Abstract
The efficacy of voriconazole in 107 patients with scedosporiosis was analyzed. Principal infection sites were the lungs/sinuses (24%), central nervous system (CNS) (20%), and bone (18%), while 21% of patients had disseminated infection. Solid organ transplantation (22%), hematological malignancy (21%), and surgery/trauma (15%) were the predominant underlying conditions. A successful therapeutic response was achieved in 57% of patients (median, 103 therapy days), with >98% of those responding receiving ≥28 days of therapy. Patients receiving primary therapy showed a 61% response versus 56% for the others. The best therapeutic responses were seen for skin/subcutaneous (91%) or bone (79%) infections, and the lowest for CNS infections (43%). Patients without major immune suppression (72%) or those with solid organ transplantation (63%) or various hematological conditions (60%) showed the best responses by underlying condition. Median known survival time was 133 days (therapy successes, 252 days; failures, 21 days). In all, 43 (40%) patients died, 73% due to scedosporiosis. Patients with Scedosporium prolificans infection had significantly reduced survival times (P = 0.0259) and were more likely to die from fungal infection (P = 0.002) than were Scedosporium apiospermum-infected patients. In a subset of 43 patients where voriconazole baseline MICs were available, response to voriconazole was higher for S. apiospermum-infected patients (54% response; MIC50, 0.25 μg/ml) than for S. prolificans-infected patients (40% response; MIC50, 4.0 μg/ml). Voriconazole demonstrated clinically useful activity in the treatment of both S. apiospermum and S. prolificans infections and was well tolerated.
Scedosporium apiospermum (sexual form, Pseudallescheria boydii) and Scedosporium prolificans are significant fungal pathogens of immunocompromised or otherwise debilitated patients but are also capable of causing severe disease in immunocompetent individuals (6, 16, 20, 36, 30, 45). They may be misidentified in the laboratory as Aspergillus spp. or other filamentous fungi, which can be problematic, as Scedosporium spp. are resistant to many antifungal agents, including amphotericin B and itraconazole (8, 30, 36). Despite therapy, mortality is high (50 to 70%), especially in S. prolificans infection (6, 20, 21, 27, 29). There is, therefore, a need for more effective antifungal strategies.
The broad-spectrum azole voriconazole exhibits activity in vitro against both Scedosporium species (4, 30, 45). Voriconazole may show fungicidal activity against S. apiospermum and is efficacious in both mouse and guinea pig models of scedosporiosis (4, 5, 10). In humans, numerous case reports have detailed its clinical effectiveness against Scedosporium infections, including those involving the central nervous system (CNS) and bone (6, 12, 13, 14, 20, 22, 23, 34, 35, 39, 40, 47). Most describe the use of voriconazole as the sole antifungal agent, sometimes combined with surgery. Successful control of infection with voriconazole in combination with terbinafine or an echinocandin has been described, particularly for S. prolificans, where voriconazole is less potent in vitro (3, 15, 19, 25, 32, 46, 50); however, no large series of cases have been published to date and the long-term efficacy of voriconazole in treating scedosporiosis has not been clearly established. One small (n = 10 patients) study of voriconazole use as salvage therapy noted a 30% response rate (38). Thus, treatment remains largely driven by anecdotal experience. This report summarizes the efficacy of voriconazole in the treatment of 107 Scedosporium infections in patients registered in the primary, salvage, and compassionate/named patient programs from the Pfizer global voriconazole clinical trials database and postmarketing cases from Australia. The in vitro susceptibilities of isolates from 43 of these patients to voriconazole and standard antifungal agents were also determined.
MATERIALS AND METHODS
Study design.
The voriconazole global clinical trials database (Pfizer), consisting of demographic, clinical, and microbiological details of over 2,000 patients, was queried for all infections due to Scedosporium or Pseudallescheria. This survey (completed in August 2005) included previously published cases from the primary/salvage therapy (38) and compassionate/named patient (13, 14, 23, 48) programs. The data for all these patients were combined with those from a postmarketing epidemiological and clinical survey of patients treated in Australia and analyzed retrospectively. In all instances, patient data were collected on standardized case report forms designed for the purposes of the respective surveys. The entire study adopted investigator-based diagnosis of Scedosporium infection and assessments of efficacy. Approvals for the studies were obtained from the institutional review boards of the participating centers (on an individual basis for compassionate cases) affiliated with the authors (listed in the Acknowledgments). All patients provided informed consent, according to the Declaration of Helsinki.
Definitions.
Successful therapy was defined as a complete or partial clinical response at the end of treatment/last visit. All other responses were considered therapy failures (18). Stable disease was defined as “no significant change in clinical, imaging or microbiological results” (18). Known survival time was defined as the last date on which a patient was reported to be alive. In total, 117 cases were identified; however, only 107 cases that satisfied the criteria for “probable” or “definite” infection according to published European Organization for Research and Treatment of Cancer/Mycoses Study Group guidelines (1) were evaluated.
Microbiology.
Scedosporium isolates were identified to species level (as S. prolificans or S. apiospermum) by standard colonial and mycological methods (9). In vitro susceptibility data were obtained for baseline isolates from 43 patients. MICs of amphotericin B, itraconazole, and voriconazole were determined using a broth microdilution method, according to the Clinical and Laboratory Standards Institute (formerly NCCLS) M38-A guidelines using a MIC endpoint of complete growth inhibition (33). Confirmation of species identification and susceptibility testing were carried out at the two Voriconazole Mycology Reference Laboratories (11) (Pfizer study isolates) or at the Mycology Unit of the Women's and Children's Hospital, Adelaide, South Australia (Australian isolates).
Statistical analysis.
The overall global response to treatment (success or failure) and patient survival end points were summarized by site of infection and by underlying disease. In addition, survival and global responses to treatment were compared for subjects infected with S. prolificans and subjects infected with S. apiospermum. For these two groups, Kaplan-Meier and logistic regression analyses were performed and the impact of two covariates, site of infection and underlying disease, was evaluated. Logistic regression analyses, controlling for the above factors, were conducted for the global overall response, survival, and death due to fungal infection. Data were analyzed using SAS software (43).
RESULTS
The demographic features and geographical distribution of the 107 voriconazole-treated patients with scedosporiosis are summarized in Table 1. Demographic, clinical, and outcome data were available for all patients, with species identification and data on prior antifungal therapy available in 106 (99.1%) and 85 (79.4%) cases, respectively.
TABLE 1.
Patient demographic data
| Demographic parameter | Value |
|---|---|
| No. of patients | 107 |
| Age range (median), in yr | <1.0-85 (50) |
| No. (%) of patients by parameter | |
| Pediatric (<18 yr) | 22 (21) |
| Gender | |
| Male | 69 (64) |
| Female | 38 (36) |
| Race | |
| Caucasian | 100 (93) |
| Other | 7 (7) |
| Geographic distribution | |
| Australia | 44 (41) |
| Americas | 23 (22) |
| Europe | 39 (36) |
| Middle East | 1 (1) |
| Infection type | |
| Probable | 36 (34) |
| Definite | 71 (66) |
| Voriconazole therapy type | |
| Primary | 28 (26) |
| Salvage/compassionate | 79 (71) |
Most patients were adults, but 22 (21%) were <18 years of age. The majority (77 [72%]) came from the voriconazole compassionate/named patient/salvage therapy programs. Definite infection was present in 71 (66%) patients and probable infection in the remainder (Table 1), and 57 (53%) were known to have received prior antifungal therapy for scedosporiosis. Prior therapy included one or more conventional or lipid formulations of amphotericin B, itraconazole, terbinafine, miconazole, or an echinocandin, sometimes in combination; treatment durations were unrecorded.
All patients received voriconazole at standard doses, at least initially (6 mg/kg of body weight intravenously twice daily on day 1, followed by 4 mg/kg intravenously twice daily and then a switch to oral therapy at 200 mg twice daily). Dose escalation due to insufficient response to therapy was identified in 4/77 (5%) patients, but no information was available for the remainder. The overall duration of voriconazole therapy ranged from 1 to 802 days (median, 103 days) with 88 (82%) patients receiving >14 days of therapy, while 23 (21%) received treatment for a year or more (Tables 2 and 3).
TABLE 2.
Scedosporium infection: clinical response and site of infection
| Site(s) of infection, no. (%) | Range of therapy in days (median) | No. with successful outcome/total no. (%) | Known survival range in days (median) | No. of patients alive/total no. (no. of deaths due to scedosporiosis) |
|---|---|---|---|---|
| CNS, 21 (20) | 4-600 (115) | 9/21 (43) | 8->2,000 (116) | 10/21 (8) |
| Disseminated, 23 (21) | 1-691 (108) | 11/23 (48) | 2-816 (108) | 10/23 (12) |
| Bone, 19 (18) | 15-322 (140) | 15/19 (79) | 20-1,440 (147) | 18/19 (1) |
| Lung, sinus,a 26 (24) | 1-802 (101) | 14/26 (54) | 1-802 (101) | 14/26 (9) |
| Other body sites,b 7 (7) | 3-274 (42) | 2/7 (29) | 3-1,800 (275) | 5/7 (2) |
| Skin/subcutaneous, 11 (10) | 13-463 (100) | 10/11 (91) | 13-720 (178) | 8/11 (0) |
| Total, 107 | 1-802 (103) | 61/107 (57) | 1->2,000 (133) | 64/107 (32 IFIc) |
Lungs (25), sinus (one).
Other body sites: eye (three), ear (two), and vocal cord (one).
IFI, invasive fungal infection.
TABLE 3.
Scedosporium infection: clinical response and underlying conditiond
| Underlying disease, no. (%) | Range of therapy in days (median) | No. with successful outcome/total no. (%) | Known survival range in days (median) | No. of patients alive/total no. (no. of deaths due to IFI) |
|---|---|---|---|---|
| HSCT, 10 (9) | 1-799 (73) | 4/10 (40) | 1-799 (94) | 4/10 (4) |
| Hematological malignancy, 22 (21) | 1-540 (30) | 10/22 (45) | 2-720 (34) | 7/22 (11) |
| SOT, 24 (22) | 13-487 (139) | 15/24 (63) | 13-720 (140) | 15/24 (7) |
| Other hematological conditions,a 5 (5) | 32-691 (138) | 3/5 (60) | 32-816 (138) | 4/5 (1) |
| Other malignancy,b 7 (7) | 8-150 (90) | 3/7 (43) | 9-1,440 (108) | 3/7 (2) |
| Other,c 29 (27) | 9-802 (179) | 21/29 (72) | 9->2,000 (221) | 26/29 (3) |
| High-dose steroids, 10 (9) | 3-495 (96) | 5/10 (50) | 3-720 (96) | 5/10 (4) |
| Total, 107 | 1-802 (103) | 61/107 (57) | 1->2,000 (133) | 64/107 (32) |
Other hematological conditions: chronic granulomatous disease (four) and angioimmunoblastic lymphadenopathy (one).
Other malignancy: non-Hodgkin's lymphoma (three), laryngeal carcinoma (one), prostate cancer (one), Waldenstrom's macroglobulinemia (one), and choroid plexus tumor (one).
Other: trauma/postsurgery (16), diabetes (five), none (three), near-drowning (two), idiopathic pulmonary fibrosis (one), vasculitis (one), and chronic immune deficiency (one).
Abbreviations: HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; SOT, solid organ transplant.
Site of infection, underlying medical conditions, and risk factors.
The main sites of infection were the lungs and/or sinuses (24%), CNS (20%), and bone (18%), although disseminated disease (more than one noncontiguous site, excluding the CNS, or one deep site in addition to blood) was present in 21% of patients (Table 2). Skin and/or subcutaneous tissuse alone was the main infection site in 10% of patients (Table 2).
Cancer was a common underlying comorbidity (29/107 [27%]), with most cases affecting those with hematological malignancies (22/29 [76%]) (Table 3). Patients with underlying conditions categorized as “other” formed a large group (27%) and included 16 who had recent trauma or surgery, five with diabetes mellitus, and two with near-drowning incidents (Table 3). In total, 24/34 (71%) organ transplant recipients had received solid organ transplants (11 lung, nine kidney, three heart or heart-lung, and one liver) and 9% had hematopoietic cell transplants.
Scedosporium species.
A total of 70 (65%) patients were diagnosed by the investigator as infected with S. apiospermum, and 35 (33%) were diagnosed as infected with S. prolificans. One patient was infected with both species (considered S. prolificans for analyses since this species is less susceptible to voriconazole and other antifungal agents and more difficult to treat), while for the other no species identification was available.
Response to therapy.
A successful global response to therapy was achieved in 61 (57%) patients, with 33 (31%) considered to have a complete response, 28 (26%) to have a partial response, six (6%) to have stable disease, and 40 (37%) assigned as treatment failures. The median voriconazole therapy duration in treatment success cases was 180 days (range, 13 to 799 days). In contrast, the median was 19 days (range, 1 to 802 days) for the therapy failures (n = 46). There was no difference in success rates between patients diagnosed as having definite and those diagnosed as having probable infections (57% versus 56%, respectively). Some patients (28/107 [26%]) received no prior therapy or only fluconazole prophylaxis and were regarded as receiving primary voriconazole therapy (Table 1). Their response rate to voriconazole therapy was 61% compared with 56% for the other 79 patients who had received one or more antifungal agents (described above). Infections of the skin/subcutaneous tissues had the highest success rate (91%), followed by bone (79%) and lungs/sinuses (54%). For the other body sites, the success rate was <50%, including 43% for CNS infections (Table 2).
Response to voriconazole therapy varied by underlying condition. The highest success rate was observed for patients with conditions described as “other” (72%) (patients with relatively minor or no defined compromise), followed by solid organ transplantation (63%) and other hematological conditions (60%) (Table 3). Conversely, patients with cancer and hematopoietic stem cell transplantation had the poorest success rate (40 to 45%) (Table 3). Of note, 21/30 patients (70%) with skin/subcutaneous tissue or bone infections had “other” or solid organ transplantation as their underlying condition. Species (P = 0.0456) and site of infection (P = 0.0316) were shown to be statistically significant prognostic factors for overall global response.
Successful responses to therapy were observed for 45/70 (64%) of S. apiospermum- and 16/36 (44%) of S. prolificans-infected patients. This difference in rate of success between the two groups was barely not significant (P = 0.052); however, when adjusting for site of infection, the rate of success difference between the two groups became significant (P = 0.046; data not shown). All nine successfully treated CNS infections were due to S. apiospermum, although one patient was infected with both species.
Patient survival.
Known patient survival ranged from 1 to >2,000 days (median, 131 days). It exceeded 1 year in 21% of patients, including six with cerebral, four with disseminated, and five with pulmonary infection. Successfully treated patients had a median survival time of 252 days (range, 13 to >2,000 days), but in therapy failures the median was only 21 days (range, 1 to 802 days) (P = 0.001). The all-cause mortality was 40% (43/107) with 74% of deaths attributed to Scedosporium infection. All 19 patients who received ≤14 days of therapy died, 79% from fungal infection. Of 64 patients not known to have died, the length of the observation period varied from 18 to >2,000 days (median, 246 days) from start of therapy. It exceeded 365 days in 21 (33%) cases.
Patients with infection identified at “other” body sites (275 days), skin/subcutaneous tissue (178 days), or bone (147 days) had the longest median survival times, while those with lung, disseminated, or CNS infection had the lowest (101 to 116 days; Table 2). Of cancer patients, those with hematological malignancy had substantially shorter median survival times than those with other cancer types (median of 34 versus 108 days). Patients with underlying conditions assigned as “other” (221 days) and solid organ transplantation (140 days) had the best median survival times (Table 3).
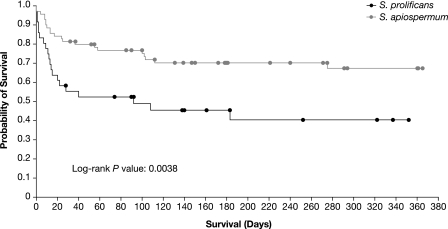
Both species (P = 0.0247) and underlying disease (P = 0.0440), but not site of infection, were shown to be statistically significant prognostic factors for survival. The odds ratios are shown in Table 4, and a Kaplan-Meier analysis by species is shown in Fig. 1. There were significant differences in the overall number of patients surviving, both with (P = 0.0247) and without (P = 0.0259) adjustment for the effect of underlying disease. Similar results were observed for the number of patients dying due to fungal infection (P = 0.0020/P = 0.0029).
TABLE 4.
Logistic regression analysis of survival by Scedosporium species for primary underlying disease
| Group and prognostic factore | Odds ratioa | 95% Confidence interval |
|---|---|---|
| Pathogen | ||
| S. apiospermum vs S. prolificans | 3.349 | 1.166-9.618 |
| Underlying disease vs other | ||
| HSCTb | 0.059 | 0.007-0.497 |
| Hematological malignancy | 0.062 | 0.009-0.416 |
| High-dose steroids | 0.156 | 0.022-1.132 |
| Other hematological conditionc | 0.784 | 0.046-13.364 |
| Other malignancyd | 0.043 | 0.004-0.459 |
| SOT | 0.170 | 0.028-1.043 |
Refers to S. apiospermum as the reference category.
Other: trauma/postsurgery (16), diabetes (five), none (three), near-drowning (two), idiopathic pulmonary fibrosis (one), vasculitis (one), and chronic immune deficiency (one).
Other hematological conditions: chronic granulomatous disease (four) and angioimmunoblastic lymphadenopathy (one).
Other malignancies: non-Hodgkin's lymphoma (three), laryngeal carcinoma (one), prostate cancer (one), Waldenstrom's macroglobulinemia (one), and choroid plexus tumor (one).
HSCT, hematopoietic stem cell transplantation; SOT, solid organ transplant.
FIG. 1.
Kaplan-Meier plot of all-cause death within the first year for S. apiospermum versus S. prolificans.
In vitro susceptibility.
The in vitro susceptibilities of 43 baseline isolates from voriconazole-treated patients are summarized in Table 5. In 11/28 (36%) S. apiospermum and 13/14 (93%) S. prolificans isolates the itraconazole MICs were ≥1.0 μg/ml. For amphotericin B, 26/28 (93%) S. apiospermum isolates had MICs of ≥2.0 μg/ml, while all 14 S. prolificans isolates had MICs of ≥4.0 μg/ml. Voriconazole was more active than itraconazole or amphotericin B against S. apiospermum (MIC90, 1 μg/ml) and was as active as, or more active than, both comparator agents against S. prolificans (5/15 isolates had MICs of <4.0 μg/ml). Not only were S. apiospermum isolates more susceptible to voriconazole in vitro than were S. prolificans isolates, but the 28 patients infected with these S. apiospermum isolates had a higher rate of response to voriconazole therapy (MIC50, 0.25 μg/ml; successful response rate, 54%) than did the 15 S. prolificans-infected patients (MIC50, 4.0 μg/ml; response rate, 40%) (Table 5).
TABLE 5.
In vitro susceptibilities of incident baseline Scedosporium isolates to voriconazole, itraconazole, and amphotericin B and responses to voriconazole therapy
| First isolate/species | No. of successful therapy responses/total no. (%) | MIC range of drug, μg/ml (MIC50/MIC90)
|
||
|---|---|---|---|---|
| Voriconazole | Itraconazole | Amphotericin | ||
| S. apiospermum | 15/28 (54) | 0.0625-8.0 (0.25/1.0) | 0.125->16.0 (0.5/2.0) | 0.5->16 (4.0/>8.0) |
| S. prolificans | 6/15 (40) | 0.125-8.0 (4.0/8.0) | 0.5->16.0a (16/16) | 4.0-16.0a (8.0/16) |
n = 14 isolates.
In six patients, multiple Scedosporium cultures were available during receipt of voriconazole therapy (therapy range, 5 to 156 days; median, 41 days). When tested for in vitro susceptibility, there was no increase (greater than two doubling dilutions) in MIC over time on therapy.
Safety and toleration.
Safety data were available for 86 (80.3%) patients (70 from the global clinical trials database and 16 from the Australian epidemiology study). Overall, 84 patients experienced 655 adverse events (77 posttreatment) including three Australian patients. Of the adverse events, 220 were serious and 175 severe. All events labeled as “serious” met the regulatory definition of serious adverse events. Only 133 (20.3%) events were considered possibly voriconazole related. In 15 patients voriconazole was discontinued temporarily or the dose was reduced and in 10 patients it was stopped permanently. Treatment-emergent, voriconazole-related serious adverse events included elevations in liver function tests (six), hallucinations (one), visual disturbances (one), abdominal pain (one), hypotension (one), eosinophilia (one), and relapse of acute myelogenous leukemia (one), accounting for 4 of the 10 permanent study drug-related discontinuations.
DISCUSSION
Scedosporium spp. are an increasingly important cause of life-threatening infections, not just in immunocompromised patients after cancer chemotherapy or organ transplantation (6, 20) but also in immunocompetent individuals (37, 45). Based on increasing numbers of published case reports, voriconazole alone or in combination with another antifungal agent such as terbinafine is becoming the therapy of choice to treat scedosporiosis; however, isolated case reports provide a limited indication of the potential of a drug and may represent publication bias. Furthermore, not all clinical reports have documented successful outcomes. The current study of 107 primary therapy, salvage therapy, and compassionate/named patient cases is representative of a diverse patient group from four continents and is the largest published to date. The overall response rate of 57% provides a better indication of the clinical effectiveness of voriconazole against these problematic pathogens. Importantly, efficacy and patient survival were dependent on the causative Scedosporium species: patients with infection due to S. prolificans had significantly reduced survival times and were more likely to die from their fungal infection than were those with S. apiospermum infection. The results also confirm that voriconazole has clinically useful activity against both S. apiospermum and S. prolificans.
Pharmacokinetic data show that voriconazole is distributed throughout the body, including the brain, eyes, and other tissues (17, 26, 42). These data are supported by the results of this study, where voriconazole was used successfully to treat a wide range of Scedosporium infections. The findings are consistent with efficacy results for voriconazole for other fungal infections of the CNS (44), bone (31), and other body sites (18, 38). Moreover, we note that for CNS, pulmonary, bone, and subcutaneous/skin infections, efficacy is better than or similar to that published for invasive aspergillosis (18, 31, 44).
Overall, 57% of patients responded successfully to therapy, a higher percentage than that reported in other studies with salvage or compassionate therapy in patients with various fungal infections (28, 38, 49). Possible explanations include the differences in patient mix or causative fungal species or the fact that 26% of patients in the current study received voriconazole as primary therapy and thus may have been less seriously ill. The overall mortality rate (40%) in this study also compares favorably with those reported previously (2, 3, 12).
Unsurprisingly, patients with superficial infection (involving skin without dissemination) or single primary sites of infection (bone) showed better responses and survival times than those with dissemination or involvement of deep organ sites. Brain and/or disseminated disease due to both S. apiospermum and S. prolificans has previously been associated with high mortality rates (60 to 100%) (2, 3, 12, 24, 34); however, in the current study, survival times exceeding 1 year were recorded in some patients with cerebral, disseminated, or deep organ infections. To determine if these favorable survival data extend into the longer term (>2 years) requires a prolonged period of observation post-commencement of therapy. Although not uniform in our study, this period extended to a median duration of >8 months (246 days).
A significant challenge in determining therapeutic efficacy in this complex patient population is the high mortality related to underlying illness (e.g., cancer) and the influence of host immunity. The poorest responses were seen in hematology patients receiving cancer chemotherapy and in hematopoietic stem cell transplant recipients, consistent with the high mortality reported previously (24, 27, 45). Conversely, patients with no or relatively low levels of immune suppression had superior responses to voriconazole therapy, suggesting that both an effective drug and effective host immunity are required for a favorable response. However, solid organ transplant patients also responded well (63% success rate); in two other reports, voriconazole showed promise as an antifungal agent in a small number of transplant patients (6, 20). We also noted longer survival times in solid organ transplant recipients than in patients who were otherwise immunosuppressed, adding to the experience of Husain et al. (20), where receipt of voriconazole was associated with a trend toward better survival in this patient group. The role of host immunological recovery, reduction of immunosuppression, or surgery was not assessed in this series.
Importantly, the causative Scedosporium species influenced both response to voriconazole therapy and patient survival. Patients with S. apiospermum showed a higher response to voriconazole than did those with S. prolificans infection (66% versus 44%, respectively; P = 0.052). Others have noted that S. apiospermum infection is, in general, more likely to respond to antifungal therapy than S. prolificans infection (20, 24); however, differences in response rates to voriconazole between infections with the two species have largely been limited to individual case reports. Although the differences in the species-specific response rates in this study were not statistically significant, after adjusting for body site of infection, patients with S. prolificans infection were significantly less likely to achieve a successful clinical response. In addition, survival times were significantly lower for infection due to this species (P = 0.026) and the attributable mortality was higher (P = 0.002) than that for S. apiospermum infection.
It is noteworthy that all but one of the 61 patients who had a successful therapeutic response received 28 days or more of voriconazole therapy and that these patients also achieved significantly longer survival times than the 46 therapy failures, the majority of whom received <28 days of therapy. Successful responders included those with CNS, systemic, or deep organ infections. Since patients who failed to achieve successful responses were more likely to die within the first 28 days, and because of the presence of other confounding factors, we were unable to draw any firm conclusions regarding the impact of duration of therapy on outcome. Other limitations of the study include the inclusion of cases enrolled in different clinical (salvage/compassionate and postmarketing) settings and the use of investigator-based reported outcomes. Every effort was made to include only data that were comparable between studies and to provide guidelines for assigning outcome responses according to established clinical trial practice (18).
The in vitro susceptibility results confirm that voriconazole has good activity against S. apiospermum isolates recovered in different patient populations from a wide geographic distribution and that the drug was more potent than amphotericin B and itraconazole (current study) (8). Although MIC breakpoints to define resistance to voriconazole have not yet been published for molds, the MIC90 for S. apiospermum of 1 μg/ml is well within achievable serum voriconazole concentrations in humans (41). As observed previously (8, 30), S. apiospermum was more susceptible than S. prolificans to all drugs tested, including voriconazole, for which MICs for S. prolificans were slightly lower than those of amphotericin B. As a result, some authors have questioned the efficacy of voriconazole in treating S. prolificans infections (8); however, the results from the current study indicate that voriconazole has clinically useful activity against this species. In parallel with the greater in vitro potency of voriconazole against S. apiospermum, there was a higher response rate for S. apiospermum infection (54%) than for S. prolificans infection (40%). Further studies correlating in vitro susceptibility with in vivo outcome are warranted.
Given the inherent resistance of S. prolificans to currently available antifungal agents, combination therapy of an azole with terbinafine has been recommended by some authors (3, 7, 15, 19, 25, 32, 45, 50). Successful use of voriconazole with an echinocandin has also been reported (46). We are unable to support these recommendations as data on combination therapy were not available for the majority of patients in this data set.
Finally, where the data were available, voriconazole was well tolerated in these seriously ill patients despite prolonged treatment (median duration, 103 days), with 14% receiving more than 1 year of therapy. We acknowledge the absence of safety data for just under 20% of patients, including almost two-thirds of Australian patients. However, overall, only four patients discontinued therapy because of clinically significant serious adverse events possibly related to voriconazole.
In conclusion, this large study reporting the efficacy of voriconazole treatment for invasive scedosporiosis is encouraging. Efficacy was shown for S. apiospermum but also for the less-susceptible S. prolificans. Many patients had relatively long-term, successful outcomes. This was particularly notable for S. apiospermum infections, including sites considered difficult to treat such as the CNS and eye. Whether an alternative new azole, posaconazole, will prove as effective as or more effective than voriconazole in treating scedosporiosis remains to be established. Independently of the antifungal agent selected for therapy, early diagnosis to species level remains of paramount importance in overall patient management.
Acknowledgments
Scedosporium Global Study Group members: in Australia, R. Benn (Royal Prince Alfred Hospital, Sydney), L. Dalla Poza (New Children's Hospital, Westmead, Sydney), D. de Witt (Gosford Hospital, Gosford), J. Dyer (Fremantle Hospital, Fremantle), C. Franklin (Alfred Hospital, Melbourne), J. Flexman (Royal Perth Hospital, Perth), T. Gottlieb (Concord Hospital, Sydney), P. Georghiou (Wesley Medical Center, Brisbane), D. Gordon (Flinders Medical Centre, Adelaide), R. Henry (Sydney Children's Hospital, Sydney), B. Johnson (Princess Alexandra Hospital, Brisbane), T. Korman (Monash Medical Centre, Melbourne), D. Marriott (St. Vincent's Hospital, Sydney), M. Malouf (St. Vincent's Hospital, Sydney), A. Mijch (The Alfred Hospital, Melbourne), J. Morton (Queensland Heart and Lung Transplant Unit, Brisbane), P. Palasanthrian (Sydney Children's Hospital, Sydney), G. Playford (Princess Alexandra Hospital, Brisbane), K. Rowlands (Royal Adelaide Hospital, Adelaide), T. Sorrell (Westmead Hospital, Sydney), B. Speed (Austin and Repatriation Hospitals, Melbourne), L. Tierney (John Hunter Hospital, Newcastle), A. Watson (The Canberra Hospital, Canberra), K. Weeks (Royal North Shore Hospital, Sydney), and M. Whitby (Princess Alexandra Hospital, Brisbane); in Belgium, G. Bricteux (Centre Hospitalaire Universitaire, Liege) and F. Jacob (Hopital Universitaire, Brussels); in Canada, P. Phillips (St. Paul's Hospital, Vancouver, British Columbia); in Chile, F. Barriga (Universitad Católica de Chile, Santiago de Chile); in France, V. Baclet (Hôpital Gustav Dron, Tourcoing), L. Crevon (HIA Desgenettes, Lyons), A. Jabado (Hôpital Necker, Paris), F. Metge (Centre Hôpital National d'Ophthalmologie des Quinz-Vingts, Paris), N. Milpied (CHU de Nantes, Nantes), and E. Senneville (Hôpital Bron, Tourcoing); in Germany, H. Bertz (Freiburg), G. Faetkenheuer (Cologne), and K. Potthoff (University of Freiburg Medical Center, Freiburg); in Italy, E. Gotti (Ospedali Riuniti, Bergano); in The Netherlands, M. Heuvel-Elbrink (Sophia Children's Hospital, Rotterdam); in Saudi Arabia, A. Rusheed (Riyadh Armed Forces Hospital, Riyadh); in Spain, P. Bastida-Nila (Hospital Materno-Infantil Vall d'Hebron, Barcelona), E. Bouza (Hospital General Universitario Gregorio Marañón, Madrid), A. Delgado (Carretera de Pozuelo a Majadahonda, Madrid), C. Ferra (Hospital Duran I Reynals, Barcelona), J. Martinez-Montauti (Hospital de Barcelona, Barcelona), J. Monfa (Hospital General Rio Carrión, Palencia), G. Poza (Hospital Virgin de la Arrixaca, Madrid), J. Vidal (Hospital Nuestra Senõra de Aranzazu, San Sebastian), and P. Fernandez-Viladricht (Hospital de Bellvitge, Barcelona); in Sweden, M. Studhal (Sahlgrenska Universitaetssjukhuset, Göteborg); in Switzerland, A. Gratwohl (University Hospital, Basel); in the United Kingdom, P. Bullock (Kings College Hospital, London), K. Orr (Freeman Hospital, Newcastle-upon-Tyne), and G. Spicket (Royal Victoria Infirmary, Newcastle); in the United States, C. Cunningham (SUNY Upstate Medical Hospital, Syracuse, NY), H. Dannawi (Medical Center of Central Georgia, Macon, GA), S. Davis (St. Paul Medical Center, Dallas, TX), D. Dealy (Mission St. Joseph's Hospital, Asheville, NC); T. Driscoll (Duke University Medical Center, Durham, NC), R. Fisher (Children's Hospital of The King's Daughters, Norfolk, VA), P. Flomenberg (Thomas Jefferson University, Philadelphia, PA), A. Gamis (The Children's Mercy Hospital, Kansas City, KS), S. Kusne (University of Pittsburgh Medical Center, Pittsburgh, PA), M. Joyce (Wolfson Children's Hospital, Jacksonville, FL), J. Martin (Children's Hospital of Pittsburgh, Pittsburgh, PA), N. Markowitz (Henry Ford Hospital, Detroit, MI), M. Nesky (North Carolina Baptist Hospital, Winston-Salem, NC), S. Rajf (University of Texas Medical Center, Houston, TX), L. Rickman (University Campus Southern California Medical Center, San Diego, CA), R. Rubin (Massachusetts General Hospital, Boston, MA), P. Tebas (Washington University, St. Louis, MO), J.-A. van Burik (University of Minnesota, Minneapolis, MN), and J. Yao (St. Luke's Hospital, Jacksonville, FL).
We also thank the many other infectious diseases physicians, clinical microbiologists, and hospital scientists for their assistance in case contribution and submission of isolates.
This study was sponsored by Pfizer Inc. P.T. was previously an employee of, and is currently a consultant to, Pfizer Inc. He received an honorarium from Pfizer in connection with the development of the manuscript. C.A. is currently an employee of Pfizer Inc. C.H.H., M.S., and D.E. are serving/have served on Antifungal Advisory Boards for Gilead Sciences, Pfizer, Australia; Merck, Sharp and Dohme, Australia; and Schering-Plough, Australia. I.L. was previously an employee of, and is currently a consultant to, Pfizer Inc. S.C.A.C. is/has been a member of the Antifungal Advisory Board of Gilead Sciences and Pfizer Inc., Australia. Editorial assistance was provided by T. Wetter of Parexel and was funded by Pfizer Inc.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh on behalf of the Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, J. V. Martinez-Suarez, et al. 1997. Deep infections caused by Scedosporium prolificans: a report on 16 cases in Spain and a review of the literature. Medicine (Baltimore) 76:256-265. [DOI] [PubMed] [Google Scholar]
- 3.Bhat, S. V., D. L. Paterson, M. G. Rinaldi, and P. J. Veldkamp. 2007. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand. J. Infect. Dis. 39:87-90. [DOI] [PubMed] [Google Scholar]
- 4.Capilla, J., and J. Guarro. 2004. Correlation between in vitro susceptibility of Scedosporium apiospermum to voriconazole and in vivo outcome of scedosporiosis in guinea pigs. Antimicrob. Agents Chemother. 48:4009-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capilla, J., C. Serena, F. J. Pastor, M. Ortoneda, and J. Guarro. 2003. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47:3976-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castiglioni, B., D. A. Sutton, M. G. Rinaldi, J. Fung, and S. Kusne. 2002. Pseudallescheria boydii (Anamorph Scedosporium apiospermum): infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine 81:333-348. [DOI] [PubMed] [Google Scholar]
- 7.Charles, P. G. P., and B. R. Speed. 2002. A case of Scedosporium prolificans septic arthritis responding to combined oral voriconazole and terbinafine. Intern. Med. J. 32:A60. [Google Scholar]
- 8.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 9.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed., p. 899-901. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 10.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., E. Johnson, H. Hockey, and P. Troke. 2008. Activities of voriconazole, itraconazole and amphotericin B in vitro against 590 moulds from 323 patients in the voriconazole Phase III clinical studies. J. Antimicrob. Chemother. 61:616-620. [DOI] [PubMed] [Google Scholar]
- 12.Farina, C., E. Gotti, F. Suter, and A. Goglio. 2006. Scedosporium apiospermum soft-tissue infection: a case report and review of kidney transplant literature. Transplant. Proc. 38:1333-1335. [DOI] [PubMed] [Google Scholar]
- 13.German, J. W., S. M. Kellie, M. P. Pai, and P. T. Turner. 2004. Treatment of a chronic Scedosporium apiospermum vertebral osteomyelitis. Case report. Neurosurg. Focus 17:E9. [DOI] [PubMed] [Google Scholar]
- 14.Girmenia, C., G. Luzi, M. Monaco, and P. Martino. 1998. Use of voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 36:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosbell, I. B., V. Toumasatos, J. Yong, R. S. Kuo, D. H. Ellis, and R. C. Perrie. 2003. Cure of orthopedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233-236. [DOI] [PubMed] [Google Scholar]
- 16.Guarro, J., A. S. Kantarcioglu, R. Horre, J. L. Rodriguez-Tudela, M. Cuenca Estrella, J. Berenguer, and G. Sybren De Hoog. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 44:295-327. [DOI] [PubMed] [Google Scholar]
- 17.Hariprasad, S. M., W. F. Mieler, E. R. Holz, H. Gao, J. E. Kim, J. Chi, and R. A. Prince. 2004. Determination of vitreous, aqueous, and plasma concentration of orally administered voriconazole in humans. Arch. Ophthalmol. 122:42-47. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 19.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 20.Husain, S., P. Munoz, G. Forrest, B. D. Alexander, J. Somani, K. Brennan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 21.Idigoras, P., E. Perez-Trallero, L. Pineiro, J. Larruskain, M. C. Lopez-Lopategui, N. Rodriguez, and J. M. Gonzalez. 2001. Disseminated infection and colonization by Scedosporium prolificans: a review of 18 cases, 1990-1999. Clin. Infect. Dis. 32:E158-E165. [DOI] [PubMed] [Google Scholar]
- 22.Jabado, N., J. L. Casanova, E. Haddad, F. Dulieu, J. C. Fournet, B. Dupont, A. Fischer, C. Hennequin, and S. Blanche. 1998. Invasive pulmonary infection due to Scedosporium apiospermum in two children with chronic granulomatous disease. Clin. Infect. Dis. 27:1437-1441. [DOI] [PubMed] [Google Scholar]
- 23.Kanafani, Z. A., Y. Comair, and S. S. Kanj. 2004. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 23:836-840. [DOI] [PubMed] [Google Scholar]
- 24.Lamaris, G. A., G. Chamilos, R. E. Lewis, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2006. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin. Infect. Dis. 43:1580-1584. [DOI] [PubMed] [Google Scholar]
- 25.Li, J. Y., T. Y. Yong, D. I. Grove, and P. T. Coates. 2008. Successful control of Scedosporium prolificans septic arthritis and probable osteomyelitis without radical surgery in a long-term renal transplant recipient. Transpl. Infect. Dis. 10:63-65. [DOI] [PubMed] [Google Scholar]
- 26.Lutsar, I., S. Roffey, and P. Troke. 2003. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin. Infect. Dis. 37:728-732. [DOI] [PubMed] [Google Scholar]
- 27.Maertens, J., K. Lagrou, H. Deweerdt, I. Surmont, G. E. Verhoef, J. Verhaegen, and M. A. Boogaerts. 2000. Disseminated infection by Scedosporium prolificans: an emerging fatality among haematology patients: case report and review. Ann. Hematol. 79:340-344. [DOI] [PubMed] [Google Scholar]
- 28.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh for the Caspofungin Salvage Aspergillosis Study Group. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 29.Marco de Lucas, E., P. Sadaba, P. Lastra Garcia-Baron, M. L. Ruiz Delgado, J. Cuevas, R. Salesa, A. Bermudez, A. Gonzalez Mandly, A. Gutierrez, F. Fernandez, F. Marco de Lucas, and C. Diez. 2006. Cerebral scedosporiosis: an emerging fungal infection in severe neutropenic patients: CT features and CT pathologic correlation. Eur. Radiol. 16:496-502. [DOI] [PubMed] [Google Scholar]
- 30.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mouas, H., I. Lutsar, B. Dupont, O. Fain, R. Herbrecht, F. X. Lescure, and O. Lortholary. 2005. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin. Infect. Dis. 40:1141-1147. [DOI] [PubMed] [Google Scholar]
- 32.Mursch, K., S. Trnovec, H. Ratz, D. Hammer, R. Horre, A. Klinghammer, S. de Hoog, and J. Behnke-Mursch. 2006. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 22:189-192. [DOI] [PubMed] [Google Scholar]
- 33.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of molds: approved standard M38-A. NCCLS, Wayne, PA.
- 34.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 35.Nulens, E., C. Eggink, A. J. Rijs, P. Wesseling, and P. E. Verweij. 2003. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J. Clin. Microbiol. 41:2261-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panackal, A. A., and K. A. Marr. 2004. Scedosporium/Pseudallescheria infections. Semin. Respir. Crit. Care Med. 25:171-181. [DOI] [PubMed] [Google Scholar]
- 37.Panichpisal, K., K. Nugent, and J. C. Sarria. 2006. Central nervous system pseudallescheriasis after near-drowning. Clin. Neurol. Neurosurg. 108:348-352. [DOI] [PubMed] [Google Scholar]
- 38.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 39.Porte, L., S. Khatibi, L. E. Hajj, S. Cassaing, A. Berry, P. Massip, M. D. Linas, J. F. Magnaval, N. Sans, and B. Marchou. 2006. Scedosporium apiospermum mycetoma with bone involvement successfully treated with voriconazole. Trans. R. Soc. Trop. Med. Hyg. 100:891-894. [DOI] [PubMed] [Google Scholar]
- 40.Poza, G., J. Montoya, C. Redondo, J. Ruiz, N. Vila, J. L. Rodriguez-Tudela, A. Ceron, and E. Simarro. 2000. Meningitis caused by Pseudallescheria boydii treated with voriconazole. Clin. Infect. Dis. 30:981-982. [DOI] [PubMed] [Google Scholar]
- 41.Purkins, L., N. Wood, P. Ghahramani, K. Greenhalgh, M. J. Allen, and D. Kleinermans. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 43.SAS Institute, Inc. 1989. SAS/STAT users guide, version 8.2 ed. SAS Institute, Inc., Cary, NC.
- 44.Schwartz, S., M. Ruhnke, P. Ribaud, L. Corey, T. Driscoll, O. A. Cornely, U. Schuler, I. Lutsar, P. Troke, and E. Thiel. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641-2645. [DOI] [PubMed] [Google Scholar]
- 45.Steinbach, W. J., and J. R. Perfect. 2003. Scedosporium species infections and treatments. J. Chemother. 15:16-27. [DOI] [PubMed] [Google Scholar]
- 46.Steinbach, W. J., W. A. Schell, J. L. Miller, and J. R. Perfect. 2003. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 41:3981-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symoens, F., C. Knoop, M. Schrooyen, O. Denis, M. Estenne, N. Nolard, and F. Jacobs. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J. Heart Lung Transplant. 25:603-607. [DOI] [PubMed] [Google Scholar]
- 48.Torre-Cisneros, J., A. Gonzalez-Ruiz, M. R. Hodges, and I. Lutsar. 2000. Voriconazole (VORI) for the treatment of S. apiospermum and S. prolificans infection, abstr. 305. 38th Annu. Meet. Infect. Dis. Soc. Am., New Orleans, LA.
- 49.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 50.Whyte, M., H. Irving, P. O'Regan, M. Nissen, D. Siebert, and R. Labrom. 2005. Disseminated Scedosporium prolificans infection and survival of a child with acute lymphoblastic leukemia. Pediatr. Infect. Dis. J. 24:375-377. [DOI] [PubMed] [Google Scholar]