antivir. Ther. 9:385-393. [PubMed] [Google Scholar]
- 46.Viengchareun, S., M. Caron, M. Auclair, M. J. Kim, P. Frachon, J. Capeau, M. Lombes, and A. Lombes. 2007. Mitochondrial toxicity of indinavir, stavudine and zidovudine involves multiple cellular targets in white and brown adipocytes. Antivir. Ther. 12:919-929. [PubMed] [Google Scholar]
- 47.Villarroya, F., P. Domingo, and M. Giralt. 2005. Lipodystrophy associated with highly active antiretroviral therapy for HIV infection: the adipocyte as a target of antiretroviral-induced mitochondrial toxicity. Trends Pharmacol. Sci. 26:88-93. [DOI] [PubMed] [Google Scholar]
- 48.Vittecoq, D., C. Jardel, C. Barthélémy, L. Escaut, N. Cheminot, S. Chapin, D. Sternberg, T. Maisonobe, and A. Lombès. 2002. Mitochondrial damage associated with long-term antiretroviral treatment: associated alteration or causal disorder? J. Acquir. Immune Defic. Syndr. 31:299-308. [DOI] [PubMed] [Google Scholar]
- 49.Walker, U. A., M. Bickel, S. I. Lutke Volksbeck, U. P. Ketelsen, H. Schofer, B. Setzer, N. Venhoff, V. Rickerts, and S. Staszewski. 2002. Evidence of nucleoside analogue reverse transcriptase inhibitor-associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J. Acquir. Immune Defic. Syndr. 29:117-121. [DOI] [PubMed] [Google Scholar]
- 50.Wilson-Fritch, L., A. Burkart, G. Bell, K. Mendelson, J. Leszyk, S. Nicoloro, M. Czech, and S. Corvera. 2003. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell. Biol. 23:1085-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao, Q., J. Wang, I. V. Levichkin, S. Stasinopoulos, M. T. Ryan, and N. J. Hoogenraad. 2002. A mitochondrial specific stress response in mammalian cells. EMBO J. 21:4411-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]