Abstract
Daptomycin pharmacokinetics were evaluated for burn patients. Burn patients had decreases in the maximum concentration of the drug in serum (44%) and the area under the concentration-time curve (47%) and increases in the volume of distribution (64%) and total clearance (77%) compared to healthy volunteers. In burn patients, daptomycin at 10 to 12 mg/kg of body weight/day would be required to achieve drug exposures similar to those for healthy volunteers receiving 6 mg/kg.
Daptomycin is a novel lipopeptide antibiotic with potent in vitro activity against gram-positive bacteria, including antimicrobial-resistant strains (10). Daptomycin is approved for the treatment of complicated skin and skin structure infections and bloodstream infections, including right-sided endocarditis caused by methicillin-susceptible and -resistant Staphylococcus aureus strains. Patients with thermal burn injury are at risk of developing burn wound infections and bacteremia caused by gram-positive organisms, including drug-resistant organisms (11). Evaluation of the effect of burn injury on the deposition of daptomycin is essential to appropriate dosing in this special population. The purpose of this study was to describe daptomycin pharmacokinetics for patients with thermal burn injury and compare those parameters with those previously reported for healthy volunteers.
(These data were presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 2007.)
This was an open-label, single-center, pharmacokinetic study in patients with ≥18% body surface area burns who were at least 7 days after burn injury and had completed their initial fluid resuscitation. The study was approved by the Committee for the Protection of Human Subjects, the Institutional Review Board for the University of Texas Health Science Center at Houston. Patients were excluded if their actual body weight was >30% of their ideal body weight for height or if they had serum glutamic pyruvic transaminase or serum glutamic oxaloacetic transaminase values of ≥4 times the upper normal limit of normal, creatine phosphokinase values of ≥5 times the upper limit of normal, or estimated creatinine clearance of ≤30 ml/min or were receiving continuous renal replacement therapy. Patients received a single 30-min intravenous infusion of 6 mg of daptomycin/kg of body weight based on the patient's actual body weight via infusion pump. Plasma samples were obtained at 0.5, 1, 2, 4, 8, 12, and 24 h after the start of the infusion. Serum samples were obtained at 1, 2, and 8 h to determine protein binding. All urine was collected and refrigerated from drug administration through 48 h after dosing. All samples were stored at −70°C until analysis.
Daptomycin plasma and urine concentrations were analyzed using reverse-phase high-performance liquid chromatography with UV detection at 220 nm over a range of concentrations from 2 to 100 μg/ml (7). The intrarun percent coefficients of variation for the low-quality control of 4 μg/ml and the high-quality control of 80 μg/ml were 6.20% and 4.69%, respectively. The interrun percent coefficients of variation were 5.74% and 2.56%, respectively. The correlation coefficient for each calibration curve was greater than 0.999. Daptomycin protein binding was evaluated as previously described (8).
Pharmacokinetic parameters were calculated utilizing PK Solutions 2.0 (Summit Research Services, Montrose, CO). The maximum plasma concentration (Cmax) was determined directly from the serum concentration-time plot without interpolation. The elimination rate constant (ke) was determined from the terminal portion of the concentration-time curve, and the half-life (t1/2) was calculated as follows: t1/2 = 0.693/ke. The apparent volume of distribution (V) was calculated as dose/(area under the concentration-time curve from 0 h to infinity [AUC0-∞]·ke) and normalized to actual body weight in kilograms. The AUC was determined by the linear trapezoidal rule and was extrapolated to infinity, where AUC0-∞ = AUC0-t + CT/ke. Total clearance (CLT) was calculated as follows: CLT = dose/AUC0-∞. Urinary recovery of daptomycin was expressed as the percentage of the total amount recovered in the urine/dose administered. Renal clearance (CLR) was calculated as follows: CLR = amount of daptomycin recovered in urine in 48 h/AUC0-48.
Demographics of the nine patients are in Table 1 and the pharmacokinetic parameters are in Table 2. The pharmacokinetics of daptomycin in healthy volunteers were previously reported (1). Patients with burn injury had a 44% reduction in Cmax (53.5 ± 14.5 μg/ml versus 95.7 ± 30 μg/ml; P < 0.01), a 64% increase in V (0.18 ± 0.05 liter/kg versus 0.11 ± 0.01 liter/kg; P < 0.01), a 77% increase in CLT (17.5 ± 7.0 ml/h/kg versus 9.9 ± 1.2 ml/h/kg; P = 0.02), and a 47% decrease in AUC0-∞ (388 ± 137 μg·h/ml versus 729 ± 234 μg·h/ml; P < 0.01) compared to healthy volunteers. Renal elimination ranged from 20% to 73% and protein binding averaged 86.5%.
TABLE 1.
Patient demographics
| Patient | Sex | Age (yr) | Weight (kg) | Height (cm) | % Second-degree/third-degree burns | % TBSABa | Albumin (g/dl) | Days after burn |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 50 | 54.5 | 168 | 21/0 | 21 | 2.1 | 27 |
| 2 | Male | 31 | 120 | 188 | 0/50 | 50 | 1.6 | 18 |
| 3 | Male | 57 | 92.72 | 170 | 8/10 | 18 | 1.9 | 7 |
| 4 | Male | 38 | 77.2 | 183 | 15/13 | 28 | 1.7 | 8 |
| 5 | Female | 32 | 97.7 | 178 | 20/16 | 36 | 2.4 | 9 |
| 6 | Male | 24 | 61.3 | 170 | 25/10 | 35 | 1.6 | 10 |
| 7 | Male | 34 | 70 | 175 | 28/14 | 42 | 1.1 | 13 |
| 8 | Male | 26 | 73 | 178 | 20/4 | 24 | 2.2 | 7 |
| 9 | Female | 64 | 79 | 155 | 40/10 | 50 | 1.7 | 7 |
| Mean (SD) | 39 (14) | 81 (20) | 174 (10) | 20/14 | 34 (12) | 1.8 (0.4) | 12 (7) |
TBASB, total body surface area burned.
TABLE 2.
Daptomycin pharmacokinetic parameters for patients with thermal burn injury
| Patient | CLCRa (ml/min) | Cmax (μg/ml) | V (liter/kg) | t1/2 (h) | Protein binding (%) | AUC0-∞ (μg·h/ml) | CLT (ml/h/kg) | CLR (ml/h/kg) | % Renal elimination |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | 68.1 | 0.27 | 15.4 | 84.9 | 655 | 11.9 | 5.8 | 47 |
| 2 | 140 | 71.8 | 0.18 | 13.7 | 87.2 | 502 | 9.2 | 1.7 | 52 |
| 3 | 107 | 75 | 0.10 | 5.4 | 86.9 | 462 | 12.9 | 7.6 | 20 |
| 4 | 109 | 42.4 | 0.17 | 6.7 | 87.0 | 345 | 17.4 | 9.5 | 61 |
| 5 | 208 | 54 | 0.11 | 4.4 | 86.8 | 337 | 17.8 | 11.1 | 58 |
| 6 | 123 | 49.4 | 0.17 | 7.5 | 90.0 | 392 | 15.4 | 12.0 | 65 |
| 7 | 172 | 38.6 | 0.23 | 4.8 | 81.7 | 179 | 33.5 | 21.6 | 73 |
| 8 | 165 | 40.3 | 0.20 | 5.8 | 87.6 | 304 | 20.0 | 11.2 | 67 |
| 9 | 79 | 42.3 | 0.20 | 6.6 | 86.1 | 317 | 19.0 | 13.7 | 54 |
| Mean (SD) | 132 (43) | 53.5 (14.5) | 0.18 (0.05) | 7.8 (4.0) | 86.5 (2.2) | 388 (137) | 17.5 (7.0) | 10.5 (5.5) | 55 (14) |
CLCR, creatinine clearance.
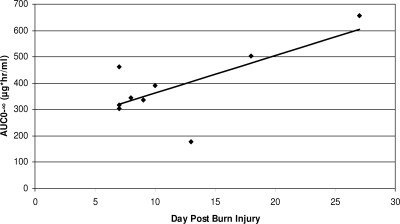
CLT was associated with creatinine clearance (r2 = 0.409, P = 0.05) and CLR (r2 = 0.09, P < 0.001). A significant association was found between AUC0-∞ and the number of days from burn injury by simple linear regression (r2 = 0.493, P = 0.03) (Fig. 1). There was not a statistically significant association between AUC and weight, age, or percent total body surface area burns by simple linear regression. There was not a significant association between protein binding and CLT and CLR (r2 = 0.414, P = 0.06, and r2 = 0.240, P = 0.18, respectively).
FIG. 1.
Relationship between days after burn injury and daptomycin AUC for patients with burn injury.
Physiological changes resulting in altered pharmacokinetics of antimicrobials are evident in patients with thermal burn injury (4, 9). We evaluated pharmacokinetics of daptomycin for burn patients and compared them to those seen for healthy volunteers, and we found that they were significantly altered. The decreased AUC and the increased V and CLT of daptomycin are consistent with other antibiotics whose pharmacokinetics have been evaluated for burn patients (2, 3, 5, 6). Although daptomycin is primarily renally eliminated, the mean percentage of daptomycin recovered in the urine was 55%, which is similar to the 54% reported for healthy subjects, suggesting increased nonrenal clearance (8). The increased V observed coupled with the association between the number of days from burn injury and AUC, with the AUCs approaching those seen for healthy volunteers later in the burn injury, supports the hypothesis that daptomycin is cleared through the burn wounds. Patients with burn injury experience protein loss across burn wounds (12). Since daptomycin is highly protein bound, it may be being eliminated in conjunction with the protein loss during this early postburn phase. However, we also observed that 86.5% of the drug was bound to serum proteins in burn patients, which is lower than the 91.7% that has been reported for healthy volunteers (8). This may have also contributed to the observed increase in CLT as a result of a higher unbound drug concentration. Regardless, the pharmacokinetics of daptomycin are linear and the AUC was reduced by 47% for burn patients with a 6-mg/kg dose; thus, an increase in the daptomycin dose to 10 to 12 mg/kg for burn patients would be needed to achieve drug exposures similar to those achieved for volunteers.
While our data are applicable only to patients between 7 and 27 days after burn injury, this is the time frame where infections caused by Staphylococcus aureus are most prevalent and the treatment with daptomycin would be most useful. We also observed a relationship between the daptomycin AUC and the number of days from burn injury, and as patients approach 30 days postburn, the pharmacokinetic parameters begin to approach those seen for healthy individuals. However, we had only two patients in the analysis who received daptomycin more than 2 weeks after their burn injury. Nonetheless, increasing the dose of daptomycin in patients with thermal burn injury to account for the altered pharmacokinetics may not be necessary after 4 weeks after burn injury, and further investigations are warranted.
In conclusion, the pharmacokinetics of daptomycin are altered in patients with thermal burn injury. Due to the linear pharmacokinetics of daptomycin, a dose of 10 to 12 mg/kg/day in burn patients would be required to achieve drug exposures comparable to those reported for healthy volunteers receiving 6 mg/kg, the suggested dose for the treatment of bacteremia and right-sided endocarditis.
Acknowledgments
This study was supported, in part, by Cubist Pharmaceuticals, Lexington, MA.
We acknowledge the University of Texas General Clinical Research Center, grant UL1 RR024148 (CTSA), for their contributions to the study. We thank the entire burn unit staff at Memorial Hermann Hospital, Houston, TX, for their assistance and dedication to this study. We are grateful to David Nicolau and Christina Sutherland at the Center for Anti-Infective Research & Development at Hartford Hospital, Hartford, CT, for their assistance with the high-performance liquid chromatography. Finally, we thank David Benziger at Cubist Pharmaceuticals and Linyee Shum at Avantix Laboratories, Inc., for their assistance with the protein binding experiments and Larry Friedrich at Cubist Pharmaceuticals for his review of the manuscript.
Footnotes
Published ahead of print on 25 February 2008.
REFERENCES
- 1.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonapace, C. R., R. L. White, L. V. Friedrich, E. D. Norcross, and J. A. Bosso. 1999. Pharmacokinetics of cefepime in patients with thermal burn injury. Antimicrob. Agents Chemother. 43:2848-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher, B. A., W. L. Hickerson, D. A. Kuhl, A. M. Bombassaro, and G. S. Jaresko. 1990. Imipenem pharmacokinetics in patients with burns. Clin. Pharmacol. Ther. 48:130-137. [DOI] [PubMed] [Google Scholar]
- 4.Boucher, B. A., D. A. Kuhl, and W. L. Hickerson. 1992. Pharmacokinetics of systemically administered antibiotics in patients with thermal injury. Clin. Infect. Dis. 14:458-463. [DOI] [PubMed] [Google Scholar]
- 5.Bourget, P., A. Lesne-Hulin, R. Le Reveille, H. Le Bever, and H. Carsin. 1996. Clinical pharmacokinetics of piperacillin-tazobactam combination in patients with major burns and signs of infection. Antimicrob. Agents Chemother. 40:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dailly, E., M. Pannier, P. Jolliet, and M. Bourin. 2003. Population pharmacokinetics of ceftazidime in burn patients. Br. J. Clin. Pharmacol. 56:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dandekar, P. K., P. R. Tessier, P. Williams, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405-411. [DOI] [PubMed] [Google Scholar]
- 8.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaehde, U., and F. Sorgel. 1995. Clinical pharmacokinetics in patients with burns. Clin. Pharmacokinet. 29:15-28. [DOI] [PubMed] [Google Scholar]
- 10.LaPlante, K. L., and M. J. Rybak. 2004. Daptomycin—a novel antibiotic against gram-positive pathogens. Expert Opin. Pharmacother. 5:2321-2331. [DOI] [PubMed] [Google Scholar]
- 11.Santucci, S. G., S. Gobara, C. R. Santos, C. Fontana, and A. S. Levin. 2003. Infections in a burn intensive care unit: experience of seven years. J. Hosp. Infect. 53:6-13. [DOI] [PubMed] [Google Scholar]
- 12.Waxman, K., T. Rebello, L. Pinderski, K. O'Neal, N. Khan, S. Tourangeau, E. Himes, and K. Cordill. 1987. Protein loss across burn wounds. J. Trauma 27:136-140. [DOI] [PubMed] [Google Scholar]