Abstract
An outbreak of cephalosporin-resistant Klebsiella pneumoniae occurred in a neonatal intensive care unit in São Paulo, Brazil. Of the 10 pulsotypes identified during the outbreak and follow-up periods, nine produced CTX-M-2 or its new variant CTX-M-59 and one produced SHV-5. blaCTX-M-2/59 genes were located on closely related plasmids that were transferable.
Klebsiella pneumoniae has long been known to cause outbreaks of infections in neonatal intensive care units (NICUs). Since the advent of extended-spectrum β-lactamases (ESBLs), many such outbreaks have been associated with ESBL-producing strains (7). Most outbreak investigations of ESBL-producing K. pneumoniae in NICUs have attributed the epidemics to dissemination of single clones, whereas only a few studies reported involvement of more than one strain in an outbreak setting (2, 6). We here describe the molecular epidemiology of a multiclonal outbreak in a Brazilian NICU caused by K. pneumoniae that produced CTX-M-type ESBLs including CTX-M-59, a novel variant of CTX-M-2.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The outbreak occurred in the NICU of a hospital near São Paulo in October 2003. A total of 10 cephalosporin-resistant K. pneumoniae strains were isolated at the time. Five were obtained from clinical specimens (one each from blood, cerebrospinal fluid, catheter tip, urine, and oropharynx), four from perirectal swabs, and one from a mother's milk. Subsequently, 37 nonrepetitive, cephalosporin-resistant K. pneumoniae strains were isolated from perirectal, axillary, and oropharyngeal swabs during a follow-up investigation between May and August 2004. Additionally, two more strains were identified from a catheter tip and blood culture during the follow-up period. Thus, a total of 49 strains were included in the study.
Escherichia coli DH10B was used as the host for cloning experiments. Chloramphenicol-resistant pBC-SK(−) plasmid (Stratagene, La Jolla, CA) was used as the cloning vector. E. coli XL1-Blue Rifr NAr was used as the recipient for conjugation and transformation experiments. Bacterial cultures were routinely grown in Luria-Bertani (LB) broth at 37°C.
PFGE.
Pulsed-field gel electrophoresis (PFGE) analysis was performed using restriction enzyme XbaI (New England Biolabs, Ipswich, MA) and a CHEF III DR electrophoresis system (Bio-Rad, Hercules, CA). Relatedness of the strains was determined according to the criteria of Tenover et al. (9).
Susceptibility testing.
MICs of cefotaxime with and without clavulanic acid, ceftazidime with and without clavulanic acid, cefepime, aztreonam, ertapenem, ciprofloxacin, and gentamicin were determined for all study strains by use of Etest strips according to the instructions from the manufacturer (AB Biodisk, Solna, Sweden). Production of ESBL was confirmed by the disk diffusion method endorsed by the Clinical and Laboratory Standards Institute (CLSI) (3).
PCR analysis and sequencing.
Primer sets to detect blaTEM (5), blaSHV (10), and blaCTX-M (4) were used for PCR analysis to detect the ESBL genes in the study strains. Nucleotide sequencing was performed with an ABI3100 genetic analyzer. The full sequences of blaCTX-M genes were obtained using a set of primers flanking the genes (Table 1).
TABLE 1.
Primers used for the study
| Gene | Primer name | Primer sequence | Product size (bp) | Reference |
|---|---|---|---|---|
| blaSHV | SHV-F-S1 | 5′-ATTTGTCGCTTCTTTACTCGC-3′ | 1,051 | 10 |
| SHV-R-S2 | 5′-TTTATGGCGTTACCTTTGACC-3′ | |||
| blaTEM | TEM-F | 5′-ATGAGTATTCAACATTTCCGTG-3′ | 840 | 5 |
| TEM-R | 5′-TTACCAATGCTTAATCAGTGAG-3′ | |||
| blaCTX-M | CTX-M/F′ | 5′-TTTGCGATGTGCAGTACCAGTAA-3′ | 544 | 4 |
| CTX-M/R′ | 5′-CGATATCGTTGGTGGTGCCATA-3′ | |||
| blaCTX-M-2 | CTXM2F | 5′-AAATGTGCTGCTCCTTTCGTGAGC-3′ | 1,122 | This study |
| CTXM2R | 5′-AGGGTTCGTTGCAAGACAAGACTG-3′ | |||
| CTXM2F XbaIa | 5′-CGTCTAGAATGATGACTCAGAGCA-3′ | 892 | This study | |
| CTXM2R BamHIa | 5′-CGGGATCCTCAGAAACCGTGGGTT-3′ |
Restriction sites are underlined in the sequences.
PCR cloning of blaCTX-M-2/59.
To assess whether the amino acid substitution observed with CTX-M-59 affected the level of resistance to β-lactams, E. coli clones producing either CTX-M-2 or CTX-M-59 were constructed. The structural genes corresponding to the enzymes were amplified with primers CTXM2F-XbaI and CTXM2R-BamHI (Table 1). The PCR product was digested with XbaI and BamHI (New England Biolabs) and ligated with pBC-SK(−). E. coli DH10B was then transformed by these recombinant plasmids by electroporation. Transformants were selected on LB agar plates containing 50 μg/ml of ampicillin and 30 μg/ml of chloramphenicol. After confirmation of the nucleotide sequences, E. coli DH10B(pCTX-M-2) producing CTX-M-2 and E. coli DH10B(pCTX-M-59) producing CTX-M-59 were used to determine the MICs of β-lactams.
Conjugation and transformation.
The standard broth mating method was employed for conjugation experiments (10). Transconjugants were selected on LB agar plates containing 50 μg/ml of ampicillin, 50 μg/ml of rifampin, and 50 μg/ml of nalidixic acid. For the strains that did not yield transconjugants, the plasmids were extracted by the modified alkaline lysis method described previously (8). E. coli XL1-Blue Rifr NAr was transformed with the plasmids by electroporation. Transformants were selected on LB agar plates containing 50 μg/ml of ampicillin.
DNA hybridization.
Plasmids were extracted from the transconjugants and transformants by the modified alkaline lysis method (8) and digested with EcoRI, HindIII, or PstI (New England Biolabs). The plasmids were then subjected to electrophoresis in an 0.8% agarose gel. The plasmids digested with PstI were hybridized with a digoxigenin-labeled DNA probe specific for blaCTX-M-2/59 using the PCR DIG detection system (Roche Diagnostics, Indianapolis, IN).
Nucleotide sequence accession numbers.
The nucleotide sequences of blaCTX-M-59 and blaSHV-85 have been deposited in the GenBank/EMBL/DDBJ database under accession no. DQ408762 and DQ322460, respectively.
RESULTS AND DISCUSSION
PFGE.
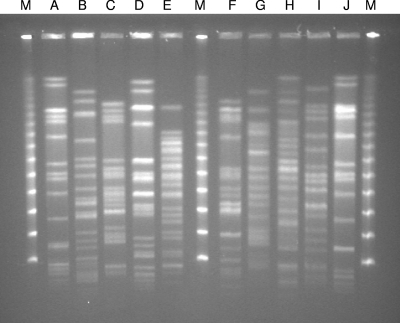
PFGE revealed the presence of 10 pulsotypes (Table 2; Fig. 1). Pulsotypes A and J were detected solely during the outbreak and were possibly related to each other, while pulsotype B was present both during and after the outbreak. Pulsotypes C and D were commonly recovered during the follow-up period. Taken together, these five pulsotypes accounted for 82% of the study strains.
TABLE 2.
MICs and ESBL types of K. pneumoniae strains according to PFGE pulsotypea
| Pulsotype | No. of isolates | Yr | Origin(s) | Median MIC (range) (μg/ml) of drug:
|
ESBL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | CAZ/CLA | CTX | CTX/CLA | ATM | FEP | ERT | GEN | CIP | |||||
| A | 7 | 2003 | Blood, CSF, urine, surveillance | 6 (4-16) | 0.5 (0.38-2) | >256 (96->256) | 0.094 (0.064-0.125) | 24 (16-64) | 16 (4-64) | 1.5 (0.19-4) | >256 (0.5->256) | 0.023 (0.023-0.047) | CTX-M-2 |
| B | 8 | 2003/2004 | Catheter tip, oropharynx, surveillance | 24 (6-48) | 0.75 (0.38-4) | >256 (>256) | 0.094 (0.064-1) | 48 (12-256) | 128 (16->256) | 1 (0.25-2) | >256 (96->256) | 0.032 (0.016-0.047) | CTX-M-2 |
| C | 13 | 2004 | Surveillance | 4 (3-8) | 0.38 (0.25-1) | >256 (256->256) | 0.094 (0.094-0.19) | 32 (12-256) | 256 (12->256) | 1.5 (0.19-1.5) | >256 (16->256) | 0.032 (0.023-0.032) | CTX-M-59 |
| D | 12 | 2004 | Surveillance | 256 (96->256) | 0.25 (0.19-2) | 48 (8-256) | 0.047 (0.023-0.125) | 256 (48->256) | 4 (1->256) | 0.094 (0.016-1.5) | 24 (0.5->256) | 0.023 (0.023-0.047) | SHV-5 |
| E | 2 | 2004 | Surveillance | 8 (6-8) | 0.75 (0.75) | >256 (>256) | 0.125 (0.094-0.125) | 32 (16-32) | 16 (16) | 1.5 (0.38-1.5) | >256 (>256) | 0.032 (0.032) | CTX-M-59 |
| F | 1 | 2004 | Surveillance | 8 | 1.5 | >256 | 0.094 | 256 | >256 | 1.5 | >256 | 0.25 | CTX-M-2 |
| G | 2 | 2004 | Surveillance | 12 (6-12) | 1.0 (0.5-1) | >256 (256->256) | 0.5 (0.094-0.5) | 64 (16-64) | 32 (16-32) | 1.5 (0.25-1.5) | >256 (256->256) | 0.125 (0.023-0.125) | CTX-M-59 |
| H | 2 | 2004 | Blood, catheter tip | 256 (96-256) | >4 (>4) | >256 (>256) | 1.0 (1.0) | 48 (32-48) | >256 (48->256) | 2 (0.5-2) | >256 (128->256) | 0.023 (0.023) | CTX-M-2 |
| I | 1 | 2004 | Surveillance | 4 | 0.5 | >256 | 0.094 | 24 | 256 | 1 | 256 | 0.032 | CTX-M-2 |
| J | 1 | 2003 | Surveillance | 6 | 0.75 | >256 | 0.094 | 128 | 12 | 1.5 | 96 | 0.023 | CTX-M-2 |
Abbreviations: CAZ, ceftazidime; CAZ/CLA, ceftazidime plus clavulanic acid; CTX, cefotaxime; CTX/CLA, cefotaxime plus clavulanic acid; ATM, aztreonam; FEP, cefepime; ERT, ertapenem; GEN, gentamicin; CIP, ciprofloxacin; CSF, cerebrospinal fluid.
FIG. 1.
PFGE profiles of the 10 pulsotypes identified in the study. M, marker.
Susceptibilities of the study strains.
All of the study strains were confirmed as ESBL producers by the disk diffusion method. Strains belonging to all pulsotypes except D had median cefotaxime MICs of greater than 256 μg/ml. Strains with pulsotype D had a median ceftazidime MIC of 256 μg/ml but a lower median cefotaxime MIC (48 μg/ml). All strains were susceptible to ertapenem and ciprofloxacin. They were variably resistant to gentamicin.
PCR analysis and sequencing of β-lactamase genes.
All PFGE pulsotypes except D gave amplicons consistent with blaCTX-M. The sequences corresponded to CTX-M-2 for pulsotypes A, B, F, H, I, and J and CTX-M-59 for pulsotypes C, E, and G (Table 2). CTX-M-59 is a novel variant of CTX-M-2 with an H89L substitution according to the Ambler numbering scheme (1). Pulsotype D carried blaSHV, which was confirmed to encode SHV-5 upon sequencing of the full open reading frame. All the other pulsotypes except F were also positive for blaSHV, which encoded various non-ESBL SHV enzymes including SHV-1, -11, -77, and -85. SHV-85 is a novel variant of SHV-11 with an L19M substitution, which is located within the signal peptide. All pulsotypes except D were positive for blaTEM. The deduced amino acid sequences were consistent with that of TEM-1.
Phenotype conferred by CTX-M-59.
The β-lactam MICs of E. coli DH10B(pCTX-M-59) did not differ significantly from those of E. coli DH10B(pCTX-M-2) (Table 3). Both clones displayed susceptibility patterns typical for organisms producing CTX-M-type enzymes, with higher levels of resistance to cefotaxime than to ceftazidime. These results suggested that the kinetic properties of CTX-M-59 were not likely altered by the H89L substitution compared with its progenitor CTX-M-2.
TABLE 3.
MICs of E. coli DH10B with recombinant plasmids encoding blaCTX-M-2/59
| E. coli strain | MIC (μg/ml) of druga:
|
||||||
|---|---|---|---|---|---|---|---|
| CAZ | CAZ/CLA | CTX | CTX/CLA | ATM | FEP | ERT | |
| DH10B(pCTX-M-2) | 8 | 0.38 | 128 | 0.094 | 12 | 4 | 0.016 |
| DH10B(pCTX-M-59) | 8 | 0.38 | 96 | 0.094 | 12 | 4 | 0.016 |
Abbreviations: CAZ, ceftazidime; CAZ/CLA, ceftazidime plus clavulanic acid; CTX, cefotaxime; CTX/CLA, cefotaxime plus clavulanic acid; ATM, aztreonam; FEP, cefepime; ERT, ertapenem.
Mating experiments.
Transconjugants were obtained from representative strains of all pulsotypes at frequencies of 2 × 10−5 or higher per recipient cell except for pulsotypes A and J, for which no transconjugant was obtained. All transconjugants except for that from pulsotype D were resistant to cefotaxime but not ceftazidime. PCR analysis for these transconjugants yielded amplicons with primers specific for blaCTX-M-2 and blaTEM, indicating successful transfer of blaCTX-M-2/59 and blaTEM-1. The transconjugant from pulsotype D was resistant to ceftazidime but not cefotaxime and was positive for blaSHV by PCR analysis, indicating conjugal transfer of blaSHV-5. The plasmids harboring blaCTX-M-2 in representative strains from profiles A and J were successfully transferred to the recipient strain by transformation, which was confirmed by PCR analysis likewise. The MICs for the transconjugants and transformants are shown in Table 4.
TABLE 4.
MICs and PCR results for the transconjugants/transformants from each PFGE pulsotype
| Pulsotype | Median MIC (μg/ml) of druga:
|
β-Lactamase(s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CAZ | CAZ/CLA | CTX | CTX/CLA | ATM | FEP | ERT | GEN | ||
| A | 8 | 0.19 | >256 | 0.094 | >256 | >256 | 0.125 | >256 | CTX-M-2, TEM-1 |
| B | 12 | 0.19 | >256 | 0.094 | 256 | 256 | 0.125 | >256 | CTX-M-2, TEM-1 |
| C | 8 | 0.25 | >256 | 0.19 | >256 | >256 | 0.064 | >256 | CTX-M-59, TEM-1 |
| D | >256 | 0.19 | 12 | 0.47 | >256 | 1.5 | 0.012 | 24 | SHV-5 |
| E | 4 | 0.19 | >256 | 0.25 | 48 | >256 | 0.094 | >256 | CTX-M-59, TEM-1 |
| F | 16 | 0.38 | >256 | 0.125 | >256 | >256 | 0.125 | >256 | CTX-M-2, TEM-1 |
| G | 8 | 0.38 | >256 | 0.19 | >256 | >256 | 0.125 | >256 | CTX-M-59, TEM-1 |
| H | 8 | 0.25 | >256 | 0.94 | >256 | >256 | 0.094 | >256 | CTX-M-2, TEM-1 |
| I | 6 | 0.19 | >256 | 0.125 | >256 | >256 | 0.032 | >256 | CTX-M-2, TEM-1 |
| J | 12 | 0.38 | >256 | 0.125 | >256 | >256 | 0.094 | 128 | CTX-M-2, TEM-1 |
Abbreviations: CAZ, ceftazidime; CAZ/CLA, ceftazidime plus clavulanic acid; CTX, cefotaxime; CTX/CLA, cefotaxime plus clavulanic acid; ATM, aztreonam; FEP, cefepime; ERT, ertapenem; GEN, gentamicin.
Plasmid analysis and DNA hybridization.
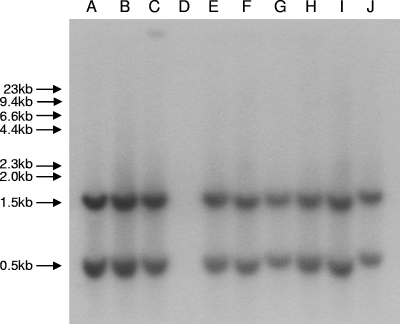
Plasmids obtained from the transconjugants and transformants encoding blaCTX-M-2/59 shared identical or very similar banding patterns with any of the three restriction enzymes, whereas that from the transconjugant encoding blaSHV-5 was distinct (results not shown). The sizes of these plasmids were estimated to be ca. 50 kb. The probe for blaCTX-M-2/59 hybridized with two bands with similar sizes, ca. 1.5 kb and 0.5 kb, for the plasmids from all pulsotypes except D (Fig. 2). Two bands were generated because blaCTX-M-2/59 contains a PstI restriction site. This finding suggested that the genetic support of blaCTX-M-2/59 in this outbreak was likely uniform regardless of pulsotypes. Taken together, the outbreak phase was initiated by three genomic clones carrying similar plasmids, all encoding blaCTX-M-2. It appears that one of the plasmids then further disseminated to other clones and produced a variant plasmid encoding blaCTX-M-59. Thus, we speculate that these ESBL-encoding plasmids persisted, evolved, and disseminated among different clones of K. pneumoniae in the confined environment of a NICU over the 10-month period.
FIG. 2.
DNA hybridization with a blaCTX-M-2-specific probe. blaCTX-M-2/59 contains a PstI restriction site. All the plasmids hybridized with the probe, except for that from pulsotype D, which is blaCTX-M-2/59 negative and blaSHV-5 positive.
Conclusion.
We described a multiclonal outbreak of K. pneumoniae producing CTX-M-2 and its variant CTX-M-59 ESBLs which took place in a Brazilian NICU. In addition to the spread of each clonal group, dissemination of identical or related plasmids harboring the CTX-M-type ESBL genes among different clonal groups was responsible for the persistence of ESBL-producing K. pneumoniae over time.
Acknowledgments
We thank Hanna Sidjabat for expert technical assistance.
D.D.O.G. and part of this study were supported by a Fogarty International Center Global Infectious Diseases Research Training Program grant, National Institutes of Health (D43TW006592; principal investigator, Lee H. Harrison). Y.D. is supported by NIH training grant T32AI007333. D.L.P. has received prior research funding from Pfizer, Elan, Merck, and AstraZeneca and is supported in part by NIH grant R01AI070896.
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukadida, J., N. Salem, N. Hannachi, K. Monastiri, and N. Snoussi. 2002. Genotypic exploration of a hospital neonatal outbreak due to Klebsiella pneumoniae producing extended-spectrum-β-lactamase. Arch. Pediatr. 9:463-468. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Edelstein, M., M. Pimkin, I. Palagin, I. Edelstein, and L. Stratchounski. 2003. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrob. Agents Chemother. 47:3724-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kruger, T., D. Szabo, K. H. Keddy, K. Deeley, J. W. Marsh, A. M. Hujer, R. A. Bonomo, and D. L. Paterson. 2004. Infections with nontyphoidal Salmonella species producing TEM-63 or a novel TEM enzyme, TEM-131, in South Africa. Antimicrob. Agents Chemother. 48:4263-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebessi, E., H. Dellagrammaticas, P. T. Tassios, L. S. Tzouvelekis, S. Ioannidou, M. Foustoukou, and N. J. Legakis. 2002. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit in the high-prevalence area of Athens, Greece. J. Clin. Microbiol. 40:799-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi, S., and Y. Nagano. 1984. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J. Clin. Microbiol. 20:608-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagi, T., H. Kurokawa, N. Shibata, K. Shibayama, and Y. Arakawa. 2000. A preliminary survey of extended-spectrum β-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Japan. FEMS Microbiol. Lett. 184:53-56. [DOI] [PubMed] [Google Scholar]