Abstract
l-Buthionine (S,R)-sulfoximine (BSO) at a dose of 220 mg/kg of body weight/day showed an anti-Trypanosoma cruzi effect in infected mice, increasing their survival rate and decreasing the parasitemias and parasite burden in the hearts. Treatment with BSO plus nifurtimox caused an increase in the survival rate in comparison to the rates with treatment with each drug alone.
Chagas' disease is a clinical condition caused by Trypanosoma cruzi. The limited success and side-effect liabilities of Chagas' disease therapy have led to a continued search for new antitrypanosomal treatments (16, 17).
The currently used drugs are nifurtimox (Nx) and benznidazole, which act through the formation of free radicals and electrophilic metabolites, respectively (12, 14). Both drugs are trypanocidal against all forms of the parasite (16).
Aerobic organisms, such as Trypanosoma cruzi, are exposed to reactive oxygen species generated by their metabolism. T. cruzi cells have to handle their endogenously produced reactive oxygen species and also cope with the oxidative burst delivered by the host's immune system and the free radicals and electrophilic metabolites generated by the antichagasic drugs (2, 12, 15).
The redox metabolism of trypanosomatids is based on a bis-glutathionylspermidine conjugate, trypanothione, and on the flavoenzyme trypanothione reductase (1, 15).
Trypanothione is synthesized by an ATP-dependent reaction catalyzed by trypanothione synthetase using the tripeptide glutathione (GSH) and the polyamine spermidine as substrates. GSH synthesis involves two ATP-requiring enzymes. The first is γ-glutamylcysteine synthetase and is the rate-limiting step (10). The second is GSH synthetase. GSH synthesis is regulated primarily through γ-glutamylcysteine synthetase by cysteine availability and GSH feedback inhibition (10).
The glutamate analog l-buthionine (S,R)-sulfoximine (BSO) inhibits the synthesis of glutathionylcysteine and, thus, of GSH and trypanothione. The results of a previous report indicate that BSO increased the effects of Nx and benznidazole in an in vitro model of Chagas' disease (7).
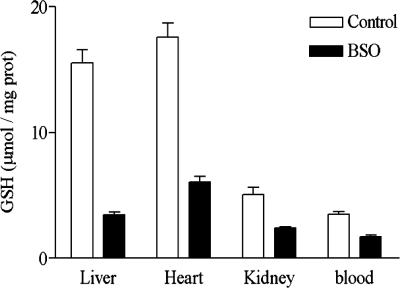
In agreement with the results of a previous report (9), a significant decrease in GSH content in liver, heart, blood, and kidney in BALB/c mice treated intraperitoneally with 220 mg/kg of body weight/day of BSO (Fig. 1) was observed after 20 days of treatment. The BSO treatment did not show toxic effect. The concentration of GSH was determined by high-pressure liquid chromatography separation and quantification of monobromobimane derivatives (6, 13). In the host, BSO can decrease GSH levels (Fig. 1), but mammalian cells exert several efficient GSH-independent antioxidant mechanisms (3).
FIG. 1.
Effect of BSO upon GSH content in BALB/c mice. Bars indicate standard error of the means.
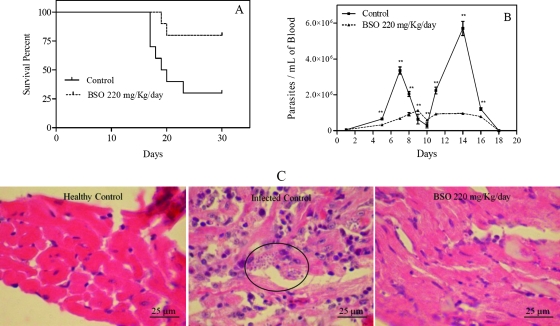
Figure 2 shows the effect of BSO upon mice infected with 30,000 T. cruzi metacyclic trypomastigotes of clone Dm 28c. A dose of 220 mg/kg/day of BSO during 20 days significantly increased the survival rate of the mice, from 25% to 80% (Fig. 2A). The Kaplan-Meier nonparametric method was used to estimate the survival functions of the different experimental groups. Rank tests (log rank) were used to compare them (P = 0.0199). Figure 2B shows a significant decrease in parasitemias (P value of <0.0001 by two-way analysis of variance and Bonferroni's posttest). Figure 2C shows the decrease in parasite burden in the hearts of infected mice, dyed with hematoxylin and eosin stain. BSO at 30 mg/kg/day had no significant effect on the survival rate, parasitemia, or parasite burden in treated mice in comparison to those in control mice. Survival with a 440-mg/kg/day dose of BSO showed a difference from survival with 220 mg/kg/day, but this was not significant, most probably because the effects were close to the maximum possible survival. The data shown in Fig. 2 are the results of at least three independent experiments.
FIG. 2.
BSO increases the survival rate and decreases parasitemia and parasite burden in hearts of Trypanosoma cruzi-infected mice. Error bars show standard deviations. An amastigote nest is circled in panel C.
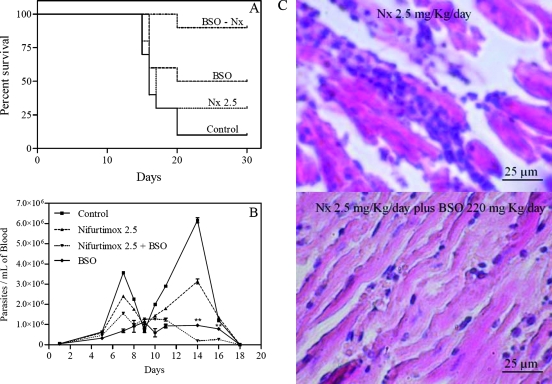
Figure 3 shows the effects of BSO in combination with Nx on T. cruzi-infected mice. Doses of 0.1 to 10 mg/kg/day of Nx were tested in survival assays. The 2.5-mg/kg/day dose was chosen because mice treated with that dose showed a moderate decrease in survival (25%). In contrast, mice treated with 10 mg/kg/day of Nx showed 100% survival rates, masking any enhancing effect in the BSO combination. Figure 3A shows that Nx at a dose of 2.5 mg/kg/day had no significant effect on survival compared to the survival of controls (P = 0.3589). Mice treated with BSO showed a significant difference in survival rate compared with the survival rate of controls (P = 0.0407), and mice treated with Nx plus BSO showed a significant difference in survival rate compared with the survival rates of controls (P = 0.0002), of mice treated with Nx alone (P = 0.0043), and of mice treated with BSO alone (P = 0.0467), indicating an increase in the survival rate when the two drugs are used in combination. When parasitemia data were analyzed by two-way analysis of variance and Bonferroni's posttest, it was observed that Nx at 2.5 mg/kg/day had a significant effect on parasitemia compared with the parasitemia in the control mice (P < 0.0001), contrary to the negative result for the survival rate. Mice treated with BSO alone and BSO plus Nx had significant decreases in parasitemia compared with the parasitemia in the control mice (P < 0.0001); also, treatment with BSO plus Nx decreased parasitemia significantly compared to treatment with Nx (P < 0.001). Nevertheless, there were differences between the results of treatment with BSO and with BSO plus Nx (P < 0.0001) at days 14 and 16 (Fig. 3B). Figure 3C shows the decrease in parasite burden in the hearts of infected mice, dyed with hematoxylin and eosin stain, that were treated with BSO plus Nx compared with the burden in hearts of mice treated with Nx alone and that in untreated controls (Fig. 2C). All images of parasite burdens in hearts are photographs representative of three random slices each of heart tissue from at least three surviving mice for each experimental condition.
FIG. 3.
BSO increases the effect of Nx in Trypanosoma cruzi-infected mice. Error bars show standard deviations.
Mice infected with Trypanosoma brucei brucei have been cured with BSO alone (2). Similar approaches with an in vitro model using megazol in T. brucei (4) and Leishmania infections have been reported (11).
In humans, the BSO-GSH synthesis inhibition strategy used to overcome resistance and to potentiate the effect of antineoplastic agents, such as doxorubicin, melphalan, or cyclophosphamide, has been successful. In phase I trials, the concentration of BSO in blood reached 0.5 to 1 mM, reducing by 80% the GSH level in white blood cells (5).
BSO alone shows an important trypanocidal effect (Fig. 2A, B, and C), which may be explained by higher thiol decreases in the parasites than in the host.
In addition, BSO increases the effect of Nx on survival of treated mice (Fig. 3A), but BSO plus Nx did not show a significant modification of parasitemia in comparison to the parasitemia with BSO alone (Fig. 3B). The same effect was observed for the heart parasitic burden (not shown).
Our studies suggest that the use of BSO alone or in combination with Nx could lower the dose of the drug needed to obtain the same clinical effect and, consequently, should diminish its side effects and/or the duration of therapy. Nevertheless, the decrease of thiol groups in the host might increase the mutagenic effects of Nx (8).
Acknowledgments
We acknowledge the critical review of this paper by Bruce Cassels from the Faculty of Sciences, University of Chile.
FONDECYT-Chile, grant 1061072, and Proyecto Bicentenario Anillo, grant ACT29, supported this research.
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Ariyanayagam, M. R., and A. H. Fairlamb. 2001. Ovothiol and trypanothione as antioxidants in trypanosomatids. Mol. Biochem. Parasitol. 115:189-198. [DOI] [PubMed] [Google Scholar]
- 2.Arrick, B. A., O. W. Griffith, and A. Cerami. 1981. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. J. Exp. Med. 153:720-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dröge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47-95. [DOI] [PubMed] [Google Scholar]
- 4.Enanga, B., M. R. Ariyanayagam, M. L. Stewart, and M. P. Barrett. 2003. Activity of megazol, a trypanocidal nitroimidazole, is associated with DNA damage. Antimicrob. Agents Chemother. 47:3368-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrela, J. M., A. Ortega, and E. Obrador. 2006. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43:143-181. [DOI] [PubMed] [Google Scholar]
- 6.Fairlamb, A. H., G. B. Henderson, C. J. Bacchi, and A. Cerami. 1987. In vivo effects of difluoromethylornithine on trypanothione and polyamine levels in bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 24:185-191. [DOI] [PubMed] [Google Scholar]
- 7.Faúndez, M., L. Pino, P. Letelier, C. Ortiz, R. López, C. Seguel, J. Ferreira, M. Pavani, A. Morello, and J. D. Maya. 2005. Buthionine sulfoximine increases the toxicity of nifurtimox and benznidazole to Trypanosoma cruzi. Antimicrob. Agents Chemother. 49:126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorla, N. B., O. S. Ledesma, G. P. Barbieri, and I. B. Larripa. 1989. Thirteen fold increase of chromosomal aberrations non-randomly distributed in chagasic children treated with nifurtimox. Mutat. Res. 224:263-267. [DOI] [PubMed] [Google Scholar]
- 9.Griffith, O. W., and A. Meister. 1979. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254:7558-7560. [PubMed] [Google Scholar]
- 10.Griffith, O. W. 1999. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 27:922-935. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor, P., M. Sachdev, and R. Madhubala. 2000. Inhibition of glutathione synthesis as a chemotherapeutic strategy for leishmaniasis. Trop. Med. Int. Health 5:438-442. [DOI] [PubMed] [Google Scholar]
- 12.Maya, J. D., B. K. Cassels, P. Iturriaga-Vásquez, J. Ferreira, M. Faúndez, N. Galanti, A. Ferreira, and A. Morello. 2007. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. A 146:601-620. [DOI] [PubMed] [Google Scholar]
- 13.Maya, J. D., S. Bollo, L. J. Núñez-Vergara, J. A. Squella, Y. Repetto, A. Morello, J. Perie, and G. Chauvière. 2003. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem. Pharmacol. 65:999-1006. [DOI] [PubMed] [Google Scholar]
- 14.Moreno, S. N., R. Docampo, R. P. Mason, W. Leon, and A. O. Stoppani. 1982. Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi microsomal preparations. Arch. Biochem. Biophys. 218:585-591. [DOI] [PubMed] [Google Scholar]
- 15.Muller, S., E. Liebau, R. D. Walter, and R. L. Krauth-Siegel. 2003. Thiol-based redox metabolism of protozoan parasites. Trends Parasitol. 19:320-328. [DOI] [PubMed] [Google Scholar]
- 16.Rodriques Coura, J., and S. L. de Castro. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira, A. R., N. Nitz, M. C. Guimaro, C. Gomes, and C. A. Santos-Buch. 2006. Chagas disease. Postgrad. Med. J. 82:788-798. [DOI] [PMC free article] [PubMed] [Google Scholar]