Abstract
The effects of quinoline antimalarials on endocytosis by Plasmodium falciparum was investigated by measuring parasite hemoglobin levels, peroxidase uptake, and transport vesicle content. Mefloquine, quinine, and halofantrine inhibited endocytosis, and chloroquine inhibited vesicle trafficking, while amodiaquine shared both effects. Protease inhibitors moderated hemoglobin perturbations, suggesting a common role for heme binding.
Despite problems associated with drug resistance and side effects, quinolines remain widely used for the treatment of severe malaria and malaria prophylaxis, in artemisinin combination therapy regimens, and for the development of novel quinoline drug candidates (1, 2, 11, 16, 19). The mechanism of action of the 4-aminoquinoline chloroquine (CQ) has been extensively investigated. Intraerythrocytic malarial parasites ingest the erythrocytic cytosol by endocytosis and deliver it to the parasite food vacuole via hemoglobin (Hb) transport vesicles. Hb digestion in the vacuole releases ferriprotoporphyrin IX (FP), which is detoxified by incorporation into inert hemozoin crystals (6). CQ concentrates in the food vacuole and is thought to dimerize with FP to cause an inhibition of hemozoin formation and the lethal accumulation of toxic FP or FP-CQ complexes (5, 9, 14, 21, 22). Other quinolines have also been found to inhibit hemozoin formation in vitro at concentrations that correlate with their parasite-inhibitory concentrations (4, 5, 7-9, 13, 14), which supplies a strong argument that they share the mechanism of action of CQ and produce toxic levels of FP by disrupting hemozoin formation. This conclusion is confounded, however, by their disparate effects on the parasite Hb endocytic pathway (10). We have previously found that CQ inhibits Hb transport vesicle trafficking, with a resultant accumulation of Hb and vesicles, while mefloquine (MQ) inhibits Hb endocytosis (15). In this study, we investigated the extent to which these differential effects extend to other therapeutically relevant quinolines, i.e., quinine (Q), halofantrine (H), and amodiaquine (AQ).
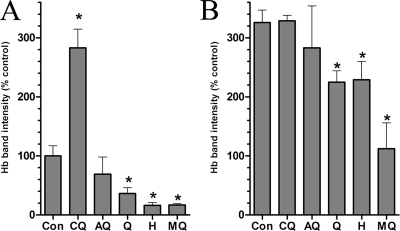
Early trophozoite-stage cultures of Plasmodium falciparum (strain 3D7) were incubated for 8 h with the quinolines at concentrations 5 times their 50% inhibitory concentration values, the parasites were released from the infected erythrocytes by saponin lysis, and the parasite Hb content was determined by Western blotting (15, 20) (Fig. 1A). Consistent with the notion that MQ inhibits fluid phase endocytosis in malaria parasites (10, 15), the Hb content in MQ-treated parasites was reduced by 83%. The structurally related quinoline-methanols Q and H also reduced Hb content, by 64% and 84%, respectively. By contrast, the Hb content in CQ-treated parasites increased to 283% compared to that in the untreated controls. Being a 4-aminoquinoline related to CQ, AQ was expected to act similarly (10). However, the Hb levels in AQ-treated parasites were comparable to that in the controls or slightly reduced (statistically insignificant; P = 0.37). In parallel cultures, parasites were incubated with the quinolines in combination with 40 μM of the protease inhibitors (PIs) ALLN and E64 to inhibit Hb digestion (12) and were subjected to Hb Western blotting (20) (Fig. 1B). Predictably, the addition of the PIs increased the Hb levels in the control parasites more than threefold. CQ or AQ did not significantly affect Hb levels in parasites in the presence of PIs. Although a reduction in Hb levels was still found with Q and H, it was less profound than that obtained in the absence of PIs (31% and 30% versus 64% and 84% for Q and H, respectively). Hb uptake inhibition by MQ was also alleviated by the PIs (66% versus 83% inhibition). This moderation of quinoline effects on Hb levels in the presence of the PIs agrees with published reports that Hb antagonize quinoline action (18). It supports the notion that quinolines share FP binding as a mode of action and suggests that their differential effects on the Hb endocytic pathway may be manifestations of differences in the physiochemical properties of the individual quinoline-FP complexes. The residual endocytosis inhibition by quinoline-methanols in the presence of PIs may be due to their additional propensity to bind phospholipid membranes (3, 13, 23), which could disrupt the plasma membrane properties required for endocytic vesicle formation. The Hb dependence of quinoline action may potentially be explored by using erythrocyte-free parasites (15). However, reevaluation of the procedure suggests that the removal of parasites from their intraerythrocytic environment usually terminates endocytic activity, precluding the reproducible application of this technique.
FIG. 1.
Hemoglobin levels in drug-treated parasites. (A) Parasite cultures were untreated (control [Con]) or incubated with amodiaquine (AQ), chloroquine (CQ), halofantrine (H), mefloquine (MQ), or quinine (Q) at concentrations 5 times their 50% inhibitory concentrations for 8 h. The concentrations used were 137 nM CQ, 156 nM MQ, 665 nM Q, 102 nM AQ, and 27 nM H. (B) In parallel cultures, the quinoline drugs were added in combination with the protease inhibitors ALLN and E64. Following treatment, parasite hemoglobin levels were detected by Western blotting. The intensities of the hemoglobin bands on the blots were determined using the histogram function of Adobe Photoshop (version 7.0) and normalized to control intensities. Error bars indicate standard deviations. An asterisk (*) indicates a significant change from the results for the controls (P < 0.05; 95% confidence interval [CI]).
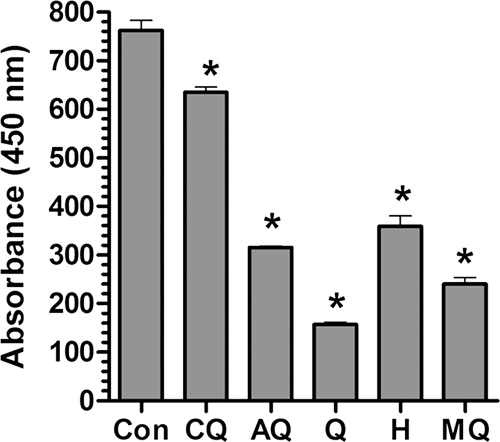
To further investigate the effects of the quinolines on endocytosis, an assay was performed using an exogenous indigestible endocytic tracer, horseradish peroxidase (HRP) (17). Erythrocytes were preloaded with HRP by hypotonic lysis and infected by incubation with enriched schizont-infected erythrocytes. Parasites in the HRP-loaded erythrocytes were exposed to quinolines for 10 h, released with saponin, and lysed with Triton X-100, and their HRP content was measured by a colorimetric peroxidase assay (20). Consistent with the Hb Western blotting results, MQ, Q, and H inhibited HRP uptake by the parasites (Fig. 2). Surprisingly, AQ produced a comparable inhibition of HRP uptake, while CQ had a weak inhibitory effect.
FIG. 2.
Effect of quinoline drugs on horseradish peroxidase endocytosis by malaria parasites. Parasites were allowed to invade erythrocytes preloaded with HRP. They were then left untreated (control [Con]) or incubated with amodiaquine (AQ), chloroquine (CQ), halofantrine (H), mefloquine (MQ), or quinine (Q) for 10 h. Following incubation, parasites were released from erythrocytes by saponin treatment, washed repeatedly, and lysed with Triton X-100, and the HRP levels were measured by enzymatic activity following the addition of O-phenylenediamine (absorbance of the product at 450 nm). Error bars indicate standard errors. An asterisk (*) indicates a significant difference from the results for the controls (P < 0.05; 95% CI).
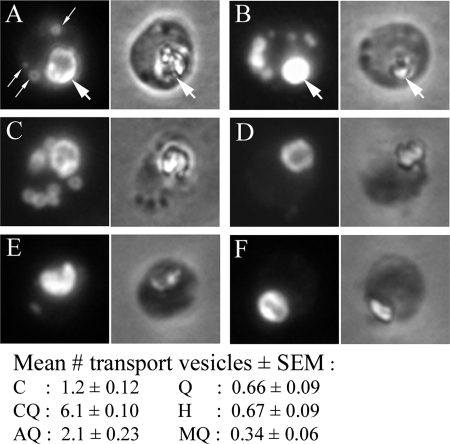
The apparent incongruity between the inhibition of HRP endocytosis by AQ and the lack of a significant effect on Hb levels by this compound was clarified by a subsequent Hb immunofluorescence microscopy assay. Following quinoline treatment, parasites were released with saponin and fixed on polylysine-coated coverslips, using paraformaldehyde and glutaraldehyde. After being permeated in Triton X-100, the parasites were incubated with rabbit anti-Hb antiserum followed by tetramethyl rhodamine isocyanate-conjugated goat anti-rabbit antibodies and viewed by fluorescence microscopy (15, 20). The majority of the Hb colocalized with the hemozoin crystal in the parasite food vacuole (Fig. 3A and B). Additional fluorescent puncta represent Hb transport vesicles in transit from the plasma membrane to the food vacuole (Fig. 3A). Enumeration of the vesicles showed that Q, H, and MQ caused a reduction in endocytic vesicle content per parasite, supporting the conclusion that they inhibit endocytosis and, consequently, endocytic vesicle formation (Fig. 3D to F). CQ treatment markedly increased the vesicle content, consistent with a disruption of normal vesicle trafficking (Fig. 3B). Unexpectedly, AQ also caused an increase in vesicle content, often strikingly, in individual parasites (Fig. 3C). This finding suggests that AQ produces a combination of the cellular effects of CQ on the one hand and of Q, H, and MQ on the other: when Hb trafficking and endocytosis are inhibited simultaneously, the net content of Hb in AQ-treated parasites remains largely unaffected. Further investigation of the molecular mechanisms underlying quinoline effects is required to determine if the endocytosis perturbations result from upstream primary effects or are directly related to the parasiticidal mechanisms of the drugs.
FIG. 3.
Effect of quinoline drugs on hemoglobin transport vesicle numbers in malaria parasites, as determined by immunofluorescence microscopy. Parasite cultures were left untreated (A) or incubated with chloroquine (B), amodiaquine (C), quinine (D), halofantrine (E), or mefloquine (F) for 8 h. Following saponin lysis, parasites were immobilized, fixed onto glass coverslips, incubated with anti-hemoglobin antiserum followed by tetramethyl rhodamine isocyanate-labeled secondary antibodies, and examined by fluorescence microscopy. The panels on the left are fluorescence micrographs, and the panels on the right are the corresponding phase-contrast light micrographs. The large arrows in panels A and B indicate the position of the parasite food vacuole, identifiable by the presence of the prominent hemozoin crystal in the phase-contrast images. The smaller arrows in panel A denote individual hemoglobin transport vesicles. The mean number of hemoglobin transport vesicles per parasite (± the standard error of the means) in the control and treated samples, determined by counting 100 randomly selected parasites per sample, is tabulated below the micrographs. All values for the treated samples were significantly different from that for the control (P < 0.05; 95% CI).
Acknowledgments
This work was supported by a Wellcome Trust Senior International Research Fellowship to H.C.H. and a Medical Research Council postgraduate scholarship to L.R.
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Bray, P. G., S. A. Ward, and P. M. O'Neill. 2005. Quinolines and artemisinin: chemistry, biology and history. Curr. Top. Microbiol. Immunol. 295:3-38. [DOI] [PubMed] [Google Scholar]
- 2.Chen, L. H., M. E. Wilson, and P. Schlagenhauf. 2007. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA 297:2251-2263. [DOI] [PubMed] [Google Scholar]
- 3.Chevli, R., and C. D. Fitch. 1982. The antimalarial drug mefloquine binds to membrane phospholipids. Antimicrob. Agents Chemother. 21:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou, A. C., and C. D. Fitch. 1993. Control of heme polymerase by chloroquine and other quinoline derivatives. Biochem. Biophys. Res. Commun. 195:422-427. [DOI] [PubMed] [Google Scholar]
- 5.Dorn, A., S. R. Vippagunta, H. Matile, C. Jaquet, J. L. Vennerstrom, and R. G. Ridley. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 55:727-736. [DOI] [PubMed] [Google Scholar]
- 6.Egan, T. J., J. M. Combrinck, J. Egan, G. R. Hearne, H. M. Marques, S. Ntenteni, B. T. Sewell, P. J. Smith, D. Taylor, D. A. Van Schalkwyk, and J. C. Walden. 2002. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 365:343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egan, T. J., and H. M. T. Marques. 1999. The role of haem in the activity of chloroquine and related antimalarial drugs. Coord. Chem. Rev. 192:493-517. [Google Scholar]
- 8.Egan, T. J., and K. K. Ncokazi. 2005. Quinoline antimalarials decrease the rate of β-hematin formation. J. Inorg. Biochem. 99:1532-1539. [DOI] [PubMed] [Google Scholar]
- 9.Egan, T. J., D. C. Ross, and P. A. Adams. 1994. Quinoline antimalarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 352:54-57. [DOI] [PubMed] [Google Scholar]
- 10.Famin, O., and H. Ginsburg. 2002. Differential effects of 4-aminoquinoline-containing antimalarial drugs on hemoglobin digestion in Plasmodium falciparum-infected erythrocytes. Biochem. Pharmacol. 63:393-398. [DOI] [PubMed] [Google Scholar]
- 11.Foley, M., and L. Tilley. 1998. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 79:55-87. [DOI] [PubMed] [Google Scholar]
- 12.Francis, S. E., D. J. Sullivan, and D. E. Goldberg. 1997. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97-123. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg, H., and R. A. Demel. 1984. Interactions of hemin, antimalarial drugs and hemin-antimalarial complexes with phospholipid monolayers. Chem. Phys. Lipids 35:331-347. [DOI] [PubMed] [Google Scholar]
- 14.Hawley, S. R., P. G. Bray, M. Mungthin, J. D. Atkinson, P. M. O'Neill, and S. Ward. 1998. Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 42:682-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe, H. C., D. A. van Schalkwyk, U. I. M. Wiehart, S. A. Meredith, J. Egan, and B. W. Weber. 2004. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob. Agents Chemother. 48:2370-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra, S. K., S. Mohanty, A. Mohanty, and B. S. Das. 2006. Management of severe and complicated malaria. J. Postgrad. Med. 52:281-287. [PubMed] [Google Scholar]
- 17.Montgomery, R. R., P. Webster, and I. Mellman. 1991. Accumulation of indigestible substances reduces fusion competence of macrophage lysosomes. J. Immunol. 147:3087-3095. [PubMed] [Google Scholar]
- 18.Mungthin, M., P. G. Bray, R. G. Ridley, and S. A. Ward. 1998. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob. Agents Chemother. 42:2973-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sirima, S. B., and A. Gansané. 2007. Artesunate-amodiaquine for the treatment of uncomplicated malaria. Expert Opin. Investig. Drugs 16:1079-1085. [DOI] [PubMed] [Google Scholar]
- 20.Smythe, W. A., K. A. Joiner, and H. C. Hoppe. 2008. Actin is required for endocytic trafficking in the malaria parasite Plasmodium falciparum. Cell. Microbiol. 10:452-464. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, D. J. 2002. Theories on malarial pigment formation and quinoline action. Int. J. Parasitol. 32:1645-1653. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, J., M. Krugliak, and H. Ginsburg. 1999. The fate of ferriprotorphyrin IX in malaria infected erythrocytes in conjunction with the mode of action of antimalarial drugs. Mol. Biochem. Parasitol. 99:129-141. [DOI] [PubMed] [Google Scholar]
- 23.Zidovetzki, R., I. W. Sherman, A. Atiya, and H. De Boeck. 1989. A nuclear magnetic resonance study of the interactions of the antimalarials chloroquine, quinacrine, quinine and mefloquine with dipalmitoylphosphatidylcholine bilayers. Mol. Biochem. Parasitol. 35:199-207. [DOI] [PubMed] [Google Scholar]