Abstract
Production of E,E-farnesol (FOH) and biofilm formation were studied under various conditions in 56 strains of eight Candida spp. FOH production differed significantly not only between Candida spp. but within Candida albicans strains as well. FOH concentrations and biofilm formation were the highest for C albicans.
Candida albicans is a major human fungal pathogen, causing both superficial and invasive tissue infections. The ability of C. albicans to form biofilms on medical devices has a profound impact on its capacity to cause human disease. C. albicans and other members of the genus Candida are able to grow in different forms as budding yeast, pseudohyphae, and true hyphae, which is called dimorphism (1, 8). This transition from yeast to hyphal growth can be induced by various conditions (2, 25). Progression to a mature biofilm is dependent on cell adhesion, extracellular matrix production, and the yeast-to-hypha transition in C. albicans (2, 3). However, biofilm development in non-C. albicans Candida spp. (NCAC) is not well understood.
Suppression of biofilm formation in C. albicans may be achieved by quorum-sensing molecules (5). E,E-Farnesol (FOH) has been reported to inhibit the induction of hyphal growth and biofilm formation in C. albicans (10, 11). In this study, FOH secretion by C. albicans and eight NCAC was examined under various culture conditions. In addition, the development of biofilms was studied. Finally, the correlation between FOH secretion and biofilm formation was analyzed for all of the isolates studied.
We studied 56 strains of eight Candida species (Table 1). All isolates were cultivated in RPMI 1640 medium with or without the addition of 10% fetal calf serum (FCS) at 37°C for 24 h under continuous rotation at 125 rpm. Two milliliters of sterile filtered (0.45 μm) culture supernatant was extracted with 5 ml n-hexane-ethanol (90:10, vol/vol) and derivatized with 9-anthroylnitrile as previously described (13). Quantification was done with n-butanol as an internal standard (50 ng added to each sample). Reverse-phase high-performance liquid chromatography was done with a YMC Hydrosphere C18 column (5 μm, 150 by 2.1 mm [inside diameter]). A linear gradient of acetonitrile-water (85% to 100% over 20 min) was used as the mobile phase. Standard concentrations ranged from 0.004 μM to 40 μM FOH. Detection, determination of recovery, and calculations were performed as previously described (4, 13). The development of biofilms by all of the Candida spp. was studied according to Krom et al. (6), with minor differences such as using 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt instead of 2,3-bis[2-methoxy-4-nitro-5-sulfophenyl]-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide. In addition to visual reading at 450 nm, optical density was measured at 405 nm for data validation (15). All tests were done twice and analyzed with the two-tailed paired Student t test comparing 0 and 10% FCS cultivations (significance was set at a P value of ≤0.05).
TABLE 1.
Candida spp. used in this study and concentrations of secreted FOH
| Straina | FOH concn [μM]
|
|
|---|---|---|
| 0% FCS | 10% FCS | |
| C. albicans strains | ||
| ATCC 90028 | 13.7 | 1.7 |
| ATCC 24433 | 32.0 | 2.4 |
| ATCC 36801 | 34.6 | 0.5 |
| ATCC 44373 | 16.9 | 0.9 |
| ATCC 44374 | 51.2 | 3.0 |
| ATCC 76615 | 58.5 | 2.4 |
| Y0119b | 42.2 | 2.3 |
| Mean ± SD | 35.6 ± 16.5 | 2.0 ± 0.9 |
| C. glabrata strains | ||
| ATCC 90030 | 0.8 | 0.5 |
| Y3390b | 0.5 | 0.4 |
| RKI 04-0388 | 0.2 | 0.2 |
| RKI 05-0284.01 | 0.4 | 0.3 |
| RKI 05-0445.01 | 0.1 | 0.2 |
| RKI 05-0559.02 | 0.4 | 0.1 |
| RKI 06-0367 | 0.3 | 0.2 |
| Mean ± SD | 0.4 ± 0.2 | 0.3 ± 0.1 |
| C. parapsilosis strains | ||
| Y0501b | 0.3 | 0.4 |
| ATCC 90018 | 1.2 | 0.8 |
| ATCC 22019 | 0.8 | 0.9 |
| RKI 00-0438 | 0.5 | 0.4 |
| RKI 02-0579 | 0.5 | 0.5 |
| RKI 04-0241 | 0.4 | 0.6 |
| RKI 06-0220 | 0.2 | 0.1 |
| Mean ± SD | 0.6 ± 0.3 | 0.6 ± 0.3 |
| C. tropicalis strains | ||
| ATCC 750 | 1.2 | 0.4 |
| ATCC 90874 | 2.2 | 0.4 |
| RKI 03-0108 | 0.3 | 0.1 |
| RKI 04-0326 | 0.4 | 0.8 |
| RKI 05-559.01 | 0.6 | 0.6 |
| RKI 98-0463 | 0.5 | 0.4 |
| RKI 99-0499.02 | 1.2 | 0.4 |
| Mean ± SD | 1.0 ± 0.7 | 0.5 ± 0.02 |
| C. kefyr strains | ||
| Y0601b | 0.8 | 0.7 |
| RKI 01-0709 | 0.7 | 0.1 |
| RKI 95-0329.02 | 0.2 | 1.1 |
| RKI 95-1915.02 | 0.5 | 2.8 |
| RKI 95-2066 | 0.1 | 2.6 |
| RKI 97-0656 | 0.2 | 5.6 |
| RKI 97-0768.02 | 0.5 | 0.2 |
| Mean ± SD | 0.5 ± 0.3 | 1.9 ± 1.8 |
| C. krusei strains | ||
| MB16b | 0.5 | 0.4 |
| ATCC 6258 | 0.7 | 0.8 |
| ATCC 90878 | 0.8 | 0.6 |
| RKI 03-450.02 | 0.5 | 2.5 |
| RKI 04-0167.04 | 0.5 | 0.9 |
| RKI 05-0126 | 0.6 | 0.9 |
| RKI 06-0365 | 0.6 | 0.6 |
| Mean ± SD | 0.6 ± 0.1 | 1.0 ± 0.6 |
| C. guilliermondii strains | ||
| ATCC 90877 | 1.3 | 0.7 |
| RKI 01-0546 | 0.8 | 0.6 |
| RKI 02-0043 | 0.4 | 0.3 |
| RKI 04-0347 | 0.9 | 0.6 |
| RKI 05-0091 | 0.4 | 0.4 |
| RKI 95-1889 | 0.5 | 0.3 |
| RKI 95-2865 | 0.7 | 0.1 |
| Mean ± SD | 0.8 ± 0.3 | 0.5 ± 0.2 |
| C. dubliniensis strains | ||
| CBS 8500 | 17.5 | 2.1 |
| CBS 8501 | 7.8 | 2.9 |
| RKI 01-0170.01 | 6.0 | 0.1 |
| RKI 01-0265 | 12.5 | 2.5 |
| RKI 01-0268.02 | 10.2 | 1.7 |
| RKI 05-0037 | 6.7 | 0.4 |
| RKI 06-0019 | 9.2 | 1.4 |
| Mean ± SD | 8.7 ± 3.8 | 1.5 ± 1.0 |
Shown are the strains used and the concentrations of FOH secreted by them when they were cultivated in RPMI 1640 medium with 10% FCS and without FCS (n = 2; agreement of ≤25%) and the mean ± SD of each Candida species, respectively. Strains were obtained from the Centraal Bureau voor Schimmelcultures (CBS), Baarn, The Netherlands; the American Type Culture Collection (ATCC), Manassas, VA; and the Robert Koch Institute (RKI), Berlin, Germany.
C. albicans Y0119, C. glabrata Y3390, C. parapsilosis Y0501, C. kefyr Y0601, and C. krusei MB16 were supplied by Pfizer Laboratories, Illertissen, Germany.
The quantification and standardization of FOH showed good linearity with R2 = 0.99. The recovery rate was determined as approximately 95%. The FOH concentrations for all 56 Candida strains are shown in Table 1. Under FCS-free conditions, the highest concentrations of FOH were measured for C. albicans isolates, with a mean of 35.6 μM (range, 13.7 to 58.5 μM). The quantity was up to 35 times higher than for NCAC, except for C. dubliniensis. The mean FOH concentration for C. dubliniensis was 8.3 μM (range, 6.0 to 17.5 μM). Individual C. albicans isolates varied remarkably in their ability to produce FOH. FOH concentrations were almost four times as high as for C. dubliniensis. All other NCAC showed relatively low concentrations of FOH (mean, 0.6 ± 0.2 μM), independently of whether the Candida strains were cultivated with or without supplementation with 10% FCS.
Significant decreases in the secretion of FOH were observed for C. albicans (P = 0.001), C. dubliniensis (P = 0.0007), and C. guilliermondii (P = 0.01) under FCS-supplemented culture conditions. The concentration of FOH for C. albicans decreased to 2.0 ± 0.9 μM (mean). The comparison of the FCS-free and 10% FCS cultivations indicated a significant disparity (P = 0.003).
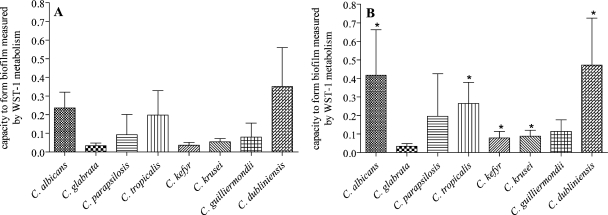
The investigation of biofilm formation showed comparable results for both techniques (Fig. 1). An optical density cutoff of ≥0.2 (>35% transmission blocked) was used to discriminate biofilm producers according to recent suggestions (14, 15). With both media, C. albicans and C. dubliniensis formed good biofilms. C. dubliniensis isolates produced 48% more biofilm under FCS-free conditions than did C. albicans (no statistically significant difference). Addition of 10% FCS to the growth medium induced significantly better biofilm formation in five of the eight Candida species tested (Fig. 1; P < 0.05). With C. albicans, increased biofilm formation (by 76%) was observed with 10% FCS in the medium. Increased biofilm formation was also obtained for C. dubliniensis (34%), C. tropicalis (34%), and C. krusei (61%).
FIG. 1.
Biofilm formation by Candida species. Fifty-six Candida spp. were incubated for 24 h in RPMI 1640 medium containing 0% (A) or 10% (B) FCS. Biofilm formation was measured as described in the text. The average for all of the isolates of a species in Table 1 is shown as the result of 14 values calculated for each column. *, Significantly increased biofilm (P < 0.05) when cultured in medium with 10% FCS. WST-1, 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt.
Quorum-sensing molecules, and FOH in particular, are considered to play an important role in the development of biofilms by C. albicans on the surfaces of medical devices (10-12). In this study, we found that C. dubliniensis can produce significant amounts of FOH but still less than C. albicans. In contrast, biofilm formation was more pronounced in C. dubliniensis than in any of the other Candida spp. tested. It is unclear whether this is related to the phylogenetic relationship between the two species or to other conditions (7). C. dubliniensis is a rare human pathogen and causes much less common invasive Candida infections in humans than do C. albicans and other NCAC. Furthermore, we have observed that several other NCAC do not produce any notable FOH but may form a biofilm (e.g., C. tropicalis, C. parapsilosis). The mechanism of reduced FOH production after supplementation with 10% FCS is unclear, but it is known that culture conditions may affect the global transcriptional response of Candida spp. (C. albicans) (9). It is assumed that the ability to form a biofilm is linked to FOH secretion as the major quorum-sensing molecule in C. albicans and C. dubliniensis, as shown in this study. We found that other Candida spp. can form biofilms without high levels of FOH, and it may be concluded that these NCAC use other pathways or quorum-sensing molecules for biofilm formation without FOH secretion.
Biofilm formation by NCAC is not well understood and requires further exploration with other models (e.g., gene regulation, detection of regulatory pathways).
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Calderone, R. A. 2002. Taxonomy and biology of Candida, p. 15-28. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, DC.
- 2.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 3.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 4.Glowka, F. K., M. Karazniewicz, and E. Lipnicka. 2006. RP-HPLC method with fluorescence detection for determination of small quantities of triamcinolone in plasma in presence of endogenous steroids after derivatization with 9-anthroyl nitrile; pharmacokinetic studies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 839:54-61. [DOI] [PubMed] [Google Scholar]
- 5.Hornby, J. M., and E. C. Jensen. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krom, B. P., J. B. Cohen, G. E. Elhaney Feser, and R. L. Cihlar. 2007. Optimized candidal biofilm microtiter assay. J. Microbiol. Methods 68:421-423. [DOI] [PubMed] [Google Scholar]
- 7.Mähnss, B., F. Stehr, W. Schafer, and K. Neuber. 2005. Comparison of standard phenotypic assays with a PCR method to discriminate Candida albicans and C. dubliniensis. Mycoses 48:55-61. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 9.Murillo, L. A., G. Newport, C. Y. Lan, S. Habelitz, J. Dungan, and N. M. Agabian. 2005. Genome-wide transcription profiling of the early phase of biofilm formation by Candida albicans. Eukaryot. Cell 4:1562-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickerson, K. W., A. L. Atkin, and J. M. Hornby. 2006. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 72:3805-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage, G., K. VandeWalle, B. L. Wickes, and J. L. López-Ribot. 2001. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. Micol. 18:163-170. [PubMed] [Google Scholar]
- 13.Saisho, Y., A. Morimoto, and T. Umeda. 1997. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal. Biochem. 252:89-95. [DOI] [PubMed] [Google Scholar]
- 14.Shin, J. H., S. J. Kee, M. G. Shin, S. H. Kim, D. H. Shin, S. K. Lee, S. P. Suh, and D. W. Ryang. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumbarello, M., B. Posteraro, E. M. Trecarichi, B. Fiori, M. Rossi, R. Porta, K. de Gaetano Donati, M. La Sorda, T. Spanu, G. Fadda, R. Cauda, and M. Sanguinetti. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45:1843-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]