Abstract
The basis of the β-lactam resistance of 39 multidrug-resistant Acinetobacter baumannii isolates recovered from hospitalized patients was studied. These isolates were collected from 2001 to 2005 at the Sahloul Hospital in Sousse, Tunisia. They belonged to two distinct clones. One clone that grouped 19 isolates produced a carbapenem-hydrolyzing oxacillinase, OXA-97, that differed from OXA-58 by a single amino acid substitution and conferred the same β-lactam resistance profile as OXA-58. The blaOXA-97 gene was located on plasmids that varied in size in 18 isolates and was chromosomally located in a single isolate. Cloning and sequencing identified genetic structures surrounding the blaOXA-97 gene similar to those reported to be adjacent to the blaOXA-58 gene. In addition, the novel ISAba8 element (which is of the IS21 family) was identified. This is the first report of the nosocomial spread of carbapenemase producers in A. baumannii isolates in Africa.
Acinetobacter baumannii is an opportunistic pathogen that is increasingly being reported to be a cause of nosocomial infections (9, 16, 17). Carbapenem resistance is now observed worldwide in A. baumannii isolates, leading to limited therapeutic options. Several mechanisms are responsible for resistance to carbapenems in A. baumannii. These are reduced outer membrane permeability, penicillin binding protein changes, and mostly, the production of carbapenemases (21).
The carbapenem-hydrolyzing β-lactamases in A. baumannii are either metallo-β-lactamases (MBLs) (31) or oxacillinases (carbapenem-hydrolyzing class D β-lactamases [CHDLs]) (21). Three major subgroups of acquired CHDLs have been identified in A. baumannii and are represented by the OXA-23, OXA-24/OXA-40, and OXA-58 β-lactamases. The blaOXA-58 gene has been identified in France, Italy, Belgium, the United Kingdom, Austria, Turkey, Greece, Kuwait, Brazil, Argentina, and Australia (1, 4, 5, 8, 10, 23, 25).
The OXA-58 β-lactamase was first identified in Europe from a carbapenem-resistant A. baumannii isolate recovered in France in 2003 that was at the origin of a nosocomial outbreak (24). The blaOXA-58 gene was located on a plasmid, and its activity was inhibited by NaCl (14, 24). Genetic investigations showed that the blaOXA-58 gene was bracketed by insertion sequences (ISs), which were likely the origin of its acquisition and expression (22).
The aim of the study described here was to analyze multidrug-resistant A. baumannii isolates that were resistant to carbapenems and that were recovered from the same hospital over 5 years for their β-lactamase contents. This work constitutes the first analysis of an outbreak of CHDL-producing A. baumannii strains in Africa.
(This study was presented in part at the 17th European Congress of Clinical Microbiology and Infectious Diseases [O497], 2007, Munich, Germany.)
MATERIALS AND METHODS
Bacterial isolates.
The 39 A. baumannii clinical isolates were identified by using the API 32GN system (bioMérieux SA, Marcy l'Etoile, France) and sequencing of the rpoB gene, as described previously (7). Electrocompetent Escherichia coli TOP10 and A. baumannii CIP7010 were used as recipient strains in the transformation experiments, as described previously (14). A. baumannii MAD, which carries the blaOXA-58 gene, was used as an OXA-58-producing reference strain (24). E. coli NCTC 50192, which harbors four plasmids of 154, 66, 38, and 7 kb, respectively, was used as a size marker.
Susceptibility testing and screening for MBL-producing strains.
The antibiotic susceptibilities of the A. baumannii isolates were first determined by the disk diffusion method on Mueller-Hinton agar plates with β-lactam antibiotic- and non-β-lactam antibiotic-containing disks (Sanofi Diagnostics Pasteur, Marnes-La-Coquette, France), according to the guidelines of the Clinical and Laboratory Standards Institute (3). MBL production was evaluated by using Etest strips with imipenem and EDTA (AB Biodisk, Solna, Sweden) for all strains studied. The susceptibility to colistin and tigecycline was also evaluated by Etest (AB Biodisk) (31). The breakpoints used for tigecycline susceptibility testing were those recommended by the EUCAST for members of the family Enterobacteriaceae, with susceptibility being an MIC ≤1 μg/ml and resistance being an MIC >2 μg/ml.
PCR amplification for detection of CHDL genes and sequencing.
Under standard PCR conditions (26), a series of primers was used for detection of CHDL-encoding genes, including blaOXA-23, blaOXA-24/OXA-40, and blaOXA-58, as reported previously (14). Primers for the detection of genetic structures identified at the 5′ or 3′ end of blaOXA-58 (including the ISAba2 and ISAba3 elements) were used in combination with primers OXA-58A and OXA-58B (24). Additionally, the naturally occurring blaOXA-51/OXA-69 gene of A. baumannii was amplified (with primers OXA-69A and OXA-69B) (12). PCR combinations were performed with blaOXA-69-specific primers on one side and ISAba1-specific primers on the other side (2, 30). Sequencing reactions were performed with an automated sequencer (ABI 3130; Applied Biosystems, Foster City, CA). The nucleotide and deduced protein sequences were analyzed with software available over the Internet (http://www.ncbi.nlm.hih.gov).
Cloning experiments and analysis of recombinant plasmids.
Cloning into E. coli TOP10 was performed as described previously (19) by using EcoRI-digested whole-cell DNA of blaOXA-97-positive A. baumannii strain A10 ligated into EcoRI-restricted plasmid pBK-CMV (Stratagene, La Jolla, CA), followed by selection on plates containing 50 μg/ml of amoxicillin and 30 μg/ml of kanamycin.
In addition, PCR amplicons encompassing the entire sequence of the blaOXA-97 gene and blaOXA-58 were obtained with primers pre-OXA-58 prom+ and pre-OXA-58B (22) from whole-cell DNA of A. baumannii isolates A1 and MAD, respectively, and were subsequently cloned by use of a ZeroBlunt TOPO PCR cloning kit (Invitrogen, Cergy-Pontoise, France). Recombinant strains E. coli TOP10(pOXA-97) and E. coli TOP10(pOXA-58) were used for MIC and specific activity comparisons (24).
PFGE and Southern hybridization.
Pulsed-field gel electrophoresis (PFGE) analysis was done, and the results were interpreted as described previously (18, 29). DNA-DNA hybridizations were performed as described by Sambrook et al. (26) with a probe consisting of a 528-bp PCR fragment internal to blaOXA-97 generated from A. baumannii A1 (24). Labeling of the probe and signal detection were carried out with a nonradioactive enhanced chemiluminescence labeling and detection kit according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Transformation and plasmid DNA content analysis.
Transformation experiments were performed with clinical isolates A. baumannii A1 and A. baumannii A2 as plasmid donors and A. baumannii CIP7010 as the recipient strain (14). Transformants were selected on Trypticase soy agar plates containing 50 μg/ml of ticarcillin.
To assess the chromosomal location of the β-lactamase gene, we used the homing endonuclease I-CeuI (Ozyme; New England Biolabs) (15) and separated the fragments by PFGE, as reported previously (18). After transfer onto a nylon membrane, the DNA was UV cross-linked (Stratalinker; Stratagene) and hybridized with two different probes: a 1,504-bp PCR-generated probe specific for the 16S and 23S rRNA genes (18) and a 528-bp probe specific for blaOXA-97, as indicated above.
Measurement of β-lactamase specific activities.
Crude cell extracts were obtained by sonication from A. baumannii clinical isolates and E. coli TOP10 recombinant strains expressing β-lactamases OXA-97 and OXA-58, respectively, as described previously (13, 20). The β-lactamase specific activities were determined for imipenem, meropenem, cephalothin, ceftazidime, oxacillin, and benzylpenicillin, which were each used at a final concentration of 100 μM, as described previously (13).
Nucleotide sequence accession number.
The nucleotide sequence data reported in this work have been deposited in the GenBank nucleotide database under accession no. EF102240.
RESULTS AND DISCUSSION
Epidemiology of the carbapenem-resistant A. baumannii isolates.
From October 2001 to September 2005, 445 nonrepetitive A. baumannii isolates were identified at the Sahloul Hospital, Sousse, Tunisia; 222 (50%) of these isolates were resistant to carbapenems. Among these isolates, all 39 nonrepetitive isolates that had been kept frozen were retained for this study. A single isolate was recovered in 2001, 5 isolates were recovered in 2002, 8 isolates were recovered in 2003, 7 isolates were recovered in 2004, and 18 isolates were recovered in 2005. These isolates had been recovered from different specimens (mostly blood cultures and pus) of patients hospitalized in five wards. Two main antibiotic resistance phenotypes were observed. Nineteen isolates (isolates A1 to A19) exhibited phenotype I (resistance to all β-lactams, including carbapenems, fluoroquinolones, tigecycline, and gentamicin, and susceptibility to tobramycin and colistin). Twenty isolates exhibited phenotype II (resistance to ceftazidime, carbapenems, and fluoroquinolones; intermediate susceptibility to ticarcillin, cefepime, and tigecycline; and susceptibility to gentamicin, tobramycin, and colistin). PFGE analysis showed that the 19 A. baumannii isolates of phenotype I were clonally related, whereas the other carbapenem-resistant isolates (phenotype II) were distinct but clonally related (data not shown). The isolates belonging to phenotype II were recovered in 2004 (3 of 7 isolates) and 2005 (17 of 18 isolates). Hydrolytic assays showed that isolates of phenotype I had weak carbapenemase activities, whereas those of phenotype II did not (Table 1).
TABLE 1.
MICs of β-lactams and specific activitiesa against different β-lactams for clinical isolates of A. baumannii belonging to antibiotic resistance phenotypes I and II, A. baumannii MAD, E. coli TOP10(pOXA-58), E. coli TOP10(pOXA-97), A. baumannii CIP7010, A. baumannii CIP7010(pOXA-97), E. coli DH10B, and E. coli DH10B(pOXA-97)
| β-Lactam | MIC (μg/ml)
|
Sp act (mU/mg of protein)c
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii (phenotype I) | A. baumannii (phenotype II) | A. baumannii MAD | E. coli TOP10(pOXA-58) | E. coli TOP10(pOXA-97) | A. baumannii CIP7010 | A. baumannii CIP7010(pOXA-97) | E. coli DH10B | E. coli DH10B(pOXA-97) | A. baumannii (phenotype I) | A. baumannii (phenotype II) | A. baumannii MAD | E. coli TOP10(pOXA-58) | E. coli TOP10(pOXA-97) | A. baumannii CIP7010 | A. baumannii CIP7010(pOXA-97) | E. coli DH10B | E. coli DH10B(pOXA-97) | |
| Ampicillin | >512 | >512 | >512 | >512 | >512 | >512 | >512 | 4 | >512 | |||||||||
| Ampicillin + CLAb | >512 | >512 | >512 | 128 | 128 | 512 | >512 | 4 | 128 | |||||||||
| Ticacillin | >512 | 64 | >512 | >512 | >512 | 4 | >512 | 4 | >512 | |||||||||
| Ceftazidime | >512 | >512 | 128 | 0.06 | 0.06 | 2 | 2 | 0.06 | 0.12 | 1 | 0.4 | 2 | 1.5 | 0.5 | — | — | ND | — |
| Cefepime | 64 | 256 | 256 | 0.06 | 0.06 | 1 | 1 | 0.06 | 0.12 | |||||||||
| Aztreonam | 128 | 128 | 32 | 0.06 | 0.12 | 64 | 64 | 0.12 | 0.06 | |||||||||
| Imipenem | 32 | 16 | 32 | 2 | 2 | 0.25 | 2 | 0.06 | 1 | 5.2 | ND | 4.5 | 3 | 4 | — | — | — | — |
| Meropenem | 16 | 16 | >64 | 0.12 | 0.12 | 0.25 | 2 | 0.06 | 0.5 | 1.7 | ND | 1.5 | 1 | 1 | — | — | ND | — |
| Cephalothin | 20 | 10 | 15 | 15 | 20 | — | — | ND | — | |||||||||
| Oxacillin | 230 | ND | 230 | 210 | 220 | — | — | ND | — | |||||||||
| Penicillin G | 220 | 210 | 170 | 150 | 220 | — | — | — | — | |||||||||
Data are the means of three independent experiments. Standard deviations were within 10% of the means.
CLA, clavulanic acid (4 μg/ml).
ND, not detectable; —, not determined.
Identification of CHDL-encoding A. baumannii isolates.
The results obtained with Etest strips combining imipenem and EDTA did not identify MBL production by the phenotype I or II isolates. PCR experiments with primers specific for CHDL-encoding genes were performed with all 39 clinical isolates; and blaOXA-58-like genes were identified only in isolates exhibiting phenotype I, but no blaOXA-23- or blaOXA-40-like gene was detected in the whole collection. The carbapenem resistance in the phenotype II isolates was therefore not mediated by β-lactamase and may be related to permeability defects and/or the overexpression of efflux. Overall, the isolates positive for the blaOXA-58-like gene were recovered from multiple wards and were detected during the entire study period.
Sequencing of the blaOXA-58-positive amplicons obtained from all isolates of phenotype I identified a gene with a single base pair substitution with respect to the sequence of blaOXA-58. This substitution gave rise to OXA-97 with an Ala-to-Gly substitution at position DBL35 (corresponding to amino acid 53 of the premature OXA-58 sequence) (24). Cloning experiments were performed in identical plasmid and strain backgrounds to compare the OXA-97 and the OXA-58 hydrolysis spectra toward β-lactams. Analysis of the MICs of β-lactams and measurement of the specific activities with imipenem and benzylpenicillin as the substrates showed very similar results for both recombinant strains, E. coli DH10B(pOXA-97) and E. coli DH10B(pOXA-58), suggesting an identical hydrolysis spectrum (Table).
Screening of the naturally occurring blaOXA-51/OXA-69 gene of A. baumannii was performed with the blaOXA-97-positive isolates that gave positive results. PCR was also performed to search for the ISAba1 element and gave a positive result for all these isolates, in agreement with previous observations showing the ubiquity of that IS element in A. baumannii (11, 28). However, PCR experiments did not identify ISAba1 upstream of the blaOXA-97 gene, thus ruling out the ISAba1-mediated overexpression of this naturally occurring CHDL gene.
Genetic location of blaOXA-97.
Plasmid analysis identified two or three plasmids with different sizes in the 19 blaOXA-97-positive isolates. Hybridization with a blaOXA-97-specific probe gave a single positive signal, corresponding to a 60-kb plasmid for 12 isolates, a 50-kb plasmid for 4 isolates, and a 45-kb plasmid for 2 isolates; no hybridization signal was obtained for isolate A2. The chromosomal location of the blaOXA-97 gene in isolate A2 was confirmed by use of the I-CeuI technique. Thus, β-lactamase OXA-97 was plasmid encoded in all except one of the isolates; in the latter isolate, integration of the blaOXA-97-positive plasmid or mobilization of this gene from the plasmid to the chromosome may have occurred.
Transfer of the ticarcillin resistance marker was successful by use of all the blaOXA-97-positive A. baumannii clinical isolates as donors (except for isolate A2) and an A. baumannii reference strain as the recipient but not an E. coli strain as the recipient. A. baumannii CIP7010 transformants harboring the blaOXA-97-positive natural plasmids exhibited a β-lactam resistance pattern consistent with that resulting from the expression of OXA-58/OXA-97, with MICs identical to those already obtained with natural plasmid pMAD (14). The blaOXA-97-positive plasmids did not confer additional resistance to antibiotics, except for resistance to tetracycline for plasmids obtained from four isolates.
Genetic structures surrounding the blaOXA-97 gene.
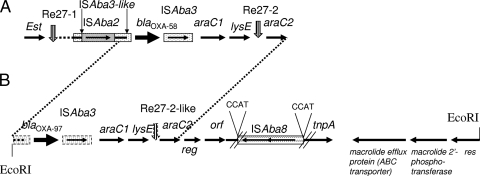
Cloning of the EcoRI-restricted DNA of A. baumannii A1 gave rise to recombinant plasmid pA1WM, which expressed the blaOXA-97 gene. Sequencing of the 13,122-bp insert revealed that an ISAba3-like element was located 20 bp upstream of blaOXA-97, as previously identified in other structures upstream of the blaOXA-58 gene (23, 24). PCR mapping showed that this ISAba3-like element was not truncated by an IS26, ISAba1, or ISAba2 element, as opposed to what has previously been found (2, 24, 25). An entire copy of ISAba3 was identified downstream of the blaOXA-97 gene, followed by the araC1 and the lysE genes, as observed in natural plasmid pMAD (25). A sequence similar to what has been defined as the Re27-2 structure in A. baumannii MAD was identified downstream of the lysE gene (3 nucleotides of those 27 bp). That structure was likely involved in a homologous recombination process at the origin of the acquisition of the blaOXA-58 gene in the latter strain (22). An araC2-like gene was identified in A. baumannii MAD (Fig. 1). A gene encoding a putative regulator was found downstream of araC2, followed by an orf corresponding to a putative 160-amino-acid protein and by a gene encoding a putative transposase. The latter gene was truncated by the insertion of a novel IS element named ISAba8 (see below). An operon containing genes involved in macrolide resistance was identified downstream of the putative transposase gene and was identical to that found on pRSB105, a plasmid isolated from sewage water treatment plants (27). It included a gene encoding a 491-amino-acid efflux protein also identified in E. coli and Citrobacter freundii (GenBank accession numbers AAN87714.1 and ABG33795.1, respectively) and a 294-amino-acid macrolide 2′-phosphotransferase. The gene encoding a 203-amino-acid resolvase/site-specific recombinase identified in pRSB105 was also found (Fig. 1). Overall, the genetic structures surrounding the blaOXA-97 gene were similar to those identified in association with the blaOXA-58 gene in a series of isolates identified from France, Italy, and Greece (2, 22, 23), indicating that a common structure has been at the origin of the dissemination of the blaOXA-58 and blaOXA-97 genes.
FIG. 1.
Schematic map of the genetic structure containing the blaOXA-58 and blaOXA-97 genes. (A) The structure identified in blaOXA-58-positive isolate MAD (22, 24); (B) the structure identified in A. baumannii A1 (this study). The genes and their corresponding transcription orientations are indicated by horizontal arrows. The transcription regulator genes (araC1 and araC2), the threonine efflux protein gene (lysE), the esterase gene (est), the putative regulatory gene (reg), the gene encoding an open reading frame of unknown function (orf), the putative transposase gene (tnpA), and the putative resolvase gene (res) are indicated. Vertical arrows are for the Re27-like sequences. The genetic structure which is highly similar between panels A and B is indicated with the dotted lines. The EcoRI restriction sites bracketing the insert of pA1WM are indicated. The figure is not to scale.
Identification of ISAba8, a novel IS.
ISAba8 is 1,867 bp and belongs to the IS21 family (http://www-is.biotoul.fr/). It possesses 27-bp inverted repeats, and its transposition has likely generated the observed 4-bp target site duplication (CCAT in the structure identified). ISAba8 contains two open reading frames (Orf1 and Orf2) encoding proteins of 339 and 257 amino acids, respectively. The deduced amino acid sequences of the Orf1 and Orf2 proteins had 72% and 79% amino acid identities to the transposase subunits of ISPsy4 and ISRso19, respectively.
Conclusion.
This work identified carbapenemase-producing A. baumannii isolates as sources of nosocomial infections in Tunisia. An identical OXA-97-producing A. baumannii isolate was identified from 2001 to 2005, indicating its persistence in the hospital and its environment. This novel β-lactamase, OXA-97, is the second member of the OXA-58 subgroup of CHDLs, whose production confers the same β-lactam resistance profile as OXA-58. This report constitutes the first report of the nosocomial dissemination of a CHDL-producing A. baumannii strain in Africa, after the identification of single OXA-23-producing A. baumannii isolates from Algeria, Libya, and South Africa (6, 28). The current worldwide emergence of multiresistant A. baumannii isolates is mostly associated with carbapenemase producers. Therefore, such carbapenemases may be considered the main targets in the development of inhibitors.
Acknowledgments
This work was partially funded by grants from the Ministère de l'Education Nationale et de la Recherche (grant UPRES-EA3539), Université Paris XI, Paris, France, and the Ministry of Scientific Research Technology and Competence Development of Tunisia and was mostly funded by a grant from the European Community (grant LSHM-CT-2005-018705).
Footnotes
Published ahead of print on 25 February 2008.
REFERENCES
- 1.Bertini, A., A. Giordano, P. Varesi, L. Villa, C. Mancini, and A. Carattoli. 2006. First report of the plasmid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter baumannii isolates in Italy. Antimicrob. Agents Chemother. 50:2268-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertini, A., L. Poirel, S. Bernabeu, D. Fortini, L. Villa, P. Nordmann, and A. Carattoli. 2007. Multicopy blaOXA-58 gene as a source of high-level resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:2324-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement. M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Coelho, J., N. Woodford, M. Afzal-Shah, and D. M. Livermore. 2006. Occurrence of OXA-58-like carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob. Agents Chemother. 50:756-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbella, X., A. Montero, M. Pujol, M. A. Domínguez, J. Ayats, M. J. Argerich, F. Garrigosa, J. Ariza, and F. Gudiol. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corvec, S., L. Poirel, T. Naas, H. Drugeon, and P. Nordmann. 2007. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-23 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dortet, L., P. Legrand, C. J. Soussy, and V. Cattoir. 2006. Bacterial identification, clinical significance, and antimicrobial susceptibility of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J. Clin. Microbiol. 44:4471-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giordano, A., P. Varesi, A. Bertini, L. Villa, A. M. Dionisi, M. Venditti, P. Carfagna, I. Luzzi, C. Mancini, and A. Carattoli. 2007. Outbreak of Acinetobacter baumannii producing the carbapenem-hydrolyzing oxacillinase OXA-58 in Rome, Italy. Microb. Drug Resist. 13:37-43. [DOI] [PubMed] [Google Scholar]
- 9.Hanlon, G. W. 2005. The emergence of multidrug resistant Acinetobacter species: a major concern in the hospital setting. Lett. Appl. Microbiol. 41:375-378. [DOI] [PubMed] [Google Scholar]
- 10.Héritier, C., A. Dubouix, L. Poirel, N. Marty, and P. Nordmann. 2005. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolyzing oxacillinase OXA-58. J. Antimicrob. Chemother. 55:115-118. [DOI] [PubMed] [Google Scholar]
- 11.Héritier, C., L. Poirel, and P. Nordmann. 2006. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12:123-130. [DOI] [PubMed] [Google Scholar]
- 12.Héritier, C., L. Poirel, P. E. Fournier, J. M. Claverie, D. Raoult, and P. Nordmann. 2005. Characterization of the naturally occurring oxacillinase of Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:4174-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Héritier, C., L. Poirel, D. Aubert, and P. Nordmann. 2003. Genetic and functional analysis of the chromosome-encoded carbapenem-hydrolyzing oxacillinase OXA-40 of Acinetobacter baumannii. Antimicrob. Agents Chemother. 47:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Héritier, C., L. Poirel, T. Lambert, and P. Nordmann. 2005. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:3198-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navon-Venezia, S., R. Ben-Ami, and Y. Carmeli. 2005. Update on Pseudomonas aeruginosa and Acinetobacter baumannii infections in the healthcare setting. Curr. Opin. Infect. Dis. 18:306-313. [DOI] [PubMed] [Google Scholar]
- 17.Paterson, D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl. 2):S43-S48. [DOI] [PubMed] [Google Scholar]
- 18.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel, L., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel, L., M. Guibert, D. Girlich, T. Naas, and P. Nordmann. 1999. Cloning, sequence analyses, expression, and distribution of ampC-ampR from Morganella morganii clinical isolates. Antimicrob. Agents Chemother. 43:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel, L., and P. Nordmann. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin. Microbiol. Infect. 12:826-836. [DOI] [PubMed] [Google Scholar]
- 22.Poirel, L., and P. Nordmann. 2006. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene blaOXA-58 in Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:1442-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel, L., E. Lebessi, C. Héritier, A. Patsoura, M. Foustoukou, and P. Nordmann. 2006. Nosocomial spread of OXA-58-positive carbapenem-resistant Acinetobacter baumannii isolates in a pediatric hospital in Greece. Clin. Microbiol. Infect. 12:1138-1141. [DOI] [PubMed] [Google Scholar]
- 24.Poirel, L., S. Marqué, C. Héritier, C. Segonds, G. Chabanon, and P. Nordmann. 2005. OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49:202-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pournaras, S., A. Markogiannakis, A. Ikonomidis, L. Kondyli, K. Bethimouti, A. N. Maniatis, N. J. Legakis, and A. Tsakris. 2006. Outbreak of multiple clones of imipenem-resistant Acinetobacter baumannii isolates expressing OXA-58 carbapenemase in an intensive care unit. J. Antimicrob. Chemother. 57:557-561. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 27.Schlüter, A., R. Szczepanowski, N. Kurz, S. Schneiker, I. Krahn, and A. Pühler. 2007. Erythromycin resistance-conferring plasmid pRSB105, isolated from a sewage treatment plant, harbors a new macrolide resistance determinant, an integron-containing Tn402-like element, and a large region of unknown function. Appl. Environ. Microbiol. 73:1952-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal, H., S. Garny, and B. G. Elisha. 2005. Is ISAba1 customized for Acinetobacter? FEMS Microbiol. Lett. 243:425-429. [DOI] [PubMed] [Google Scholar]
- 29.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turton, J. F., M. E. Ward, N. Woodford, M. E. Kaufmann, R. Pike, D. M. Livermore, and T. L. Pitt. 2006. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258:72-77. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. R., A. Bolmström, A. Qwärnström, and A. Gales. 2002. Evaluation of a new Etest for detecting metallo-β-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]