Abstract
ST-246 is a novel, potent orthopoxvirus egress inhibitor that is being developed to treat pathogenic orthopoxvirus infections of humans. This phase I, double-blind, randomized, placebo-controlled single ascending dose study (first time with humans) was conducted to determine the safety, tolerability, and pharmacokinetics of ST-246 in healthy human volunteers. ST-246 was administered in single oral doses of 500, 1,000, and 2,000 mg to fasting healthy volunteers and 1,000 mg to nonfasting healthy volunteers. ST-246 was generally well tolerated with no serious adverse events, and no subject was withdrawn from the study due to ST-246. The most commonly reported drug-related adverse event was neutropenia, which was found, upon further analysis, not to be treatment related. ST-246 was readily absorbed following oral administration with mean times to maximum concentration from 2 h to 3 h. Absorption was greater in nonfasting volunteers than in fasting volunteers. Administration of ST-246 resulted in exposure levels predicted to be sufficient for inhibiting orthopoxvirus replication compared to exposure levels in nonhuman primates in which ST-246 protected animals from lethal orthopoxvirus infection.
Historically, variola virus, the etiologic agent of smallpox, has been estimated to have killed, crippled, or disfigured nearly 10% of the human population prior to eradication (5). Smallpox is highly communicable and carries exceptionally high morbidity. Secondary attack rates among unvaccinated members of households in which someone had smallpox have been reported to range from 30% to 80%. Mortality rates range from 1% for variola minor to 30% for variola major. With the advent of biowarfare as an instrument of terrorism, smallpox can no longer be thought of as a disease of historic impact only (7, 13).
There are currently no therapies other than early vaccination that can alter the outcome of disease or potentially prevent disease in a population that has been exposed to smallpox. Vaccination carries an inherent risk of adverse events for certain immunosuppressed recipients and even some healthy recipients (2, 6). Moreover, vaccination is effective only if administered within 4 days postexposure (10). Thus, antiviral drugs used alone or potentially in combination with vaccination can be used to treat individuals during the window of vulnerability which occurs prior to development of protective immunity. Additionally, antiviral drugs could also be used in the treatment of zoonotic poxvirus disease in humans, such as monkeypox.
ST-246 (Tecovirimat; 4-trifluoromethyl-N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl)-benzamide) was discovered as part of a program to identify orally available antiviral drugs to prevent and treat smallpox viral infection (14). An initial hit was identified from a high-throughput screen of over 350,000 chemical compounds designed to identify inhibitors of vaccinia virus replication. This hit was potent (50% effective concentration of 1 μM), selective (cytotoxic concentration of drug that reduced the viable cell number by 50% of >50 μM), and active against a panel of orthopoxviruses, including variola virus in cell culture (1, 14). Approximately 200 analogs of the initial hit were synthesized in order to improve potency and metabolic stability. ST-246 emerged from this optimization program as being more potent (50% effective concentration of 25 nM) and stable (half-life [t1/2] of >200 min in an S9 in vitro assay) compared with the other analogs (1). ST-246 is chemically unique and unrelated to other substances shown to be active against orthopoxviruses.
Given the potential utility of ST-246 antiviral therapy against smallpox and the fact that there are no FDA-approved medications for the treatment of smallpox infection, there is clearly a need for a safe medication that can be taken by mouth that is highly active against variola virus. In this regard, ST-246 meets these specifications. Oral administration of ST-246 protected mice from lethal infection with vaccinia virus, cowpox virus, and ectromelia virus (11, 14). In addition, ST-246 was also found to be active against monkeypox virus in a ground squirrel model of severe monkeypox virus disease and against monkeypox and the authentic smallpox (variola) viruses in nonhuman primate lesional disease models (12) (unpublished results). The compound was well tolerated after 28-day multiple-dose administration in mice and nonhuman primates. After a single oral administration of radiolabeled ST-246 (100 mg/kg of body weight) in mice, there was very little difference in the rates and routes of excretion with approximately 95% of the delivered dose excreted by 96 h postadministration and approximately 58 to 77% of the radioactivity excreted within the first 24 h. Most of the excreted radioactivity was associated with the feces (71 to 77% of the administered dose) with the remainder recovered in urine (18 to 24% of the administered dose). Less than 0.25% of the dose remained in tissues 96 h postadministration.
The aims of this study were to determine the safety, tolerability, and clinical pharmacokinetics (PK) of ST-246 following single oral dose administration in healthy human subjects.
MATERIALS AND METHODS
Study design and population.
This was a randomized, double-blind, placebo-controlled study to compare the safety, tolerability, and PK of three different doses of ST-246 (500 mg, 1,000 mg, and 2,000 mg) and placebo when orally administered to fasting, healthy male and female volunteer subjects as a single dose in an ascending fashion, groups 1, 2, and 3, respectively. Additionally, a fourth group was evaluated for the safety, tolerability, and PK of ST-246 (1,000 mg) and placebo when orally administered to nonfasting, healthy male and female volunteer subjects as a single dose. This dose was included on the basis of the observation that in investigational new drug-enabling pharmacology studies in nonhuman primates, a significant (50%) increase in exposure was noted in the nonfasting versus fasting states. Nonfasting volunteers were fed a typical American breakfast prior to compound administration that contained approximately 53% fat (57 g and 513 fat calories), 32% carbohydrate (78 g and 312 carbohydrate calories), 15% protein (37 g and 148 protein calories) as suggested by the FDA food effect guidelines for a high-fat meal. Males and nonpregnant females from 18 to 50 years old receiving no concomitant medications were eligible to enroll in the study.
A total of 40 eligible subjects were sequentially enrolled into one of four study groups consisting of 10 subjects each. Within each study group of 10 subjects, 8 were randomly assigned to receive a single oral dose of ST-246 and 2 were randomly assigned to receive placebo. The SAS procedure PROC PLAN was used to randomly assign 10 subjects within each of the study groups in an allocation ratio of 4:1 and block size of five to ST-246 and placebo, respectively. Additionally, for each dose level in the study, the SAS computer program also generated one additional group of 10 subjects to be utilized in the event that a subject(s) needed to be replaced.
Subjects were screened within 28 days prior to enrollment to determine eligibility for participation. At this time, a medical history, physical examination, vital signs, routine laboratory tests, pregnancy test, drug screen, and electrocardiogram (ECG) were conducted. Women of child-bearing age were required to remain abstinent or use two forms of contraception to prevent pregnancy, since the full range of preclinical teratogenicity trials had not yet been completed. A subsequent visit was scheduled to update history, perform a physical exam, and to establish safety baseline values (laboratory tests and ECG) occurred within 7 days prior to receiving study drug. No concomitant medications were permitted during the study.
On day 0 (day of study drug administration), subjects had an intravenous catheter inserted for collection of various venous blood samples for PK assays (predose 0 h and specified times postdose over 48 h for groups 1 through 3 and over 72 h for group 4). A predose 0-h urine sample was collected for all subjects. Subjects then received study drug under direct observation of site staff. A 24-hour urine collection schedule, obtained as three 8-hour samples, was then initiated. An ECG was also performed on day 1 (24 h postdose). Follow-up visits occurred at week 1 (days 7 to 9) and week 4 (days 26 to 31) during which updates to medical history and physical exams were performed, and the study safety assessments of adverse events, vital signs, laboratory tests, and ECG (only at the week 4 visit) were performed. The safety results of each dose cohort were evaluated by the principal investigator and medical monitor prior to initiation of dosing for the next higher dose group.
Safety assessments.
Clinical laboratory tests, including hematology, chemistries (electrolytes, liver function tests, amylase, and lipase), urinalysis, and urine drug and alcohol screens, were conducted and reviewed prior to each dosing period. In addition, thyroid function tests and cholesterol panels were obtained prior to dosing. Clinical laboratory tests were repeated prior to discharge from each dosing period.
Dose selection.
Initial nonclinical efficacy evaluations have established an effective dose in mice at 100 mg/kg of body weight and in nonhuman primates of 30 mg/kg in lethal models of orthopoxvirus infection (14) (data not shown). Based on body surface area calculations in mice, this dose level represents a human equivalent dose of approximately 8 mg/kg (300 mg/m2) or 500 mg. The nonclinical safety of ST-246 has been established in acute and repeated-dose oral toxicity studies in mice, rats, and monkeys, and the genotoxic potential was assessed in vitro and in vivo (unpublished data). Based on the single-dose no observable adverse effect level (NOAEL) of 2,000 mg/kg and the multiple-dose no observable effect level (NOEL) of 300 mg/kg in monkeys and a 10-fold safety margin, 500 mg was selected as the starting human dose.
Blood and urine sample collection.
Serial venous blood samples for the determination of ST-246 concentrations in plasma were collected during each treatment period at the following times: predose and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 12 (±10 min), 24 (±1 h), and 48 (±1 h) hours postdose for groups 1 through 4. For group 4 only, an additional PK time point of 72 (±1 h) hours postdose was used. In addition, a predose urine sample and a 24-hour postdose urine sample (collected as three 8-hour collections) were used to evaluate urinary excretion of the drug for subjects in all groups. Samples were immediately centrifuged at 4°C for 10 min at 2,000 × g, and plasma samples were collected. Plasma samples were stored at −70°C until analyzed.
Bioanalysis.
ST-246 was quantified from human plasma specimens by a validated liquid chromatography and tandem mass spectrometry method using an analog of ST-246 as an internal standard. Human plasma (10 μl) was extracted with ammonium hydroxide-methanol solution, and the supernatant was injected onto a Phenomenex LUNA C18 column (30 by 2 mm; 5-μm particle size). The mobile phase consisted of water containing 0.05% acetic acid and 0.05% NH4OH. ST-246 was eluted using a 20% to 90% gradient of methanol containing 0.05% acetic acid and 0.05% NH4OH from 0.2 to 0.5 min, with a flow rate of 300 μl/min. Fractions were analyzed on a Sciex API 4000 triple quadrupole mass spectrometer, using turbospray ionization in the negative ion mode. Analytes were detected by multiple reactions monitoring the 375.1 m/z to 282.9 m/z transition for ST-246 and the 340.9 m/z to 248.9 m/z transition for the internal standard. The calibration curves were validated and were linear over the concentration range of 0.5 to 1,000 ng/ml.
Pharmacokinetic evaluation.
Using WinNonlin Professional version 3.1 (Pharsight Corporation, Mountain View, CA), noncompartmental PK parameters were derived from each subject's data using the actual sampling times. The maximum concentration of drug in serum (Cmax) (ng/ml) and time to Cmax (Tmax) (h) represent the maximum plasma drug concentration and maximum plasma drug concentration time, respectively, and were obtained directly from the observed concentration-versus-time data. AUClast (ng·h/ml) is the area under the plasma drug concentration-time curve from time zero until the last quantifiable time point and was calculated by the trapezoid rule using a minimum of four data points. AUC0-∞ (ng·h/ml) is the total area under the plasma drug concentration-time curve from time zero to infinity and was calculated by the trapezoid rule and extrapolated to infinity by adding the last quantifiable plasma drug concentration divided by the elimination rate constant (kel). The kel (1/h) was determined by linear regression of the terminal points on a semilogarithmic scale from the natural log (ln) of the plasma drug concentration-versus-time curve. Visual assessment was used to identify the terminal linear phase of the ln plasma drug concentration-time profile with a minimum of three data points used for the determination. The t1/2 (h) is the time needed to eliminate half of the drug from the blood at the terminal linear phase (terminal half-life). This parameter was determined by fitting a straight line through the set of points of the terminal linear phase on semilogarithmic scale. The t1/2 is equal to the ln(2)/kel.
The apparent oral clearance (CL [liter/h]) was calculated as the dose (D) divided by AUC0-∞. Provided that D is measured in mg and AUC0-∞ is measured in ng·h/ml, the following unit conversion formula was derived: CL (liter/h) = 1,000 × D (mg)/AUC0-∞ (ng·h/ml). Ae(0-24) (ng) corresponds to the cumulative amount of drug excreted from time zero to 24 h postdose and was calculated as the summation of the amounts (product of urine volume and urine concentration) excreted in subsequent collection intervals (24-hour urinary drug excretion).
These parameters were not calculated for a subject if the array of nonmissing concentrations was inadequate.
Statistical analysis.
PK parameters were summarized for each dose level of ST-246 (500 mg, 1,000 mg, and 2,000 mg) for the fasting subjects (groups 1 through 3) and for the nonfasting subjects in group 4 for ST-246 (1,000 mg). All summaries and listings were produced by the treatment the subject received. No formal multiple-comparison adjustments were employed. The PK population was used for all PK summaries. Subjects with partial data were evaluated on a case-by-case basis to determine whether sufficient data were available for meaningful analysis.
Plasma drug concentrations were summarized using descriptive statistics for each study group and nominal time point (population size, mean, standard deviation [SD], coefficient of variation [as a percentage], median, minimum, and maximum values). Below limit of quantification (BLQ) concentrations were treated as zero for descriptive statistics. Prior to the estimation of the PK parameters, BLQ concentrations were assigned a value of zero if they preceded quantifiable samples prior to Tmax. A BLQ concentration that was embedded between two quantifiable points was assigned a value of missing. BLQ values that occurred at the end of the collection interval were assigned a value of zero. If consecutive BLQ concentrations were followed by quantifiable concentrations, these quantified values were excluded from the PK analysis by assigning them a value of missing. Actual elapsed time from dosing was used in the final PK analysis to estimate all individual plasma drug PK parameters. Descriptive statistics were used to summarize the following PK parameters: Cmax, Tmax, AUClast, AUC0-∞, kel, t1/2, CL, and Ae(0-24). PK analyses were performed for ST-246 in both plasma and urine samples.
Plasma and urine drug PK parameters were summarized by treatment using descriptive statistics (population size, mean, SD, percent coefficient of variation, geometric mean, median, minimum, and maximum values). Individual PK parameters and appropriate descriptive statistics were reported to three significant figures.
RESULTS
Demographics.
A total of 38 subjects were randomized and treated with ST-246 or placebo. A summary of the demographic characteristics of the subjects is presented in Table 1. The mean age of subjects in the study ranged from approximately 30 to 33 years. There were more males than females in the fasting groups with a total of 56.5% males receiving active treatment and 60.0% receiving placebo; however, the distribution by gender varied for each dose group. Males and females were equally represented in the nonfasting treatment groups (50.0% each). Distribution by race also varied by dose group, with the majority of subjects in the fasting group given active treatment being white (56.5%) and the majority of subjects in the fasting group given placebo being black (80.0%) or of Hispanic or Latino ethnicity (56.5% receiving active treatment and 80.0% receiving placebo). In the nonfasting treatment group (group 4), the majority of subjects were black (62.5% receiving active treatment, 100% receiving placebo) or of Hispanic or Latino ethnicity (62.5% receiving active treatment, 50% receiving placebo). Most baseline vital signs were similar across treatment groups; in some cases, the mean values for subjects receiving placebo were slightly higher than for subjects receiving active treatment (pulse, systolic blood pressure, and the QT interval, a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle).
TABLE 1.
Demographic and baseline characteristics of study population
| Characteristic | Value for group
|
||||||
|---|---|---|---|---|---|---|---|
| Fasting subjects given placebo (n = 5) | Fasting subjects given the following dose of ST-246:
|
All fasting subjects given ST-246 (500 to 2,000 mg) (n = 23) | Nonfasting subjects (group 4) given:
|
||||
| 500 mg (n = 8) (group 1) | 1,000 mg (n = 8) (group 2) | 2,000 mg (n = 7) (group 3) | Placebo (n = 2) | ST-246 (1,000 mg) (n = 8) | |||
| Gender (no. of subjects [%])a | |||||||
| Male | 3 (60.0) | 7 (87.5) | 3 (37.5) | 3 (42.9) | 13 (56.5) | 1 (50.0) | 4 (50.0) |
| Female | 2 (40.0) | 1 (12.5) | 5 (62.5) | 4 (57.1) | 10 (43.5) | 1 (50.0) | 4 (50.0) |
| Race (no. of subjects [%])a | |||||||
| White | 1 (20.0) | 3 (37.5) | 5 (62.5) | 5 (71.4) | 13 (56.5) | 0 (0.0) | 3 (37.5) |
| Black | 4 (80.0) | 4 (50.0) | 2 (25.0) | 2 (28.6) | 8 (34.8) | 2 (100.0) | 5 (62.5) |
| Asian | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (4.3) | 0 (0.0) | 0 (0.0) |
| American Indian or native Alaskan | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (4.3) | 0 (0.0) | 0 (0.0) |
| Ethnicity (no. of subjects [%])a | |||||||
| Hispanic or Latino | 4 (80.0) | 3 (37.5) | 4 (50.0) | 6 (85.7) | 13 (56.5) | 1 (50.0) | 5 (62.5) |
| Not Hispanic or Latino | 1 (20.0) | 5 (62.5) | 4 (50.0) | 1 (14.3) | 10 (43.5) | 1 (50.0) | 3 (37.5) |
| Age (yr)b | |||||||
| Mean | 31.6 | 35.0 | 36.6 | 27.7 | 33.3 | 30.0 | 32.5 |
| SD | 5.2 | 7.1 | 13.4 | 8.5 | 10.4 | 1.4 | 6.7 |
| Median | 32.0 | 36.0 | 39.5 | 23.0 | 35.0 | 30.0 | 34.5 |
| Range | 25-39 | 21-45 | 19-51 | 20-42 | 19-51 | 29-31 | 23-44 |
| Wt (kg)c | |||||||
| Mean | 120.3 | 82.5 | 83.7 | 77.9 | 81.5 | 86.0 | 75.9 |
| SD | 50.4 | 14.9 | 23.4 | 14.8 | 17.6 | 21.8 | 9.0 |
| Median | 91.8 | 84.1 | 73.2 | 76.4 | 76.4 | 86.0 | 74.6 |
| Range | 75-190 | 58-107 | 67-136 | 61-106 | 58-136 | 71-101 | 66-96 |
| Ht (cm) | |||||||
| Mean | 169.1 | 168.6 | 165.6 | 170.2 | 168.1 | 179.5 | 170.5 |
| SD | 5.7 | 8.5 | 10.2 | 7.6 | 8.7 | 2.1 | 8.0 |
| Median | 168.0 | 169.5 | 165.0 | 166.0 | 168.0 | 179.5 | 168.0 |
| Range | 163-176 | 154-183 | 150-180 | 163-184 | 150-184 | 178-181 | 162-182 |
Note that percentages are based on the number of subjects in each treatment group.
Age was calculated as follows: (number of days between screening visit date and birth date)/365.25].
Weight at screening.
The mean weights (±SD) were similar for all groups except for the fasting placebo-treated group, which was higher (120.3 ± 50.4 kg) than for the other treatment groups (mean weights ranged from 75.9 ± 9.0 kg to 86.0 ± 21.8 kg).
Safety.
Single doses of ST-246 were generally well tolerated throughout the study. No serious adverse events (SAEs) were observed, and no drug-related AEs were reported. The most commonly reported AEs are summarized in Table 2. No subjects were withdrawn from the study, and no dose-related changes or trends in clinical lab values were noted.
TABLE 2.
Incidence of TEAEsa
| AE | No. (%)b of subjects with AE by treatment
|
|||||
|---|---|---|---|---|---|---|
| Fasting subjects given placebo (n = 5) | Fasting subjects given the following dose of ST-246
|
Nonfasting subjects (group 4)
|
||||
| 500 mg (n = 8) (group 1) | 1,000 mg (n = 8) (group 2) | 2,000 mg (n = 7) (group 3) | Placebo (n = 2) | 1,000 mg ST-246 (n = 8) | ||
| Any AE | 2 (40.0) | 2 (25.0) | 1 (12.5) | 0 | 0 | 1 (12.5) |
| TEAE total | 2 | 3 | 2 | 0 | 0 | 1 |
| Neutropenia | 2 (40.0) | 0 | 1 (12.5) | 0 | 0 | 0 |
| Constipation | 0 | 0 | 1 (12.5) | 0 | 0 | 0 |
| Feeling abnormal | 0 | 1 (12.5) | 0 | 0 | 0 | 0 |
| Back pain | 0 | 1 (12.5) | 0 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 0 | 1 (12.5) | 0 | 0 | 0 | 1 (12.5) |
Treatment-emergent adverse events (TEAEs) are nonserious adverse events (AEs) on or after day 0 after treatment with ST-246 through week 1 follow-up (days 7 to 9) or serious adverse events (SAEs) on or after day 0 after treatment with ST-246 through week 4 (study completion [days 26 to 31]). Additionally, in computing the number of subjects with TEAEs, subjects were counted once for each system organ class and once for each preferred term. Adverse events are coded in accordance with MedDRA dictionary (version 9.0).
Percentages are based on the number of subjects in each treatment group.
Six of the 38 subjects (15.8%) reported at least one treatment-emergent adverse events (TEAEs). Most TEAEs (seven/eight [87.5%]) were mild in intensity. No deaths, SAEs, or TEAEs leading to discontinuation occurred during the course of the study. There was a trend for white blood cells (WBCs) and neutrophils to decrease slightly 1 day after treatment in all fasting treatment groups (active treatment and placebo). Abnormalities in WBCs or neutrophils were noted in eight subjects (data not shown), as an AE for three subjects (one subject given active treatment and two subjects given placebo) or as grade 3 or 4 laboratory values for five other subjects (given active treatment). The grading system is based upon the Division of AIDS table for grading the severity of adult and pediatric adverse events (4). Abnormalities in lactate dehydrogenase levels were noted in three subjects (two subjects given active treatment and one given placebo) categorized as grade 3 or 4 laboratory values (data not shown). There were no clinically meaningful changes in vital signs. ECG analysis did not reveal any clinically significant QTcB (QT interval corrected for heart rate using Bazett's formula) changes (none of the QTcB changes from screening/baseline were greater than 30 ms) or changes in interpretation in individual subjects.
Pharmacokinetics.
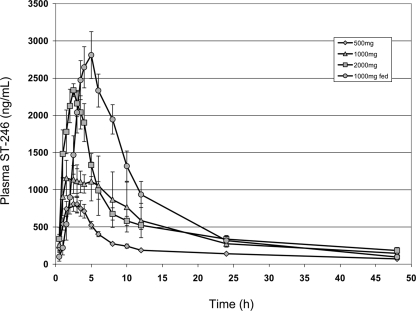
Individual plasma drug concentrations of ST-246 were measurable in all 31 subjects given active treatment through all postdose sampling time points, at the first time point measured of 0.50 h through 48 and 72 h postdose. No ST-246 levels were measured in the predose sample. The mean ST-246 concentration-versus-time profiles obtained from all human subjects at doses of 500, 1,000 (nonfasting and fasting), and 2,000 mg/day declined in a biexponential manner postadministration (Fig. 1). Intersubject variability was generally low (<50%), with the exception of early and late time points in the plasma drug concentration-time profiles. Plasma Cmax was proportional over the dose levels of 500 and 1,000 mg (mean [median] values of 934.0 [805] and 1,813.6 [1,615] ng/ml, respectively) but less than proportional between dose levels of 1,000 and 2,000 mg (mean [median] concentrations of 2,552.9 [2,610] ng/ml for the latter group) (Table 3). However, median Tmax values were relatively similar across all three fasting dose groups (2.0, 2.8, and 2.5 h at dose levels of 500, 1,000, and 2,000 mg, respectively), suggesting little dose effect on Tmax. The median t1/2s were also relatively similar (20.9, 19.8, 1 and 8.9 h) in the fasting 500-, 1,000-, and 2,000-mg dose groups, respectively. Similar to the Cmax results, AUC0-∞ was proportional over the dose levels of 500 and 1,000 mg, demonstrated most by median AUC0-∞ values (10,008 and 19,891 ng·h/ml, respectively). Dose proportionality in AUC0-∞ was not observed between dose levels of 1,000 and 2,000 mg (median of 26,027 ng·h/ml). Plasma clearance (CL) was independent of dose for the 500- and 1,000-mg levels (median values of 51.6 and 50.3 liters/h, respectively) but was increased for the 2,000-mg dose level (77.3 liters/h). At the 1,000-mg dose level, nonfasting subjects had greater apparent Cmax (3,125 ng/ml), Tmax (5.0 h), and AUC0-∞ (32,489 ng·h/ml) values than fasting subjects did but lower t1/2 (15.8 h) and CL (30.8 liters/h) than fasting subjects did (median values are given).
FIG. 1.
Mean plasma ST-246 concentration-time profiles following single-dose administration of ST-246. Symbols represent oral administration of ST-246 at 500 mg (diamonds), 1,000 mg (triangles), and 2,000 mg (squares) of ST-246 to fasting volunteers and 1,000 mg (circles) to nonfasting (fed) volunteers.
TABLE 3.
Summary of PK parameters of fasting and nonfasting subjects
| PK parametera | Fasting groups treated with the following dose of ST-246:
|
Group 4 treated with1,000 mg of ST-246 (nonfasting) (n = 8)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 500 mg (n = 8) (group 1)
|
1,000 mg (n = 8) (group 2)
|
2,000 mg (n = 7) (group 3)
|
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| kel (h) | 18 | 7 | 18 | 11 | 17 | 6 | 13 | 4 |
| t1/2 (h) | 26 | 16 | 40 | 45 | 22 | 9 | 17 | 6 |
| Tmax (h) | 2 | 1 | 3 | 2 | 3 | 1 | 5 | 1 |
| Cmax (ng/ml) | 934 | 361 | 1814 | 907 | 2553 | 589 | 2933 | 871 |
| AUClast (h·ng/ml) | 9,879 | 2,099 | 21,088 | 11,797 | 24,548 | 5,720 | 34,056 | 11,035 |
| AUC0-∞ (h·ng/ml) | 9,808 | 2,550 | 24,937 | 14,186 | 25,746 | 3,893 | 35,886 | 10,952 |
| CL (liter/h) | 53 | 14 | 49 | 22 | 79 | 12 | 30 | 9 |
kel, elimination rate constant; t1/2, half-life; Cmax, maximum plasma drug concentration; Tmax, time of maximum plasma drug concentration; AUC, area under the concentration-time curve; CL, clearance rate. Calculations assume a 60-kg human.
DISCUSSION
ST-246 appears safe and well-tolerated when administered orally as a single dose to healthy human volunteers in a fasting state (500 to 2,000 mg) or nonfasting state (1,000 mg). The pharmacokinetics of ST-246 in plasma were well characterized for all dose levels, 500, 1,000, and 2,000 mg in fasting subjects and 1,000 mg in nonfasting subjects. In this first human study, the single-dose plasma PK of ST-246 in fasting subjects showed dose proportionality over the dose levels of 500 and 1,000 mg, but not over the 1,000- and 2,000-mg dose levels (Table 3). At the 1,000-mg dose level of ST-246, nonfasting subjects had greater apparent Cmax, Tmax, and AUC0-∞ than fasting subjects did (Table 3).
Six of the 38 enrolled subjects (15.8%) reported at least one TEAE, which upon learning which subject received which treatment, occurred in 4/31 (12.9%) subjects given active treatment and also in 2/7 (28.6%) subjects who received placebo. Fewer TEAEs were reported with increasing dose. In total, 5/38 (13.2%) subjects (3 actively treated subjects and 2 placebo-treated subjects) reported at least one TEAE considered by the investigator, while still unaware of which subjects were given which treatment, to be drug related. The most common of these was neutropenia, which was observed on day 1 after study drug administration in the fasting state in three subjects, all of black ethnicity. Interestingly, upon learning which subject received which treatment, two of these subjects had received placebo and 1 had received 1,000 mg ST-246; therefore, this finding of neutropenia is not considered relevant to ST-246 administration. Of the other subjects who experienced drug-related TEAEs, two were in the fasting 500-mg treatment group (with headache or back pain), and one was in the fasting 1,000-mg treatment group (with constipation). Thus, there appeared to be no dose relationship in the incidence of TEAEs or drug-related TEAEs.
There was a trend for WBC counts and neutrophil percentages to decrease slightly 1 day after treatment in all fasting treatment groups (active treatment and placebo). This was not apparent in the nonfasting treatment group for which the tests were performed the same day as the blood draws and whose plasma drug levels were highest. Among the six subjects given active treatment who had low WBCs or neutrophil percentages reported, three had a single low value noted 4 weeks after treatment and had entered the study with low or relatively low WBCs or neutrophil percentages. Among the three actively treated subjects who had low WBCs or neutrophil results on day 1 (24 h postdose), all had normal results by day 2. Thus, these low WBC and neutrophil results were observed transiently postdose (lasting <2 days) or were not associated with ST-246 administration. Moreover, no hematological abnormalities were observed in preclinical toxicological evaluations. On the basis of these observations, it is likely that the transient decreases in WBC counts and neutrophils were the result of a testing artifact. Overall, changes in laboratory variables (hematology and clinical chemistry) and vital signs were small and not considered significant. ECG analysis did not reveal any clinically significant QTcB changes (none of the QTcB changes from screening/baseline were greater than 30 ms) or changes in interpretation in individual subjects. No deaths, SAEs, or TEAEs leading to discontinuation occurred during the course of the study.
The plasma PK of ST-246 were well characterized for all dose levels, 500 mg, 1,000 mg, and 2,000 mg in fasting subjects and 1,000 mg in nonfasting subjects, for the postdose blood sampling times evaluated (through 48 and 72 h postdose). Individual plasma concentrations of ST-246 were measurable in all 31 actively treated subjects through all postdose sampling time points, as early as 0.50 h through 48 and 72 h postdose (Fig. 1). In fasting subjects, the Cmax and AUC0-∞ of ST-246 in plasma were dose proportional for the 500- to 1,000-mg dose levels but less than proportional for the 1,000- to 2,000-mg dose levels.
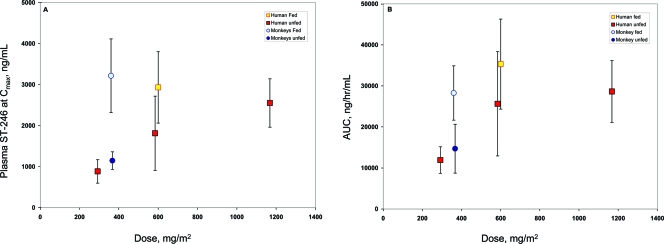
Food improved the rate and extent of oral plasma exposure of ST-246. At the 1,000-mg dose level, nonfasting subjects had 1.6-fold-greater apparent Cmax and AUC0-∞ than fasting subjects did. This food effect was observed in mice and nonhuman primates also (data not shown and Fig. 2). ST-246 is a weak base having a pKa of 9.0 and is a lipophilic drug with cLogP (logarithm of its partition coefficient between n-octanol and water) of 2.94. ST-246 is poorly soluble in physiologically relevant pH buffers. Coadministration of lipophilic drug with food can significantly enhance the gastrointestinal absorption and oral bioavailability (3). The enhanced rate and extent of oral absorption in the presence of a high-fat meal could be due to combined mechanism of prolonged gastric retention time, increased gut fluid volume, dissolution rate of ST-246, and micellar solubilization in the gut.
FIG. 2.
Pharmacokinetic values for oral administration of ST-246 in humans and nonhuman primates. The Cmax values (A) and AUC0-∞ values (B) for humans and nonhuman primates given ST-246 orally are shown. Doses of 500 mg (300 mg/m2), 1,000 mg (600 mg/m2), and 2,000 mg (1,200 mg/m2) of ST-246 were given to fasting human volunteers (red squares), a dose of 1,000 mg (600 mg/m2) was given to nonfasting (fed) human volunteers (yellow square), and 90 mg (360 mg/m2) was given to fasting (dark blue circles) or nonfasting (light blue circles) nonhuman primates. The means ± standard deviations (error bars) are shown. The 1,000-mg symbol representing the human nonfasting condition is slightly offset for clarity.
Since smallpox has been eradicated, human clinical trials designed to link antiviral efficacy to clinical outcome have been replaced by antiviral efficacy evaluations in animal models of orthopoxvirus disease. A nonhuman primate model of orthopoxvirus disease is being developed to evaluate smallpox vaccines and therapeutics (8, 9). Administration of 107 PFU of monkeypox virus to nonhuman primates delivered by intravenous injection produces a lethal infection that reproduces the lesional disease characteristic of smallpox and monkeypox in humans. Typically, animals die between 10 and 14 days postinfection with as many as 750 poxvirus lesions.
To establish the effective human dose, the exposure levels in humans will have to be comparable to exposure levels in nonhuman primates that protect animals from lethal orthopoxvirus infection. On the basis of data from our preliminary animal efficacy studies using survival as a primary end point, an oral dose of approximately 10 mg/kg (120 mg/m2) in nonfasting nonhuman primates confers 100% protection from death following intravenous injection of 1 × 107 PFU of monkeypox virus strain Zaire (J. Huggins, personal communication). In investigational new drug-enabling pharmacology studies in nonhuman primates, a dose of 30 mg/kg (360 mg/m2) results in blood exposure levels comparable to levels attained in humans administered a 1,000-mg (600-mg/m2) dose in the fed (eating or nonfasting) state (Fig. 2). This dose level is well below the no observed adverse effect level (NOAEL) of 2,000 mg/kg (24,000 mg/m2) in a single-dose experiment in nonhuman primates and the no observable effect level (NOEL) of 300 mg/kg (3,600 mg/m2) in a 28-day repeated-dose experiment in nonhuman primates.
Given the variability in exposure levels in monkeys and humans in the nonfasting and fasting states, we predict that doses of 400 mg (230 mg/m2) and 800 mg (460 mg/m2) for humans who are nonfasting will encompass plasma drug exposure levels comparable to those that provide protective efficacy in the nonhuman primate model of orthopoxvirus disease (Fig. 2). Furthermore, planned dose-response experiments in the nonhuman primate model where ST-246 plasma concentration is measured in infected animals will define the lower limit of exposure that provides protective efficacy. This lower limit may be below the 120-mg/m2 dose that has been shown in preliminary studies to provide protection against lethal orthopoxvirus infection.
In conclusion, ST-246 is safe and well tolerated when administered orally as a single dose to healthy human volunteers in a fasting state (500 to 2,000 mg) or nonfasting state (1,000 mg). The single-dose plasma PK of ST-246 in fasting subjects showed dose proportionality over the dose levels of 500 and 1,000 mg, with saturation of exposure occurring above the 2,000-mg dose level. At the 1,000-mg dose level of ST-246, nonfasting subjects had greater apparent Cmax, Tmax, and AUC0-∞ than fasting subjects.
Acknowledgments
We acknowledge our colleagues at the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Office of Biodefense Research Affairs for their continued support of this program.
Robert Jordan, Deborah Tien, Tove' C. Bolken, Kevin F. Jones, Shanthakumar R. Tyavanagimatt, and Dennis E. Hruby are shareholders of SIGA Technologies, Inc.
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Bailey, T. R., S. R. Rippin, E. Opsitnick, C. J. Burns, D. C. Pevear, M. S. Collett, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, E. R. Kern, K. A. Keith, D. Dai, G. Yang, D. Hruby, and R. Jordan. 2007. N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2-(1H)-yl)carboxamides: identification of novel orthopoxvirus egress inhibitors. J. Med. Chem. 50:1442-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antivir. Res. 58:101-114. [DOI] [PubMed] [Google Scholar]
- 3.Charman, W. N., C. J. Porter, S. Mithani, and J. B. Dressman. 1997. Physiochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J. Pharm. Sci. 86:269-282. [DOI] [PubMed] [Google Scholar]
- 4.Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf.
- 5.Fenner, F., D. A. Henderson, I. Arita, Z. Jazek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland.
- 6.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 7.Henderson, D. A., T. V. Inglesby, J. G. Bartlett, M. S. Ascher, E. Eitzen, P. B. Jahrling, J. Hauer, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. Perl, P. K. Russell, and K. Tonat for the Working Group on Civilian Biodefense. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127-2137. [DOI] [PubMed] [Google Scholar]
- 8.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huggins, J. W., D. F. Smee, M. Martinez, and M. Bray. 1998. Cidofovir (HPMPC) treatment of monkeypox. Antivir. Res. 37:A37. [Google Scholar]
- 10.Mortimer, P. P. 2003. Can postexposure vaccination against smallpox succeed? Clin. Infect. Dis. 36:622-629. [DOI] [PubMed] [Google Scholar]
- 11.Quenelle, D. C., R. M. Buller, S. Parker, K. A. Keith, D. E. Hruby, R. Jordan, and E. R. Kern. 2007. Efficacy of delayed treatment with ST-246 given orally against systemic orthopoxvirus infections in mice. Antimicrob. Agents Chemother. 51:689-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sbrana, E., R. Jordan, D. E. Hruby, R. I. Mateo, S.-Y. Xiao, M. Siirin, P. C. Newman, A. P. A. Travassos da Rosa, and R. B. Tesh. 2007. Efficacy of the antipoxvirus compound ST-246 for treatment of severe orthopoxvirus infection. Am. J. Trop. Med. Hyg. 76:768-773. [PubMed] [Google Scholar]
- 13.Whitley, R. J. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 57:7-12. [DOI] [PubMed] [Google Scholar]
- 14.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]