Abstract
Rapid emergence of resistance to monotherapy with virus-specific inhibitors necessitates combination therapy. ACH-806 is a hepatitis C virus NS4A inhibitor with a novel mechanism of action and resistance pathway. This compound was synergistic with NS3 protease inhibitors and NS5B nucleoside and nonnucleoside polymerase inhibitors.
Significant progress has been made in the discovery and testing of novel inhibitors of hepatitis C virus (HCV) replication (4). The majority of the compounds evaluated in vitro and in early clinical trials have belonged to one of three classes of HCV inhibitors: NS3 protease inhibitors (PI) (11, 17, 29), NS5B nucleoside polymerase inhibitors (21, 22, 24), and NS5B nonnucleoside (NNI) polymerase inhibitors (2, 7, 8, 12). Importantly, resistance to each of these compound classes has been described with some resistance mutations conveying cross-resistance to several inhibitors within a given class (e.g., A156T in HCV protease) (15, 18, 20, 28). Analogous to the experience with human immunodeficiency virus type 1 therapy, combinations of several classes of viral inhibitors with unique mechanisms of action and resistance pathways will be integral to the success of small-molecule-based antiviral therapy for chronic HCV infection.
ACH-806 [1-(4-pentyloxy-3-trifluoromethylphenyl)-3-(pyridine-3-carbonyl)thiourea] is a novel acylthiourea compound with a 50% effective concentration (EC50) of 14 nM in the genotype 1b replicon system and 30 nM in a genotype 1a replicon system (13). A phase 1b proof-of-concept study showed significant antiviral activity at the lowest dose tested (23). ACH-806 possesses a unique mechanism of action. It selectively binds to the NS4A protein, resulting in altered protein composition and inactivation of the replicase complex (13). Given its unique mechanism of action, we sought to evaluate ACH-806 in combination with other small-molecule inhibitors of HCV replication, as well as alpha interferon, in a genotype 1b luciferase reporter replicon system.
(A portion of the results described here was presented as an abstract at the XVI International HIV Drug Resistance Workshop, Hilton Barbados, Bridgetown, Barbados, 12 to 16 June 2007.)
Replicon constructs.
The BM4-5 replicon is a subgenomic HCV genotype 1b replicon which contains a deletion of a serine in NS5A (10). The firefly luciferase gene was inserted into the BM4-5 replicon, in a manner we and others have previously described (26, 27), to generate the BM4-5 FEO replicon. The sequence of the replicon was verified by DNA sequencing.
Cell culture and luciferase compound assays.
Cell culture and luciferase compound assays were performed as previously described in detail (9, 27). Briefly, 10,000 BM4-5 FEO cells/well were seeded into 96-well plates and incubated for 4 h. Medium was then aspirated and replaced with 100 μl of complete medium containing a single compound or combinations at the desired concentration(s). Plates with compounds were incubated for 48 h and then assayed for luciferase expression (Bright-Glo; Promega). All conditions were run in triplicate, and the number of relative light units for each condition was reported as the mean ± the standard error of the mean of the three wells.
Compounds tested.
The Achillion NS4A antagonist ACH-806 (Fig. 1) (John Pottage, Achillion Pharmaceuticals, New Haven, CT) was dissolved in dimethyl sulfoxide to a concentration of 2 mM; further serial 10-fold dilutions were made in complete medium. The additional compounds tested included two peptidomimetic HCV PI, BILN 2061 (14) and a Vertex PI which is a close structural analog of VX-950 (16) (Vicki Sato, Vertex Pharmaceuticals, Cambridge, MA); a trans-lactam GSK-PI active-site mimic (compound 4d in reference 1) (Karen Romines, GlaxoSmithKline, Research Triangle Park, NC); one nucleoside analog HCV RNA-dependent RNA polymerase inhibitor, 2′-C-methyladenosine (6) (William Lee, Gilead Sciences, Foster City, CA); one GSK-NNI benzothiadiazine RNA polymerase inhibitor (compound 4 in reference 5) (Karen Romines, GlaxoSmithKline); and human recombinant alpha interferon A/D (Sigma-Aldrich I4401).
FIG. 1.
Chemical structure of ACH-806 [1-(4-pentyloxy-3-trifluoromethylphenyl)-3-(pyridine-3-carbonyl)thiourea].
The EC50 of each compound was determined independently and used to determine the range of concentrations used for the synergy experiments. ACH-806 was tested singly and in combination with each of the compounds listed above at two twofold serial dilutions above and below the EC50. The ratio of the two compounds, based on the compound's EC50, remained fixed across the dosing range. The potential cytotoxicity of individual compounds and all combinations was assessed with a luminescent ATP-based cell viability assay (Cell Titer-Glo; Promega). All compounds were assessed for cytotoxicity at the highest concentration used both singly and in combination.
Data analysis.
Compound interactions were quantified by the approach described by Chou and Talalay (3), relying on the median effect principle and the multiple-drug effect equation. Isobolograms were generated for each combination tested and were used to determine the additivity, synergism, or antagonism of inhibitor combinations. Combination indices (CI) were determined with Calcusyn (Biosoft) for each experiment at the EC50, EC70, and EC90 of the combination. In total, six combinations were evaluated with three to eight experiment replicates per condition. By convention, a CI of <0.9 was considered synergistic, a CI of ≥0.9 or ≤1.1 was considered additive, and a CI of >1.1 was deemed antagonistic.
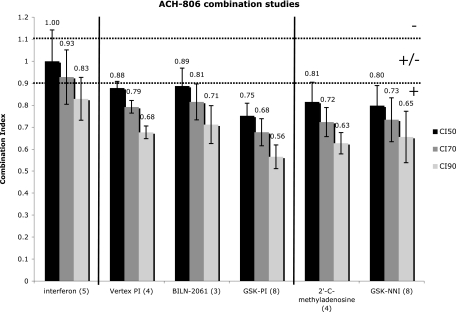
The EC50 (± the standard error of the mean) of ACH-806 in the BM4-5 FEO replicon system was 116.8 ± 5.4 nM. The EC50s of the other compounds used in this study were as follows: interferon, 4.45 ± 0.6 IU/ml; Vertex PI, 310.3 ± 48.4 nM; BILN-2061, 9.33 ± 0.7 nM; GSK-PI, 301 ± 23.9 nM; 2′-C-methyladenosine, 446.8 ± 46.2 nM; GSK-NNI, 3.5 ± 0.4 μM. ACH-806 was additive with alpha interferon at the CI50 and CI70; at the CI90, the CI was 0.83 with the 95% confidence interval crossing 0.9 (additivity). Combinations of ACH-806 and either NS3 PI or NS5B polymerase inhibitors (nucleoside and NNI) showed consistent synergy (Fig. 2). No individual compounds or compound combinations showed cytotoxicity at the highest concentrations used in the activity and synergy studies (data not shown).
FIG. 2.
CI of ACH-806 in combination with various anti-HCV compounds. Numerical values above the bars are mean CI. Error bars represent the standard error of the mean of the CI calculated from the experimental replicates (in parentheses). The dotted lines at 0.9 and 1.1 represent the bounds of an additive interaction. +, synergy; ±, additivity; −, antagonism.
We have shown that an HCV NS4A antagonist, ACH-806, is synergistic with other small-molecule inhibitors of HCV replication in an HCV genotype 1b replicon system. In vitro, ACH-806 binds directly to NS4A and inhibits HCV replicon replication by altering the composition of the replication complex, resulting in nonfunctional complexes (13). ACH-806-resistant mutants contain mutations in the portion of NS3 which interacts with NS4A; importantly, no cross-resistance has been shown in vitro between ACH-806 and NS3 PI such as VX-950.
The various inhibitors tested in combination with ACH-806 are representative of the major classes of HCV therapeutics currently being developed. Given its error-prone RNA polymerase and high rate of viral turnover (19) and the early appearance of resistant mutants seen both in vitro (15, 18, 20) and in vivo (25) during monotherapy, we believe that combination therapy with several inhibitors will be needed to avoid selection of preexisting viral mutants and obtain durable virus inhibition. To that end, inhibitors which possess complementary actions in vitro (i.e., show synergy) and have divergent resistance pathways should be prioritized for study in clinical trials of combination therapy for HCV infection. NS4A antagonists, such as ACH-806, are attractive compounds to potentially combine with both protease and polymerase inhibitors.
Acknowledgments
We thank John Pottage and Achillion Pharmaceuticals, Inc., for providing the NS4A antagonist used in this study. Additionally, we thank Milind Deshpande (Achillion Pharmaceuticals) for reviewing the manuscript.
D.L.W. and the research presented here were supported in part by NIAID grant AI069989.
This publication's contents are solely our responsibility and do not necessarily represent the official views of the NIAID.
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Andrews, D. M., M. C. Barnes, M. D. Dowle, S. L. Hind, M. R. Johnson, P. S. Jones, G. Mills, A. Patikis, T. J. Pateman, T. J. Redfern, J. E. Robinson, M. J. Slater, and N. Trivedi. 2003. Pyrrolidine-5,5-trans-lactams. 5. Pharmacokinetic optimization of inhibitors of hepatitis C virus NS3/4A protease. Org. Lett. 5:4631-4634. [DOI] [PubMed] [Google Scholar]
- 2.Chan, L., T. J. Reddy, M. Proulx, S. K. Das, O. Pereira, W. Wang, A. Siddiqui, C. G. Yannopoulos, C. Poisson, N. Turcotte, A. Drouin, M. H. Alaoui-Ismaili, R. Bethell, M. Hamel, L. L'Heureux, D. Bilimoria, and N. Nguyen-Ba. 2003. Identification of N,N-disubstituted phenylalanines as a novel class of inhibitors of hepatitis C NS5B polymerase. J. Med. Chem. 46:1283-1285. [DOI] [PubMed] [Google Scholar]
- 3.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 4.De Francesco, R., and G. Migliaccio. 2005. Challenges and successes in developing new therapies for hepatitis C. Nature 436:953-960. [DOI] [PubMed] [Google Scholar]
- 5.Dhanak, D., K. J. Duffy, V. K. Johnston, J. Lin-Goerke, M. Darcy, A. N. Shaw, B. Gu, C. Silverman, A. T. Gates, M. R. Nonnemacher, D. L. Earnshaw, D. J. Casper, A. Kaura, A. Baker, C. Greenwood, L. L. Gutshall, D. Maley, A. DelVecchio, R. Macarron, G. A. Hofmann, Z. Alnoah, H. Y. Cheng, G. Chan, S. Khandekar, R. M. Keenan, and R. T. Sarisky. 2002. Identification and biological characterization of heterocyclic inhibitors of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:38322-38327. [DOI] [PubMed] [Google Scholar]
- 6.Eldrup, A. B., M. Prhavc, J. Brooks, B. Bhat, T. P. Prakash, Q. Song, S. Bera, N. Bhat, P. Dande, P. D. Cook, C. F. Bennett, S. S. Carroll, R. G. Ball, M. Bosserman, C. Burlein, L. F. Colwell, J. F. Fay, O. A. Flores, K. Getty, R. L. LaFemina, J. Leone, M. MacCoss, D. R. McMasters, J. E. Tomassini, L. D. Von, B. Wolanski, and D. B. Olsen. 2004. Structure-activity relationship of heterobase-modified 2′-C-methyl ribonucleosides as inhibitors of hepatitis C virus RNA replication. J. Med. Chem. 47:5284-5297. [DOI] [PubMed] [Google Scholar]
- 7.Gopalsamy, A., A. Aplasca, G. Ciszewski, K. Park, J. W. Ellingboe, M. Orlowski, B. Feld, and A. Y. Howe. 2006. Design and synthesis of 3,4-dihydro-1H-[1]-benzothieno[2,3-c]pyran and 3,4-dihydro-1H-pyrano[3,4-b]benzofuran derivatives as non-nucleoside inhibitors of HCV NS5B RNA dependent RNA polymerase. Bioorg. Med. Chem. Lett. 16:457-460. [DOI] [PubMed] [Google Scholar]
- 8.Gopalsamy, A., K. Lim, G. Ciszewski, K. Park, J. W. Ellingboe, J. Bloom, S. Insaf, J. Upeslacis, T. S. Mansour, G. Krishnamurthy, M. Damarla, Y. Pyatski, D. Ho, A. Y. Howe, M. Orlowski, B. Feld, and J. O'Connell. 2004. Discovery of pyrano[3,4-b]indoles as potent and selective HCV NS5B polymerase inhibitors. J. Med. Chem. 47:6603-6608. [DOI] [PubMed] [Google Scholar]
- 9.Grünberger, C., D. L. Wyles, K. A. Kaihara, and R. T. Schooley. 2008. 3-Drug synergistic interactions of small molecular inhibitors of hepatitis C virus replication. J. Infect. Dis. 197:42-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo, J. T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinrichsen, H., Y. Benhamou, H. Wedemeyer, M. Reiser, R. E. Sentjens, J. L. Calleja, X. Forns, A. Erhardt, J. Cronlein, R. L. Chaves, C. L. Yong, G. Nehmiz, and G. G. Steinmann. 2004. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology 127:1347-1355. [DOI] [PubMed] [Google Scholar]
- 12.Howe, A. Y., J. Bloom, C. J. Baldick, C. A. Benetatos, H. Cheng, J. S. Christensen, S. K. Chunduru, G. A. Coburn, B. Feld, A. Gopalsamy, W. P. Gorczyca, S. Herrmann, S. Johann, X. Jiang, M. L. Kimberland, G. Krisnamurthy, M. Olson, M. Orlowski, S. Swanberg, I. Thompson, M. Thorn, A. Del Vecchio, D. C. Young, M. van Zeijl, J. W. Ellingboe, J. Upeslacis, M. Collett, T. S. Mansour, and J. F. O'Connell. 2004. Novel nonnucleoside inhibitor of hepatitis C virus RNA-dependent RNA polymerase. Antimicrob. Agents Chemother. 48:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, M., Y. Sun, W. Yang, X. Hou, J. Fabrycki, X. Nie, A. Sanchez, Y. Zhao, A. Phadke, and M. Deshpande. 2007. ACH-806: a potent inhibitor of HCV replication with a novel mechanism of action. J. Hepatol. 46:S221. [Google Scholar]
- 14.Lamarre, D., P. C. Anderson, M. Bailey, P. Beaulieu, G. Bolger, P. Bonneau, M. Bos, D. R. Cameron, M. Cartier, M. G. Cordingley, A. M. Faucher, N. Goudreau, S. H. Kawai, G. Kukolj, L. Lagace, S. R. LaPlante, H. Narjes, M. A. Poupart, J. Rancourt, R. E. Sentjens, R. St George, B. Simoneau, G. Steinmann, D. Thibeault, Y. S. Tsantrizos, S. M. Weldon, C. L. Yong, and M. Llinas-Brunet. 2003. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426:186-189. [DOI] [PubMed] [Google Scholar]
- 15.Lin, C., C. A. Gates, B. G. Rao, D. L. Brennan, J. R. Fulghum, Y. P. Luong, J. D. Frantz, K. Lin, S. Ma, Y. Y. Wei, R. B. Perni, and A. D. Kwong. 2005. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J. Biol. Chem. 280:36784-36791. [DOI] [PubMed] [Google Scholar]
- 16.Lin, K., A. D. Kwong, and C. Lin. 2004. Combination of a hepatitis C virus NS3-NS4A protease inhibitor and alpha interferon synergistically inhibits viral RNA replication and facilitates viral RNA clearance in replicon cells. Antimicrob. Agents Chemother. 48:4784-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin, K., R. B. Perni, A. D. Kwong, and C. Lin. 2006. VX-950, a novel hepatitis C virus (HCV) NS3-4A protease inhibitor, exhibits potent antiviral activities in HCV replicon cells. Antimicrob. Agents Chemother. 50:1813-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migliaccio, G., J. E. Tomassini, S. S. Carroll, L. Tomei, S. Altamura, B. Bhat, L. Bartholomew, M. R. Bosserman, A. Ceccacci, L. F. Colwell, R. Cortese, R. De Francesco, A. B. Eldrup, K. L. Getty, X. S. Hou, R. L. LaFemina, S. W. Ludmerer, M. MacCoss, D. R. McMasters, M. W. Stahlhut, D. B. Olsen, D. J. Hazuda, and O. A. Flores. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164-49170. [DOI] [PubMed] [Google Scholar]
- 19.Neumann, U., G. Puhl, M. Bahra, T. Berg, J. M. Langrehr, R. Neuhaus, and P. Neuhaus. 2006. Treatment of patients with recurrent hepatitis C after liver transplantation with peginterferon alfa-2B plus ribavirin. Transplantation 82:43-47. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien, C., E. Godofsky, M. Rodriguez-Torres, N. Afdhal, S. Pappas, P. Pockros, E. Lawitz, N. Bzowej, V. Rustgi, M. Sulkowski, K. Sherman, I. Jacobson, G. Chao, S. Knox, K. Pietropaolo, and N. Brown. 2005. Randomized trial of valopicitabine, alone or with peg-interferon, versus retreatment with peg-interferon plus ribavirin in hepatitis C patients with previous non-response to peg-interferon plus ribavirin: first interim results. Hepatology 42:234A. [Google Scholar]
- 22.Olsen, D. B., A. B. Eldrup, L. Bartholomew, B. Bhat, M. R. Bosserman, A. Ceccacci, L. F. Colwell, J. F. Fay, O. A. Flores, K. L. Getty, J. A. Grobler, R. L. LaFemina, E. J. Markel, G. Migliaccio, M. Prhavc, M. W. Stahlhut, J. E. Tomassini, M. MacCoss, D. J. Hazuda, and S. S. Carroll. 2004. A 7-deaza-adenosine analog is a potent and selective inhibitor of hepatitis C virus replication with excellent pharmacokinetic properties. Antimicrob. Agents Chemother. 48:3944-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pottage, J., E. Lawitz, D. Mazur, D. L. Wyles, H. Vargas, R. Ghalib, R. Gugliotti, M. Donohue, and H. Robison. 2007. Short-term antiviral activity and safety of ACH-806 (GS-9132), an NS4A antagonist, in HCV genotype 1 infected individuals. J. Hepatol. 46:S294-S295. [Google Scholar]
- 24.Roberts, S., G. Cooksley, G. Dore, R. Robson, D. Shaw, H. Berns, M. Brandl, S. Fettner, G. Hill, E. Ipe, K. Klumpp, M. Mannino, E. O'Mara, I. Najera, Y. Tu, and C. Washingtion. 2006. Results of a phase 1B, multiple dose study of R1626, a novel nucleoside analog targeting HCV polymerase in chronic HCV genotype 1 patients. Hepatology 44:692A. [Google Scholar]
- 25.Sarrazin, C., T. Kieffer, D. Bartels, B. Hanzelka, U. Muh, M. Welker, D. Wincheringer, C. Lin, T. Grossman, S. Purdy, C. Weegink, H. Reesink, S. Zeuzem, and A. D. Kwong. 2005. Characterization of viral variants in the HCV NS3 protease domain of genotype 1 patients that are selected during 14 days of dosing with VX-950. Annual Meeting of the American Association for the Study of Liver Disease. Hepatology 42:751A. [Google Scholar]
- 26.Tanabe, Y., N. Sakamoto, N. Enomoto, M. Kurosaki, E. Ueda, S. Maekawa, T. Yamashiro, M. Nakagawa, C. H. Chen, N. Kanazawa, S. Kakinuma, and M. Watanabe. 2004. Synergistic inhibition of intracellular hepatitis C virus replication by combination of ribavirin and interferon-α. J. Infect. Dis. 189:1129-1139. [DOI] [PubMed] [Google Scholar]
- 27.Wyles, D. L., K. A. Kaihara, F. Vaida, and R. T. Schooley. 2007. Synergy of small molecular inhibitors of hepatitis C virus replication directed at multiple viral targets. J. Virol. 81:3005-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yi, M., X. Tong, A. Skelton, R. Chase, T. Chen, A. Prongay, S. L. Bogen, A. K. Saksena, F. G. Njoroge, R. L. Veselenak, R. B. Pyles, N. Bourne, B. A. Malcolm, and S. M. Lemon. 2006. Mutations conferring resistance to SCH6, a novel hepatitis C virus NS3/4A protease inhibitor. Reduced RNA replication fitness and partial rescue by second-site mutations. J. Biol. Chem. 281:8205-8215. [DOI] [PubMed] [Google Scholar]
- 29.Zeuzem, S., C. Sarrazin, R. Rouzier, A. Tarral, N. Brion, N. Forestier, S. Gupta, D. Deckman, K. Fellows, M. Hussain, D. Cutler, and J. Zhang. 2005. Anti-viral activity of SCH 503034, a HCV protease inhibitor, administered as monotherapy in hepatitis C genotype-1 patients refractory to pegylated interferon. Hepatology 42:233A-234A. [Google Scholar]