Abstract
AMD070, a CXCR4 antagonist, has demonstrated antiretroviral activity in human immunodeficiency virus-infected patients. Since AMD070 is a substrate of cytochrome P450 3A4 and P-glycoprotein, both of which may be affected by ritonavir, we tested for a ritonavir effect on AMD070 pharmacokinetics. Subjects were given a single 200-mg dose of AMD070 on days 1, 3, and 17. Ritonavir (100 mg every 12 h) was dosed from day 3 to day 18. Blood samples to test for AMD070 concentrations were collected over 48 h after each administration of AMD070. Twenty-three male subjects were recruited. Among them, 21 completed the study, and 2 were discontinued for reasons other than safety. All adverse events were grade 2 or lower. AMD070 alone had the following pharmacokinetic features, given as medians (ranges): 3 h (0.5 to 4 h) for the time to peak blood concentration, 256 ng/ml (41 to 845 ng/ml) for the peak concentration (Cmax), 934 h·ng/ml (313 to 2,127 h·ng/ml) for the area under the concentration-time curve from 0 h to infinity (AUC0-∞), 214 liters/h (94 to 639 liters/h) for apparent body clearance, and 4,201 liters (1,996 to 9,991 liters) for the apparent volume of distribution based on the terminal phase. The initial doses of ritonavir increased the Cmax of AMD070 [geometric mean (90% confidence interval)] by 39% (3 to 89%) and the AUC0-∞ by 60% (29 to 100%). After 14 days of ritonavir dosing, the pharmacokinetic changes in AMD070 persisted. The plasma pharmacokinetics of ritonavir were consistent with previous reports. It is concluded that AMD070 concentrations were increased with concomitant ritonavir dosing for 14 days in healthy volunteers.
Despite the availability of many effective antiretroviral drugs, treatment options are still challenged by drug toxicities and the emergence of drug-resistant human immunodeficiency virus (HIV). The identification of a new class of drugs with a novel mechanism of action, durable efficacy, and lack of cross-resistance with existing antiretroviral drug classes remains a continuing therapeutic need. AMD070 belongs to a novel class of antiretroviral entry inhibitors (AnorMED, Inc., data on file). It is a specific and reversible antagonist of the CXCR4 chemokine receptor, the coreceptor used by T-cell tropic (X4), syncytium-inducing HIVs, and has a potent and selective ability in vitro to inhibit X4 viral replication by blocking fusion and viral entry into the cell. The investigational CXCR4 antagonist AMD3100 given intravenously has been shown to reduce the X4 viral load in HIV-infected subjects; however, its development for HIV infection was terminated due to poor oral bioavailability and adverse effects (7, 8). AMD3100 is currently in phase III clinical trials as an intravenous adjunct for stem cell mobilization in malignant diseases. In contrast, orally administered AMD070 is well absorbed and well tolerated and displays a terminal elimination half-life of 11 to 16 h (19). A majority of the subjects dosed with 200 mg twice daily attained plasma concentrations at or near the in vitro 90% effective concentration 24 h after dosing. The combined results of two related 10-day phase II studies of HIV-infected subjects receiving 200 mg AMD070 twice daily demonstrated an antiviral effect, defined as a 1-log reduction in X4 relative luminescence units in the profile assay, in 7 of 15 subjects (13, 17). Furthermore, like its predecessor, AMD3100, AMD070 showed a dose response for leukocytosis which may serve as a surrogate marker for CXCR4 inhibition and a second potential indication for use of the drug.
AMD070 is cleared primarily by metabolism, with <1% of the oral dose appearing unchanged in the urine (AnorMED, Inc., data on file). In vitro studies using human liver microsomes have shown that AMD070 is metabolized by cytochrome P450 3A4 (CYP3A4) and, to a lesser extent, CYP2D6 enzymes; it is a substrate of P-glycoprotein (P-gp) (AnorMED, Inc., data on file). CYP3A4 is a monooxygenase encoded by the CYP3A gene. Many CYP3A enzyme substrates can also be metabolized by other CYP enzymes. Ritonavir is a substrate for multiple CYP enzymes, including CYP3A4 and CYP2D6. It is also an inhibitor and inducer of several CYP enzymes, such as CYP3A4 and CYP2C8. Ritonavir is commonly given with other protease inhibitors to “boost” their plasma concentrations through hepatic or enterocytic CYP enzyme inhibition (4). Additionally, ritonavir inhibits P-gp, which enhances the absorption and alters the distribution of substrate drugs (3, 21). In rat studies, the coadministration of ritonavir increased the area under the concentration-time curve (AUC), the peak concentration in blood plasma (Cmax), and the oral bioavailability (F) of AMD070 by 58%, 158%, and 66%, respectively. In dog studies, there was no significant increase in the AUC for AMD070 when it was coadministered with either a single dose of ritonavir or at ritonavir steady state. In vitro and clinical studies have also shown that AMD070 is a weak inhibitor of the CYP2D6 and CYP3A4 enzymes (AnorMED, Inc., data on file) (14).
Because of the potential for an AMD070-ritonavir interaction based on in vitro studies, we performed this pharmacokinetic drug interaction study to assess the interactions of AMD070 with ritonavir given both as a single dose and twice a day for 2 weeks. We hypothesized that the coadministration of a low dose of ritonavir would increase the oral bioavailability of AMD070.
(The results of this study were presented previously at the Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA, 2007.)
MATERIALS AND METHODS
Study design.
This was a phase I, open-label, multiple-dose, sequential-design drug interaction study that consisted of one cohort of healthy male volunteers. Volunteers were dosed initially with 200 mg AMD070 alone on day 1. On days 1 to 3, blood samples were taken for analysis of the AMD070 concentrations. Volunteers did not receive any drug on day 2 to allow for the clearance of nearly all (estimated to be 93%) of the first AMD070 dose. On day 3, volunteers began taking ritonavir (100 mg twice daily) and continued through day 18 (for a total of 16 consecutive days and 32 total doses). A single 200-mg dose of AMD070 was also given simultaneously with the morning ritonavir dose on day 3. On days 3 to 5, frequent blood samples were taken for pharmacokinetic analysis of the AMD070 concentrations. Blood was collected to measure ritonavir concentrations within 6 h after dosing on day 10 or 11 to indirectly assess the adherence to the ritonavir dosing regimen. On day 17, volunteers received another 200-mg dose of AMD070 simultaneously with their morning ritonavir dose. The evening ritonavir dose on day 18 was the last dose. On days 17 to 19, blood samples were again taken for analysis of the AMD070 concentrations. The safety of taking AMD070 alone and during coadministration with ritonavir was assessed throughout the study period.
The study was conducted at two research sites, Johns Hopkins University and the University of Washington. Subjects were healthy men with no active medical illness, as determined by history, physical, or laboratory evaluation. Uninfected women were not enrolled due to the lack of reproductive toxicity data from preclinical studies. The inclusion criteria, by which each subject was evaluated within 28 days prior to commencing the study drug, included the following. (i) The subjects were healthy males age 18 to 55 years (inclusive). (ii) If participating in sexual activity that could lead to pregnancy, the subjects agreed that two reliable methods of contraception would be used simultaneously while participating in the study and for 2 weeks after stopping the medication. (iii) The subjects had acceptable laboratory values, including an HIV-seronegative result determined by any licensed enzyme-linked immunosorbent assay; a white blood cell count within normal limits; a hemoglobin level of ≥12.5 g/dl; a platelet count of ≥150,000 cells/mm3; pertussis toxin level, partial thromboplastin time, and total bilirubin level within normal limits; aspartate transaminase (serum glutamic oxaloacetic transaminase) and alanine aminotransferase (serum glutamic pyruvic transaminase) levels of ≤1.3× the upper limit of normal; and calcium and magnesium levels within normal limits. (iv) The subjects had electrocardiograms deemed normal or with abnormalities not considered clinically significant by the site investigators. (v) The subjects had body weights within 33% of the ideal weight for their height based on the Metropolitan Life table (20). (vi) The subjects had a willingness to abstain from exercise for at least 24 h prior to study entry. Subjects were excluded if they (i) had participated in a previous AMD070 study with any adverse event of grade 3 or higher; (ii) had used prescription medications, herbal supplements, or aspirin within 7 days prior to study entry or nonsteroidal anti-inflammatory drugs, over-the-counter medications, or other supplements, including multivitamins, within 1 day prior to study entry; (iii) had received any immunizations within 30 days prior to study entry or been treated with radiation therapy or cytotoxic chemotherapeutic agents or immunomodulating agents within 30 days prior to study entry; (iv) had experienced an active infection or acute illness of any kind within 14 days prior to study entry; (v) had experienced active drug or alcohol abuse or dependence; (vi) had a history of gastrointestinal bleeding or ulcer or had chronic diarrhea (defined as >3 stools/day) for more than 4 weeks prior to study entry; (vii) had a history of cardiac conduction abnormalities, cardiac arrhythmias, cardiomyopathy, repolarization delay (rate-corrected QT interval, >500 ms), or additional risk factors for torsade de pointes (e.g., heart failure, hypokalemia); and (viii) had any medical or psychological condition that, in the opinion of the site investigator, might interfere with participation in the study or put the volunteer at undue risk. The protocol was approved by the institutional review board at both research sites, and all study participants provided written informed consent prior to enrollment.
After screening and enrollment, the subjects were admitted to the General Clinical Research Center for 5 nights to determine the pharmacokinetics of the first two doses of AMD070 (days 1 to 5) and 3 nights to determine the pharmacokinetics of the last AMD070 dose (days 17 to 19). In the evening (on day 0 and day 16), subjects were admitted to the General Clinical Research Center and concomitant medications and vital signs were obtained. A targeted physical exam, including signs, symptoms, diagnosis, and body weight, was performed, and an indwelling intravenous catheter was inserted before the standardized breakfast on the second day. A single, 200-mg dose of AMD070 was given 30 min after breakfast. Ritonavir was given on days 3 to 18 twice a day, 30 min after breakfast and 30 min after dinner. Urine for a urine dipstick test with microscopic exam was collected for 24 h following AMD070 dosing. Blood was collected into heparinized tubes predose and at 0.5, 1, 1.5, 2, 3, 4, 8, 12, 24, 34, and 48 h following AMD070 dosing. Food and drink were given ad libitum 4 h after AMD070 dosing. Breakfast, lunch, and dinner were served at approximately 8:00 a.m., 12:00 p.m., and 5:00 p.m. during hospitalization. A follow-up safety visit was conducted approximately 2 weeks after discharge (on day 19) or upon premature discontinuation of the study. A review of the subject's medical history and a targeted physical exam were performed daily during hospitalization and on either day 10 or 11. Similarly to the screening visit, the final follow-up visit included a history review, a targeted physical exam, and laboratory evaluations.
Blood samples were kept on ice and sent to the clinical laboratory for processing within 1 h of collection. During processing, the sample was centrifuged at 2 to 8°C at 900 × g for 10 min. The blood plasma was aliquoted and frozen at −20°C. After the sample from the last volunteer had been collected, all samples were shipped on dry ice to the BAS Northwest Laboratory (McMinnville, OR) for the AMD070 assay.
AMD070 assay.
Concentrations of AMD070 in plasma and urine were determined by high-performance liquid chromatography-mass spectrometry (HPLC-MS) using validated methods. Validations included assessments of linearity; within-run and between-run precision and accuracy; selectivity; long-term (frozen), short-term (room temperature), autosampler, freeze-thaw, and processed sample stability; extraction efficiency; carryover; the effect of dilution; and potential over-the-counter and HIV drug interferences. The validated calibration range was 0.5 to 500 ng/ml for both plasma and urine and was selected based on linearity (R2 > 0.95), accuracy, and precision criteria for the validation runs. The acceptance criteria were a 15% (20% at the lower limit of quantification) deviation for accuracy and a ≤15% (≤20% at the lower limit of quantification) coefficient of variation (CV) for precision. Actual within-run precision and accuracy for the validation runs ranged from 3.7 to 14% CV and −11 to 15% deviation. Between-run precision and accuracy ranged from 6.6 to 11% CV and −5.2 to 12% deviation. The maximum validated dilution factor was 100-fold. Sample runs were accepted or rejected based on the results obtained for quality control samples included in each run: at least 67% (e.g., six out of nine) of the quality control samples were required to be within ±15% of their respective nominal values.
Standard calibrators, quality control samples, and study samples were prepared and analyzed in identical manners. Briefly, samples (100 μl) were heated for 30 min at 57°C, followed by the addition of 50 μl of a 0.5-mg/ml internal standard solution (AMD025, a structural analog of AMD070) and 50 μl of a 1 N sodium hydroxide (NaOH) solution. After the samples were mixed briefly, 1.0 ml of methyl tert-butyl ether (MTBE) was added, and the samples were vortexed (10 min) and then centrifuged (3 min, >10,000 rpm). Samples were then frozen at ≤−60°C for approximately 60 min. The MTBE layer was decanted into a second tube and evaporated to dryness in a water bath at 30°C under nitrogen. Following its reconstitution in 200 μl of 5% acetonitrile-95% water-0.1% trifluoroacetic acid, the samples were analyzed by reversed-phase HPLC with tandem MS detection (mobile phase, 7% acetonitrile-93% water-0.1% trifluoroacetic acid; flow rate, 0.5 ml/min; run time, 4.5 min). Detection by tandem MS incorporated an electrospray interface in the positive ion mode.
Ritonavir assay.
The concentration of ritonavir was determined by the Pharmacology Support Laboratory at the University of North Carolina at Chapel Hill using an HPLC/UV method. Briefly, 200 μl of plasma was combined with 50 μl of a 5-μg/ml midazolam internal standard working solution. Samples were subjected to liquid-liquid extraction using MTBE, and the organic layer was evaporated to dryness under a gentle stream of nitrogen. Samples were reconstituted in the mobile phase before injection onto an Agilent (Wilmington, DE) 1100 HPLC system. Samples were separated on a Zorbax C18 analytical column (3.5 μm, 150 by 4.6 mm; Agilent, Wilmington, DE) with a Zorbax C18 guard column (3.5 μm, 12.5 by 4.6 mm; Agilent) and facilitated via a gradient elution. Calibration standard curves ranged from 25 to 10,000 ng/ml. Intraday and interday CVs were less than 6% (16).
Pharmacokinetic analysis of AMD070.
Noncompartmental analysis was performed using WinNonlin professional software (version 5.0.1; Pharsight Corp., Cary, NC). The AUC, Cmax, time to maximum concentration (Tmax), apparent total clearance (CL/F), and apparent volume of distribution based on the terminal phase (Vz/F) were estimated. The AUC was calculated with the linear/log trapezoidal method. CL/F was calculated as the quotient of the dose to the AUC0-∞, where the AUC0-∞ was the AUC0-48 plus the quantity of C48 divided by λz (AUC0-48 is the AUC from 0 to 48 h post-AMD070 dosing, C48 is the AMD070 concentration at 48 h postdosing, and λz is the slope of the linear regression of the log concentration versus time for the terminal portion of the log concentration-time curve). Vz/F was calculated by dividing the dose by AUC0-∞·λz. The AMD070 concentration during the acute ritonavir phase was corrected for any residual concentration from the AMD070-alone phase, assuming exponential decay with time based on the C48 during the AMD070-alone phase. Only the AUC0-∞ was reported since the percentage of extrapolation from the time of last measurable concentration to infinity is relatively small [the medians (ranges) were 6% (2 to 10%) for the first AMD070 dose, 8% (4 to 17%) for the second AMD070 dose, and 6% (3 to 24%) for the last AMD070 dose].
Pharmacokinetic analysis of ritonavir.
Similar methods were used for ritonavir pharmacokinetic analysis as were used for AMD070 except that the summary parameters were estimated only for 0 to 12 h postdosing since ritonavir was given twice daily. CL/F for the initial ritonavir dose and Vz/F were not estimated due to the relatively large Tmax, the limited number of concentration points during the decay phase, and the still-high concentration at 12 h (C12) postdosing. For the first dose of ritonavir, the concentrations of the samples collected within the individual lag time were set to zero for the calculation of the AUC. CL/F for steady-state ritonavir was estimated by dividing the dose by the AUC at steady state.
Statistical consideration.
Based on the data from our previous study (19), we determined that with a paired study design, we would need 19 subjects to detect a 35% change in the AUC of AMD070 (80% power; 5% two-sided significance level; logarithmically transformed data). Assuming that 25% of the volunteers would be lost or not evaluable, we planned to enroll up to 26 volunteers. In order to be evaluable, volunteers would have to have taken at least 80% of their scheduled ritonavir doses, including the last three scheduled doses prior to the last AMD070 dose.
Data are presented as either medians and ranges or geometric means and 95% confidence intervals (CI), except for the ratios of pharmacokinetic parameters, which were presented as geometric means and 90% CI computed with the Proc Mixed program of SAS v9.1.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Subjects.
A total of 23 subjects were enrolled: 17 subjects at Johns Hopkins University and 6 subjects at the University of Washington. Twenty-one subjects completed the study and were included in the final pharmacokinetic analysis; one subject discontinued in the first week due to consent withdrawal, and one discontinued in the second week due to unwillingness to come to the clinic. The ages and weights of the 21 subjects included in the final pharmacokinetic analysis, given as medians (ranges), were 42 years (18 to 53 years) and 77.8 kg (55.2 to 101.2 kg), respectively. Ten subjects were white (non-Hispanic), and 13 subjects were black (non-Hispanic).
Safety.
All subjects tolerated the study drugs well. Reversible, grade 1 to grade 2 adverse events were seen in 7 subjects (30%) on AMD070 alone (a 2-day period) and in 16 subjects (70%) on ritonavir, sometimes in association with an AMD070 dose during a longer 16-day period. Among nine of the grade 2 adverse events, five were assigned this grade solely because either ibuprofen or acetaminophen was prescribed. All adverse events were resolved. There was no grade 3- or grade 4-related adverse event or finding of hepatotoxicity. Since the study was powered for potential ritonavir-induced changes in AMD070 pharmacokinetics, not for safety endpoints, further statistical analysis of the frequency of adverse events was not performed.
Pharmacokinetics.
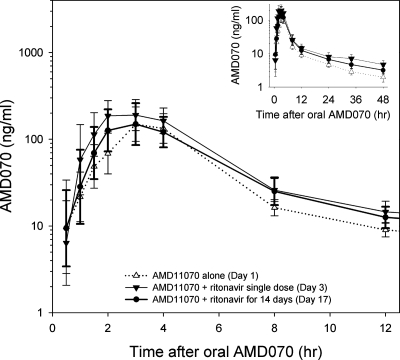
The blood plasma pharmacokinetic parameters of a single 200-mg oral dose of AMD070 are listed in Table 1. Ritonavir increased the concentration of AMD070 in blood plasma (Fig. 1). The Cmax and AUC0-∞ of AMD070 coadministered with the first dose of ritonavir were increased [90% geometric mean (90% CI)] by 39% (3 to 89%) and 60% (29 to 100%), respectively, compared to those with the administration of AMD070 alone (Table 2). These changes remained after ritonavir had been administered twice daily for 14 days (Table 2).
TABLE 1.
AMD070 pharmacokinetic parametersa
| Day of AMD070 doseb |
Tmax (h)
|
Cmax (ng/ml)
|
AUC0-∞ (h·ng/ml)
|
CL/F (liters/h)
|
Vz/F (liters)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (range) | Mean (% CV) | Median (range) | Mean (% CV) | Median (range) | Mean (% CV) | Median (range) | Mean (% CV) | Median (range) | Mean (% CV) | |
| 1 | 3 (0.5-4) | 3 (28) | 256 (41-845) | 292 (64) | 934 (313-2,127) | 1,029 (46) | 214 (94-639) | 244 (55) | 4,201 (1,996-9,991) | 5,329 (45) |
| 3 | 2 (0.5-8) | 3 (64) | 362 (40-1,458) | 405 (76) | 1,643 (286-4,095) | 1,723 (52) | 122 (49-698) | 167 (87) | 3,452 (1,473-10,218) | 4,223 (61) |
| 17 | 2 (1-8) | 3 (61) | 333 (76-985) | 384 (50) | 1,205 (406-4,386) | 1,456 (64) | 166 (46-493) | 178 (53) | 3,931 (1,293-27,885) | 5,364 (104) |
The number of subjects who completed the study was 21.
AMD070 was given on day 1 (no ritonavir treatment), day 3 (first dose of ritonavir), and day 17 (29th dose of ritonavir).
FIG. 1.
Concentration-time profile of AMD070 in blood plasma. The inset shows the full time course of measured AMD070 concentrations. Data are geometric means with 95% CIs.
TABLE 2.
Comparison of AMD070 pharmacokinetic parameters
| Parameter | Geometric mean ratio (90% CI)
|
||
|---|---|---|---|
| Day 3/day 1 | Day 17/day 1 | Day 17/day 3 | |
| Cmax | 1.39 (1.03-1.89) | 1.47 (1.08-2.01) | 1.06 (0.81-1.38) |
| AUC0-∞ | 1.60 (1.29-2.00) | 1.37 (1.08-1.75) | 0.85 (0.70-1.05) |
There was a lag of 1 h (0 to 4 h) [median (range)] for ritonavir to reach a detectable blood plasma concentration after the first dose of ritonavir. The Cmax and AUC0-12 following the first dose of ritonavir, given as geometric means (95% CI), were 184 ng/ml (103 to 329 ng/ml) and 1,209 ng·h/ml (565 to 2,586 ng·h/ml), respectively. The Cmax, Cmin, and AUC at steady state following the morning dose of ritonavir on day 17 were 741 ng/ml (354 to 1,551 ng/ml), 242 ng/ml (133 to 440 ng/ml), and 5,014 ng·h/ml (2,048 to 12,277 ng·h/ml), respectively. The trough ritonavir concentration appeared stable starting at the second dosing interval. The blood samples for the indirect assessment of ritonavir regimen adherence at day 10 or 11, after the first week of ritonavir dosing, were taken between 1.3 and 5.3 h postdosing. The ritonavir concentration in these samples, given as the geometric mean (95% CI), was 642 ng/ml (424 to 973 ng/ml), which was not different from those obtained 1 week later when the subjects were dosed in the hospital [geometric mean (95% CI), 617 ng/ml (525 to 725 ng/ml) for the postdosing time between 1 and 4 h and 616 ng/ml (535 to 711 ng/ml) for the postdosing time between 1 and 8 h].
DISCUSSION
We have shown that low-dose ritonavir caused weak increases in the Cmax and AUC of oral AMD070, during both the acute and steady-state phases of ritonavir treatment. In addition, the combination of single-dose AMD070 with low-dose ritonavir in our study appeared safe, as the subjects tolerated the study regimen well and no adverse events of grade 3 or 4 were reported. The exclusion of female subjects is a limitation of this study in that the study results may not be generalizable to females.
The common mechanisms contributing to the increase of the AUC of oral drugs include increasing oral bioavailability (by increasing either absorption or decreasing presystemic clearance or both) and decreasing elimination (by inhibiting metabolism and/or excretion). The apparent decrease of Tmax and Vz/F and the increase of Cmax in our study were consistent with an increase in oral bioavailability. Thus, ritonavir may inhibit P-gp (thus increasing AMD070 absorption) and intestinal CYP3A4 (thus decreasing presystemic clearance). The inhibition of the hepatic CYP3A4 enzyme may also be involved. Alteration of renal clearance is unlikely since less than 1% of AMD070 is excreted unchanged in the urine (AnorMED, Inc., data on file).
In our study, ritonavir was given for 14 days at a low dose. In a study with HIV-infected subjects (11), ritonavir (given as a single agent) was administered in dosing regimens of 200 mg to 500 mg twice daily; the trough ritonavir concentration decreased from day 8 to day 16, whereas the AUC and Cmax were relatively time invariant. One of the proposed mechanisms was the induction of CYP3A4. Based on the model of the time course, the authors concluded that the half-life of the potential ritonavir-mediated CYP3A4 induction was ∼3.5 days. Their data show that the trough concentrations of ritonavir tended to stabilize by the end of a 2-week dosing period. Apparently, ritonavir in our study reached steady state and the ritonavir pharmacokinetic parameters were similar to those reported by Aarnoutse et al. (1). Since ritonavir induces CYP3A4 enzyme activity, we had expected slightly higher CYP3A4 activity after 2 weeks of ritonavir treatment. Although the geometric mean ratio of AMD070 Cmax and AUC0-∞ with both acute (day 3) and chronic (day 17) ritonavir dosing to that with no ritonavir dosing showed the lack of bioequivalence of AMD070, it is still not clear whether the impacts of acute and chronic ritonavir treatment on AMD070 are different. An in vitro study has shown that AMD070 weakly inhibits CYP2D6, CYP3A4, and CYP1A2 (AnorMED, Inc., data on file), which can metabolize ritonavir and add to the complexity of the AMD070-ritonavir drug interaction.
Single-dose rather than steady-state pharmacokinetics of AMD070 were studied because dosing once or twice daily as anticipated in future efficacy studies would yield an accumulation less than or equal to two times the single-dose concentrations only during the terminal elimination phase, with little or no detectable difference in peak concentration and AUC (19). Furthermore, concentrations achieved with single 200-mg doses are near the range expected to be clinically relevant (19). The AMD070 dose chosen (200 mg) was based on demonstrated safety in the study of up to 400 mg twice daily (AIDS Clinical Trials Group study A5191) (19).
AMD070 has modest antiretroviral activity in some patients with dosing of 200 mg every 12 h (17); the doses used in the antiretroviral studies are also below the concentrations associated with maximal leukocyte mobilization (19). The pharmacokinetic interaction we report here may provide a way to increase concentrations of AMD070, and perhaps the antiviral activity, without increasing the AMD070 dose, as occurs for numerous other antiretroviral drugs, primarily protease inhibitors. However, after the clinical phase of this study was completed, the FDA placed AMD070 on clinical hold due to liver histology changes observed in longer-term preclinical toxicity experiments. Currently, AMD070 is still on clinical hold. However, it has been reported that disrupting the CXCR4 receptor-mediated trafficking of hematopoietic progenitor cells produces a stem cell mobilization effect (5, 12, 15). Therefore, the favorable ritonavir interaction may also have relevance in another clinical setting, as AMD070 has leukocyte-mobilizing activity similar to that of AMD3100, another CXCR4 inhibitor which is in advanced clinical development as a stem-cell-mobilizing drug (2, 18). AMD070, however, has the significant advantage of being orally bioavailable (19).
In conclusion, the coadministration of ritonavir with AMD070 weakly increased the Cmax and AUC of AMD070 in blood plasma. The increased Cmax and AUC of AMD070 due to concomitant ritonavir dosing may result in clinically beneficial effects.
Acknowledgments
This work was supported in part by NIH General Clinical Research Center grants M01RR000052 (Johns Hopkins University) and M01RR00037 (University of Washington) and AIDS Clinical Trials Unit grants U01AI027668 (Johns Hopkins University), U01AI27664 (University of Washington), and AI038855.
We recognize the sustained excellence of all members of the ACTG A5191 study team and the essential contributions of our healthy volunteers.
Footnotes
Published ahead of print on 19 February 2008.
AIDS Clinical Trial Group study A5191.
REFERENCES
- 1.Aarnoutse, R. E., J. Kleinnijenhuis, P. P. Koopmans, D. J. Touw, J. Wieling, Y. A. Hekster, and D. M. Burger. 2005. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin. Pharmacol. Ther. 78:664-674. [DOI] [PubMed] [Google Scholar]
- 2.Cashen, A. F., B. Nervi, and J. DiPersio. 2007. AMD3100: CXCR4 antagonist and rapid stem cell-mobilizing agent. Future Oncol. 3:19-27. [DOI] [PubMed] [Google Scholar]
- 3.Drewe, J., H. Gutmann, G. Fricker, M. Torok, C. Beglinger, and J. Huwyler. 1999. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem. Pharmacol. 57:1147-1152. [DOI] [PubMed] [Google Scholar]
- 4.Flexner, C. 2000. Dual protease inhibitor therapy in HIV-infected patients: pharmacologic rationale and clinical benefits. Annu. Rev. Pharmacol. Toxicol. 40:649-674. [DOI] [PubMed] [Google Scholar]
- 5.Flomenberg, N., J. DiPersio, and G. Calandra. 2005. Role of CXCR4 chemokine receptor blockade using AMD3100 for mobilization of autologous hematopoietic progenitor cells. Acta Haematol. 114:198-205. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Hendrix, C. W., A. C. Collier, M. M. Lederman, D. Schols, R. B. Pollard, S. Brown, J. B. Jackson, R. W. Coombs, M. J. Glesby, C. W. Flexner, G. J. Bridger, K. Badel, R. T. MacFarland, G. W. Henson, and G. Calandra. 2004. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr. 37:1253-1262. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix, C. W., C. Flexner, R. T. MacFarland, C. Giandomenico, E. J. Fuchs, E. Redpath, G. Bridger, and G. W. Henson. 2000. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob. Agents Chemother. 44:1667-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Reference deleted.
- 11.Hsu, A., G. R. Granneman, G. Witt, C. Locke, J. Denissen, A. Molla, J. Valdes, J. Smith, K. Erdman, N. Lyons, P. Niu, J.-P. Decourt, J.-B. Fourtillan, J. Girault, and J. M. Leonard. 1997. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob. Agents Chemother. 41:898-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapidot, T., and O. Kollet. 2002. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 16:1992-2003. [DOI] [PubMed] [Google Scholar]
- 13.Moyle, G., E. DeJesus, M. Boffito, R. Wonf, E. Coakley, K. Badel, G. Calandra, G. Bridger, and S. Becker. 2007. CXCR4 antagonism: proof of activity with AMD11070, abstr. 511. 14th Conf. Retrovir. Oppor. Infect., Los Angeles, CA.
- 14.Nyunt, M., S. Becker, R. MacFarland, S. Everts, P. Chee, R. Scarborough, and C. W. Hendrix. 2007. Pharmacokinetic interaction between AMD11070 and substrates of CYP3A4 and 2D6 enzymes in healthy volunteers, abstr. 569. 14th Conf. Retrovir. Oppor. Infect., Los Angeles, CA.
- 15.Pello, O. M., C. Moreno-Ortiz Mdel, J. M. Rodriguez-Frade, L. Martinez-Munoz, D. Lucas, L. Gomez, P. Lucas, E. Samper, M. Aracil, C. Martinez, A. Bernad, and M. Mellado. 2006. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood 108:3928-3937. [DOI] [PubMed] [Google Scholar]
- 16.Rezk, N. L., R. D. Crutchley, R. F. Yeh, and A. D. Kashuba. 2006. Full validation of an analytical method for the HIV-protease inhibitor atazanavir in combination with 8 other antiretroviral agents and its applicability to therapeutic drug monitoring. Ther. Drug Monit. 28:517-525. [DOI] [PubMed] [Google Scholar]
- 17.Saag, M., S. Rosenkranz, S. Becker, K. Klingman, B. Kallungal, A. Zadzilka, E. Coakley, E. Acosta, G. Calandra, and V. Johnson. 2007. Proof of concept of antiretroviral activity of AMD11070 (an orally administered CXCR4 entry inhibitor): results of the first dosing cohort A studied in ACTG protocol A5210, abstr. 512. 14th Conf. Retrovir. Oppor. Infect., Los Angeles, CA. [DOI] [PMC free article] [PubMed]
- 18.Shepherd, R. M., B. J. Capoccia, S. M. Devine, J. Dipersio, K. M. Trinkaus, D. Ingram, and D. C. Link. 2006. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood 108:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone, N. D., S. B. Dunaway, C. Flexner, C. Tierney, G. B. Calandra, S. Becker, Y.-J. Cao, I. P. Wiggins, J. Conley, R. T. Macfarland, J.-G. Park, C. Lalama, S. Snyder, B. Kallungal, K. L. Klingman, and C. W. Hendrix. 2007. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects (ACTG A5191). Antimicrob. Agents Chemother. 51:2351-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.University of Washington Academic Medical Centers and Harborview Medical Center. 1998. Appendix E: 1983 vs. 1959 Metropolitan height-weight tables for men, p. 53-54. In Clinical nutrition: a resource book for delivering enteral and parenteral nutrition for adults. University of Washington Medical Science Center, Seattle, WA.
- 21.Washington, C. B., G. E. Duran, M. C. Man, B. I. Sikic, and T. F. Blaschke. 1998. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:203-209. [DOI] [PubMed] [Google Scholar]