Abstract
Searching the database for mouse homologs of the antimicrobial peptide human beta-defensin-3 (hBD-3) revealed highest identity (69%) to mouse beta-defensin-14 (mBD-14). Recombinant mBD-14 exhibited broad-spectrum, nanomolar microbicidal activity. Treatment of keratinocytes with gamma interferon or transforming growth factor alpha increased mBD-14 gene expression. These data suggest that mBD-14 is the functional ortholog of hBD-3.
Antimicrobial proteins are effector molecules of the innate immune system and offer a fast response to invading microorganisms by exhibiting potent antimicrobial activity (19). A major family of antimicrobial proteins in mammals comprises the beta-defensins. Beta-defensins are small (4 to 5 kDa), cationic proteins which exhibit a potent antimicrobial activity at micro- to nanomolar concentrations (12). Human beta-defensin-3 (hBD-3) was originally isolated from lesional psoriatic skin extracts, and it is inducibly expressed in many epithelia, such as the skin, respiratory tract, and gut (4, 5, 12). hBD-3 is characterized by its potent antibacterial activity against many bacteria, including multiresistant strains (5, 8, 15). A BLAST search of the mouse protein database with the amino acid sequence of hBD-3 revealed highest identity (69%) to mouse beta-defensin-14 (mBD-14, Defb14) (Fig. 1). The next-closest hit was mBD-3 (Defb3), with only 44% identity, followed by mBD-6, with only 38% identity. Interestingly, a BLAST search with the mature hBD-3 peptide revealed only one hit, mBD-14. This suggests that mBD-14 could be the mouse ortholog of hBD-3. However, no data exist regarding the biological function of mBD-14, and its potential role as an antimicrobial protein has not yet been evaluated. Therefore, we decided to recombinantly express mBD-14 in Escherichia coli and to analyze its antimicrobial activity.
FIG. 1.
Amino acid sequence alignment of hBD-3 and mBD-14 (single-letter code). The signal peptide and the recombinantly expressed mature peptide are indicated.
We used the software program SignalP 3.0 (2) to determine the putative cleavage site in the mBD-14 amino acid sequence to generate the mature protein (Fig. 1). The corresponding DNA encoding mBD-14 was amplified from mouse keratinocyte cDNA using the forward primer 5′-ATCCAGATCTGGGTACCGACGACGACGACAACTTCCTACCAAAAACCCTCC-3 and reverse primer 5′-ATTTGCGGGCGCCTACTTCTTCTTTCGGCAGC-3′. The resulting fragment was cloned into the expression vector pET32a(+) (Novagen, Madison, WI) to generate a fusion protein containing an N-terminal His tag sequence allowing purification of the fusion protein by the use of a nickel affinity column. After expression in E. coli BL21(DE3)pLysS (Novagen), the fusion protein was purified using a nickel affinity column (Macherey-Nagel, Dueren, Germany), followed by preparative C8 reversed-phase high-pressure liquid chromatography as described previously for the purification of human beta-defensin-3 (5). The N-terminal part of the purified fusion protein was cleaved off by incubation for 1 h at 37°C with enterokinase (Invitrogen, Carlsbad, CA), and the resulting mature mBD-14 protein was purified by C4 reversed-phase high-pressure liquid chromatography, similar to the method described previously (5). Mass analysis using electrospray ionization mass spectrometry (QTOF-II hybrid mass spectrometer; Micromass, Manchester, United Kingdom) yielded a mass of 5,184.3 Da, which is 6 Da less than the theoretical mass calculated from the deduced amino acid sequence (5,190.3 Da), suggesting that the six cysteyl residues of mBD-14 are connected through three disulfide bridges.
To test the antimicrobial activity of mBD-14, a microdilution assay was used as previously described for RNase 8 (14). Briefly, microorganisms were incubated at 37°C with different concentrations of mBD-14 in 10 mM sodium phosphate buffer containing 1% Trypticase soy broth. After 2 h, the antimicrobial activity of mBD-14 was analyzed by plating serial dilutions of the incubation mixture and determining the CFU the following day. Results are given either as the minimal bactericidal concentration (≥99.9% killing) or as the concentration necessary to kill 90% of the microorganisms (90% lethal dose [LD90]). mBD-14 exhibited a broad spectrum of potent, nanomolar antimicrobial activity against various microorganisms, including gram-positive and gram-negative bacteria and the yeast Candida albicans (Table 1). mBD-14 was also able to efficiently kill methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis. Burkholderia cepacia was not susceptible to mBD-14, an observation also made for hBD-3 (15). Together, these data show that mBD-14 exhibits antimicrobial activity which is comparable to the antimicrobial activity reported for hBD-3 (8, 15). Moreover, the killing activities of mBD-14 and hBD-3 are influenced in a similar manner by higher salt concentrations and at a lower pH, thus further confirming their functional similarity (Table 2). Interestingly, both peptides were able to kill S. aureus at 100 mM NaCl but completely lost their activity against E. coli at 100 mM NaCl (Table 2). In contrast, the susceptibility of E. coli to mBD-14 and hBD-3 was enhanced at pH 5.5, whereas the susceptibility of S. aureus was reduced. The high susceptibility of E. coli to mBD-14 and hBD-3 at pH 5.5 may be caused by synergistic killing effects, because we observed that a pH of 5.5 alone exhibited a slight killing effect against E. coli.
TABLE 1.
Antimicrobial activities of mBD-14 and hBD-3 against various microorganisms
| Straina | MBCb (μM) of mBD-14 | LD90 (μM) of protein
|
|
|---|---|---|---|
| mBD-14 | hBD-3c | ||
| Gram-positive bacteria | |||
| Staphylococcus aureus ATCC 12600 | 1.2 | 0.08-0.15 | 0.15 |
| Staphylococcus aureus ATCC 33593 (MRSA) | 2.4 | 0.30 | 0.6 |
| Staphylococcus epidermidis ATCC 14990 | 2.4 | 1.2 | 0.15 |
| Streptococcus pyogenes ATCC 12344 | 1.2 | 0.15 | 1.2 |
| Enterococcus faecalis ATCC 29212 | 1.2 | 0.08-0.15 | |
| Enterococcus faecalis ATCC 51299 (VRE) | 2.4 | 0.30 | |
| Gram-negative bacteria | |||
| Escherichia coli ATCC 11775 | 0.60-1.2 | 0.08-0.15 | |
| Escherichia coli ATCC 25922 | 1.2 | 0.30 | 0.02 |
| Escherichia coli ESBL 4 (clinical isolate) | 0.60 | 0.15 | 0.15 |
| Salmonella enterica serovar Typhimurium ATCC 13311 | 1.2 | 0.15 | |
| Pseudomonas aeruginosa ATCC 10145 | 1.2-2.4 | 0.30 | 0.30 |
| Pseudomonas aeruginosa Va 6201/2000 (multiresistant; clinical isolate) | 4.8 | 0.60 | 0.15 |
| Burkholderia cepacia ATCC 25416 | >19 | >19 | >19 |
| Burkholderia cepacia ATCC 17759 | >19 | >19 | |
| Burkholderia stabilis NCTC 13011 | >19 | >19 | |
| Yeast | |||
| Candida albicans ATCC 24433 | 1.21 | 0.60 | |
MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococcus.
MBC, minimal bactericidal concentration.
LD90s of hBD-3 were calculated based on the work of Sahly et al. (15).
TABLE 2.
Antimicrobial activities of mBD-14 and hBD-3 at different ionic strengths and pHs
| Strain | Special condition | LD90 (μM) of protein
|
|
|---|---|---|---|
| mBD-14 | hBD-3 | ||
| Staphylococcus aureus | None | 0.08-0.15 | 0.15 |
| ATCC 12600 | 100 mM NaCl | 0.3 | 0.6 |
| pH 5.5 | 2.4 | 4.8 | |
| 2 mM MgCl2 | 0.08 | NDa | |
| Escherichia coli | None | 0.08-0.15 | 0.15 |
| ATCC 11775 | 100 mM NaCl | >19 | >19 |
| pH 5.5 | 0.02 | 0.08 | |
| 2 mM MgCl2 | 1.2 | ND | |
ND, not determined.
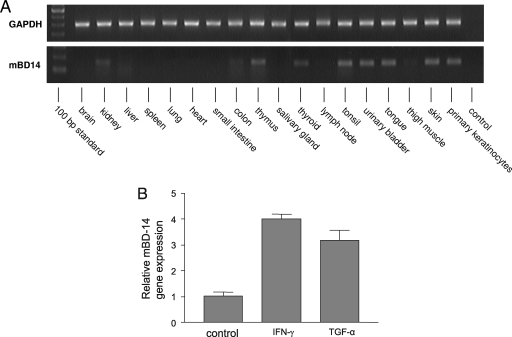
To obtain additional insight into the biological role of mBD-14, we analyzed its gene expression in various mouse tissues (Fig. 2A). Reverse transcription-PCR analyses revealed gene expression of mBD-14 in the tongue, thymus, tonsil, and kidney. All of these tissues have also been reported to express hBD-3 (5). As shown in Fig. 2A, mBD-14 mRNA was also expressed in skin and in primary keratinocytes, suggesting a role for mBD-14 in cutaneous defense. This is in concordance with several studies indicating that hBD-3 participates in skin innate immunity (5, 7, 16). In addition, we found that gene expression of mBD-14 in primary mouse keratinocytes was induced by treatment of the cells with gamma interferon (IFN-γ) or transforming growth factor alpha (TGF-α) (Fig. 2B), which were also identified as stimuli for the induction of hBD-3 expression in human keratinocytes (4, 6, 11). Further parallelisms in the gene regulation of mBD-14 and hBD-3 can be deduced from comparison of the promoter regions of these genes. It has been shown that the transcription factors NF-κB, STAT-1, and AP-1 are involved in both TNF-α/IFN-γ- and bacterium-mediated hBD-3 gene induction (1, 9). Interestingly, analysis of the mBD-14 promoter region using the Genomatix software program revealed that the promoter region of mBD-14 also contains putative binding sites for NF-κB, STAT-1, and AP-1 (not shown).
FIG. 2.
Analysis of mBD-14 gene expression. (A) Reverse transcription-PCR analysis of mBD-14 and glycerolaldehyde-3-phosphate dehydrogenase (GAPDH) gene expression in various tissues. The following intron-spanning primers were used: mBD-14, forward primer, 5′-TCTTGTTCTTGGTGCCTGCT-3; reverse primer, 5′-CGACCGCTATTAGAACATCGAC-3′; GAPDH, forward primer, 5′-TGTTCCTACCCCCAATGTGT-3; reverse primer, 5′-TGTGAGGGAGATGCTCAGTG-3. (B) Mouse primary keratinocytes were stimulated with IFN-γ or TGF-α (50 ng/ml) for 24 h, and mBD-14 mRNA expression was analyzed by real-time PCR using the primers for mBD-14 and GAPDH as shown above. Bars represent the relative mBD-14 transcript levels normalized to GAPDH transcript levels. Results are from one representative experiment of three independent experiments and are presented as means ± standard deviations.
In summary, this is the first report demonstrating that mBD-14 exhibits potent antimicrobial activity, with an antimicrobial spectrum similar to that reported for hBD-3. In addition, as seen for hBD-3, the expression of mBD-14 in keratinocytes is induced by IFN-γ and TGF-α. These results, together with the high sequence identity between mBD-14 and hBD-3, indicate that mBD-14 is the functional ortholog of hBD-3.
Mouse models are useful for studying the in vivo relevance of antimicrobial proteins. The knockout mouse for the human cathelicidin LL-37 ortholog CRAMP has been extensively used to study the role of cathelicidins in innate immunity (3, 10). Another study showed that matrilysin-deficient mice lacked mature Paneth cell-derived cryptdins and were more susceptible to orally administered bacteria (18). Therefore, studying mBD-14 with mouse models could help us to further understand the role of antimicrobial peptides such as hBD-3 in host defense.
During revision of the manuscript, two other studies reported the identification of mBD-14 as the mouse ortholog of hBD-3 (13, 17). The work of Taylor et al. (17) reported that hBD-3 and mBD-14 exhibit similar potent antimicrobial activities against S. aureus and Pseudomonas aeruginosa which are independent of intramolecular disulfide bonds. Röhrl et al. (13) reported that mBD-14 is active against E. coli but not against S. aureus, a finding that contrasts with our findings. However, it should be noted that whereas we used the mature peptide for analysis of antimicrobial activity, Röhrl et al. used an mBD-14-immunoglobulin fusion protein of approximately 37 kDa. In addition, both reports showed that mBD-14 exhibits chemotactic activities similar to those of hBD-3. Taken together, both reports confirmed that mBD-14 is the functional ortholog of hBD-3.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 617 and a Heisenberg fellowship to J.H.).
We thank Graziella Francesca Podda, Sylvia Voss, and Andrea Hoelzgen for excellent technical assistance and Grace Chen for help with the manuscript.
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Albanesi, C., H. R. Fairchild, S. Madonna, C. Scarponi, O. De Pita, D. Y. Leung, and M. D. Howell. 2007. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J. Immunol. 179:984-992. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Chromek, M., Z. Slamova, P. Bergman, L. Kovacs, L. Podracka, I. Ehren, T. Hokfelt, G. H. Gudmundsson, R. L. Gallo, B. Agerberth, and A. Brauner. 2006. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 12:636-641. [DOI] [PubMed] [Google Scholar]
- 4.Garcia, J. R., F. Jaumann, S. Schulz, A. Krause, J. Rodriguez-Jimenez, U. Forssmann, K. Adermann, E. Kluver, C. Vogelmeier, D. Becker, R. Hedrich, W. G. Forssmann, and R. Bals. 2001. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 306:257-264. [DOI] [PubMed] [Google Scholar]
- 5.Harder, J., J. Bartels, E. Christophers, and J. M. Schröder. 2001. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J. Biol. Chem. 276:5707-5713. [DOI] [PubMed] [Google Scholar]
- 6.Harder, J., U. Meyer-Hoffert, K. Wehkamp, L. Schwichtenberg, and J. M. Schröder. 2004. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J. Investig. Dermatol. 123:522-529. [DOI] [PubMed] [Google Scholar]
- 7.Kisich, K. O., M. D. Howell, M. Boguniewicz, H. R. Heizer, N. U. Watson, and D. Y. Leung. 2007. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on beta-defensin 3. J. Investig. Dermatol. 127:2368-2380. [DOI] [PubMed] [Google Scholar]
- 8.Maisetta, G., G. Batoni, S. Esin, W. Florio, D. Bottai, F. Favilli, and M. Campa. 2006. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 50:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzies, B. E., and A. Kenoyer. 2006. Signal transduction and nuclear responses in Staphylococcus aureus-induced expression of human beta-defensin 3 in skin keratinocytes. Infect. Immun. 74:6847-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 11.Nomura, I., E. Goleva, M. D. Howell, Q. A. Hamid, P. Y. Ong, C. F. Hall, M. A. Darst, B. Gao, M. Boguniewicz, J. B. Travers, and D. Y. Leung. 2003. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J. Immunol. 171:3262-3269. [DOI] [PubMed] [Google Scholar]
- 12.Pazgier, M., D. M. Hoover, D. Yang, W. Lu, and J. Lubkowski. 2006. Human beta-defensins. Cell Mol. Life Sci. 63:1294-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Röhrl, J., D. Yang, J. J. Oppenheim, and T. Hehlgans. 2008. Identification and biological characterization of mouse β-defensin 14, the orthologue of human β-defensin 3. J. Biol. Chem. 283:5414-5419. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph, B., R. Podschun, H. Sahly, S. Schubert, J. M. Schröder, and J. Harder. 2006. Identification of RNase 8 as a novel human antimicrobial protein. Antimicrob. Agents Chemother. 50:3194-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahly, H., S. Schubert, J. Harder, P. Rautenberg, U. Ullmann, J. Schröder, and R. Podschun. 2003. Burkholderia is highly resistant to human beta-defensin 3. Antimicrob. Agents Chemother. 47:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen, O. E., D. R. Thapa, A. Rosenthal, L. Liu, A. A. Roberts, and T. Ganz. 2005. Differential regulation of β-defensin expression in human skin by microbial stimuli. J. Immunol. 174:4870-4879. [DOI] [PubMed] [Google Scholar]
- 17.Taylor, K., D. J. Clarke, B. McCullough, W. Chin, E. Seo, D. Yang, J. Oppenheim, D. Uhrin, J. R. Govan, D. J. Campopiano, D. Macmillan, P. E. Barran, and J. R. Dorin. 7 January 2008, posting date. Analysis and separation of residues important for the chemoattractant and antimicrobial activities of beta-defensin 3. J. Biol. Chem. [Epub ahead of print.] doi: 10.1074/jbc.M709238200. [DOI] [PubMed]
- 18.Wilson, C. L., A. J. Ouellette, D. P. Satchell, T. Ayabe, Y. S. Lopez-Boado, J. L. Stratman, S. J. Hultgren, L. M. Matrisian, and W. C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117. [DOI] [PubMed] [Google Scholar]
- 19.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]