Abstract
Phosphinothricin tripeptide (PTT) is a peptide antibiotic produced by Streptomyces viridochromogenes Tü494, and it is synthesized by nonribosomal peptide synthetases. The PTT biosynthetic gene cluster contains three peptide synthetase genes: phsA, phsB, and phsC. Each of these peptide synthetases comprises only one module. In neither PhsB nor PhsC is a typical C-terminal thioesterase domain present. In contrast, a single thioesterase GXSXG motif has been identified in the N terminus of the first peptide synthetase, PhsA. In addition, two external thioesterase genes, theA and theB, are located within the PTT biosynthetic gene cluster. To analyze the thioesterase function as well as the assembly of the peptide synthetases within PTT biosynthesis, several mutants were generated and analyzed. A phsA deletion mutant (MphsA) was complemented with two different phsA constructs that were carrying mutations in the thioesterase motif. In one construct, the thioesterase motif comprising 45 amino acids of phsA were deleted. In the second construct, the conserved serine residue of the GXSXG motif was replaced by an alanine. In both cases, the complementation of MphsA did not restore PTT biosynthesis, revealing that the thioesterase motif in the N terminus of PhsA is required for PTT production. In contrast, TheA and TheB might have editing functions, as an interruption of the theA and theB genes led to reduced PTT production, whereas an overexpression of both genes in the wild type enhanced the PTT yield. The presence of an active single thioesterase motif in the N terminus of PhsA points to a novel mechanism of product release.
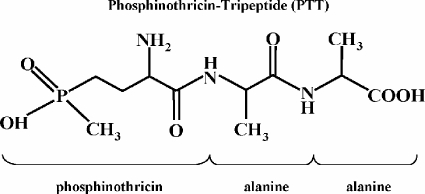
The antibiotic phosphinothricin tripeptide (PTT; also called bialaphos) is produced by Streptomyces viridochromogenes Tü494 and Streptomyces hygroscopicus, respectively (4, 20). It consists of two molecules, l-alanine and the unusual amino acid phosphinothricin (PT) (Fig. 1). The bioactive compound PT shows bactericidal, fungicidal, and herbicidal activity, as it is similar to the structure of glutamic acid and, thus, competitively inhibits glutamine synthetase.
FIG. 1.
Chemical structure of PTT and relevant compounds.
A biosynthetic pathway for bialaphos was postulated previously (44) based on the analysis of blocked mutants. In addition, the biosynthesis of PTT has been investigated by genetic and biochemical means (6, 17, 35, 36, 50). The precursor peptide N-acetyl-demethylphosphinothricin (N-Ac-DMPT) tripeptide is synthesized in at least 13 steps from intermediates of the primary metabolism and assembled by nonribosomal peptide synthetases (NRPSs). During or after assembly, N-Ac-DMPT is converted to PT by methylation and deacylation (1, 12, 35, 50). Within the completely sequenced PTT cluster, 24 genes were identified that are involved in the biosynthesis of PTT (5, 36). The PTT biosynthetic gene cluster harbors three genes, phsA, phsB, and phsC, which encode NRPSs (Fig. 2A) (36).
FIG. 2.
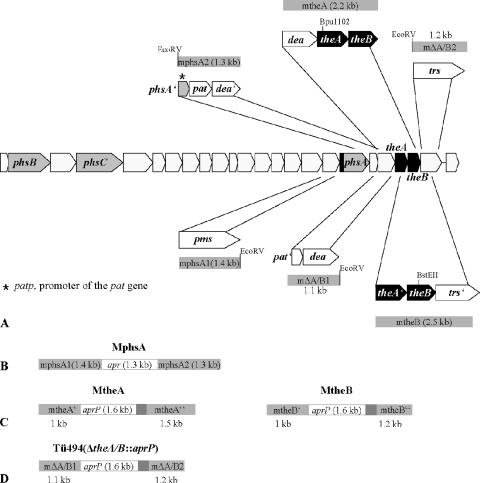
Localization of the peptide synthetase genes phsA, phsB, and phsC and the TE genes theA and theB in the PTT biosynthetic gene cluster from S. viridochromogenes Tü494. (A) The 5′ end of phsA containing the sequence of the TE motif is highlighted. pat, phosphinothricin N-acetyltransferase gene; pat′, part of the pat gene; dea, deacylase gene; dea′, part of the dea gene; trs, putative transporter gene; trs′, part of the trs gene; and phsA′, part of the phsA gene. The PCR fragments mphsA1, mphsA2, mtheA, and mtheB, which were used for the construction of the mutants MphsA, MtheA, and MtheB, are schematically illustrated. (B) Replacement of 1.7 kb of the phsA gene with the apramycin cassette (apr), resulting in the mutant MphsA. (C) Insertion of the apramycin-ermEp cassette (aprP) within theA and theB, respectively, resulting in the mutants MtheA and MtheB. (D) Replacement of the theA and theB genes with the apramycin-ermEp cassette (aprP), resulting in the mutant Tü494(ΔtheA/theB::aprP).
NRPSs are involved in the biosynthesis of many pharmacologically important natural products, such as penicillin and vancomycin (44, 48). These peptides are synthesized via a thiotemplate mechanism (25, 39). NRPSs are characterized by their modular organization. Each module is responsible for the handling of one amino acid. Furthermore, each module is subdivided into various domains. The adenylation domain (A domain) is required for the specific recognition and activation of an amino acid to form an aminoacyladenylate. The activated amino acid is converted into a thioester attached to the 4′-phosphopantetheine (4′PP) arm of the peptide carrier protein (PCP or T domain). The elongation of the peptide chain takes place at the condensation domain (C domain), where the amino acids are covalently bound and transferred to the next module. After the final peptide chain length has been achieved, the thioesterase domain (TE domain), which normally is localized at the C terminus of the last amino acid-activating module, cleaves off the peptide chain from the enzyme complex (7, 43). This type of TE is a member of the so-called type I TEs (TEIs), which show high sequence similarity to the TEs of fatty acid synthases of vertebrates (25).
The modules are classified by their function and arrangement within the peptide synthesis. The first amino acid-activating module (initiation module) consists of only the A and PCP domains. In many bacterial systems, such initiation modules stand alone, whereas later modules are organized as multimodular enzyme units. These multimodular enzymes have a C-A-PCP arrangement and represent the elongation modules. The so-called termination module carries an additional TE domain at the C terminus of the last module (27). The nonribosomal biosynthesis of PTT differs from that of the known bacterial systems. Sequence analysis has revealed that all three peptide synthetase genes encode only one peptide synthetase module. Furthermore, these genes are not juxtaposed in the biosynthetic gene cluster (35, 37). An in silico analysis, as well as biochemical and genetic experiments, proved that PhsA is responsible for the activation of the first amino acid, N-Ac-DMPT, the precursor of PT, whereas PhsB and PhsC each activate one alanine (37). PhsA consists of A and PCP domains; PhsB of PCP, C, and A domains; and PhsC of C, A, and PCP domains (Fig. 3A) (12, 37). Currently, it is not possible to determine which of the two alanylylation steps is catalyzed by PhsB and PhsC. The fact that the two enzymes cannot replace each other suggests a defined positioning for each protein in the PTT assembly line. However, in PTT synthetases, short communication-mediating domains (COM domains) that mediate interactions between peptide synthetases (14, 15) could not be identified (37).
FIG. 3.
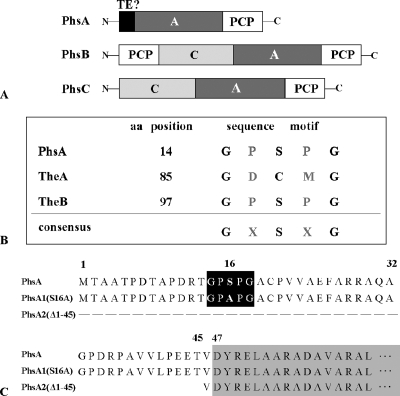
(A) Domain organization of the peptide synthetases PhsA, PhsB, and PhsC. (B) Localization of the TE motif GXSXG in PhsA, TheA, and TheB. In TheA, the active serine site is replaced by a cysteine residue. (C) Schematic representation of the primary sequence of the native PhsA protein as well as the mutated PhsA variants PhsA1(S16A) and PhsA2(Δ1-45). The TE motifs in PhsA and PhsA1(S16A) in which the conserved serine residues were changed to alanines are highlighted by a black box. The beginning of the adenylation domain of PhsA at aa 47 is marked by a gray box.
Surprisingly, a typical TE domain at the C terminus of PhsB or PhsC is missing. However, a highly conserved TE GXSXG motif at amino acids (aa) 14 to 18 was localized at the N terminus of PhsA (Fig. 3A and B). Furthermore, the two genes theA and theB (formerly known as the1 and the2 [36]) were identified, and their gene products showed high similarity to type II TEs (TEII). TEIIs are autonomous, monomeric proteins, while TEIs typically are integrated in the last module of peptide synthetases. Within polyketide and nonribosomal polypeptide biosyntheses, it was shown that TEII enzymes have editing roles (16, 38).
The PTT-specific TheA and TheB proteins belong to the TE family of α/β hydrolases. Members of this family contain the characteristic serine-aspartate-histidine (Ser-Asp-His) catalytic triad (45). TheB possesses the active-site Ser and His residues as part of the highly conserved GXSXG and GXHF motifs, respectively. In TheA, the GXHF motif also was identified, but the active-site serine in the GXSXG motif, which normally is involved in the catalytic activity of TEs, is replaced by a cysteine (Fig. 3B). It has been shown that the exchange of the serine residue for a cysteine can lead to the loss of TE activity (46). However, it also has been described that the same substitution had no significant effect on TE activity (49). Furthermore, the presence of a conserved Asp residue in both proteins was not apparent. The absence of the His or Asp residues also was observed in some other TEs (23, 32). The essential catalytic residue is the active-site serine, whereas the His residue, stabilized by Asp, acts as a catalytic base to remove a proton from Ser, which attacks the acyl carrier protein-bound thioester substrate (45).
In this study, we describe experimental approaches to clarify the function of the three putative TEs within PTT biosynthesis. A primary model of product release from the PTT assembly system is postulated.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains, cosmids, and plasmids used in this work are listed in Table 1. The morphological and physiological properties of wild-type S. viridochromogenes Tü494 and of the phsA, theA, and theB mutants were examined on yeast malt medium (YM) (35). Cultivation was carried out at 30°C; liquid cultures were incubated in 100 ml of medium in an orbital shaker (180 rpm) in 500-ml Erlenmeyer flasks with steel springs. Spores were isolated as previously described (19).
TABLE 1.
Bacterial strains, plasmids, and cosmids used in this study
| Bacterial strain, plasmid, or cosmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| S. viridochromogenes | ||
| Tü494 | PTT-producing wild type | 4 |
| MphsA | Replacement of 1.7 kb of phsA; apr; no PTT production | This study |
| MtheA | Gene interruption of theA; apr; reduced PTT production | This study |
| MtheB | Gene interruption of theB; apr; reduced PTT production | This study |
| Tü494(ΔtheA/theB::aprP) | Replacement of theA and theB; apr; reduced PTT production | This study |
| E. coli | ||
| XL1 Blue | recA1 end A1 gyrA96 thi-1 hsdR17 supE44 relA1lac[F′ proAB lac1qZΔ M15 Tn10(tetr)] | 8 |
| Cosmids | ||
| pPtcos2 | Carries a part of the PTT biosynthetic gene cluster | 1 |
| Plasmids | ||
| pK18/19 | pUC derivative; aphII; lacZ′ complementation system | 29 |
| pRSETB | T7 promoter expression system, 6× His tag, bla | Invitrogen |
| pJOE890 | bla, two multiple cloning sites, two ter genes | 2 |
| pGEM-T-Easy | pUC-derivative, lacZ′ bla | Promega |
| pDRIVE | lacZ′ complementation system, ampicillin and kanamycin resistance, multiple cloning site | Qiagen |
| pGM190 | Streptomyces-E. coli shuttle vector, aphII tsr, inducible tipA promoter, medium copy number | G. Muth, personal communication |
| pWHM3 | Streptomyces-E. coli shuttle vector, trs bla | 47 |
| pDS1 | pUC18 derivative carrying the phsA gene | 35 |
| pDS3 | pQE30 derivative carrying the mutated phsA gene phsA2(Δ1-45), in which the first 135 bp are deleted | 34 |
| pEH13 | pUC21 derivative carrying the aprP resistance cassette | 17 |
| pEH15 | pK18 derivative carrying constitutive ermEp | 17 |
| pSE1 | pJOE890 derivative carrying mphsA1, the fragment upstream of phsA | This study |
| pSE2 | pJOE890 derivative carrying mphsA2, the fragment downstream of phsA | This study |
| pSE3 | pK18 derivative carrying mphsA1 | This study |
| pSE4 | pSE3 derivative carrying mphsA2 behind mphsA1 | This study |
| pSE5 | pSE4 derivative, carrying aprP inserted between mphsA1 and mphsA2 | This study |
| pSE6 | pK18 derivative carrying the 622-bp PstI fragment (phsA′) of the 5′ end of phsA | This study |
| pSE6* | pSE6 derivative carrying phsA1′(S16A) | This study |
| pSE7 | pDS1 derivative carrying phsA1(S16A) | This study |
| pSE10 | pGEM-T-Easy derivative carrying the fragment mtheA | This study |
| pSE11 | pJOE980 derivative carrying the fragment mtheB | This study |
| pSE12 | pK19 derivative carrying the fragment mtheA | This study |
| pSE13 | pK18 derivative carrying the fragment mtheB | This study |
| pSE14 | pSE12 derivative carrying aprP inserted into mtheA | This study |
| pSE15 | pSE13 derivative carrying aprP inserted into mtheB | This study |
| pSE16 | pGEM-T-Easy derivative carrying the theA′ gene | This study |
| pSE17 | pGEM-T-Easy derivative carrying the theB′ gene | This study |
| pSE18 | pRSETB derivative carrying the theA′ gene | This study |
| pSE19 | pRSETB derivative carrying the theB′ gene | This study |
| pSE20 | pEH15 derivative carrying the RBS of pRSETB followed by the theA′ gene | This study |
| pSE21 | pEH15 derivative carrying the RBS of pRSETB followed by the theB′ gene | This study |
| pSE22 | pWHM3 derivative carrying ermEp theA′ | This study |
| pSE23 | pWHM3 derivative carrying ermEp theB′ | This study |
| pSE31 | pK18 derivative carrying the mutated phsA gene phsA2(Δ1-45), in which the first 135 bp are deleted | This study |
| pSE32 | pRSETB derivative carrying phsA2(Δ1-45) | This study |
| pSE34 | pGM190 derivative carrying the mutated phsA gene phsA2(Δ1-45) | This study |
| pSE40 | pDRIVE derivative carrying the phsA gene | This study |
| pSE41 | pGM190 derivative carrying the native phsA gene | This study |
| pSE42 | pDRIVE derivative carrying phsA1(S16A) | This study |
| pSE43 | pGM190 derivative carrying the point-mutated phsA1(S16A) gene | This study |
| pSE44 | pDRIVE derivative carrying the fragment mΔA/B1 | This study |
| pSE45 | pDRIVE derivative carrying the fragment mΔA/B2 | This study |
| pSE46 | pK18 derivative carrying mΔA/B1 | This study |
| pSE47 | pSE46 derivative carrying mΔA/B2 behind mΔA/B1 | This study |
| pSE48 | pSE47 derivative carrying aprP inserted between mΔA/B1 and mΔA/B2 | This study |
Amplification, cloning, restriction mapping, and in vitro manipulation of DNA.
For the amplification of DNA fragments by PCR, the following reaction mixture was used: 0.5 μg of template DNA (see Table 3), 1.0 μg of each primer (Tables 2 and 3 ), 10 μl of 10× reaction buffer (containing 20 mM MgCl2), 5% dimethylsulfoxide, 0.2 mM deoxynucleoside triphosphates, and 1 μl of polymerase (Pwo polymerase; Roche, Mannheim, Germany) or Herculase (Stratagene) (Table 3). After a denaturation step (5 min at 98°C), 25 cycles of amplification (90 s at 94°C, 90 s at the annealing temperature of the primer pair [Table 3], and 2 min at 72°C) were performed. Each resulting PCR fragment (Table 3) was sequenced to exclude any mutations during PCR. DNA isolation methods were used as previously described (19, 42). Restriction nucleases were purchased from various suppliers and used according to their instructions. The transformation of Escherichia coli was performed by the CaCl2 method (31). E. coli XL1 Blue (8) was used for standard cloning experiments.
TABLE 3.
Amplified fragments and PCR conditions
| Fragmenta | Primer pair | Annealing temp (°C) | Polymerase | Template |
|---|---|---|---|---|
| mphsA1 (1.4 kb) | P1 and P2 | 64 | Pwo | Genomic DNA of Tü494 |
| mphsA2 (1.3 kb) | P2 and P3 | 60 | Herculase | Genomic DNA of Tü494 |
| phsA1′ (S16A) (622 bp) | P5 and P6 | 68 | Pwo | pSE6 |
| phsA/phsA1(S16A) (1.8 kb) | P7 and P8 | 58 | Pwo | Genomic DNA of Tü494/pSE7 |
| mtheA (2.5 kb) | P9 and P10 | 60 | Herculase | Genomic DNA of Tü494 |
| mtheB (2.2 kb) | P11 and P12 | 70 | Pwo | Genomic DNA of Tü494 |
| theA (0.75 kb) | P13 and P14 | 60 | Herculase | pPtcos2 |
| theB (0.78 kb) | P15 and P16 | 62 | Herculase | pPtcos2 |
| mΔA/B1 (1.1 kb) | P17 and P18 | 62 | Pwo | Genomic DNA of Tü494 |
| mΔA/B2 (1.2 kb) | P19 and P20 | 70 | Pwo | Genomic DNA of Tü494 |
The value given in parentheses is the expected size of the PCR fragment.
TABLE 2.
Primer sequences
| Primer | Sequence (5′-3′) | Significant propertya |
|---|---|---|
| P1 | ATGACCATCCACAACCCCGAGGAAC | |
| P2 | AAGATATCTCAGCTCGCCTCTTCCTCGGTG | EcoRV restriction site |
| P3 | AAGATATCAGGTGGGTGAGATCTG | EcoRV restriction site |
| P4 | ATGCGGAGCAGGTCCTCGGTGTCCAG | |
| P5 | ACCGGCCCGGCCCCGGGGGCCTGCCCGGTCGTCGCGGAG | Exchange T for G |
| P6 | CTCCGCGACGACCGGGCAGGCCCCCGGCCGGGCCGGT | Exchange A for C |
| P7 | ATCATATGACCGCAGCGACACCG | NdeI restriction site |
| P8 | ATAAGCTTCTACGTCCCCTTCAGTTCG | HindIII restriction site |
| P9 | AACTGGCACACCCGCAACGGCGATGTG | |
| P10 | AGGTCCTTCGTGTGCGAGAACAGGTAG | |
| P11 | ACCTCCTCTTCGGCGACTGCATGGGCG | |
| P12 | AGGGTGGAAAGCCCTGCCATGATGTGG | |
| P13 | AAAGATCTGTGACCGACTGGATCCAGAG | BglII restriction site |
| P14 | AATAAGCTTTCACGCGGTGCTCCCCGGCTCCG | HindIII restriction site |
| P15 | AAGGATCCGTGAGCGGGCGGGGCG | BamHI restriction site |
| P16 | ATAAGCTTTCACTGCCACGTCCGGTC | HindIII restriction site |
| P17 | TACACCCACCTGCTGAAGTCC | |
| P18 | AAGATATCTCACCGCTCGCCCTCCTCGG | EcoRV restriction site |
| P19 | AAGATATCGTGAGAGACACCGGCCCGGC | EcoRV restriction site |
| P20 | TCACCGCGCACAGTGCCGCACG |
The sequences from which the properties are derived are underlined in the primer sequence.
Biological assay for PTT production.
PTT production was analyzed in a biological assay using Bacillus subtilis and E. coli XL1 Blue as test organisms. Equal amounts of the strains to be tested were cultivated in YM medium for 5 days. Fifty-milliliter samples of the cultures were centrifuged, the cell pellet was resuspended in 1 ml YM medium, and the resulting suspension was spread uniformly on the surface of a defined YM agar plate (45 ml per plate; diameter, 9 cm). After 5 days of incubation at 30°C, blocks of agar were cut out and applied to B. subtilis and E. coli test media (1, 34). The plates were incubated overnight at 37°C, and the antibiotic production was assayed by the area of the inhibition zone around the agar blocks.
Gene replacement mutagenesis of phsA.
For the replacement of phsA (1.9 kb) with an apramycin resistance cassette, two phsA gene-flanking fragments, mphsA1 (1.4 kb) and mphsA2 (1.3 kb) (Fig. 2A; Table 3), were amplified by PCR using the primer pairs P1/P2 and P3/P4 (Tables 2 and 3), respectively. mphsA1 and mphsA2 carry a synthetic EcoRV restriction site at the 3′ end and 5′ end, respectively. It was considered important to maintain a region of 215 bp in front of the stop codon of phsA, which overlaps with the pat promoter, to ensure the transcription of the pat gene (Fig. 2A). The fragments mphsA1 and mphsA2 were cloned into pJOE890 (2), resulting in pSE1 and pSE2. mphsA1 then was isolated as an EcoRI fragment from pSE1 and cloned into the E. coli vector pK18 (29), resulting in pSE3. In the next step, mphsA2 was cloned downstream of mphsA1 as an EcoRV/EheI fragment into the EcoRV site of pSE3. Finally, the resulting plasmid pSE4 was digested with EcoRV, and the apramycin resistance cassette apr, isolated as an EcoRV/SmaI fragment from pEH13 (17), was inserted into the EcoRV site between mphsA1 and mphsA2, resulting in pSE5. The plasmid was transferred into S. viridochromogenes Tü494 by polyethylene glycol-mediated transformation of protoplasts as previously described (19, 42). By a double-crossover event, 1.7 kb of the chromosomal phsA gene was replaced with an apramycin resistance cassette. To exclude an integration of the whole plasmid into the genome by a single-crossover event, which would confer kanamycin resistance, the apramycin-resistant clones were tested for kanamycin sensitivity. The PTT production of the resulting mutant MphsA (Fig. 2B) was tested in a biological assay against E. coli XL1 blue.
Site-specific mutagenesis.
In order to replace serine with alanine in the TE motif of PhsA, site-specific mutagenesis was performed using QuikChange site-directed mutagenesis kits (Stratagene Europe, Amsterdam, The Netherlands). The plasmid pDS1 (35), carrying the phsA gene was digested with PstI, and the resulting 622-bp PstI fragment (phsA′), which represents the 5′ end of phsA comprising the sequence for the TE motif, were cloned into pK18. The resulting plasmid pSE6 served as the template for PCR using the mutagenic primer pair P5/P6 (Tables 2 and 3). By introducing a base substitution, a new SfiI restriction site was generated, which facilitated the identification of plasmids in which site-specific mutagenesis was effected. Plasmids carrying the point-mutated 622-bp phsA fragment [phsA1′(S16A)] were named pSE6*. phsA1′(S16A) then was isolated as a 622-bp PstI fragment from pSE6*. pDS1 then was digested with PstI, and the native 622-bp fragment was exchanged for the point-mutated phsA1′(S16A) fragment in pDS1. The resulting plasmid, carrying the point-mutated phsA gene [phsA1(S16A)] (Fig. 3C), was designated pSE7.
Complementation of the mutant MphsA.
For the complementation of MphsA, the native phsA and the point-mutated phsA1(S16A) genes, each carrying an NdeI and HindIII restriction site at its 5′ and 3′ ends, respectively, were isolated by PCR using the primer pair P7/P8 (Tables 2 and 3). For the amplification of phsA and phsA1(S16A), genomic DNA of wild-type S. viridochromogenes and the plasmid pSE7, respectively, served as templates.
The amplified fragments were subcloned into the vector pDRIVE (Qiagen, Hilden, Germany), resulting in the plasmids pSE40 and pSE42. These plasmids were digested with NdeI/HindIII and subsequently cloned into the replicative medium-copy-number Streptomyces-E. coli shuttle vector pGM190 (G. Muth, personal communication) behind the tipA promoter, resulting in pSE41 and pSE43. Furthermore, the complementation plasmid pSE34, carrying a phsA fragment in which the first 135 bp were deleted, was generated. This mutated phsA fragment, phsA2(Δ1-45) (Fig. 3C) (originally called phsA* [34]), was isolated as a BamHI/HindIII fragment from the plasmid pDS3 (34) and cloned into BamHI/HindIII-restricted pK18 (pSE31). Subsequently, phsA2(Δ1-45) was isolated from pSE31 as a EcoRI/HindIII fragment and was cloned into the vector pRSETB (Invitrogen, Karlsruhe, Germany), resulting in pSE32. This cloning step was required to obtain the appropriate restriction sites for the insertion of phsA2(Δ1-45) into the vector pGM190. In the final step, phsA2(Δ1-45) was isolated from pSE32 as an NdeI/HindIII fragment and cloned into the pGM190 vector, resulting in pSE34. The plasmids pSE34, pSE41, and pSE43 were used for the transformation of MphsA. Finally, PTT production in the complemented MphsA, MphsA(pSE34), MphsA(pSE41), and MphsA(pSE43) mutants was tested by a biological assay. As the complemented MphsA mutants were cultivated in YM medium with 10 μg/ml kanamycin, a kanamycin-resistant E. coli strain was used as a test organism for the biological assay.
Immunoblot analysis of PhsA1(S16A).
For the detection of the gene product of phsA1(S16A), an immunoblot experiment was performed. S. viridochromogenes Tü494, MphsA, MphsA(pSE41), and MphsA(pSE43) cells were cultivated in YM medium for 5 days. The harvested cells were resuspended in lysis buffer and subsequently broken twice using a French press (at 10,000 lb/in2). The protein lysates were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis. The transfer of separated proteins to a nitrocellulose membrane (BioTraceNT; Pall Life Science, Dreieich, Germany) was achieved by the semidry method using the MilliBlot graphite electroblotter (Millipore, Bedford, MA). The immunoblotting analysis was performed by a standard technique using a 1:5,000 dilution of a primary PhsA antibody (35), alkaline phosphatase-conjugated goat anti-rabbit antibody (Bio-Rad Laboratories GmbH, München, Germany), and chloronaphtol as the reagent.
Gene interruption mutagenesis of theA and theB genes.
For the generation of the theA and theB mutants MtheA and MtheB, the 2.5-kb mtheA and 2.2-kb mtheB fragments comprising the theA and theB genes, respectively (Fig. 2A; Table 3), were amplified using the primer pairs P9/P10 and P11/P12 (Tables 2 and 3). MtheA was cloned into the vector pGEM-T-Easy (Promega, Madison, WI), and mtheB was cloned into pJOE890, thereby generating the plasmids pSE10 and pSE11. Subsequently, mtheA and mtheB were isolated as EcoRI fragments and then inserted in the EcoRI-restricted pK19 (29) and pK18 vectors, respectively. The resulting plasmids pSE12 and pSE13 were digested with Bpu1102 and BstEII, respectively. The generated sticky ends were filled in with Klenow polymerase (Fermentas, St. Leon-Rot, Germany) to obtain blunt ends (pSE12* and pSE13*). Afterwards, the apramycin-ermEp resistance cassette (designated aprP) (17) was isolated from pEH13 as an EcoRV/StuI blunt end fragment and was inserted into pSE12* and pSE13*. As the genes theA, theB, and trs (Fig. 2A) are translationally coupled, the presence of the ermE promoter behind the apr gene in the mutants should avoid polar effects on the genes located downstream. The resulting plasmids pSE14 and pSE15 were used for the generation of the theA and theB mutants MtheA and MtheB (Fig. 2C) by gene replacement as described above. PTT production in MtheA and MtheB was tested by a biological assay against B. subtilis.
Complementation of MtheA and MtheB.
For the complementation of MtheA and MtheB, the complementation plasmids pSE22 and pSE23 were constructed. Therefore, the theA and theB genes were amplified by PCR using the primer pairs P13/P14 and P15/P16, respectively (Tables 2 and 3). theA carries a BglII site and theB a BamHI site at their 5′ ends, and both have a HindIII site at the 3′ end. The obtained theA′ (758 bp) and theB′ (783 bp) fragments each were subcloned in the pGEM-T-Easy vector, resulting in pSE16 and pSE17. theA′ was cloned as a BglII/HindIII fragment and theB′ as a BamHI/HindIII fragment into the BglII/HindIII-restricted E. coli expression vector pRSETB. As the theA and theB genes were amplified without their own ribosome binding sites (RBS), these fragments were cloned behind an RBS sequence, which was supplied by the pRSETB vector. The resulting plasmids were named pSE18 and pSE19. Furthermore, theA′ and theB′ were isolated together with the RBS of pRSETB as XbaI/HindIII fragments and subcloned into pEH15 (17) behind the ermE promoter (ermEp), resulting in pSE20 and pSE21. Finally, the resulting ermEp theA′ and ermEp theB′ fragments were cloned as EcoRI and HindIII fragments, respectively, into the Streptomyces-E. coli shuttle vector pWHM3 (47). The constructed plasmids pSE22 and pSE23 were used for the transformation of MtheA, MtheB, and wild-type S. viridochromogenes Tü494. The PTT production of the supplemented strains Tü494(pSE22), Tü494(pSE23), MtheA(pSE22), and MtheB(pSE23) was analyzed using a biological assay against B. subtilis.
Generation of a theA/theB double mutant by gene replacement mutagenesis.
For the replacement of theA and theB with the apramycin-ermEp resistance cassette (aprP), two of the TE gene-flanking fragments, mΔA/B1 (1.1 kb) and mΔA/B2 (1.2 kb) (Fig. 2A; Table 3), were amplified by PCR using the primer pairs P17/P18 and P19/P20 (Tables 2 and 3), respectively. mΔA/B1 and mΔA/B2 carry a synthetic EcoRV restriction site at their 3′ and 5′ ends, respectively. The fragments mΔA/B1 and mΔA/B2 were cloned into pJOE890, resulting in pSE44 and pSE45. mΔA/B1 was isolated as an EcoRI fragment from pSE44 and was cloned into the E. coli vector pK18, resulting in pSE46. In the next step, mΔA/B2 was cloned downstream of mΔA/B1 as an EcoRV/HindIII fragment into pSE46. Finally, the resulting plasmid pSE47 was digested with EcoRV, and the aprP resistance cassette, which was isolated as an EcoRV/StuI fragment from pEH13, was inserted into the EcoRV restriction site between mΔA/B1 and mΔA/B2. The resulting plasmid pSE48 was used for the generation of the theA/theB double mutant strain Tü494(ΔtheA/theB::aprP) (Fig. 2D) by gene replacement as described above. PTT production in Tü494(ΔtheA/theB::aprP) was tested by a biological assay against B. subtilis.
RESULTS
Analysis of the phsA mutant MphsA.
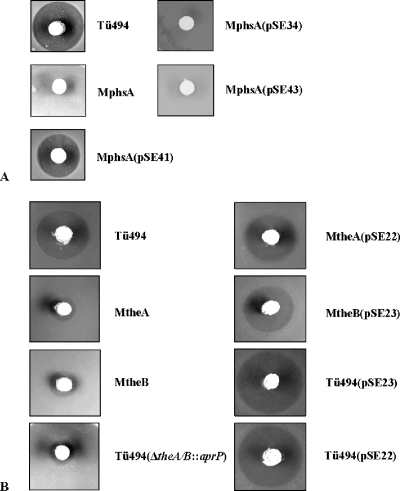
In order to analyze the functionality of the TE motif at the N terminus of PhsA, first the phsA deletion mutant MphsA was generated. Therefore, two fragments upstream and downstream of phsA were amplified, resulting in the fragments mphsA1 and mphsA2 (see Materials and Methods) (Fig. 2A). Both fragments were cloned into the pK18 vector and then were separated by the insertion of an apramycin resistance cassette isolated from the vector pEH13 (as described in Materials and Methods). The resulting plasmid, pSE5, was used for the generation of the phsA mutant MphsA. After the transformation of wild-type S. viridochromogenes Tü494, two apramycin-resistant and kanamycin-sensitive clones were obtained, indicating the replacement of the phsA gene by a double-crossover event. For one of the two clones (MphsA) (Fig. 2B) isolated, the correct integration of the apramycin resistance cassette was verified by Southern hybridization (data not shown). The PTT production of MphsA was analyzed in a biological assay, and no inhibition zone was detected (Fig. 4A), which proved that phsA is required for PTT biosynthesis. To confirm that the loss of PTT production was caused by the deletion of phsA and not by polar effects, the mutant was complemented with the native phsA gene. The phsA gene was cloned into the Streptomyces-E. coli shuttle vector pGM190 behind the inducible tipA promoter, resulting in the plasmid pSE41. As the tipA promoter has basal activity, the experiment was performed without induction. PTT production was restored in MphsA after complementation (Fig. 4A), confirming that the loss of PTT production was due to the deletion of the phsA gene and not due to polar effects on downstream genes. However, the amount of PTT in the complemented phsA mutant was less than that in the wild type. A reason for the impaired PTT production might be that the natural balance between the three peptide synthetases, which is necessary for efficient antibiotic production, cannot be achieved by the nonspecific promoter and the high-copy-number plasmid pSE41 (3, 24).
FIG. 4.
(A) Analysis of PTT production of wild-type strain Tü494, the mutant MphsA, and the phsA-, phsA2(Δ1-45)-, and phsA1(S16A)-complemented mutants MphsA(pSE41), MphsA(pSE34), and MphsA(pSE43) in a biological assay. (B) Analysis of PTT production of wild-type strain Tü494, the mutants MtheA and MtheB with the native theA and theB genes, respectively, complemented mutants MtheA(pSE22) and MtheB(pSE23), the wild-type strain overexpressing theA and theB [Tü494(pSE22) and Tü494(pSE23), respectively], and the theA/theB double mutant Tü494(ΔtheA/theB::aprP).
Analysis of the functionality of the TE motif in PhsA.
To analyze the functionality of the TE motif in the N terminus of PhsA, differently mutated PhsA derivatives were constructed.
First, PhsA2(Δ1-45) was generated, in which the first 45 aa were deleted (see Materials and Methods) (Fig. 3C). As the sequence for the TE GXSXG motif is located at aa 14 to 18, the TE motif also was affected by the deletion. Sequence analysis of PhsA by SMART (21, 33) revealed that the adenylation domain of PhsA starts at aa 47 (corresponding to bp 141). Therefore, the deletion of the first 45 aa should not affect the adenylation activity of PhsA. The phsA2(Δ1-45) gene was isolated from the plasmid pDS3 and cloned, as described in Materials and Methods, into the vector pGM190 behind the tipA promoter. The resulting plasmid, pSE34, then was used for the complementation of MphsA. In a biological test, it was shown that PhsA2(Δ1-45) cannot restore PTT production (Fig. 4A), indicating that the TE motif plays an important role during PTT biosynthesis.
To prove that the failure of PTT production in MphsA(pSE34) was exclusively due to the absence of the TE motif and not due to the deleted first 45 aa, PhsA1(S16A), in which the highly conserved serine residue is replaced by an alanine, was constructed (Fig. 3C). This should lead to the loss of TE activity (22). The replacement of serine in PhsA was performed by site-directed mutagenesis via PCR using primers in which the T of the serine-coding triplet TCC was changed to a G to generate an alanine-coding triplet. The mutated phsA gene, phsA1(S16A), was cloned behind the tipA promoter of pGM190 to give pSE43 (for details, see Materials and Methods). pSE43 then was used for the complementation of the phsA deletion mutant MphsA. MphsA(pSE43) also was not able to produce PTT (Fig. 4A).
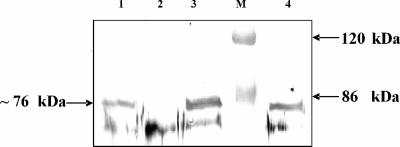
To prove that the failure to synthesize PTT after complementation with the mutated phsA gene [phsA1(S16A)] was caused by a mutation and not by a lack of expression, an immunoblotting experiment using antibodies against PhsA was performed. For immunoblotting, the lysates of MphsA(pSE43) and, as controls, wild-type S. viridochromogenes Tü494 and the mutants MphsA and MphsA(pSE41) (carrying the native phsA gene) were used. The denatured PhsA (76 kDa) described previously (12) was detected in all samples except in the phsA mutant MphsA (Fig. 5), proving that the lack of PTT production in MphsA(pSE43) was due to the mutated TE motif in PhsA. These results confirm that the TE motif in the N terminus of PhsA is required for PTT production.
FIG. 5.
Detection of PhsA in an immunoblotting experiment using antibodies against PhsA. Lane 1, Tü494; lane 2, MphsA; lane 3, MphsA(pSE41); lane M, prestained protein marker (Fermentas, St. Leon-Rot, Germany); and lane 4, MphsA(pSE43).
Analysis of the theA and theB mutants MtheA and MtheB.
In silico analysis revealed the presence of two individual TE genes, theA and theB, in the PTT biosynthetic gene cluster (36) (Fig. 2A). To elucidate the function of these two external TEs, theA as well as theB mutants were generated by the insertion of the apramycin resistance cassette equipped with the constitutive ermE promoter (aprP). The presence of the ermE promoter downstream of the apr gene should prevent polar effects on the genes, which are located downstream of theA and theB, respectively, as shown for other S. viridochromogenes Tü494 mutants in which the aprP cassette was used for the mutagenesis of PTT biosynthetic genes (17, 36, 37). Two fragments of the PTT biosynthetic gene cluster, containing theA and theB, respectively, were amplified by PCR. The resulting fragments mtheA (2.5 kb) and mtheB (2.3 kb) (Fig. 2A) were cloned into pK19 and pK18 vectors, respectively (see Materials and Methods). In the next step, the aprP cassette was inserted into each of the two genes. The resulting plasmids pSE14 and pSE15 were used for the transformation of S. viridochromogenes and the subsequent isolation of mutants MtheA and MtheB, in which the native genes were replaced by the mutated ones as described above (Fig. 2C). The correct integration of the apramycin resistance cassette in the mutants was verified by Southern hybridization (data not shown). The bioassay revealed that antibiotic activity was reduced about 80% in MtheA and about 70% in MtheB compared to that of the wild type (Fig. 4B).
To confirm that the activity was a result of the mutation and not due to polar effects on the downstream genes theA and theB, the mutants were complemented with their native genes. The theA and theB genes were amplified by PCR and cloned into the Streptomyces-E. coli shuttle vector pWHM3. The resulting constructs pSE22 and pSE23 were used for the transformation of MtheA and MtheB, respectively. In both cases, the complementation led to the restoration of PTT production to nearly the wild-type level, which proved that the reduced PTT production in MtheA and MtheB was an effect of the interruption of theA and theB genes (Fig. 4B). These results also indicate that the TEs are not essential for PTT biosynthesis but are required for efficient PTT production. Therefore, an editing role for both TEs by removing aberrant intermediates or wrongly activated amino acids from the peptide synthetase complex can be suggested.
Effect of overexpression of theA and theB in wild-type S. viridochromogenes Tü494.
If theA and theB have an editing role, the overexpression of both genes in S. viridochromogenes Tü494 should enhance antibiotic production. Therefore, the complementation plasmids pSE22 and pSE23 (see Materials and Methods) also were used to transform the wild type. The antibiotic production of the recombinant strains was assayed by measuring the inhibition zones against sensitive indicator bacteria (Fig. 4B). Tü494(pSE22) and Tü494(pSE23) each produced ∼50% more PTT or more active PTT than the wild type. This result supports the postulation that both TEs exhibit an editing role.
Analysis of the theA/theB double mutant Tü494(ΔtheA/theB::aprP).
To support the assumption that both TEIIs TheA and TheB have an editing role, a theA/theB double mutant was generated by the replacement of the theA and theB genes by aprP. As described above, the presence of the ermE promoter behind the apr gene should prevent polar effects on the genes, which are located downstream of theA and theB. Two fragments of the PTT biosynthetic gene cluster, which flank theA and theB, respectively, were amplified by PCR. The resulting fragments, mΔA/B1 (1.1 kb) and mΔA/B1 (1.2 kb) (Fig. 2A), were cloned into the vector pK18 and then were separated by the insertion of the aprP cassette. The resulting plasmid pSE48 was used for the generation of the theA/theB double mutant. After the transformation of wild-type S. viridochromogenes Tü494, two apramycin-resistant and kanamycin-sensitive clones were obtained, indicating the replacement of theA and theB by a double-crossover event. For one of the two clones [Tü494(ΔtheA/theB::aprP); Fig. 2D] isolated, the correct integration of the apramycin resistance cassette was verified by PCR (data not shown). The PTT production of Tü494(ΔtheA/theB::aprP) was analyzed in a biological assay against B. subtilis. The bioassay revealed that the antibiotic activity in Tü494(ΔtheA/theB::aprP) was reduced to 9% compared to that of the wild type (Fig. 4B). This result supports the assumption that both TE genes, theA and theB, have an editing role, as the mutant still is able to produce PTT.
DISCUSSION
PTT biosynthesis represents a special case of bacterial nonribosomal peptide synthesis. Three peptide synthetase genes, phsA, phsB, and phsC, are involved in the biosynthesis of PTT, with each encoding one peptide synthetase module. In addition, these three peptide synthetase genes are not clustered but are encoded at different positions of the biosynthetic gene cluster (35, 36). In this NRPS system, PhsA is the initiation module; however, it is not clear whether PhsB or PhsC represents the termination module. A typical TE domain in the C terminus of PhsB as well as in PhsC, which would allow classification as a final module, is missing. However, neither of these two enzymes can replace the other. Consequently, a defined positioning for PhsB and PhsC in the PTT assembly line is likely (37). Between the tyrocidine synthetases TycA, TycB, and TycC, so-called short COM domains are described that mediate the interaction between the peptide synthetases. The COM domains are classified into the accepter COM domain (COMA) and the donor COM domain (COMD). At the N terminus of the initiation module and at the C terminus of the termination module, a COM domain usually is lacking (14, 15, 26). However, in the PTT synthetases PhsA, PhsB, and PhsC, such COM domains are not apparent (37).
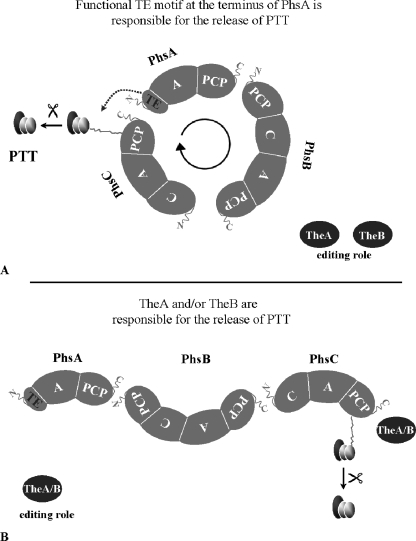
Instead of an integrative C-terminal TE domain in PhsB or PhsC, a highly conserved TE GXSXG motif in the N terminus of PhsA was identified. However, the region surrounding the motif has no overall homology to other TE domains. Usually the TE motif is part of an ∼280-aa-long region that is located at the C terminus of the terminal amino acid-activating module (13). Additionally, the PTT biosynthetic gene cluster harbors the genes theA and theB, the gene products of which show high similarity to TEIIs. A TEII is assumed to have an editing role within the biosynthesis of polypeptides and polyketides (16, 38). To our knowledge, no other biosynthetic gene cluster has been described in which two TEII genes are located. In most cases, in addition to the integrated TEI, only one autonomous TE-encoding gene is part of the cluster. An example is the biosynthetic gene cluster of the lipopeptide antibiotic surfactin, which harbors the srfA TE gene coding for TEII (32). Based on these facts, two alternatives for the release of PTT from the peptide synthetase complex can be postulated. In the first model, it is assumed that the TE motif in PhsA is functional. In this case, the three peptide synthetases arrange in a manner such that the C terminus of PhsB or PhsC and the N terminus of PhsA can interact, and the tripeptide can be cleaved off from the peptide synthetase complex with the participation of the TE motif. Here, the two TEs TheA and TheB have editing roles within PTT biosynthesis (Fig. 6A). In the second model, it is assumed that the TE motif is not functional. In this case, one of the TEIIs of PTT biosynthesis is responsible for the release of the tripeptide from the peptide synthetase complex. The other TE would have editing roles in PTT biosynthesis (Fig. 6B). A similar system is described for product release in nangchangmycin biosynthesis. In that case, a TE domain is not included in either of the two type I polyketide synthases involved in the biosynthesis of the polyether nangchangmycin. Instead, polyether chain release is catalyzed by the gene product of nanE. The nanE gene is located within the biosynthetic gene cluster and encodes a monofunctional TE (23).
FIG. 6.
Models for the interaction of PhsA, PhsB, and PhsC as well as TheA and TheB. (A) A fully functional TE motif is supposed to be present in PhsA. TheA and TheB have editing roles in PTT biosynthesis. (B) The TE motif in PhsA is not functional. One of the two TEs is involved in PTT release, and the other one has a corrective function. In both models, the order for the peptide synthetase assembly might be changed to PhsA, PhsC, and PhsB.
In order to investigate whether the TE motif in PhsA is active, first the nonpolar phsA deletion mutant MphsA was generated, which served as a recipient for the subsequent analysis of the mutated phsA genes. This mutant lost the ability to produce PTT. For the analysis of the TE motif, the first 45 aa of the N terminus of PhsA, which comprises the TE motif, were deleted. After the complementation of MphsA with this construct, no PTT production was detectable, indicating that the TE motif has a role in PTT biosynthesis. This result supports the first model of product release. Another interpretation of these results is that, because of the missing N terminus of PhsA, an interaction between the N terminus of PhsA and the C terminus of PhsB or PhsC is not possible. As mentioned above, the N and C termini of the peptide synthetase play an essential role for the selective interaction and communication between peptide synthetases mediated by COM domains (14, 15). Although no COM domains were identified in the PTT peptide synthetases (37), it cannot be excluded that there are specific sequences that exhibit the function of a COM domain and thus are responsible for the selective interaction of PhsA, PhsB, and PhsC. If the first model represents the NRPS assembly within PTT biosynthesis, the N terminus of the initiation module (PhsA) and the C terminus of the last module (PhsB/C) have to interact. Thus, the failure to produce PTT after complementation with PhsA2(Δ1-45) also might be explained by the deletion of a short COM domain-like region in the N terminus of PhsA. In this case, an interaction of PhsA and PhsC or PhsA and PhsB, which has to be postulated for the first model, would not be possible and consequently would lead to a failure of PTT production.
To determine whether the lack of PTT production was caused by the absence of the TE motif or of the N terminus of PhsA, a second variant PhsA [PhsA1(S16A)] was constructed in which only the catalytic active serine site of the TE GXSXG motif was changed to an alanine. This exchange usually leads to the loss of TE activity, as shown for the TEI of the l-α-aminoadipyl-l-cysteinyl-d-valine synthetase and the TEII Srf TE from the surfactin biosynthetic gene cluster (W. Kullow, H. von Döhren, J. Kennedy, and G. Turner, presented at Enzymology of Biosynthesis of Natural Products, Technische Universitat, Berlin, Germany, 1996) (22). After the complementation of the mutant MphsA with PhsA1(S16A), no PTT production was detectable in a biological assay. This indicates that the TE motif in the N terminus of PhsA is required for the release of PTT from the NRPS complex. However, it is not yet clear how the singular TE motif in PhsA, without the 280-aa TE region, which normally is important for activity, catalyzes product release. A sequence analysis of the first 45 aa in front of the adenylation domain in PhsA by using the bioinformatics tool Hhpred (40) revealed that the TE motif comprising the 45-aa region in PhsA has no homology to the TE. Further, it is conceivable that this N-terminal region interacts with other PTT-specific proteins that are related to TE-like proteins. Therefore, the complete PTT biosynthetic gene cluster was screened for a TE-like gene (data not shown). Except for the two TEIIs, no TE-like protein was identified. To clarify if the single TE motif is sufficient for TE activity, a biochemical analysis will be performed with PhsA in further studies.
To exclude the possibility that the TheA and/or TheB protein is involved in the product release, the nonpolar mutants MtheA and MtheB were generated. In each case, the mutation led to reduced PTT production. On the other hand, the overexpression of TheA and TheB, respectively, in the wild type led to enhanced PTT production (50% increase). The results regarding TheA indicate that the identified motif (GXCXG), in which a cysteine instead of a serine is located in the catalytic center, has no detectable effect on TE activity. A similar exchange performed for the analysis of a thioesterase within fatty acid synthesis also showed no dramatic effect on TE activity (43).
Therefore, it can be concluded that TheA and TheB might have an editing role within PTT biosynthesis. This assumption is supported by the phenotype of the theA/theB double mutant Tü494(ΔtheA/theB::aprP). The mutant still produces 9% of the amount of PTT produced by the wild type, but this amount is less than that of either the MtheA or MtheB mutant.
The functions of TEIIs have been analyzed in different assembly-line syntheses. In polyketide synthesis, it has been described that the function of TEII is to remove abnormal polyketides from the acyl carrier protein domain of the polyketide synthase (9, 16). Such molecular mistakes can appear during biosynthesis by different mechanisms. The aberrant polyketides carrying a wrong oxidation state and therefore a chain elongation cannot proceed, and the polyketide biosynthesis fails. In this case, TEII cleaves off the aberrant polyketides from the polyketide synthase complex. Besides the removal of aberrant intermediate products from the biosynthetic machinery, a further model was described for the function of TEII in which TEII is involved in the regeneration of misprimed NRPS (38). Before the synthesis of the polypeptide can start, the inactive NRPS has to be activated by attaching the prosthetic group coenzyme A to the PCP domain. This reaction is catalyzed by the 4′PP-transferase. The 4′PP-transferase also is able to recognize acyl-coenzyme A as a substrate and to attach it to the inactivated PCP. In this case, the synthesis of the polypeptide is not possible, because the thiol group of the PCP is blocked by the acyl chain. Consequently, TEII cleaves off the acyl chain, and peptide synthesis can take place. As both the removal of aberrant intermediate products from the biosynthetic machinery and the regeneration of misprimed NRPSs strongly influence the antibiotic yield, editing by TEIIs represents a rate-limiting step in antibiotic biosynthesis (9, 16, 38).
Also in the case of PTT biosynthesis, TheA and TheB are important to maintain normal levels of the antibiotic. It is conceivable that incorrect amino acids assemble during peptide elongation because of a specific feature of the A domain in PhsB and PhsC. An in silico analysis of the specificity region (10, 30, 41) of PhsB and PhsC predicted that PhsB has a serine-activating domain and PhsC a proline-activating domain. A biochemical analysis of PhsB and PhsC, however, revealed that PhsB and PhsC each preferentially activate alanine. However, besides alanine, the specificity for short-chain amino acids such as serine or aminobutyric acid, but not for proline, as predicted, also was measured (37). Therefore, it may be possible that instead of alanine, other amino acids are linked to N-Ac-DMPT, resulting in altered PTT derivatives. This may lead to decreased PTT activity against bacteria, as the di-alanine residue is needed for the transport of PT into the bacterial cell (11). It seems likely that the functions of the two TEs TheA and TheB are the release of wrongly activated amino acids and the regeneration of misprimed NRPSs. This suggestion is supported by the results of this study, as the inactivation of the TE genes theA and theB led to reduced PTT production. Furthermore, the increase in antibiotic activity caused by the overexpression of theA and theB in S. viridochromogenes Tü494 may be attributed to an enhanced editing function by the release of aberrant PTT derivatives or of the regeneration of misprimed NRPSs. It has been shown that the accessory expression of TEII for Saccharopolyspora erythraea leads to an 80% increase in the production of the erythromycin aglycon 6-deoxyerythronolide B in cultures of recombinant E. coli (28). Further biochemical studies will be carried out to analyze the substrate specificity of TheA and TheB proteins.
Considering all of the results, the first model (Fig. 6A) seems to represent the mode of PTT biosynthesis. Here, the three peptide synthetases arrange in a manner such that the N-terminal TE motif can physically interact with the C-terminal PCP of PhsB or PhsC. Consequently, the peptide chain is cleaved off the peptide synthetase complex by the action of the N-terminal TE motif of PhsA. The peptide synthesis thereby starts and ends in PhsA, which would represent both the initiation and termination module. This kind of cyclic arrangement of NRPS was not previously described; therefore, this study introduces a new strategy for NRPS arrangement.
Acknowledgments
This research was supported by a grant from the Deutsche Forschungsgemeinschaft (SPP1152: Wo 485/2-1).
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Alijah, R., J. Dorendorf, S. Talay, A. Pühler, and W. Wohlleben. 1991. Genetic analysis of the phosphinothricin-tripeptide biosynthetic pathway of Streptomyces viridochromogenes Tü494. Appl. Microbiol. Biotechnol. 34:749-755. [DOI] [PubMed] [Google Scholar]
- 2.Altenbuchner, J., P. Viell, and I. Pelletier. 1992. Positive selection vectors based on palindromic DNA sequences. Methods Enzymol. 216:457-466. [DOI] [PubMed] [Google Scholar]
- 3.Baltz, R. H. 1998. Genetic manipulation of antibiotic-producing Streptomyces. Trends Microbiol. 6:76-83. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, E., K. H. Gugel, K. Haegele, H. Hagemaier, S. Jassipow, W. A. König, and H. Zähner. 1972. Stoffwechselprodukte von Mikroorganismen. Phosphinothricin und Phosphinothricyl-Alanyl-Alanin. Helv. Chim. Acta 55:224-239. [DOI] [PubMed] [Google Scholar]
- 5.Blodgett, J. A., J. K. Zhang, and W. W. Metcalf. 2005. Molecular cloning, sequence analysis, and heterologous expression of the phosphinothricin tripeptide biosynthetic gene cluster from Streptomyces viridochromogenes DSM 40736. Antimicrob. Agents Chemother. 49:230-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blodgett, J. A., P. M. Thomas, G. Li, J. E. Velasquez, W. A. van der Donk, N. L. Kelleher, and W. W. Metcalf. 2007. Unusual transformations in the biosynthesis of the antibiotic phosphinothricin tripeptide. Nat. Chem. Biol. 3:480-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruner, S. D., T. Weber, R. M. Kohli, D. Schwarzer, M. A. Marahiel, C. T. Walsh, and M. T. Stubbs. 2002. Structural basis for the cyclization of the lipopeptide antibiotic surfactin by the thioesterase domain SrfTE. Structure 10:301-310. [DOI] [PubMed] [Google Scholar]
- 8.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. Xl1-Blue, a high efficiency plasmid transforming recA Escherichia coli strain with beta galactosidase selection. Focus 5:376-378. [Google Scholar]
- 9.Butler, A. R., N. Bate, and E. Cundliffe. 1999. Impact of thioesterase activity on tylosin biosynthesis in Streptomyces fradiae. Chem. Biol. 6:287-292. [DOI] [PubMed] [Google Scholar]
- 10.Challis, G. L., J. Ravel, and C. A. Townsend. 2000. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 7:211-224. [DOI] [PubMed] [Google Scholar]
- 11.Diddens, H., H. Zähner, E. Kraas, W. Goehring, and G. Jung. 1976. On the transport of tripeptide antibiotics in bacteria. Eur. J. Biochem. 66:11-23. [DOI] [PubMed] [Google Scholar]
- 12.Grammel, N., D. Schwartz, W. Wohlleben, and U. Keller. 1998. Phosphinothricin-tripeptide synthetases from Streptomyces viridochromogenes. Biochemistry 37:1596-1603. [DOI] [PubMed] [Google Scholar]
- 13.Grünewald, J., and M. A. Marahiel. 2006. Chemoenzymatic and template-directed synthesis of bioactive makrocyclic peptides. Microbiol. Mol. Biol. Rev. 70:121-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn, M., and T. Stachelhaus. 2004. Selective interaction between nonribosomal peptide synthetases is facilitated by short communication-mediating domains. Proc. Natl. Acad. Sci. USA 101:15585-15590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn, M., and T. Stachelhaus. 2006. Harnessing the potential of communication-mediating domains for the biocombinatorial synthesis of nonribosomal peptides. Proc. Natl. Acad. Sci. USA 103:275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heathcote, M. L., J. Staunton, and P. F. Leadlay. 2001. Role of type II thioesterases: evidence for removal of short acyl chains produced by aberrant decarboxylation of chain extender units. Chem. Biol. 8:207-220. [DOI] [PubMed] [Google Scholar]
- 17.Heinzelmann, E., G. Kienzlen, S. Kaspar, J. Recktenwald, W. Wohlleben, and D. Schwartz. 2001. The phosphinomethylmalate isomerase gene pmi, encoding an aconitase-like enzyme, is involved in the synthesis of phosphinothricin tripeptide in Streptomyces viridochromogenes. Appl. Environ. Microbiol. 67:3603-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, CT.
- 20.Kondo, Y., T. Shomura, Y. Ogawa, T. Tsuruoka, H. Watanabe, K. Totsukawa, T. Suzuki, C. Moriyama, J. Yoshida, S. Inouye, and T. Niida. 1973. Studies on a new antibiotic SF-1293. I. Isolation and physico-chemical and biological characterization of SF-1293 substances. Sci. Rep. Meiji Seika 13:34-41. [Google Scholar]
- 21.Letunic, I., R. R. Copley, B. Pils, S. Pinkert, J. Schultz, and P. Bork. 2006. SMART 5: domains in the context of genomes and networks. Nucleic Acids Res. 34:D257-D260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linne, U., D. Schwarzer, G. N. Schroeder, and M. A. Marahiel. 2004. Mutational analysis of a type II thioesterase associated with nonribosomal peptide synthesis. Eur. J. Biochem. 271:1536-1545. [DOI] [PubMed] [Google Scholar]
- 23.Liu, T., D. You, C. Valenzano, Y. Sun, J. Li, Q. Yu, X. Zhou, D. E. Cane, and Z. Deng. 2006. Identification of NanE as the thioesterase for polyether chain release in nanchangmycin biosynthesis. Chem. Biol. 13:945-955. [DOI] [PubMed] [Google Scholar]
- 24.Malpartida, F., and D. A. Hopwood. 1984. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. Nature 309:462-464. [DOI] [PubMed] [Google Scholar]
- 25.Marahiel, M. A., T. Stachelhaus, and H. D. Mootz. 1997. Modular synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 97:2651-2673. [DOI] [PubMed] [Google Scholar]
- 26.Miao, V., M. F. Coeffet-Le Gal, K. Nguyen, J. Penn, A. Whiting, J. Steele, D. Kau, S. Martin, R. Ford, T. Gibson, M. Bouchard, S. K. Wrigley, and R. H. Baltz. 2006. Genetic engineering in Streptomyces roseosporus to produce hybrid lipopeptide. Chem. Biol. 13:268-276. [DOI] [PubMed] [Google Scholar]
- 27.Mootz, H. D., D. Schwarzer, and M. A. Marahiel. 2002. Ways of assembling complex natural products on modular nonribosomal peptide synthetases. Chembiochem 3:490-504. [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer, B., Z. Hu, P. Licari, and C. Khosla. 2002. Process and metabolic strategies for improved production of Escherichia coli-derived 6-deoxyerythronolide B. Appl. Environ. Microbiol. 68:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pridmore, R. D. 1987. New and versatile cloning vectors with kanamycin resistance marker. Gene 56:309-312. [DOI] [PubMed] [Google Scholar]
- 30.Rausch, C., T. Weber, O. Kohlbacher, W. Wohlleben, and D. H. Huson. 2005. Specificity prediction of adenylation domains in nonribosomal peptide synthetases (NRPS) using transductive support vector machines (TSVMs). Nucleic Acids Res. 33:5799-5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., T. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Schneider, A., and M. A. Marahiel. 1998. Genetic evidence for a role of thioesterase domains, integrated in or associated with peptide synthetases, in non-ribosomal peptide biosynthesis in Bacillus subtilis. Arch. Microbiol. 169:404-410. [DOI] [PubMed] [Google Scholar]
- 33.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz, D. 1997. Molekulargenetische Analyse der Phosphinothricin-Tripeptid Biosynthese in Streptomyces viridochromogenes Tü494. Ph.D. dissertation, University of Tübingen, Germany.
- 35.Schwartz, D., R. Alijah, B. Nussbaumer, S. Pelzer, and W. Wohlleben. 1996. The peptide synthetase gene phsA from Streptomyces viridochromogenes is not juxtaposed with other genes involved in nonribosomal biosynthesis of peptides. Appl. Environ. Microbiol. 62:570-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz, D., S. Berger, E. Heinzelmann, K. Muschko, K. Welzel, and W. Wohlleben. 2004. Biosynthetic gene cluster of the herbicide phosphinothricin tripeptide from Streptomyces viridochromogenes Tü494. Appl. Environ. Microbiol. 70:7093-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz, D., N. Grammel, E. Heinzelmann, U. Keller, and W. Wohlleben. 2005. Phosphinothricin tripeptide synthetases in Streptomyces viridochromogenes Tü494. Antimicrob. Agents Chemother. 49:4598-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzer, D., H. D. Mootz, U. Linne, and M. A. Marahiel. 2002. Regeneration of misprimed nonribosomal peptide synthetases by type II thioesterases. Proc. Natl. Acad. Sci. USA 99:14083-14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarzer, D., R. Finking, and M. A. Marahiel. 2003. Nonribosomal peptides: from genes to product. Nat. Prod. Rep. 20:275-287. [DOI] [PubMed] [Google Scholar]
- 40.Söding, J., M. Remmert, A. Biegert, and A. N. Lupas. 2006. HHsenser: exhaustive transitive profile search using HMM-HMM comparison. Nucleic Acids Res. 34:W374-W378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stachelhaus, T., H. D. Mootz, and M. A. Marahiel. 1999. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 6:493-505. [DOI] [PubMed] [Google Scholar]
- 42.Strauch, E., W. Wohlleben, and A. Pühler. 1987. Development of a plasmid-cloning system for Streptomyces viridochromogenes Tü494. J. Basic Microbiol. 27:449-455. [DOI] [PubMed] [Google Scholar]
- 43.Süssmuth, R. D., and W. Wohlleben. 2004. The biosynthesis of glycopeptide antibiotics—a model for complex, non-ribosomally synthesized, peptidic secondary metabolite. Appl. Microbiol. Biotechnol. 64:344-350. [DOI] [PubMed] [Google Scholar]
- 44.Thompson, C. J., and H. Seto. 1995. Bialaphos, p. 197-222. In L. C. Vining and C. Stuttard (ed.), Genetics and biochemistry of antibiotic production. Butterworth Heinemann Biotechnology, Oxford, United Kingdom.
- 45.Tsai, S. C., L. J. Miercke, J. Krucinski, R. Gokhale, J. C. Chen, P. G. Foster, D. E. Cane, C. Khosla, and R. M. Stroud. 2001. Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: versatility from a unique substrate channel. Proc. Natl. Acad. Sci. USA 98:14808-14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng, C. C., S. D. Brunner, R. M. Kholi, M. A. Marahiel, C. T. Walsh, and S. A. Sieber. 2002. Characterisation of the surfactin synthetase C-terminal thioesterase domain as a cyclic depsipeptide synthase. Biochemistry 41:13350-13359. [DOI] [PubMed] [Google Scholar]
- 47.Vara, J., M. Lewandowska-Skarbek, Y.-G. Wang, S. Donadio, and C. R. Hutchinson. 1989. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J. Bacteriol. 171:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Döhren, H. 2004. Biochemistry and general genetics of nonribosomal peptide synthetases in fungi. Adv. Biochem. Eng. Biotechnol. 88:217-264. [DOI] [PubMed] [Google Scholar]
- 49.Witkowski, A., J. Naggert, H. E. Witkowska, Z. I. Randhawan, and S. Smith. 1992. Utilization of an active serine 101 → cysteine mutant to demonstrate the proximity of the catalytic Serine 101 and histidine 237 residues in thioesterases II. J. Biol. Chem. 267:18488-18492. [PubMed] [Google Scholar]
- 50.Wohlleben, W., R. Alijah, J. Dorendorf, D. Hillemann, B. Nuβbaumer, and S. Pelzer. 1992. Identification and characterization of phosphinothricin-tripeptide biosynthetic genes in Streptomyces viridochromogenes. Gene 115:127-132. [DOI] [PubMed] [Google Scholar]