Abstract
R-type pyocins are high-molecular-weight bacteriocins carried within the chromosomes of some bacterial species, such as Pseudomonas aeruginosa, and almost certainly evolved from lysogenic bacteriophages of the Myoviridae family. They contain no head structures and no DNA and are used as defense systems, usually against other strains of the same bacterial species. They bind with their tail fibers to targeted bacterial surface molecules and then kill by inserting a core or needle that dissipates the bacterial membrane potential. Their mechanism of action, high bactericidal potency (one pyocin particle can kill one bacterium), and focused spectrum suggest that R-type pyocins could be developed as antibacterial agents. In a lethal mouse peritonitis model, submicrogram quantities of pyocin prevent death from 90% lethal dose inocula of a pyocin-sensitive, clinical isolate of P. aeruginosa. We show here the dose response curves, treatment windows, or periods of response after infection and the several-log-unit acute reduction of bacterial load in blood and spleen samples, suggesting that R-type pyocins have several characteristics that one would expect from an effective therapeutic.
Many Pseudomonas species possess the genes for one or both of the two types of high-molecular-weight pyocins, the R type and the F type (17, 28). Both are carried in the bacterial genome. The R-type pyocin locus consists of 16 open reading frames including 10 structural genes plus regulatory and chaperone genes (24, 25). Morphologically and genetically, the R-type pyocins resemble the tails of Myoviridae bacteriophages but have no head structure and thus no nucleic acid content (9, 15, 16, 23). They are thought to have evolved from the tail structure of a P2 bacteriophage-related ancestor; but they are not simply defective phages. There are meaningful physical and chemical stability differences, such as differences in protease and acid resistance, between pyocins and phage tails as the former have been adapted for their role as defensive bactericidal agents (14, 22). However, similar to bacteriophages, pyocins bind to specific “receptors” on target bacteria and penetrate their membranes with a “core,” or needle-like structure (31). In contrast to bacteriophages, pyocins have no material to inject and their penetrating core remains patent. As an immediate consequence, the bacterium is killed by dissipation of its membrane potential, a bactericidal event that can result from the binding of a single pyocin particle (8, 27, 31). An R-type pyocin particle can act only once; once it kills a cell, it cannot go on to kill others. Bacteriophages and R-type pyocins share common ancestry, but in contrast to bacteriophages, pyocins kill without directly causing bacterial cell lysis, a feature likely desirable for treating systemic infections (7, 33).
Francois Jacob discovered and first described pyocins as high-molecular-weight bacteriocins (11). Over subsequent decades, much effort has been expended studying Pseudomonas aeruginosa pyocin morphology, structure, genetics, and mechanism of action. Five R-type pyocins, termed R1 to R5, have been described (10), each with a unique spectrum that is related to those of the others such that R4 encompasses the spectrum of R3, R2 encompasses the spectrum of R3 and R4, and R5 has the broadest spectrum and includes those of all the other pyocins in addition to other strains. The spectrum of R1 is distinct from those of R2, R3, and R4 but is still a subset of that of R5. So R5 is at the root of a “spectrum tree” with two branches; R1 is one branch, and R2, R4, and R3 form another branch, in that order. Although the term R-type pyocin is commonly used to describe the high-molecular-weight bacteriocins of P. aeruginosa, similar entities have been described for multiple other gram-negative bacteria (4) and even for the gram-positive organisms Listeria monocytogenes (36) and Staphylococcus aureus (30). We elected to study peritonitis in mice infected with P. aeruginosa, a target pathogen for wild-type R-2 pyocin; R-2 pyocin is lethal to many P. aeruginosa strains. Peritonitis caused by P. aeruginosa is a serious threat to children with ruptured appendices and patients undergoing continuous ambulatory peritoneal dialysis (CAPD) (1, 3, 13, 35).
The antibacterial efficacy of R-type pyocins in P. aeruginosa infections has previously been demonstrated in two early, cursory studies of peritonitis in mice (6, 19). These studies were limited in scope, did not employ a quantitative pyocin assay to determine exact doses, and did not explore dose response, time of administration, or more than a single route of administration. In this study, we significantly expanded the analyses of in vivo efficacy to assess the potential of pyocins as human therapeutics and prophylactics. We have compared the efficacies of intraperitoneal (i.p.) and intravenous (i.v.) routes of administration of known quantities of a wild-type pyocin at various times after i.p. infection of mice. Bacterial counts in blood and spleen samples before and after treatment were monitored, and a preparation of a recombinant pyocin ineffective in vitro on P. aeruginosa, as a negative control, was shown to have no efficacy in vivo.
MATERIALS AND METHODS
Pyocin assays.
Quantitative pyocin assays were performed by counting surviving bacteria, using a method slightly modified from that described by Kageyama et al. (16). In a typical assay, fivefold serial dilutions of pyocins in TN50 buffer (50 mM NaCl, 10 mM Tris, pH 7.5) were incubated in a microtiter well with a known quantity of target bacteria in Trypticase soy broth, for example, 1 × 108 CFU/100 μl, for 40 min at 37°C. The samples were then serially diluted and aliquots spotted on Trypticase soy agar plates to count surviving bacteria. A bactericidal event is the death of a bacterium from contact with a pyocin particle(s). In order to accurately count pyocin particles, one must consider the fact that multiple particles may, by chance, attach to a single cell and that some cells may not be contacted by a pyocin at all at a given pyocin-to-cell ratio. The number of bactericidal events is related to the fraction of bacterial survivors in a Poisson distribution, m = −ln(S), where m is the average number of bactericidal events per bacterial cell, and S is the fraction of survivors. The total number of bactericidal events per ml equals m times the number of bacterial cells per ml. The microtiter well that has an average of 1 pyocin particle per bacterium will yield at or near equilibrium 37% survivors, and the well with an average of 2.3 pyocin particles per bacterium will yield 10% survivors. It was within this survival range that the m values were calculated. Based on a molecular mass of 10.1 × 106 Da and the determined total protein concentration of a partially purified pyocin preparation, one can calculate the number of pyocins per ml and the number of killing events per pyocin. From multiple preparations and assays, the latter ranged from 0.4 to 0.7, assuming 100% purity of pyocins. A typical preparation of R2 pyocins ranged from 1 × 1012 to 5 × 1012 killing events per ml and the total protein concentration from 60 to 100 μg/ml. Based on these data, we equate the number of killing events to the number of pyocin particles. The target strain used in this study, 13s, is a clinical isolate of Pseudomonas aeruginosa acquired from Stanford University Medical Center. Strain 13s is sensitive (cells are killed by pyocin at a predicted 1:1 interaction) to all five R-type pyocins.
Semiquantitative assays of R-type pyocins were also performed by a spot method wherein 5-μl samples of pyocin were serially diluted 1:5 in TN50 buffer and spotted on lawns of target bacteria. After overnight incubation at 37°C, pyocin activity could be observed by a circular clear zone of killing the size of the liquid application on the lawn.
Pyocin purification.
Pyocin production and purification have been accomplished by developing protocols similar to those described by Ito et al. (10). P. aeruginosa strain PAO1 is a producer of R2 pyocin and was the source of pyocins for this study. R-type pyocins, which naturally are under recA control, were induced by adding mitomycin C to log-phase suspension cultures growing in G medium (20 g sodium glutamate, 5 g glucose, 2.23 g Na2HPO4, 100 mg MgSO4·7H2O, 250 mg KH2PO4, 500 mg yeast extract per liter) at 37°C and shaken at 200 rpm. When the cultures, typically 100 to 200 ml, reached an optical density at 600 nm of 0.250, mitomycin C was added to give a final concentration of 3 μg/ml. Cultures were incubated for an additional 2.5 h until complete lysis occurred. The culture was allowed to incubate an additional 30 min after 5 μl (1 U/μl; Invitrogen) of DNase I was added per 200-ml culture. The subsequent preparation was carried out at 4°C. Lysates were collected, and debris was removed from the lysate by centrifugation at 22,000 × g in a Beckman Coulter Avanti J-25I centrifuge with a JLA-16.250 rotor for 1 h. To each 100 ml of supernatant, 65 ml of saturated ammonium sulfate was slowly added, at a rate of 1 ml/min, with stirring on ice. The suspension was stored at 4°C overnight. The ammonium sulfate precipitate was sedimented at 22,000 × g for 1 h and the pellet resuspended in 10 ml of TN50 buffer. R-type pyocin particles were then sedimented at 65,000 × g in a Beckman JA-25.50 rotor for 1 h at 4°C and resuspended in 3 to 5 ml of TN50 buffer. Pyocin preparations were judged to be 80 to 90% pure by sodium dodecyl sulfate gel analysis and either Coomassie blue or silver staining.
A typical yield of pyocins was 100 particles per induced bacterium, and a typical density of purified, concentrated R-pyocins was 3 × 1012 per ml. R2 pyocins are stable (<10% loss) for at least 60 days at 4°C in TN50 buffer without preservative.
In vivo studies.
All animal studies were carried out by ViviSource Laboratories, Inc., Cambridge, MA, a USDA-approved facility with AAALAC accreditation pending. P. aeruginosa strain 13s, used throughout as the pathogen for infecting mice, was grown with shaking overnight at 37°C in brain heart infusion broth. The overnight culture was diluted 10-fold in brain heart infusion broth and grown with shaking for an additional 5 h at 37°C. This culture was further diluted in 8% hog gastric mucin to achieve concentrations of 106 to 107 CFU/ml. CD-1 female mice (Charles River Laboratories) weighing 18 to 22 g were injected i.p. with 0.5 ml of the P. aeruginosa strain 13s inoculum per mouse, using 25-gauge needles. Aliquots of the inocula were cultured quantitatively on Trypticase soy agar plates to obtain the actual number of CFU in each inoculum.
Treatment involved the delivery of pyocins at the indicated times and known doses in 0.1 ml of TN50 with 25-gauge needles either directly i.p. or i.v. via the dorsal tail vein. Animals were housed at five per cage with ad lib water and food and were monitored for signs of illness. If and when animals were first observed to be moribund, they were euthanized by CO2 inhalation. Twenty-four and 48 h after inoculation, surviving animals were counted.
Bacteria in the blood samples were enumerated by serially diluting the sample and plating it on MacConkey agar plates. Spleens were weighed, homogenized, serially diluted, and plated. Plates were incubated at 37°C overnight and colonies of bacteria counted.
Immunogenicity.
Mouse sera were assayed for neutralizing antibodies by determining their ability to inactivate pyocin activity in vitro. Sera were serially diluted in TN50 buffer, added to 1010 pyocin particles to give a final volume of 50 μl, and incubated for 30 min at 25°C. Residual pyocin activities were determined by the titration assay as described above. Naïve mouse sera had no effect on pyocin activity.
RESULTS
The purified pyocins used in these studies were of the R2 type produced by P. aeruginosa PA01, which is itself resistant to R2 pyocin. P. aeruginosa strain 13s is sensitive to R2 pyocins in the in vitro assays and was used throughout for the infecting inocula. The inoculum that results in the death of 90% of animals (LD90) for strain 13s administered i.p. to 18- to 22-g female CD-1 mice was determined. When survival 48 h after infection was the endpoint, the LD90 was between 1 × 106 and 3 × 106 CFU per mouse. Animals surviving for 48 h invariably survived for weeks thereafter. When death at 24 h was scored as the endpoint, slightly larger inocula were required to kill 90% of the animals. LD90 values were confirmed with groups of 10 animals six times throughout these studies and proved to be consistent: in five independent experiments, 9 of 10 animals died from inocula of 1 × 106 to 3 × 106 CFU, and in one case, all 10 animals had died at 48 h.
Bacteria in the blood and spleen samples were enumerated starting 2 h and up to 24 h after infection (Table 1) (five animals per time point). Bacteria appeared in the blood and spleen samples within 2 h and had expanded an additional 1.6 logs in blood samples and 1.9 logs in spleens after 8 h, indicating an aggressive systemic infection.
TABLE 1.
Bacterial counts
| Time point (h) | Mean bacterial count (SD) for:
|
|
|---|---|---|
| Blood (log no. of CFU/ml) | Spleen (log no. of CFU/g) | |
| 2a | 4.92 (0.37) | 5.31 (0.20) |
| 4a | 5.08 (0.38) | 6.23 (0.19) |
| 8a | 6.57 (0.37) | 7.15 (0.31) |
| 24a | 7.08 (0.60) | 8.29 (0.21) |
| 1a | 5.50 (0.52) | 6.73 (0.92) |
| 4a | 5.51 (0.26) | 7.65 (0.34) |
| 8a | 7.61 (0.40) | 8.17 (0.24) |
| 9a,b | 3.12 (0.45) | 4.22 (0.46) |
| 12a,b | 4.30 (NAc) | 5.45 (0.98) |
| 16a,b | 3.34 (0.06) | 5.85 (0.52) |
| 24a,b | 4.61 (0.66) | 7.68 (0.06) |
Bacterial counts in blood and spleen samples collected from cohorts of five animals each were determined at various times after i.p. inoculation of Pseudomonas aeruginosa strain 13s.
Mice were treated i.v. with 3 × 1011 pyocins at 8 h after inoculation of Pseudomonas aeruginosa 13s.
NA, not available.
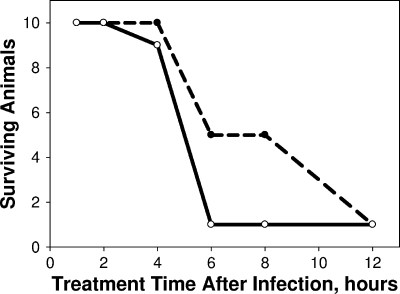
Administration of 3 × 1011 pyocins i.p. 1 hour after infection or as late as 4 h after infection protected 90 to 100% of the animals at 24 and 48 h (Fig. 1). Further delay of i.p. treatment resulted in significant reduction of survival even when observed at 24 h after infection, an interesting contrast to the effect observed at 24 h after delayed i.v. treatment (see below). As observed for the untreated animals in the LD90 studies, treated animals surviving for 48 h survived at least as long as 28 days in several extended studies. Thus, we conclude that treated survivors at 48 h have been cured.
FIG. 1.
Effective treatment window for i.p. administration of pyocin. Female CD-1 mice were infected with LD90 inocula of strain 13s P. aeruginosa. A cohort of 10 infected animals was not treated, to verify that the inocula were at the LD90 value. At each of the indicated times after infection, 10 animals were treated once i.p. with 3 × 1011 pyocins in 0.1 ml. If and when animals first appeared moribund, they were euthanized, and survivors were counted at 24 (broken line) and 48 (solid line) hours postinfection.
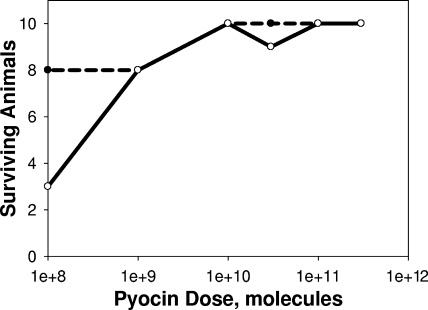
The responses of infected animals to increasing doses of pyocins administered i.p. 1 hour after infection are shown in Fig. 2 and reveal that 80% of the animals can be protected by as few as 109 pyocins. Except at the lowest dose, 108 pyocins, there was no observed difference between efficacies measured at 24- and 48-h endpoints. At the minimally (80%) effective dose, the number of pyocin molecules administered was approximately 750 per inoculated bacterium (assuming 100% survival upon inoculation). Since the doses of pyocins were administered i.p. at 1 h postinfection, there was likely limited expansion of bacterial load by the time of treatment.
FIG. 2.
Responses of infected animals to different i.p. doses of pyocins. Female CD-1 mice were infected with LD90 inocula of strain 13s P. aeruginosa. A cohort of 10 infected animals was not treated, to verify that the inocula were at the LD90 value. One hour after infection, cohorts of 10 animals each were treated once i.p. with each of the indicated doses of pyocin in 0.1 ml. If and when animals first appeared moribund, they were euthanized, and survivors were counted at 24 (broken line) and 48 (solid line) hours postinfection.
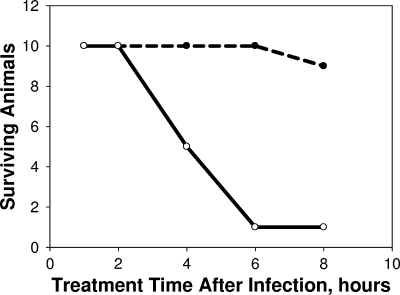
We also examined efficacy when pyocins were delivered i.v. at various doses and time intervals after infection. Survival at 48 h was 100% when the times between infection and treatment were 1 or 2 h. Survival rates fell with longer time intervals until at 6 h there was no efficacy at the 48-h endpoint (Fig. 3). However, when animals were observed at 24 h, survival was 90% for animals treated i.v. as long as 8 h after infection; in contrast, the 24-h survival rates of untreated animals or animals treated with a 10,000-fold lower dose were only 20 to 40%.
FIG. 3.
Effective treatment window for i.v. administration of pyocin. Female CD-1 mice were infected with LD90 inocula of strain 13s P. aeruginosa. At each of the indicated times after infection, cohorts of 10 animals each were treated once i.v. with 3 × 1011 pyocins in 0.1 ml. If and when animals first appeared moribund, they were euthanized, and survivors were counted at 24 (broken line) and 48 (solid line) hours postinfection.
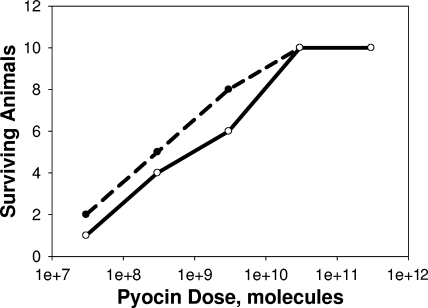
An i.v. dose response study was conducted by administering pyocins at increasing doses 1 h after infection (Fig. 4). The dose which results in 50% survival (ED50) at the 48-h endpoint was approximately 109 pyocins delivered i.v. at 1 h postinfection, a value within 5- to 10-fold of the lower ED50 for i.p. treatment, as extrapolated from Fig. 2.
FIG. 4.
Responses of infected animals to different i.v. doses of pyocins. Female CD-1 mice were infected with LD90 inocula of strain 13s P. aeruginosa. One hour after infection, cohorts of 10 animals each were treated once i.v. with each of the indicated doses of pyocin in 0.1 ml. If and when animals first appeared moribund, they were euthanized, and survivors were counted at 24 (broken line) and 48 (solid line) hours postinfection.
Bacterial counts in the blood and spleen samples of animals that were infected i.p. and treated 1 h or 8 h later by i.v. administration of 3 × 1011 pyocins were determined. In the cohort that was treated i.v. 1 h after infection, no bacteria could be detected in the blood or spleen samples after 2 hours or up to 24 h. In the group that was treated i.v. 8 h after infection, when the bacteria had increased nearly 2 logs over the preceding 6 h, there was a decrease of up to 4 logs of bacteria in the blood and spleen samples 1 hour after the delayed pyocin treatment, indicating a strong bactericidal effect (Table 1). However, the bacteria subsequently increased again, approximately 1 log in blood samples and 3 logs in spleens, resulting in death. It appears that the bacterial loads are too high to be overcome with a delayed single dose of 3 × 1011 pyocins administered 8 h after infection. Bacteria from these succumbing animals were colony purified and tested for pyocin sensitivity. Of 12 random isolates, all were still fully sensitive to R2 pyocin. This indicates that failure to rescue mice was not due to the pathogen's development of pyocin resistance but likely due to an inadequate dosing regimen.
We have engineered R-type pyocins to target other strains, species, and genera of bacteria by altering the tail fibers such that they recognize different targets (S. Williams et al., submitted for publication). One such pyocin, termed R2-P2, was made by fusing the C-terminal 511 amino acids of the bacteriophage P2 tail fiber to the 164-amino-acid portion of the R2 pyocin tail fiber. The resulting pyocin particles have no in vitro killing activity against any Pseudomonas strains, including 13s, but are active against Escherichia coli C, which is a host for phage P2. Administering 1011 R2-P2 pyocins (as determined by activity using E. coli C as the indicator) i.v. 1 h after i.p. infection did not rescue mice (1 survivor in 10) from peritoneal infection by an LD90 inoculum of P. aeruginosa strain 13s. This supports the hypothesis that pyocins rescue mice by specifically killing targeted bacteria and that the results are not due to a contaminant in the pyocin preparation or a nonspecific effect of the pyocin, such as immune stimulation.
R-type pyocins are foreign proteins to mice and may provoke immune responses which might compromise pyocin efficacy in retreated animals. To test this, mice were infected and successfully treated with an i.v. dose of 3 × 1010 pyocins at 1 hour postinfection. The recovered animals were maintained for an additional 28 days. The animals were then rechallenged i.p. with another LD90 infection by strain 13s P. aeruginosa. After the rechallenge, three groups of 10 animals each were treated again i.v. 1 hour after infection with 3 × 109, 3 × 1010, or 3 × 1011 pyocins to determine whether their sensitivities to pyocins had been altered by the previous exposure to pyocins. A cohort of reinfected animals was not retreated, in order to determine whether any protection against strain 13s P. aeruginosa was conveyed by their prior exposure to the pathogen. The results revealed a reduction in the efficacy of pyocins in animals previously infected and cured by pyocins. Between 10 and 40% of the animals originally cured with the lowest effective i.v. dose were still protected by i.v. pyocins when reinfected and then retreated, and the protection was not proportional to the retreatment dose. Sera from five of the animals that died after the second treatment were examined for neutralizing antibodies. Four out of the five animals showed low levels of neutralizing antibodies, with a 1-log reduction in pyocin activity at the 1/20 serum dilution. Sera from five survivors were also examined and exhibited little or no neutralizing activity.
DISCUSSION
P. aeruginosa is a common pathogen found in peritonitides secondary to ruptured appendices in otherwise healthy children (1, 35). It also accounts for up to 10% of cases associated with CAPD and is associated with high morbidity, CAPD failure, and late complications (12, 13, 18). Rodent models of P. aeruginosa peritonitis have been developed for the purpose of understanding the pathophysiology and evaluating various modes of intervention (2, 21, 33, 34). The literature documents that within 6 h of i.p. inoculation with P. aeruginosa, mice develop bacteremia, infected livers, and elevated serum levels of interleukin-6. The load of infectious organisms increases several orders of magnitude, and depending upon the inoculum size and virulence of the strain, death may ensue within 2 to 3 days (2).
In this study, the P. aeruginosa inocula were chosen to result in 90% mortality by 48 h after infection so as to provide an aggressive infection and clear therapeutic endpoints. The data from the LD90 determination indicate that a 10-fold reduction in the number of inoculated pathogens would result in significant reduction in mortality. The i.p. and i.v. dose responses were similar, although when delivered i.p., comparable doses of pyocins exhibited better efficacy, with a 5- to 10-fold lower ED50. At the minimally effective (80% survival) i.p. dose, the number of administered pyocin molecules relative to the number of inoculated bacteria was less than 750. The numbers of bacteria in blood and spleen samples increased when treatment was delayed, yet pyocins still killed at least 99.99% of the bacteria in the blood and spleen samples.
When delivered i.v. up to 8 h after infection, pyocins protected animals for 24 h, but the protection failed to last for 48 h. At 48 h, protection from death could be observed only if pyocins were delivered i.v. within 2 h of i.p. infection. This contrasts with i.p. administration. Up to 4 h after infection, i.p. pyocin rescued animals from death, with little difference between survival rates at 24- and 48-h endpoints. As measured at 48 h, the less durable effect of i.v. pyocins delivered 4 h or more after infection will require further exploration. However, further-delayed i.v. treatment does protect for 24 h. Perhaps diffusion of pyocin from the blood into the peritoneal space is inadequate to eliminate the load of bacteria that have expanded between 2 and 4 h in that inoculated compartment and continue to seed the bacteremia. A dose of pyocins delivered directly to the inoculated compartment could control that site while still diffusing into the vascular compartment to eliminate the early bacteremia. The latter might be predicted based on the ability of bacteriophages to enter the circulating blood rapidly after i.p. administration (5, 20, 32).
R-type pyocins are potentially immunogenic. The studies described herein have shown that neutralizing antisera can be generated by administering therapeutic doses of pyocins i.p. or i.v. to mice. However, the neutralizing titers observed 28 to 30 days afterwards are low (1:20) and do not consistently antagonize treatment. Nevertheless, it is likely that repeated systemic exposures to R-type pyocin will result in loss of efficacy. This may not be an issue for some severe, acute Pseudomonas infections requiring only short-term use but may ultimately limit chronic use. To address this, we are exploring the generation of hypoimmunogenic variants of pyocin proteins. It may be feasible to engineer “deimmunized” pyocins for human use by recently developed methods of identifying and modifying the major T-cell epitopes of the pyocin proteins (26).
R-type pyocins, compared to traditional antibiotics, have several potential advantages as antimicrobial agents. Their narrow, species- and strain-specific bactericidal spectra permit them to kill the target bacteria without disrupting the normal microbial flora, a feature advantageous for treatment and prevention of many bacterial diseases. Second, there is no known mechanism by which pyocin resistance can be spread horizontally between bacteria, a phenomenon all too common with traditional antibiotics.
R-type pyocins share many features with bacteriophages. Phages have recently been used to treat murine peritonitis and bacteremia (7, 32, 33). Watanabe et al. (33) had limited success using lytic bacteriophages to treat peritonitis, whereas Hagens et al. (7) showed better efficacy using a lysis-deficient, nonreplicating phage. It was suggested by the latter authors that the difference could be due to the fact that lysis of the bacterial cells by lytic phages led to the release of endotoxins, which may have compromised efficacy. Similarly, R-type pyocins, which leave the dead bacterial cell largely intact, should not result in release of endotoxins.
While R-type pyocins are highly specific, their specificities can also be engineered. The spectrum determinant of pyocins resides in the tail fiber protein, which binds to specific receptors on the bacterial cell surface. We have exploited this feature to engineer pyocins to create novel bactericidal agents that can be specifically targeted to other pathogens (Williams et al., submitted). In theory, we could engineer pyocins to target surface-accessible bacterial virulence factors so that in order to become pyocin resistant, bacterial mutants must lose the pyocin receptor and thereby compromise their virulence.
These studies provide evidence that pyocins delivered systemically or locally can effectively treat an acute, potentially lethal Pseudomonas infection in mice. Furthermore, the observed rapid reduction of bacterial load in blood and spleen samples, dose response curves, and therapeutic windows suggest that pyocins exhibit characteristics of therapeutic molecules. This is particularly encouraging in the context of the need for new antimicrobials (29).
Acknowledgments
This work was fully supported by AvidBiotics Corp.
We thank Steve Williams and Dana Gebhart for their technical support and Andrew Jamison for assistance in developing the pyocin assays. Dean Scholl and David Martin are employees and shareholders of AvidBiotics Corp.
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Aronoff, S. C., M. M. Olson, M. W. Gauderer, M. R. Jacobs, J. L. Blumer, and R. J. Izant. 1987. Pseudomonas aeruginosa as a primary pathogen in children with bacterial peritonitis. J. Pediatr. Surg. 22:861-864. [DOI] [PubMed] [Google Scholar]
- 2.Barekzi, N. A., K. A. Poelstra, A. G. Felts, I. A. Rojas, J. B. Slunt, and D. W. Grainger. 1999. Efficacy of locally delivered polyclonal immunoglobulin against Pseudomonas aeruginosa peritonitis in a murine model. Antimicrob. Agents Chemother. 43:1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardini J., F. Bender, T. Florio, J. Sloand, L. Palmmontalbano, L. Fried, and B. Piraino. 2005. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J. Am. Soc. Nephrol. 16:539-545. [DOI] [PubMed] [Google Scholar]
- 4.Coetzee, H. L., H. C. De Klerk, J. N. Coetzee, and J. A. Smit. 1968. Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J. Gen. Virol. 2:29-36. [DOI] [PubMed] [Google Scholar]
- 5.Dabrowska, K., K. Switała-Jelen, A. Opolski, B. Weber-Dabrowska, and A. Gorski. 2005. Bacteriophage penetration in vertebrates. J. Appl. Microbiol. 98:7-13. [DOI] [PubMed] [Google Scholar]
- 6.Haas, H., T. Sacks, and N. Saltz. 1974. Protective Effect of pyocin against lethal Pseudomonas aeruginosa infections in mice. J. Infect. Dis. 129:470-472. [DOI] [PubMed] [Google Scholar]
- 7.Hagens, S., A. Habel, U. von Ahsen, A. von Gabain, and U. Blasi. 2004. Therapy of experimental Pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob. Agents Chemother. 48:3817-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iijima, M. 1978. Mode of action of pyocin R1. J. Biochem. (Tokyo) 83:395-402. [DOI] [PubMed] [Google Scholar]
- 9.Ishii, S., Y. Nishi, and F. Egami. 1965. The fine structure of a pyocin. J. Mol. Biol. 13:428-431. [DOI] [PubMed] [Google Scholar]
- 10.Ito, S., M. Kageyama, and F. Egami. 1970. Isolation and characterization of pyocins from several strains of Pseudomonas aeruginosa. J. Gen. Appl. Microbiol. 16:205-214. [Google Scholar]
- 11.Jacob, F. 1954. Biosynthèse induite et mode d'action d'une pyocin, antibiotique de Pseudomonas pyocyanea. Ann. Inst. Pasteur 86:149-160. [PubMed] [Google Scholar]
- 12.Johnson, C. C., J. Baldessarre, and M. E. Levison. 1997. Peritonitis: update on pathophysiology, clinical manifestations, and management. Clin. Infect. Dis. 24:1035-1047. [DOI] [PubMed] [Google Scholar]
- 13.Juergensen, P. H., F. O. Finkelstein, R. Brennan, S. Santacroce, and M. J. Ahern. 1988. Pseudomonas peritonitis associated with continuous ambulatory peritoneal dialysis: a six-year study. Am. J. Kidney Dis. 11:413-417. [DOI] [PubMed] [Google Scholar]
- 14.Kageyama, M., and F. Egam. 1962. On the purification and some properties of a pyocin, a bacteriocin produced by Pseudomonas aeruginosa. Life Sci. 9:471-476. [DOI] [PubMed] [Google Scholar]
- 15.Kageyama M. 1964. Studies of a pyocin. I. Physical and chemical properties. J. Biochem. 55:49-53. [DOI] [PubMed] [Google Scholar]
- 16.Kageyama, M., K. Ikeda, and F. Egami. 1964. Studies of a pyocin. III. Biological properties of the pyocin. J. Biochem. 55:59-64. [DOI] [PubMed] [Google Scholar]
- 17.Kageyama, M. 1975. Bacteriocins and bacteriophages in Pseudomonas aeruginosa, p. 291-305. In T. Mitsuhashi and H. Hashimoto (ed.), Microbial drug resistance. University of Tokyo Press, Tokyo, Japan.
- 18.Krothapalli, R., W. B. Duffy, C. Lacke, W. Payne, H. Patel, V. Perez, and H. O. Senekjian. 1982. Pseudomonas peritonitis and continuous ambulatory peritoneal dialysis. Arch. Intern. Med. 142:1862-1863. [PubMed] [Google Scholar]
- 19.Merrikin, D. J., and C. S. Terry. 1972. Use of pyocin 78-C2 in the treatment of Pseudomonas aeruginosa infection in mice. Appl. Microbiol. 23:164-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merril, C. R., B. Biswas, R. Carlton, N. C. Jensen, G. J. Creed, S. Zullo, and S. Adhya. 1996. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 93:3188-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michéa-Hamzehpour, M., R. Auckenthaler, P. Regamey, and J.-C. Pechère. 1987. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob. Agents Chemother. 31:1803-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama, K., K. Takashima, H. Ishihara, T. Shinomiya, M. Kageyama, S. Kanaya, M. Ohnishi, T. Murata, H. Mori, and T. Hayashi. 2000. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 38:213-231. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu, Y., T. Kamazaki, and S. I. Ishii. 1982. Specific cleavage at fibers of a bacteriophage-tail-like bacteriocin, pyocin R1, by successive treatment with organomercurial compounds and trypsin. J. Virol. 44:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinomiya, T., S. Shiga, and M. Kageyama. 1983. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. I. Localization of the pyocin R2 gene cluster between the trpCD and trpE genes. Mol. Gen. Genet. 189:375-381. [DOI] [PubMed] [Google Scholar]
- 25.Shinomiya, T., S. Shiga, A. Kikuchi, and M. Kageyama. 1983. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. II. Physical characterization of pyocin R2 genes using R-prime plasmids constructed from R68.45. Mol. Gen. Genet. 189:382-389. [DOI] [PubMed] [Google Scholar]
- 26.Stickler, M., N. Rochanayon, O. J. Razo, J. Mucha, W. Gebel, N. Faravashi, R. Chin, S. Holmes, and F. A. Harding. 2004. An in vitro human cell-based assay to rank the relative immunogenicity of proteins. Toxicol. Sci. 77:280-289. [DOI] [PubMed] [Google Scholar]
- 27.Strauch, E., H. Kaspar, C. Schaudinn, P. Dersch, K. Madela, C. Gewinner, S. Hertwig, J. O. Wecke, and B. Appel. 2001. Characterization of enterocoliticin, a phage tail-like bacteriocin, and its effect on pathogenic Yersinia enterocolitica strains. Appl. Environ. Microbiol. 67:5634-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeya, K., Y. Minamishima, Y. Ohnishi, and K. Amako. 1969. Rod-shaped pyocin 28. J. Gen. Virol. 4:145-149. [DOI] [PubMed] [Google Scholar]
- 29.Talbot, G. H., J. Bradley, J. E. Edwards, D. Gilbert, M. Scheld, and J. G. Bartlett. 2006. Bad bugs need drugs: an update on the development pipeline from the antimicrobial availability. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 30.Thompson, N. E., and P. A. Pattee. 1981. Genetic transformation in Staphylococcus aureus: demonstration of a competence-conferring factor of bacteriophage origin in bacteriophage 80a lysates. J. Bacteriol. 148:294-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uratani, Y., and T. Hoshino. 1984. Pyocin R1 inhibits active transport in Pseudomonas aeruginosa and depolarizes membrane potential. J. Bacteriol. 157:632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, J., B. Hu, M. Xu, Q. Yan, S. Liu, X. Zhu, Z. Sun, E. Reed, L. Ding, J. Gong, Q. Q. Li, and J. Hu. 2006. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int. J. Mol. Med. 17:309-317. [PubMed] [Google Scholar]
- 33.Watanabe, R., T. Matsumoto, G. Sano, Y. Ishii, K. Tateda, Y. Sumiyama, J. Uchiyama, S. Sakurai, S. Matsuzaki, S. Imai, and K. Yamaguchi. 2007. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 51:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein, W. M., A. B. Onderdonk, J. G. Bartlett, and S. L. Gorbach. 1974. Experimental intra-abdominal abscesses in rats: development of an experimental model. Infect. Immun. 10:1250-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yellin, A. E., P. N. Heseltine, T. V. Berne, M. D. Appleman, M. A. Gill, C. E. Riggio, and F. C. Chenella. 1985. The role of Pseudomonas species in patients treated with ampicillin and Sulbactam for gangrenous and perforated appendicitis. Surg. Gynecol. Obstet. 161:303-307. [PubMed] [Google Scholar]
- 36.Zink, R., M. J. Loessner, and S. Schere. 1995. Characterization of cryptic prophages (monocins) in Listeria and sequence analysis of a holin/endolysin gene. Microbiology 141:2577-2584. [DOI] [PubMed] [Google Scholar]