Abstract
We evaluated interferon (IFN) and ribavirin (RBV) as dual therapy and as part of triple-combination therapies with the iminosugars N-butyl-deoxynojirimycin (NB-DNJ), N-nonyl-deoxynojirimycin, and N-7-oxanonyl-6-deoxymethyl-galactonojirimycin. The ability of these compounds to clear bovine viral diarrhea virus (BVDV), a surrogate model for hepatitis C virus (HCV), from a persistently infected Madin-Darby bovine kidney cells cell line was determined by monitoring the secretion of viral RNA and the infectivity of secreted virions. In the BVDV system, after treatment with IFN-RBV alone, viral rebound was observed immediately after removal of the drugs. In contrast, we demonstrate that a triple drug combination of IFN, RBV, and an iminosugar eradicated the BVDV infection in a time- and a dose-dependent manner, leading to sustained viral clearance. Importantly, in the case of NB-DNJ, the sustained viral clearance was achieved by using physiologically relevant and tolerated drug concentrations. Therefore, the use of a triple-combination therapy that includes an iminosugar may prove to be of greater therapeutic value for the treatment of HCV infection than the use of IFN-RBV alone.
Chronic hepatitis in humans is often caused by persistent infection with hepatitis C virus (HCV). This persistent infection commonly leads to liver fibrosis, cirrhosis, and hepatocellular carcinoma. Pegylated alpha interferon (IFN) in combination with ribavirin (RBV) is the current treatment of choice for HCV infection (15). However, the treatment outcome is viral genotype specific, and the treatment is not effective in up to 50% of cases (8). Additional therapies aimed at the total and permanent eradication of the virus from patients are urgently required.
Iminosugar derivatives are a group of compounds that have been evaluated as potential antivirals by use of the bovine viral diarrhea virus (BVDV) system (2, 6, 7, 28). Iminosugars exert their antiviral activity by one or more modes of action, depending on the sugar analogue head group and the length of the alkyl side chain that they carry. Deoxynojirimycin (DNJ)-based iminosugar derivatives exert their antiviral effects by impairing the correct folding of the viral envelope glycoproteins of BVDV (2, 6) and other enveloped viruses, including hepatitis B virus (25), human immunodeficiency virus (12), and HCV (3), via inhibition of the host-cell encoded endoplasmic reticulum (ER) α-glucosidases I and II, which prevents the crucial interaction of the viral envelope glycoproteins with the cellular chaperones calnexin and calreticulin. Treatment with DNJ-based iminosugars causes an inhibition of HCV E1-E2 assembly and the incorporation into HCV pseudoparticles, as well as a reduction in viral infectivity due to the incorporation of some misfolded glycoprotein complexes into virions (3). A second antiviral mechanism of action can be attributed to the alkyl chains attached to the sugar analogue head group of iminosugars. Long alkyl side chains attached to the head group are able to inhibit the HCV p7 ion channels that are crucial for viral infectivity (10, 21, 22). This has been demonstrated in in vitro studies with chemically synthesized HCV p7 peptides incorporated into artificial lipid membranes (19), in the BVDV cell culture system (7), and more recently, in the HCV cell culture (HCVcc) infectivity system, where long alkyl chain derivatives, such as N-nonyl-DNJ (NN-DNJ) and N-nonyl-galactonojirimycin (NN-DGJ) decrease, in a dose-dependent manner, the secretion and/or infectivity of HCV. In these experiments virus secreted from infected cells was used to reinfect naïve cells and viral infectivity was monitored over five passages (23).
Prior to the development of the HCVcc infectivity system (13, 24, 26), BVDV, which is the virus that is the most closely related to HCV, was the preferred HCV surrogate model system for studies that depended on the ability to re-create a whole infectious cycle in cell culture. Although most aspects of HCV morphogenesis, viral secretion, and reinfection can now be studied in the HCVcc system, other aspects remain problematic, most notably, the long-term culture of HCV-infected host cells. The latter is essential to enable the study of viral clearance, the emergence of viral escape mutants, and viral rebound after the cessation of extended drug treatment. For this purpose, BVDV is currently still the most robust model system available. Here we show that, in contrast to IFN-RBV treatment alone, the inclusion of iminosugars in a triple combination eradicates noncytopathic (ncp) BVDV from persistently infected Madin-Darby bovine kidney cells (MDBK) cells in a time- and a dose-dependent manner and prevents viral rebound after treatment is stopped.
MATERIALS AND METHODS
Treatment of ncp BVDV-infected MDBK cells with IFN, RBV, and iminosugar derivatives.
MDBK cells were seeded at 1 × 106 cells/35-mm dish, infected with ncp BVDV strain Pe515 (National Animal Disease Laboratory, United Kingdom) at a multiplicity of infection (MOI) of 0.1, and passaged into 2 ml of fresh RPMI 1640 medium containing 10% (vol/vol) fetal calf serum at a 1:8 dilution every 3 days. After a stable infection was achieved, IFN (1,000 IU/ml) and RBV (1 μM) (Sigma-Aldrich) were added to the cells; this passage was denoted passage 0 (P0). Simultaneously, a non-drug-treated positive control sample and mock-infected negative controls cultured in the presence and the absence of IFN and RBV (1,000 IU/ml and 1 μM, respectively) were prepared. Cells continued to be passaged every 3 days into fresh medium containing IFN and RBV. At P3, the medium was supplemented with the various iminosugar derivatives and the cells were cultured in the presence of IFN, RBV, and N-butyl-deoxynojirimycin (NB-DNJ; Sigma-Aldrich) or in the presence of N-7-oxanonyl-6-deoxymethyl-galactonojirimycin (N7-DGJ) or NN-DNJ (United Therapeutics Corporation, Silver Spring, MD). The cells were passaged every 3 days into fresh medium containing the indicated drug combinations. After five passages under triple-combination drug pressure (P8), each sample was split into three; in set 1, the drug combinations remained the same and the cells continued to be cultured in the presence of IFN-RBV and the iminosugars (continued triple combination); in set 2, all drugs were removed; and in set 3, IFN and RBV were removed; i.e., the cells continued to be cultured in the presence of the iminosugars only (iminosugar maintenance treatment). The cells were passaged as described above. At each passage the combined supernatants of cultured cells from duplicate wells were harvested (to account for biological variation), the levels of secreted viral RNA were measured (by real-time reverse transcription [RT]-PCR with technical duplicates), and the infectivity of the supernatant was determined by an immunofluorescence (IF)-based infectivity assay.
Detection of BVDV in infected cell line.
To detect the stable infection either at the beginning of the experiments or after the final passage after treatment, supernatants were harvested and the cells were probed for the presence of BVDV. The persistently infected MDBK cells were fixed with 2% (vol/vol) paraformaldehyde for 30 min. The cells were washed with phosphate-buffered saline (PBS), blocked in a 5% (wt/vol) milk-PBS solution for 30 min, and permeabilized with 1% (vol/vol) Triton X-100 for 20 min. After the cells were washed with 1% (vol/vol) Tween-PBS, the cells were incubated for 1 h with the primary antibody WB103/105 (1:500 dilution; Veterinary Laboratory Agency, Weybridge, United Kingdom), which recognizes the BVDV NS2 and NS3 proteins. After subsequent incubation with an anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma) and extensive washing in PBS, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc.). Fluorescence was observed under an inverted Nikon Eclipse TE200-U microscope.
Infectivity assays by IF analysis.
MDBK cells were grown in six-well plates to 70% confluence; the supernatant was removed and discarded. Cells were infected for 1 h at 37°C with 500 μl of the supernatants harvested from the BVDV-infected and mock-infected cells. After removal of the inoculum, the cells were washed twice with PBS and incubated overnight in fresh medium. Infectivity was determined by IF, as described above.
Viral RNA purification and real-time RT-PCR analysis.
At each passage, a 500-μl aliquot of each supernatant harvested from the cultured cells was concentrated by ultrafiltration (molecular weight cutoff, 10 kDa; Centricon filter; Millipore) to 140 μl. The RNA from the released viral particles was purified from the concentrated supernatants by using a QIAamp viral RNA purification kit (Qiagen, Crawley, United Kingdom), according to the manufacturer's instructions. Briefly, RNA was eluted in 50 μl, and samples were treated with DNase (90 min at 37°C, 20 min at 80°C). Real-time RT-PCR was carried out with a Qiagen SYBR green Quantitect RT-PCR kit. Primers amplifying a 334-bp region spanning parts of the NS2-coding sequence were used (forward primer, 5′-TAG GGC AAA CCA TCT GGA AG-3′; reverse primer, 5′-ACT TGG AGC TAC AGG CCT CA-3′). RT was achieved at 50°C for 30 min, followed by incubation at 95°C for 15 min to activate the hot start polymerase. The resulting DNA was amplified by PCR (35 cycles of 15 s at 95°C, 1 min at 50°C, and 1 min at 72°C; final extension for 7 min at 72°C). The reactions were carried out with an Opticon 2 real-time PCR machine (Bio-Rad). Internal standards prepared from a highly concentrated ncp BVDV supernatant were used to generate standard curves.
MTS cell proliferation assay.
Cellular toxicity was measured with a Cell Titer 96 aqueous nonradioactive cell proliferation assay kit, according to the manufacturer's instructions (Promega). In brief, 2 × 104 MDBK cells were taken from all three sets at P5, P10, and P15 and again at the end point of the experiments, i.e., at either P22 or P32. Then, equivalent cell numbers from all treatment regimens were subjected in a 96-well plate to a further 72 h of culture (allowing for three cell doubling times) in the presence of their respective treatment compounds. Then, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS)-phenazine methosulfate solution (40 μl) was added to each well and the samples were incubated at 37°C in a humidified 5% CO2 atmosphere for 3 h. The absorbance was read at 490 nm with a UVmax plate reader (Molecular Devices). Each sample was analyzed in triplicate, and the results were compared to those for the non-drug-treated cells (as the 100% viability control), which were passaged in parallel with the drug-treated samples.
RESULTS
Generation of an MDBK cell line persistently infected with ncp BVDV.
In order to study the abilities of antiviral compounds to eliminate BVDV infection, we prepared an MDBK cell line persistently infected with ncp BVDV. MDBK cells were infected with ncp BVDV at an MOI of 0.1 and passaged every 3 days, and the infection levels were monitored by IF microscopy. At 3 days postinfection, 95% of the cells were infected; and after an initial acute infection phase (six passages), the infection level dropped to, on average, 56%, which was maintained throughout the experiment, establishing a dynamic persistent infection (see Fig. S1 in the supplemental material). The secreted viral RNA levels mirrored this trend, reaching 2.1 × 108 copy numbers/ml at their maximum and, after the initial drop, plateauing to levels that ranged from 1.04 ×107 to 9.15 ×107 copy numbers/ml (see Table S1 in the supplemental material). In two subsequent independent viral cultures, this plateau ranged from 3.51 × 106 to 9.36 ×106 RNA copy numbers/ml in one experiment and from 11.7 ×106 to 65.8 ×106 RNA copy numbers/ml in another experiment (see Table 2 in the supplemental material).
Evaluation of antiviral effects of IFN and RBV alone or supplemented with iminosugar derivatives in persistently infected MDBK cells.
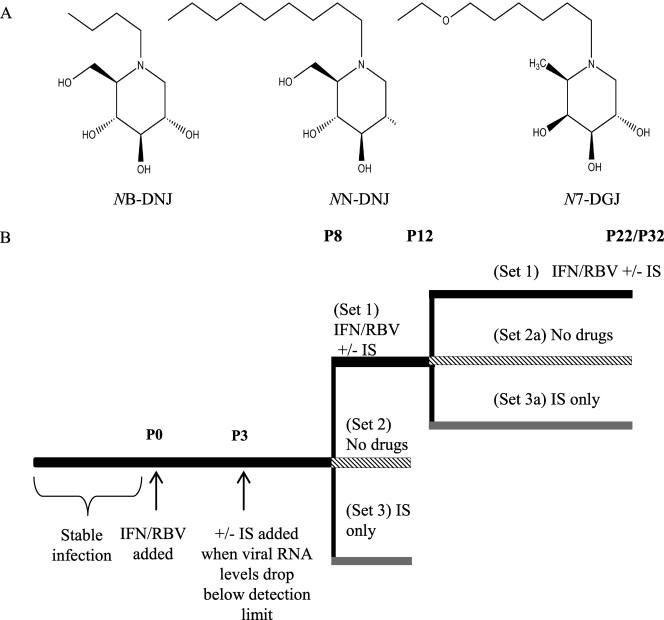
The chemical structures of the iminosugar derivatives used in this study and the experimental outline are shown in Fig. 1. The aim of this study was to evaluate whether the viral relapse observed after the cessation of treatment with the IFN-RBV double combination could be delayed or prevented by the inclusion of an iminosugar derivative in a triple combination. First, we established that addition of an iminosugar to the IFN-RBV double combination from the beginning did not cause viral RNA levels in the supernatants to decrease faster or in a synergistic fashion (data not shown). Therefore, we used IFN-RBV alone to achieve the initial fast drop in measurable virus titers. Culturing of persistently infected MDBK cells in the presence of IFN (1,000 IU/ml) and RBV (1 μM) for three passages (P0 to P3) led to a decrease in the amount of viral RNA present in the supernatant to below the detection limit of 39 RNA copies per ml of the real time (SYBR green-based) RT-PCR assay used in the experiment. Inoculation of naïve MDBK cells with this supernatant did not lead to infection, as determined by IF. In contrast, supernatant taken from the untreated control sample contained at least 3.51 × 106 RNA copies per ml and could reinfect naïve cells (data not shown).
FIG. 1.
(A) Chemical structures of the iminosugar (IS) derivatives used in the study. (B) Experimental outline. After a stable infection was established, the cells were cultured for three passages (9 days) in the presence of 1,000 IU/ml IFN and 1 μM RBV. At P3, after the viral RNA levels dropped below the detection limit, the medium was supplemented with one of the iminosugar derivatives. At the end of P8, the samples were split into three sets: set 1 (black line), all drug regimens remained the same; set 2 (cross-hatched line), all drugs were removed; set 3 (gray line), only iminosugars were continued. After P12, samples from set 1, which had been cultured for nine passages in the presence of IFN-RBV and an iminosugar, were split into sample sets 1, 2a, and 3a and treated in the same manner as described above.
At P3, after the viral titers in IFN-RBV-treated samples had dropped to below the detection limit, the cells were cultured for either an additional five passages (P3 to P8) or nine passages (P3 to P12) in the presence of IFN-RBV (control) or IFN-RBV and one of the three iminosugar derivatives, NB-DNJ (10, 50, or 100 μM), N7-DGJ (100 μM), or NN-DNJ (50 μM). This allowed us to determine both the length of time and the concentration of iminosugars needed for inclusion in a triple-combination treatment that would prevent viral relapse when treatment was subsequently stopped. At the two triple-combination-treatment end points chosen (P8 and P12, respectively), each sample was divided into three sets to allow the assessment of various follow-up treatment regimens: the cells in set 1 continued to be cultured in the presence of IFN-RBV (control) or the IFN-RBV-iminosugar triple cocktails; in set 2, all drugs were removed from the cells; and in set 3, IFN-RBV was removed; i.e., the cells continued to be cultured in the presence of the iminosugar derivative only (iminosugar maintenance therapy). MTS-based cell proliferation assays confirmed that antiviral effects observed were not due to cytotoxicity, which was not significant for any of the drug combinations tested compared to the results for the non-drug-treated controls passaged in parallel with the treated samples (data not shown).
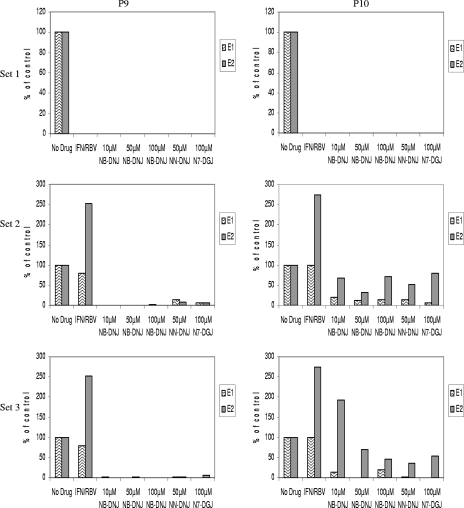
The supernatants of cells grown in the continuous presence of either the IFN-RBV double combination or any of the triple combinations that included an iminosugar (set 1) did not contain any viral RNA, as measured by real-time RT-PCR (Fig. 2, P9 and P10), or any infectious virus, as determined by infectivity studies with naïve MDBK cells (data not shown) in the two subsequent passages analyzed. In contrast, when either all three drugs (set 2) or IFN-RBV only (set 3) were removed at P8 (the earlier of the two triple-combination end points), we observed viral rebound in all samples. In set 2, one passage after drug removal, viral rebound was immediate and most pronounced for samples that had been treated for five passages with IFN-RBV only. Viral RNA was also detected in samples treated with IFN-RBV in combination with 50 μM NN-DNJ or 100 μM N7-DGJ (Fig. 2, set 2, P9). Viral rebound was delayed by one passage, to P10, for those samples that had been treated with IFN-RBV in combination with NB-DNJ (Fig. 2, set 2, P10). Viral titers were higher in samples treated with IFN-RBV only than in those treated with triple cocktails, suggesting that iminosugars may be able to control or delay viral rebound after removal of the drugs (Fig. 2, set 2, P10). This observation is supported by the results obtained with sample set 3, where, in the continued presence of an iminosugar, little or no viral RNA was detected one passage after the removal of IFN-RBV, although again, rebound was only delayed and not entirely prevented (Fig. 2, set 3, P9 and P10). In addition, infectious virus was detected, as determined by infectivity studies with naïve MDBK cells, for both set 2 (see Fig. 4) and set 3 (data not shown) in the two subsequent passages analyzed.
FIG. 2.
After eight passages of the various drug treatments, all drugs were either left on (set 1) or removed (set 2) or cells continued to be cultured in the presence of an iminosugar only for a further two passages (set 3). In all cases, for those columns denoted with an iminosugar concentration, the cells had been treated with a triple combination of IFN-RBV and an iminosugar of that concentration for five passages. The numbers of viral RNA copies from supernatants harvested at P9 (left column) and P10 (right column) were measured by real-time RT-PCR and are shown as a percentage of the numbers of copies for the non-drug-treated BVDV-infected control. The data presented are from two independent experiments (experiments E1 and E2). At P9 and P10, the supernatants from the no-drug control in experiment E1 contained 8.71 × 106 and 5.05 ×106 RNA copies/ml, respectively; and at P9 and P10 in experiment E2, the supernatants contained 5.1 ×106 and 5.5 × 106 RNA copies/ml, respectively.
FIG. 4.
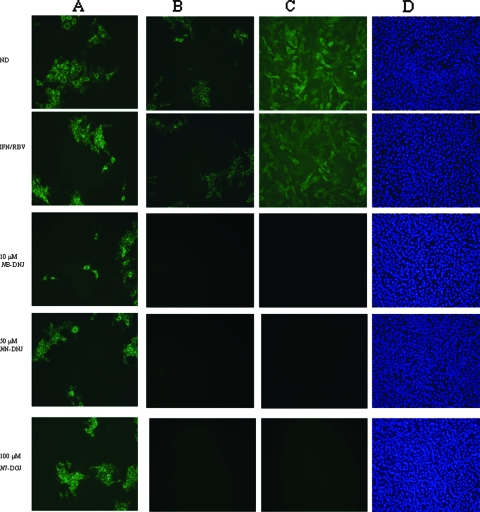
IF analysis of naïve MDBK cells incubated with supernatants from treated BVDV-infected cells (set 2) at P10 (A) and P22 (B) and of long-term-treated BVDV-infected MDBK cells (set 2) at P22 (C). The cells were fixed and probed with a monoclonal antibody against the BVDV NS2 and NS3 proteins, followed by incubation with an anti-mouse FITC-conjugated secondary antibody (green). Cell nuclei were stained with DAPI (D). ND, no drug.
Combination treatment with IFN-RBV and iminosugars eradicates BVDV infection from a persistently infected MDBK cell line in a time-dependent manner.
As combination therapy with IFN-RBV-an iminosugar for five passages was not sufficient to prevent viral relapse after the cessation of treatment, we continued treatment with the triple combination for a further four passages; i.e., these cells were treated with IFN-RBV or the various triple combinations for nine passages (27 days) in total. At P12, the samples were divided into three sets. The experiment was repeated as before, but this time, the samples were monitored for a further 10 passages (P12 to P22, 30 days).
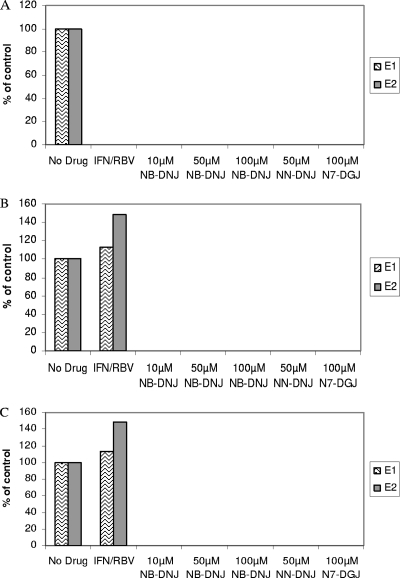
In the continued presence of all three drugs (set 1), no viral RNA was detected by RT-PCR in any of the samples under these conditions. By the conclusion of the experiment, the cells had been treated with IFN-RBV for 22 passages (or with triple cocktails for 19 passages) without any viral breakthrough occurring (Fig. 3A).
FIG. 3.
After 12 passages of the various drug treatments, all drugs were either left on (A) or removed (B) or cells continued to be cultured in the presence of an iminosugar only for a further 10 passages (30 days) (C). The numbers of viral RNA copies at P22 were measured by real-time RT-PCR and are shown as a percentage of the numbers of copies for the non-drug-treated BVDV-infected control. The data presented are from two independent experiments (experiments E1 and E2) in which the no-drug control contained 1.13 × 107 and 4.6 ×106 RNA copies/ml, respectively.
Importantly, even after the removal of all drugs (Fig. 3B, set 2a) or IFN-RBV (Fig. 3C, set 3a), no viral rebound was detected, as measured by RT-PCR, in those samples that had been treated with an IFN-RBV-an iminosugar triple cocktail, whereas viral rebound was detected in samples that had been treated with IFN-RBV only (Fig. 3B and C). These data clearly indicate that the inclusion of an iminosugar is essential to the eradication of BVDV infection entirely and the prevention of viral rebound. The RT-PCR data were confirmed by IF infectivity assays performed by infecting naïve cells with harvested supernatants. Infection was detected only in those samples that had not been drug treated at all or that had been treated with IFN-RBV alone (Fig. 4B). In addition, after the final passage (P22), the long-term-treated cells were also probed for the presence of BVDV, and the cells treated with iminosugars were found to be negative (Fig. 4C). Together, these data indicate that extended treatment with any of the IFN-RBV-NB-DNJ, IFN-RBV-NN-DNJ, or IFN-RBV-N7-DGJ triple cocktails is able to clear the BVDV infection from a persistently infected MDBK cell line.
Treatment with IFN-RBV and NB-DNJ eradicates BVDV infection from a persistently infected MDBK cell line in a dose-dependent manner and prevents viral rebound.
Having demonstrated the efficacy of iminosugars in combination with IFN and RBV, we next explored the minimum concentration of NB-DNJ required to eradicate BVDV infection from a persistently infected MDBK cell line. We performed the same cell culture experiments with triple cocktails of IFN-RBV-NB-DNJ containing NB-DNJ at lower concentrations. Infected MDBK cells were cultured in the presence of IFN-RBV for three passages until the viral RNA signal dropped below detectable levels. Subsequently, the medium was supplemented with 0.1, 1, or 10 μM NB-DNJ and the cells were cultured for a further nine passages (P3 to P12) in the presence of the triple combinations. The cells were then divided into three sets as before, and the cells receiving follow-up treatments were analyzed by both RT-PCR and infectivity assays.
For infected cells that had been treated with IFN-RBV only, removal of these two drugs led to an immediate and pronounced rebound of viral RNA in the supernatant, the levels of which fluctuated (Table 1). After a large initial surge at P13, viral RNA levels dropped for several weeks but were higher at the final reading (P32) than the viral RNA levels in the untreated controls.
TABLE 1.
Real time RT-PCR analysis of secreted viral RNA levels in the supernatants of cells under various treatment regimensa
| Seta | Treatmentb | RNA level atc:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P13 | P14 | P15 | P16 | P17 | P18 | P20 | P22 | P23 | P24 | P27 | P29 | P31 | P32 | ||
| Set 1 | No drug | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| IFN-RBV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0.1 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| IFN-RBV-10 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Set 2 | IFN-RBV | 0.7 | 967 | 17 | 42 | 21 | 34 | 21 | 118 | 84 | 80 | 86 | 138 | 90 | 130 |
| 0.1 μM NB-DNJ | 0 | 21 | 45 | 542 | 26 | 46 | 40 | 4 | 28 | 9 | 65 | 91 | 122 | 77 | |
| 1 μM NB-DNJ | 0 | 0 | 84 | 106 | 6 | 23 | 23 | 136 | 10 | 9 | 78 | 163 | 151 | 86 | |
| 10 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Set 3 | 0.1 μM NB-DNJ | 0 | 0 | 0 | 0 | 34 | 12 | 22 | 31 | 12 | 10 | 61 | 77 | 74 | 63 |
| 1 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 μM NB-DNJ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Copy no./ml (106)d | 47.92 | 20.13 | 56.96 | 70.80 | 19.73 | 38.83 | 10.43 | 78.4 | 20.49 | 40.12 | 40.38 | 15.06 | 31.20 | 30.27 | |
Set 1, all drug pressure is maintained; set 2, all drugs were removed; set 3, NB-DNJ maintenance therapy.
For those rows denoted with an iminosugar concentration, the cells had been treated with a triple concentration of IFN-RBV and an iminosugar of that concentration for nine passages.
RNA levels are expressed as the percentages of those in non-drug-treated controls. The data are representative of those from three independent experiments.
RNA copy numbers for the no-drug control samples.
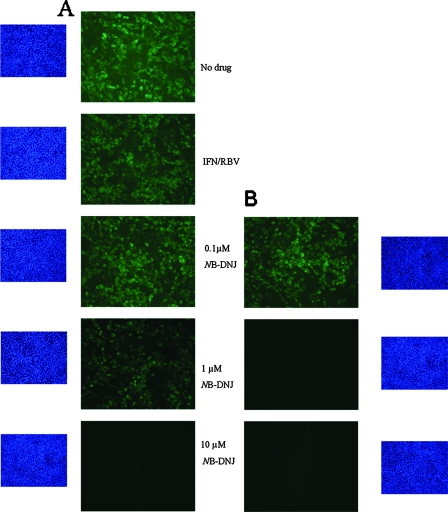
While in the continuous presence of drugs no viral RNA could be detected (Table 1, set 1), the removal of all three drugs resulted in viral rebound in those samples that had been treated with triple cocktails containing NB-DNJ at concentrations below 10 μM (Table 1, set 2). Consistent with the results of the previous experiment, neither viral RNA (Table 1) nor infectious virus (data not shown) was detected for those cells that had been treated for nine passages with IFN-RBV-10 μM NB-DNJ. In addition, when treatment with NB-DNJ at either 1 or 10 μM was maintained and only IFN and RBV were removed, no viral RNA (Table 1, set 3) or infectious virus (data not shown) was detected. Although viral rebound was delayed by four passages, it was observed under maintenance treatment with 0.1 μM NB-DNJ. These data indicate that inclusion of 10 μM NB-DNJ in the triple cocktail for the duration of nine passages is sufficient to permanently eradicate the virus even after the withdrawal of all three drugs, whereas the inclusion of 1 μM NB-DNJ in the initial triple cocktail requires continued maintenance with 1 μM NB-DNJ after the cessation of IFN-RBV treatment. The results for treated cells determined by IF mirrored the real-time PCR results; the removal of all three drugs resulted in viral rebound in those samples that had been treated with triple cocktails containing NB-DNJ at concentrations below 10 μM, as indicated by the IF detection of the BVDV NS2 and NS3 proteins in the cells (Fig. 5A). However, the continued presence of 1 μM NB-DNJ was sufficient to prevent viral rebound (Fig. 5B).
FIG. 5.
IF analysis of treated BVDV-infected MDBK cells at P32. At 20 passages (60 days) after the removal of all drugs (A) or after the removal IFN-RBV (iminosugar maintenance) (B), cells were fixed and probed with a monoclonal antibody against the BVDV NS2 and NS3 proteins, followed by incubation with an anti-mouse FITC-conjugated secondary antibody (green). Cell nuclei were stained with DAPI (blue).
DISCUSSION
Since the establishment of an HCVcc system (13, 24, 26), it has become possible to study most aspects of HCV morphogenesis and the HCV infection process. Unlike the BVDV system, though, the most permissive HCVcc system(s) suffers from low infection and viral secretion levels after the “crisis phase” of widespread cytopathology and slow subsequent recovery (27; our unpublished observations). In addition, some cells within the persistently infected HCVcc become resistant to HCV infection (27), complicating the ability to clearly distinguish between the antiviral effects of the drugs and the emerging resistance of the cells. We found that it is still not possible to consistently grow HCV-infected cells that have the secreted viral RNA levels required to study long-term treatment with more slowly acting compounds, such as iminosugars, that target the morphogenesis process of the virus rather than viral RNA replication. In this respect BVDV, a close relative of HCV which supports the secretion of infectious virions in vitro, is still the surrogate model of choice, especially since viral relapse after IFN-RBV treatment, an event frequently observed after the cessation of anti-HCV therapy (1, 20), is mirrored in the BVDV-MDBK cell system (Table 1) but has not yet been reported in the HCVcc infectivity system.
We used the BVDV model system to show that the addition of morphogenesis inhibitors to IFN-RBV has the potential to eradicate virus from persistently infected cells and to prevent viral relapse after treatment is stopped. Supplementing the drugs that comprise the current standard of care with compounds such as iminosugars, which target entirely different steps in the viral life cycle, is sufficient to cope with those undetectable (by real time RT-PCR and infectivity assays) yet undisputedly remaining viruses that lead to the quick and frequently strong viral reoccurrence and expansion after IFN and RBV are removed. DNJ-containing iminosugars cause misfolding of the viral envelope glycoproteins (via ER α-glucosidase inhibition) and the subsequent impairment of viral secretion and infectivity, as we have demonstrated for BVDV (2, 6) and HCV (23). In addition, we have shown that long alkyl chain-containing iminosugars (such as NN-DNJ, NN-DGJ, and N7-DGJ) inhibit the viral ion channel p7 (19, 23), which is crucial for the secretion of infectious virus for both BVDV (10) and HCV (11, 21, 22). Long alkyl chain-carrying DNJ compounds employ both mechanisms of action, making them attractive for future drug development.
In a previous study with the same BVDV-MDBK cell system, four passages were needed to reduce the viral signal to undetectable levels by semiquantitative RT-PCR by using 100 IU IFN and 2 μM RBV (7). In the current study we aimed to achieve a faster drop in viral RNA levels and used 1,000 IU IFN and 1 μM RBV, which also were not toxic for the duration of the experiment yet were sufficient to attain elimination of the viral signal at least two passages earlier, as determined by a more sensitive real-time PCR detection method.
In the study presented here, we focused mainly on the short alkyl chain-carrying ER α-glucosidase inhibitor NB-DNJ, as this compound, with its history of safe use (5, 18), could be a lead candidate for rapid progression to clinical anti-HCV trials.
In the BVDV-MDBK cell system, the inclusion of 10 μM NB-DNJ successfully prevented relapse after the cessation of triple therapy, and 1 μM NB-DNJ was sufficient to prevent relapse when it was administered continuously as monotherapy during maintenance treatment after the removal of IFN-RBV. This concentration range can be achieved and tolerated in human patients (5, 18). We propose that the likelihood that viruses will accumulate mutations which could enable them to become independent of either iminosugar target (the host cell-encoded ER α-glucosidases or the p7 ion channel) is much reduced compared to the demonstrated speed at which viral escape mutants emerge in the presence of inhibitors targeting virally encoded enzymes, such as the polymerase or protease (4, 16).
Ouzounov et al., who analyzed a single replication cycle of cytopathic BVDV (17), have reported that higher NB-DNJ concentrations in combination with IFN show a greater than additive antiviral effect in an experimental setting with an MOI of >1. The ncp BVDV system used in our study mirrors a chronic viral infection such as that with HCV, with a lower MOI over several viral and cellular replication cycles. In this system, no synergy was observed when physiologically achievable NB-DNJ concentrations were added to a high-concentration IFN-RBV combination; i.e., when iminosugars were added to IFN-RBV at the start of the treatment, the time needed to reduce the viral signal below the detection limits remained the same whether NB-DNJ was or was not added at the start of treatment with IFN-RBV (data not shown). We hypothesize that this is due to the low NB-DNJ concentrations used compared to those of IFN and RBV. The last two compounds are fast-acting drugs which can achieve drops in viral RNA levels on a log order scale within a few days, whereas iminosugars are slower acting drugs which do not directly affect viral RNA synthesis or replication. Therefore, we added the iminosugars after the initial IFN-RBV-induced strong decrease in viral RNA levels instead, when the potentially available mutant pool was the smallest. By our treatment protocol, all iminosugar derivatives tested showed efficacy and the potential to eradicate persistent BVDV infection from MDBK cells and prevented viral relapse after the cessation of treatment. For NB-DNJ, we showed that this was time and dose dependent.
Significantly, because of the targets involved, all HCV genotypes, including the challenging genotype 1, which is responsible for most cases of viral relapse observed in human patients (9, 14), are predicted to respond to iminosugar treatment. We have already shown in the cell culture infectivity system that HCV chimeras encoding the envelope glycoproteins and p7 of genotypes 1a, 1b, and 2a respond to NB-DNJ, NN-DNJ, and NN-DGJ to similar extents (23). Next, we aim to demonstrate that the short alkyl chain-carrying ER α-glucosidase inhibitor NB-DNJ prevents viral rebound in treated HCV-infected patients. In addition, we are improving the delivery and tolerability of long alkyl chain iminosugar derivatives to take advantage of their additional ability to inhibit the p7 ion channel and develop a dual target drug for clinical use against HCV.
Supplementary Material
Acknowledgments
This work was supported by United Therapeutics Corporation, the EU Sixth Framework Program (VIRGIL), and the Oxford Glycobiology Institute Endowment. N.Z. is a senior research fellow of Linacre College, Oxford.
We thank Jo O'Leary for critical reading of the manuscript.
Footnotes
Published ahead of print on 3 March 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Basso, M., F. Torre, A. Grasso, G. Percario, E. Azzola, S. Artioli, S. Blanchi, N. Pelli, and A. Picciotto. 2007. Pegylated interferon and ribavirin in re-treatment of responder-relapser HCV patients. Dig. Liver Dis. 39:47-51. [DOI] [PubMed] [Google Scholar]
- 2.Branza-Nichita, N., D. Durantel, S. Carrouee-Durantel, R. A. Dwek, and N. Zitzmann. 2001. Antiviral effect of N-butyldeoxynojirimycin against bovine viral diarrhea virus correlates with misfolding of E2 envelope proteins and impairment of their association into E1-E2 heterodimers. J. Virol. 75:3527-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapel, C., C. Garcia, B. Bartosch, P. Roingeard, N. Zitzmann, F. L. Cosset, J. Dubuisson, R. A. Dwek, C. Trepo, F. Zoulim, and D. Durantel. 2007. Reduction of the infectivity of hepatitis C virus pseudoparticles by incorporation of misfolded glycoproteins induced by glucosidase inhibitors. J. Gen. Virol. 88:1133-1143. [DOI] [PubMed] [Google Scholar]
- 4.Courcambeck, J., M. Bouzidi, R. Perbost, B. Jouirou, N. Amrani, P. Cacoub, G. Pepe, J. M. Sabatier, and P. Halfon. 2006. Resistance of hepatitis C virus to NS3-4A protease inhibitors: mechanisms of drug resistance induced by R155Q, A156T, D168A and D168V mutations. Antivir. Ther. 11:847-855. [PubMed] [Google Scholar]
- 5.Cox, T., R. Lachmann, C. Hollak, J. Aerts, S. van Weely, M. Hrebicek, F. Platt, T. Butters, R. Dwek, C. Moyses, I. Gow, D. Elstein, and A. Zimran. 2000. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet 355:1481-1485. [DOI] [PubMed] [Google Scholar]
- 6.Durantel, D., N. Branza-Nichita, S. Carrouee-Durantel, T. D. Butters, R. A. Dwek, and N. Zitzmann. 2001. Study of the mechanism of antiviral action of iminosugar derivatives against bovine viral diarrhea virus. J. Virol. 75:8987-8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durantel, D., S. Carrouee-Durantel, N. Branza-Nichita, R. A. Dwek, and N. Zitzmann. 2004. Effects of interferon, ribavirin, and iminosugar derivatives on cells persistently infected with noncytopathic bovine viral diarrhea virus. Antimicrob. Agents Chemother. 48:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feld, J. J., and J. H. Hoofnagle. 2005. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 436:967-972. [DOI] [PubMed] [Google Scholar]
- 9.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 10.Harada, T., N. Tautz, and H. J. Thiel. 2000. E2-p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 74:9498-9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, C. T., C. L. Murray, D. K. Eastman, J. Tassello, and C. M. Rice. 2007. Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol. 81:8374-8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Land, A., and I. Braakman. 2001. Folding of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum. Biochimie 83:783-790. [DOI] [PubMed] [Google Scholar]
- 13.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 14.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 15.National Health Service. 2007. Treatment for hepatitis C. National Health Service, United Kingdom. http://www.hepc.nhs.uk/professionals/treatment.html.
- 16.Neyts, J. 2006. Selective inhibitors of hepatitis C virus replication. Antivir. Res. 71:363-371. [DOI] [PubMed] [Google Scholar]
- 17.Ouzounov, S., A. Mehta, R. A. Dwek, T. M. Block, and R. Jordan. 2002. The combination of interferon alpha-2b and N-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: implications for hepatitis C virus (HCV) therapy. Antivir. Res. 55:425-435. [DOI] [PubMed] [Google Scholar]
- 18.Patterson, M. C., D. Vecchio, H. Prady, L. Abel, and J. E. Wraith. 2007. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 6:765-772. [DOI] [PubMed] [Google Scholar]
- 19.Pavlovic, D., D. C. Neville, O. Argaud, B. Blumberg, R. A. Dwek, W. B. Fischer, and N. Zitzmann. 2003. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc. Natl. Acad. Sci. USA 100:6104-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poynard, T., M. F. Yuen, V. Ratziu, and C. L. Lai. 2003. Viral hepatitis C. Lancet 362:2095-2100. [DOI] [PubMed] [Google Scholar]
- 21.Sakai, A., M. S. Claire, K. Faulk, S. Govindarajan, S. U. Emerson, R. H. Purcell, and J. Bukh. 2003. The p7 polypeptide of hepatitis C virus is critical for infectivity and contains functionally important genotype-specific sequences. Proc. Natl. Acad. Sci. USA 100:11646-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinmann, E., F. Penin, S. Kallis, A. H. Patel, R. Bartenschlager, and T. Pietschmann. 2007. Hepatitis C virus p7 protein is crucial for assembly and release of infectious virions. PLoS Pathog. 3:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmann, E., T. Whitfield, S. Kallis, R. A. Dwek, N. Zitzmann, T. Pietschmann, and R. Bartenschlager. 2007. Antiviral effects of amantadine and iminosugar derivatives against hepatitis C virus. Hepatology 46:330-338. [DOI] [PubMed] [Google Scholar]
- 24.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werr, M., and R. Prange. 1998. Role for calnexin and N-linked glycosylation in the assembly and secretion of hepatitis B virus middle envelope protein particles. J. Virol. 72:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong, J., P. Gastaminza, J. Chung, Z. Stamataki, M. Isogawa, G. Cheng, J. A. McKeating, and F. V. Chisari. 2006. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J. Virol. 80:11082-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitzmann, N., A. S. Mehta, S. Carrouee, T. D. Butters, F. M. Platt, J. McCauley, B. S. Blumberg, R. A. Dwek, and T. M. Block. 1999. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA 96:11878-11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.