Abstract
The enterotoxigenic Escherichia coli (ETEC) strains are major causes of morbidity and mortality due to diarrheal illness in developing countries. At present, there is no broadly protective vaccine for this diverse group of pathogens. The EtpA protein, identified in ETEC H10407 in a recent search for candidate immunogens, is a large glycosylated exoprotein secreted via two-partner secretion (TPS). Similar to structurally related molecules, EtpA functions in vitro as an adhesin. The studies reported here use a recently developed murine model of ETEC intestinal colonization to examine the immunogenicity and protective efficacy of EtpA. We report that mice repeatedly exposed to ETEC are protected from subsequent colonization and that they mount immune responses to both EtpA and its presumed two-partner secretion transporter (EtpB) during the course of experimental infection. Furthermore, isogenic etpA deletion mutants were impaired in the colonization of mice, and intranasal immunization of mice with recombinant EtpA conferred protection against ETEC H10407 in this model. Together, these data suggest that EtpA is required for optimal colonization of the intestine, findings paralleling those of previous in vitro studies demonstrating its role in adherence. EtpA and other TPS proteins may be viable targets for ETEC vaccine development.
The enterotoxigenic Escherichia coli (ETEC) strains comprise a diverse group of pathogens that are responsible for considerable morbidity in developing countries. Collectively, these organisms are thought to account for hundreds of millions of cases of diarrheal illness and as many as 500,000 deaths annually in young children (43). Perennially the most common causes of diarrheal illness in travelers (27, 38) and soldiers deployed to developing countries (7, 25), ETEC strain have also emerged in several recent large-scale outbreaks in the United States (2, 13).
ETEC strains have in common the ability to produce heat-labile and/or heat-stable enterotoxins that cause diarrhea by activation of chloride channels in the small intestine. Effective toxin delivery is thought to occur upon colonization of the small intestine and likely requires intimate association of the organism with target epithelial cells (15, 45) of the intestinal mucosa.
Colonization of the small intestine is thought to occur at least in part via fimbrial colonization factors (CFs) (39). Vaccination with CFs (16) or passive oral immunization with anti-CF immunoglobulin (22, 41) affords significant but type-specific protection against subsequent ETEC challenge (16, 22). Vaccine development to date has largely focused on the painstaking identification of CF molecules and their incorporation into a multivalent vaccine. However, recent molecular epidemiologic studies have demonstrated that many strains do not make any of the more than 20 CFs that have been identified to date (35), prompting a search for additional target antigens (5).
Another pitfall in ETEC vaccine development has been the lack of a suitable high-throughput animal model that could be used to test vaccine candidates. We have recently reported that adult immunocompetent mice can be effectively colonized with ETEC strains isolated from humans (1). We sought to further validate this model and to explore its use in examining recently identified ETEC exoproteins that might have utility in vaccine development.
One recently identified ETEC exoprotein, EtpA (21), is a member of a family of virulence proteins (generically referred to as TpsA proteins) that are secreted by TPS. TpsA exoproteins similar to EtpA play critical roles in bacterial adhesion in vitro (37) and in the colonization of mucosal surfaces in vivo (29). Furthermore, these proteins serve as protective antigens and have been incorporated in the development of highly effective acellular vaccines for other important mucosal pathogens such as Bordetella pertussis (23). Therefore, we performed additional studies to examine the contribution of EtpA to colonization of the intestine, and its potential role as a protective immunogen in the experimental mouse model.
In the studies reported here, we demonstrate that mice repeatedly challenged with ETEC are protected from subsequent colonization. These mice mount immune responses to both the secreted EtpA exoprotein and its two-partner secretion transporter, EtpB. Furthermore, we demonstrate that strains deficient in EtpA are deficient in intestinal colonization and that vaccination with recombinant EtpA affords protection from subsequent colonization in this model.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in these studies are included in Table 1. ETEC strain H10407 is a fully virulent human isolate originally isolated from a child with severe diarrheal illness in Bangladesh (17). This strain, serotype O78:H11, produces heat-labile toxin (LT), heat-stable toxin (ST), and CFAI, as well as the recently identified ETEC two-partner secretion system (21). H10407S is a spontaneous streptomycin-resistant mutant of the wild type (WT). jf1289 is an isogenic etpA deletion mutant constructed from H10407S as previously described (21). AAEC191A used as a control in these studies is an afimbriate avirulent laboratory isolated derived from E. coli K-12 (3). E. coli BL21(DE3) [F− ompT hsdSB(rB− mB−) gal dcm (DE3)] was used as the host strain for expression of recombinant proteins in these studies. To create a strain for competition assays, we first used plasmid pJL017 (containing the etpB and etpA genes cloned into pBADmycHisA; Invitrogen) as the target for in vitro transposon mutagenesis using pGPS4 (New England Biolabs) as the transposon donor. Plasmid pJMF1019 resulting from this mutagenesis contains a transposon insertion encoding a chloramphenicol resistance (Cmr) cassette at etpA nucleotide position 328. An 8,590-bp XhoI/HindIII restriction fragment from pJMF1019 containing this mutagenized etpA locus was then introduced into H10407 by lambda red-mediated allelic exchange as previously described (14) to generate the etpA-negative, Cmr mutant (jf1668) used for the competition experiments. Verification of the etpA deletion was performed by PCR with the primers jf092605.3 (5′-CAGATTGTGGCAGGTTCA-3′) and jf122205.1 (5′-CTAAAACAGAATCCCGCTATC-3′) to distinguish the mutant etpA::Cmr sequence (3,064 bp) from the WT etpA sequence (1,692 bp). jf1668 was then complemented with either pJL017 or the vector control plasmid pBADmycHisA. After induction with 0.0002% arabinose, the production of recombinant EtpA (rEtpA) was confirmed by immunoblotting trichloroacetic acid-precipitated culture supernatants (21).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or descriptiona | Source or reference |
|---|---|---|
| Bacterial strains | ||
| H10407 | ETEC serotype O78:H11, LT+ ST+ | 17 |
| H10407-S | Spontaneous Smr derivative of H10407 | |
| jf1289 | Isogenic deletion mutant of etpA | 21 |
| AAEC191A | fim deletion mutant of E. coli K-12 (MG1655) | 3 |
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | Novagen |
| jf1668 | Isogenic deletion mutant of etpA bearing a Cmr cassette in etpA | This study |
| Plasmids | ||
| pBAD/Myc-His A | Arabinose-inducible expression plasmid; Ampr | Invitrogen |
| pJL017 | etpBA cloned into pBAD/myc-His A | This study |
| pGPS4 | Transposon donor; Cmr | NEBb |
| pJMF1019 | pJL017 containing etpA mutagenized (::Cmr) with the GPS4 transposon | This study |
| pJY034 | EtpB cloned in-frame with polyhistidine tag on pET-30a(+) | This study |
| pET-30a(+) | IPTG-inducible expression plasmid; Kmr | Novagen |
Ampr, Kmr, Cmr, and Smr represent ampicillin, kanamycin, chloramphenicol, and streptomycin resistance, respectively.
NEB, New England Biolabs.
Preparation and administration of antigens.
Recombinant polyhistidine-tagged EtpA (rEtpA) was prepared by nickel affinity chromatography in the presence of 8 M urea as previously described (21). Urea was subsequently removed by sequential dialysis against 2, 1, and 0.5 M urea in phosphate-buffered saline (PBS; pH 7.4). This rEtpA molecule represents approximately the first 110 kDa of the mature EtpA molecule (∼170 kDa) and, unlike the native molecule, it is not glycosylated. The IVX908 (Protillin; ID Biomedical) used in these studies is a novel mucosal adjuvant based on Neisseria outer membrane proteins noncovalently complexed to lipopolysaccharide (10) and was kindly supplied by the laboratory of James Dale. After the mice were anesthetized with isofluorane, they were immunized with either 7.5 μg of IVX908 alone or IVX908 (7.5 μg) with rEtpA (30 μg) in a total volume of 20 μl (10 μl/nostril).
To prepare recombinant EtpB (rEtpB), the region of the etpB gene encoding amino acids 47 to 602 of EtpB was first amplified by PCR with the primers jf071405.5 (5′-AATAATAGATCTAGCTCCGGTGCTCCAGAAT-3′) and jf071405.6 (5′-AATAATGCGGCCGCTCACGTTTTCAGGGCTGACAG-3′). (The underlined regions represent the BglII and NotI sites, respectively.) The resulting amplicon was then directionally cloned into the corresponding sites on pET30a(+) in frame with the region encoding an amino-terminal polyhistidine tag to create pJY034. pJY034 was then used to transform E. coli BL21(DE3) to kanamycin resistance. After the induction of BL21(DE3)/pJY034 with IPTG (isopropyl-β-d-thiogalactopyranoside), polyhistidine-tagged rEtpB42-607 was isolated by nickel affinity chromatography as previously described (21).
Mouse challenge studies.
All experimental procedures were reviewed and approved by the University of Tennessee Health Science Center Institutional Animal Care and Use Committee. Experimental procedures were performed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (26).
Bacteria were prepared for mouse challenge experiments as previously described (1). Briefly, bacteria were grown overnight from frozen glycerol stocks in 2-ml cultures of Luria broth at 37°C and 250 rpm. On the morning of the challenge, cultures were diluted 1:100 in side-arm flasks containing 100 ml of Luria broth and incubated until the optical density at 600 nm (OD600) reached ∼0.14. A 40-ml portion of the resulting culture was then centrifuged at 4°C and 10,000 rpm for 10 min, the supernatant was discarded, and the resulting pellet was resuspended in 1.6 ml of sterile PBS to achieve a bacterial concentration of ∼109 CFU/ml. Bacteria were further diluted in PBS to achieve the final desired inoculum in 400 μl. Actual inocula were determined by plating serial dilutions of this final suspension onto Luria agar plates. These studies followed protocols previously outlined in detail in earlier work (1). Briefly, for each challenge the mice were pretreated with streptomycin and cimetidine to facilitate colonization. As before, intestinal colonization in these experiments was assessed by quantification of the bacteria in saponin lysates of segments of small intestine. For experiments in which WT ETEC strains lacking an antibiotic resistance marker (e.g., H10407) were used as the challenge organism, colony PCR for LT was used to confirm that recovered bacteria were the inoculum strain. As in prior studies (1), we used the primers jf030204.1 (5′-CCCCAGTCTATTACAGAA-3′) and jf030204.2 (5′-CTAGTTTTCCATACTGAT-3′), which flank the eltAB operon encoding the LT holotoxin of H10407.
In repeated exposures to either AAEC191A or H10407, mice were prepared as described above, followed by the administration of ∼107 CFU of bacteria by gavage. After exposure, the mice were returned to their respective cages and permitted immediate access to food and water until the next administration of bacteria. Prior to the first administration of bacteria, baseline fecal pellets and blood were obtained. Bacteria were administered on days 0, 14, and 68. Fecal and blood samples were obtained again on day 95, and then four to five mice from each group were challenged with either 6 × 107 or 6 × 103 CFU of WT H10407.
In experiments comparing single strains (e.g., the WT ETEC H10407 strain versus the isogenic etpA mutant jf1289), mice were challenged with equal numbers of organisms and sacrificed after 24 h. The degree of colonization was then determined by plating dilutions of intestinal lysates and colony counting as previously described (1).
For competition experiments, mutant strain jf1668, containing etpA interrupted by a Cmr marker (etpA::Cmr) was complemented either with the vector plasmid alone (pBAD/mycHisA) or plasmid pJL017, which expresses the etpBA locus. To examine the relative ability of these complemented etpA mutants to colonize the murine small intestine, 10 mice were each inoculated via gavage with ∼105 CFU of jf1668(pBadmycHisA)/ml and an equal number of jf1668(pJL017) CFU in a total volume of 0.4 ml of PBS containing 100 μg of ampicillin/ml and 0.0002% arabinose. The inoculum for each strain was determined by plating serial dilutions of each onto Luria agar plates containing chloramphenicol (20 μg/ml) and ampicillin (100 μg/ml) before combining them in suspension. Immediately following inoculation, the water supply for the mice was changed to include both ampicillin (50 μg/ml) and arabinose (0.0002%) to maintain plasmid selection and to promote production of rEtpA. At 24 h after inoculation, mice were sacrificed, and organisms were recovered by plating dilutions of intestinal extracts onto chloramphenicol-ampicillin plates. Colony PCR using primers jf092605.3 (5′-CAGATTGTGGCAGGTTCA-3′) and jf122205.1 (5′-CTAAAACAGAATCCCGCTATC-3′) was then performed to distinguish colonies containing only the mutant etpA::Cmr sequence (3,064 bp) from those carrying the WT etpA sequence cloned on pJL017 (1,692 bp). The competitive index (CI) for each colonized mouse was then calculated as follows: CI = {(etpA::Cmr/etpA)output PCR/[jf1668(pBADmycHisA)/jf1668(pJL017)]input CFU}, where the output fraction of mutant jf1668(pBADmycHisA) versus recombinant jf1668(pJL017) was determined by PCR, and the input fraction was determined directly by colony counting (CFU).
Immunoassays.
After the application of anesthesia with isofluorane, blood was obtained from mice by periorbital bleeding using capillary tubes or by intracardiac puncture upon sacrifice of the animals after challenge. After clotting, serum was centrifuged briefly to remove remaining cells and transferred to fresh tubes and stored at −80°C for subsequent assay. To obtain fecal antibodies, five fresh pellets were obtained for each animal. These were placed immediately into 1.5 ml of fecal reconstitution buffer containing Tris (10 mM), NaCl (100 mM), Tween 20 (0.05%), and sodium azide (5 mM) at pH 7.4. Samples were vortex mixed to homogeneity and spun at 1,500 × g at 4°C for 10 min to remove the insoluble material. Supernatants were saved and stored at −80°C for subsequent analysis.
Purified rEtpA and rEtpB, prepared by nickel affinity chromatography, were diluted in 0.1 M NaHCO3 buffer (pH 8.6) to a final concentration of ∼4 μg/ml. A total of 50 μl of each respective solution was used to coat individual wells of an enzyme-linked immunosorbent assay (ELISA) plate (Immunolon; Nunc) overnight at room temperature. After a washing step to remove excess antigen, the plate was blocked with a solution of 1% bovine serum albumin (Blocker; Pierce) in PBS containing 0.05% Tween 20 (PBST) for 1 h at room temperature. After removal, 50 μl of each antibody-containing solution diluted in PBST was added, followed by incubation for 1 h. The plate was washed with PBST and developed with horseradish peroxidase-labeled goat anti-mouse (immunoglobulin G [IgG], IgM, and IgA) antibody, followed by washing and detection with tetramethylbenzidine-H2O2 peroxidase substrate (Kierkegaard & Perry Laboratories). To detect IgA responses, horseradish peroxidase-labeled goat anti-mouse IgA antibodies (Santa Cruz) were used at a concentration of 1:2,000. Reactions were stopped after significant color development by the addition of 1 M H2SO4, and the OD405 was determined spectrophotometrically.
RESULTS
Mice repeatedly exposed to ETEC recognize ETEC-specific antigens and are protected from subsequent colonization.
In developing countries, children under the age of 5 years are disproportionately affected by diarrhea caused by ETEC, with a peak incidence in the first 2 years of life (36, 44), while adults who travel from industrialized regions to areas of endemicity are highly susceptible to infection with these pathogens (6). Detailed studies in children during the first year of life also suggest that prior infections with ETEC protect against subsequent diarrheal illness caused by these organisms (12). This suggests that naturally occurring infections are immunizing against diarrheal disease due to ETEC (40). Therefore, we set out to examine whether repeated exposure to ETEC in the murine model would afford significant protection against subsequent intestinal colonization, thereby mimicking natural infections in humans.
To examine this question, we repeatedly exposed mice to either human ETEC isolate H10407, which effectively colonizes murine intestines, or the avirulent noncolonizing (1) laboratory E. coli strain AAEC191A (3, 4). We then challenged both groups of previously exposed mice with two different doses of ETEC H10407.
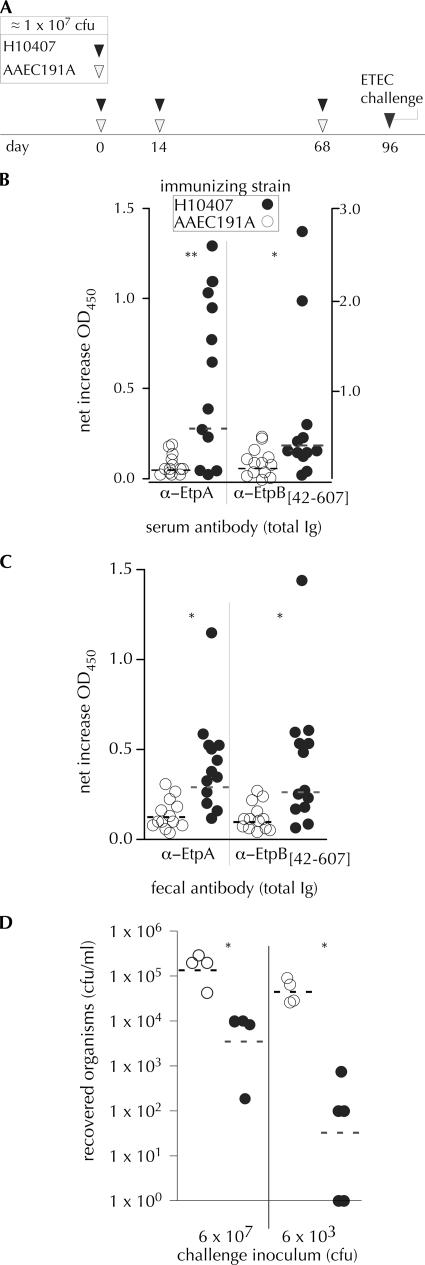
Repeated exposure to infections with ETEC H10407 resulted in significant serum (Fig. 1B) and fecal (Fig. 1C) antibody (total IgG, IgM, and IgA) responses to both EtpA and EtpB, components of the recently identified ETEC two-partner secretion system. Mice exposed to H10407 developed modest but significant increases in fecal anti-EtpA IgA responses by ELISA relative to those exposed to AAEC191A (geometric mean increases in the OD450 of 0.124 and 0.047, respectively [P = 0.01]). Together, these data suggest that EtpA and EtpB are expressed in vivo in this model and that mice recognize extracellular ETEC proteins during the course of experimental infection.
FIG. 1.
Mice repeatedly inoculated with ETEC mount immune responses to ETEC TPS proteins and are protected from subsequent challenge. (A) Mouse inoculations and challenge timeline. Mice were inoculated with ∼107 CFU of either avirulent strain AAEC191A (▿) or ETEC prototype strain H10407 (▾) on days 0, 14, and 68. Sera and stool stamples were collected on days 0 and 95 prior to challenge, and samples were frozen at −80°C for subsequent ELISA. (B) ELISA determination of total serum antibody to EtpA or EtpB after repeated inoculation with AAEC191A (○) or H10407 (•). ELISA values represent net increases in OD450 from pre- to postimmune (**, P = 0.014; *, P = 0.047). (C) ELISA of fecal antibody responses to EtpA (P = 0.012) and EtpB (P = 0.012). All values reflect determination of total IgG, IgA, and IgM. (D) Mouse challenge. Both groups of mice (those previously inoculated with H10407 [•] or AAEC191A [○]) were challenged with H10407 at two different doses: 6 × 107 CFU and 6 × 103 CFU. After 24 h, mice were sacrificed, and the degree of ileal colonization was determined by plate counting. (*, P = 0.029 and P = 0.016 for the 107 and 103 CFU challenge groups, respectively, as determined by two-tailed Mann-Whitney test). (For two mice in the 103 challenge group, no bacteria were recovered. These were arbitrarily assigned values of 1 CFU, the theoretical limit of detection.) Dashed horizontal lines represent the geometric mean values for each group. α, anti.
Interestingly, mice that were repeatedly exposed to ETEC were significantly protected from subsequent intestinal colonization with H10407 compared to those previously inoculated with the afimbriate noncolonizing AAEC191A strain (Fig. 1D). Together, these results suggest that during the course of repeated infection with ETEC, mice in this model develop protective immune responses that are directed against ETEC-specific antigens and, likewise, that this model may be used to examine the protective efficacy of candidate immunogens.
EtpA is required for optimal colonization of murine intestine.
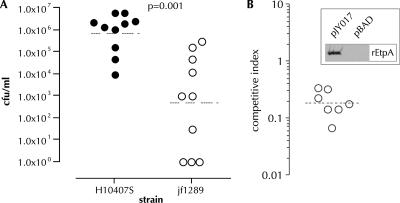
As demonstrated in Fig. 2A, an isogenic etpA mutant was deficient in colonization of murine intestine compared to the WT strain (P = 0.0014). Similarly, in direct competition assays (Fig. 2B), when etpA mutant jf1668 was complemented with EtpA expression plasmid pJL017, it exhibited a distinct colonization advantage relative to the same mutant complemented with only the vector control plasmid (P = 0.0062). Conversely, we found no difference in the levels of growth of these strains, jf1668(pJL017) and jf1668(pBAD/myc-HisA), when grown together in vitro. These studies strongly suggest that EtpA, similarly to other TpsA exoproteins, plays an essential role in the colonization of mucosal surfaces.
FIG. 2.
EtpA promotes colonization of murine small intestine. (A) Mice pretreated with cimetidine and streptomycin were challenged with either WT ETEC (H10407S) or an isogenic ΔetpA deletion mutant (jf1289). The inoculum as determined by serial dilution and plate counting was 8.8 × 102 CFU/inoculum (WT) and 9.4 × 102 CFU/inoculum (ΔetpA). Shown on the y axis are the number of CFU/ml recovered from intestinal lysates obtained approximately 24 h after challenge. In three mice challenged with the jf1289 strain no organisms were recovered from intestinal lysates, and these are arbitrarily represented at the theoretical limit of detection (1 CFU on the graph). The dashed horizontal lines for each data set indicate the respective geometric mean values. P = 0.001 by (two-tailed) Mann-Whitney analysis of the data sets. (B) Competition experiment between etpA mutant jf1668 complemented with either pBADmyc-HisA (pBAD vector control) or EtpA expression plasmid pJY017. Each point on the graph represents the CI calculated from a single mouse. To calculate the CI, the ratio of vector colonies (jf1668 complemented with the vector only) to recombinant colonies (jf1668 complemented with the pJL017 EtpA expression plasmid) was first determined by PCR of Cmr Ampr colonies grown from intestinal lysates (i.e., [vector/recombinant]output PCR). An average of nine Cmr Ampr colonies were tested per mouse. This ratio was then divided by the ratio of vector to recombinant-complemented colonies present in the initial inoculum as determined by plating dilutions of each strain (i.e., [vector/recombinant]input CFU). One mouse was colonized with jf1668(pJY017), but no jf1668(pBADmyc/HisA) colonies were identified by PCR, and this mouse is represented as having a CI of 0.07 on the graph, indicating the limits of detection for this assay. The dashed horizontal line represents the geometric mean CI (∼0.19), implying that on average the strain complemented with the EtpA expression plasmid was ∼5-fold more fit for colonization than that complemented with the vector alone under these conditions. P = 0.0062 by Mann-Whitney (two-tailed) testing of the two groups. For the inset graph, data were obtained by anti-EtpA immunoblotting of trichloroacetic acid-precipitated supernatants of jf1668 complemented with either pJL017 (shown on left) or vector control pBADmycHisA (shown on right) after induction with 0.0002% arabinose.
Immunogenicity and protective efficacy of EtpA.
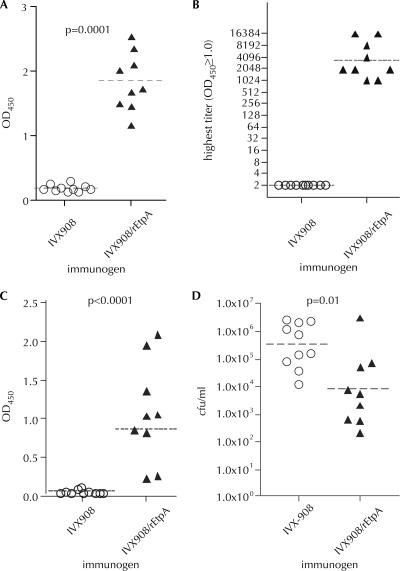
Next, we immunized mice with either a proteosome/lipopolysaccharide-based mucosal adjuvant, IVX908 (28), or IVX908 plus rEtpA. When administered intranasally, the IVX908-rEtpA formulation was highly immunogenic (Fig. 3A), resulting in high-titer (≥1:1,024) serologic responses in each of the immunized mice (Fig. 3B). We also detected significant responses to EtpA in saliva (Fig. 3C), although we failed to identify significant increases in fecal antibody in EtpA-vaccinated mice relative to controls (data not shown).
FIG. 3.
Immunogenicity and protective efficacy of recombinant EtpA tested in a murine model of ETEC colonization. Mice immunized intranasally with either IVX908 alone (controls) or IVX908 plus recombinant, nonglycosylated polyhistidine-tagged EtpA (30 μg/dose) on days 0, 22, and 42. On day 63 the mice were given 8.5 × 104 CFU of WT ETEC (H10407) by oral gavage, and the degree of colonization was assessed approximately 24 h after inoculation. (A) Anti-EtpA (total IgG, IgM, and IgA) responses (ELISA) in serum obtained on day 63 prior to challenge. Control sera have been diluted 1:50; IVX908/rEtpA sera were diluted 1:1,028. (P < 0.0001 [two-tailed Mann-Whitney]). (B) Titers of anti-EtpA antibody in mice vaccinated with either IVX908 or IVX908/rEtpA. Horizontal lines represents geometric mean titers (GMT). (C) ELISA data for salivary antibody from mice (at a saliva dilution of 1:2) immunized with either IVX908 or IVX908/rEtpA (P < 0.0001 [two-tailed Mann-Whitney]). (D) ETEC H10407 recovered from the small intestine after challenge of mice vaccinated with either IVX908 or IVX908/rEtpA (P = 0.01 [one-tailed Mann-Whitney]). Dashed horizontal bars represent geometric mean values throughout. Bacteria were recovered from the small intestine as previously described (1).
Finally, we challenged mice that had been immunized in this fashion with ∼8 × 104 CFU of WT ETEC H10407. As shown in Fig. 3D, mice immunized in this fashion were afforded significant protection from ETEC colonization relative to controls.
DISCUSSION
Studies of ETEC pathogenesis have been significantly hampered by the availability of a tractable animal model. As with most other diarrheagenic Escherichia coli pathotypes, ETEC strains exhibit significant species specificity in their ability to cause diarrheal illness. Despite these inherent shortcomings, the studies reported here, performed with a recently developed animal model of ETEC infection, are potentially relevant to the investigation of the pathogenesis of human ETEC infections for several reasons. First, the development of a protective immune response after repeated exposures to ETEC in these mice parallels previous epidemiologic observations made in children in developing countries, suggesting that prior exposures to enterotoxigenic E. coli provide protection against subsequent episodes of ETEC infection and symptomatic diarrheal illness (40). Similarly, these mouse studies are reminiscent of the results of earlier human volunteer studies demonstrating that prior infection offers protection from diarrheal illness on subsequent challenge (32). Second, to our knowledge, these are the first experiments to demonstrate that a protein other than the known toxins (1) or CFs might contribute to intestinal colonization, a critical step in the virulence of these pathogens. Likewise, these are the first studies to suggest that a virulence factor other than the intensively investigated CF molecules or toxins might serve as viable targets in vaccine development.
Our results suggest that the mouse model of colonization may be useful in evaluating potential ETEC vaccine candidates, such as EtpA. In its present form the murine model has as its major limitation the fact that mice do not get diarrhea even after prolonged exposures to high doses of ETEC that would normally cause diarrheal illness in humans. However, the ability to colonize the intestinal mucosa is thought to be a critical parameter in the development of diarrhea, and our own in vitro studies demonstrate that intimate association of the bacteria with target epithelial cells is required for effective toxin delivery (15).
The competition studies included here demonstrate that an etpA mutant strain engineered to secrete rEtpA can outcompete the same mutant bearing only the plasmid vector. The study design used here imposes some limitations that deserve discussion. The use of the same antibiotic markers in both strains and the use of PCR to distinguish vector controls from recombinants could have had a potential impact on the sensitivity of these assays. In addition, it is not clear what level of EtpA expression is required for optimal intestinal colonization. Furthermore, on the basis of these data we cannot exclude the possibility that rEtpA secreted by the recombinant partially restored the ability of the mutant vector control strain to colonize. Despite these inherent shortcomings, the competition experiments included here support a role for EtpA in intestinal colonization and may provide an avenue for future EtpA structure-function studies.
Although EtpA appears to facilitate colonization of mouse intestine, the mechanism by which this happens is not completely clear. Studies in our laboratory to date suggest that this protein is largely secreted from the organism, and it has been difficult to find EtpA associated with the outer membrane of ETEC. Indeed, EtpA lacks carboxy-terminal cysteine residues that have been shown to be important in anchoring other known TpsA adhesins to the bacterial surface (9). However, TcpF, a soluble virulence factor of Vibrio cholerae, has also been shown to enhance colonization in an in vivo mouse colonization model (30). Regardless of the mechanism by which EtpA acts to facilitate colonization, these studies suggest that EtpA contributes to the virulence of ETEC and that it may serve as a suitable target in vaccine development.
In the preliminary studies reported here we chose to administer EtpA via an intranasal route similarly to prior studies of the effect of subunit vaccines on the intestinal colonization of Campylobacter jejuni in mice (31). We recognize that direct delivery of EtpA and other antigens to the intestinal mucosa may be required to provide optimal protection (8, 24, 42), and our failure to identify significant increases in fecal immunoglobulin after intranasal immunization possibly reflects compartmentalization of secretory IgA responses. Our ability to achieve some degree of protection in these studies may reflect small amounts of antibody that leak from the lamina propria to the mucosal surface (8, 43). Administration of live, attenuated, orally delivered vaccines that express multiple relevant antigens will likely afford optimal protection against these important enteric pathogens. However, future ETEC vaccine strategies (43) will need to take into account the precedent set by highly effective parenterally administered vaccines for other enteric pathogens such as the Vi-EPA conjugate vaccine for Salmonella enterica serovar Typhi (33).
It is important to note that not all ETEC strains express EtpA. Our previous studies do suggest that EtpA may be among the more highly conserved antigens described to date, being present in ETEC strains of multiple CF groups from diverse geographic origins (21). Further studies will be required to define the true prevalence of strains expressing this molecule. However, these preliminary experiments point to the promise of novel virulence molecules that might be incorporated into a multivalent broadly protective vaccine.
In addition, while EtpA appears to contribute to virulence in the murine model, it is not clear how it might contribute to the virulence of H10407 and other EtpA-expressing strains in humans. We are intrigued by the observation that H10407 (CFAI LT+ ST+), originally isolated from a Bangladeshi child with severe cholera-like diarrheal illness (18), appears to be significantly more virulent than strain B7A (CS6 LT+ ST+), which lacks etpA and other candidate virulence loci (19, 20, 34). In human clinical challenge studies of these two organisms, the volume of diarrhea in volunteers that ingested H10407 was nearly twofold that of those who were infected with the same dose of B7A (11). Additional studies that define virulence traits associated with organisms capable of causing more severe illness would be of potential importance in the development of vaccines for deployment to developing countries, where deaths occur after rapid dehydration from voluminous ETEC-induced diarrhea.
Together, the data reported here suggest that the murine intestinal colonization model using human ETEC strains (1) can serve as a platform for examining the contribution of novel candidate virulence molecules such as EtpA to the pathogenesis of these organisms. More importantly, this model may provide a relatively high-throughput, inexpensive avenue for preclinical testing of the viability of newly defined ETEC antigens in vaccine development.
Acknowledgments
We thank James B. Dale for advice regarding the design of immunization experiments, for reagents, and for his thoughtful review of the manuscript.
This study was supported by Merit Review funding from the Department of Veterans affairs (J.M.F.) and by grant RR-16190-05 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the VA, the NCRR, or the NIH.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Allen, K. P., M. M. Randolph, and J. M. Fleckenstein. 2006. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 74869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty, M. E., P. M. Adcock, S. W. Smith, K. Quinlan, L. A. Kamimoto, S. Y. Rowe, K. Scott, C. Conover, T. Varchmin, C. A. Bopp, K. D. Greene, B. Bibb, L. Slutsker, and E. D. Mintz. 2006. Epidemic diarrhea due to enterotoxigenic Escherichia coli. Clin. Infect. Dis. 42329-334. [DOI] [PubMed] [Google Scholar]
- 3.Blomfield, I. C., M. S. McClain, and B. I. Eisenstein. 1991. Type 1 fimbriae mutants of Escherichia coli K-12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 51439-1445. [DOI] [PubMed] [Google Scholar]
- 4.Blomfield, I. C., V. Vaughn, R. F. Rest, and B. I. Eisenstein. 1991. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol. Microbiol. 51447-1457. [DOI] [PubMed] [Google Scholar]
- 5.Boedeker, E. C. 2005. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 2115-19. [PubMed] [Google Scholar]
- 6.Bouckenooghe, A. R., Z. D. Jiang, F. J. De La Cabada, C. D. Ericsson, and H. L. DuPont. 2002. Enterotoxigenic Escherichia coli as cause of diarrhea among Mexican adults and US travelers in Mexico. J. Travel Med. 9137-140. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois, A. L., C. H. Gardiner, S. A. Thornton, R. A. Batchelor, D. H. Burr, J. Escamilla, P. Echeverria, N. R. Blacklow, J. E. Herrmann, and K. C. Hyams. 1993. Etiology of acute diarrhea among United States military personnel deployed to South America and west Africa. Am. J. Trop. Med. Hyg. 48243-248. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 255467-5484. [DOI] [PubMed] [Google Scholar]
- 9.Buscher, A. Z., S. Grass, J. Heuser, R. Roth, and J. W. St. Geme. 2006. Surface anchoring of a bacterial adhesin secreted by the two-partner secretion pathway. Mol. Microbiol. 61470-483. [DOI] [PubMed] [Google Scholar]
- 10.Chabot, S., A. Brewer, G. Lowell, M. Plante, S. Cyr, D. S. Burt, and B. J. Ward. 2005. A novel intranasal Protollin-based measles vaccine induces mucosal and systemic neutralizing antibody responses and cell-mediated immunity in mice. Vaccine 231374-1383. [DOI] [PubMed] [Google Scholar]
- 11.Coster, T. S., M. K. Wolf, E. R. Hall, F. J. Cassels, D. N. Taylor, C. T. Liu, F. C. Trespalacios, A. DeLorimier, D. R. Angleberger, and C. E. McQueen. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect. Immun. 75252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cravioto, A., R. E. Reyes, F. Trujillo, F. Uribe, A. Navarro, J. M. De La Roca, J. M. Hernandez, G. Perez, and V. Vazquez. 1990. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am. J. Epidemiol. 131886-904. [DOI] [PubMed] [Google Scholar]
- 13.Daniels, N. A. 2006. Enterotoxigenic Escherichia coli: traveler's diarrhea comes home. Clin. Infect. Dis. 42335-336. [DOI] [PubMed] [Google Scholar]
- 14.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorsey, F. C., J. F. Fischer, and J. M. Fleckenstein. 2006. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell. Microbiol. 81516-1527. [DOI] [PubMed] [Google Scholar]
- 16.Evans, D. G., D. Y. Graham, and D. J. Evans, Jr. 1984. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers: response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 87934-940. [PubMed] [Google Scholar]
- 17.Evans, D. G., R. P. Silver, D. J. Evans, Jr., D. G. Chase, and S. L. Gorbach. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12656-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, D. J., Jr., and D. G. Evans. 1973. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect. Immun. 8322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckenstein, J. M., D. J. Kopecko, R. L. Warren, and E. A. Elsinghorst. 1996. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 642256-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleckenstein, J. M., L. E. Lindler, E. A. Elsinghorst, and J. B. Dale. 2000. Identification of a gene within a pathogenicity island of enterotoxigenic Escherichia coli H10407 required for maximal secretion of the heat-labile enterotoxin. Infect. Immun. 682766-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleckenstein, J. M., K. Roy, J. F. Fischer, and M. Burkitt. 2006. Identification of a two-partner secretion locus of enterotoxigenic Escherichia coli. Infect. Immun. 742245-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman, D. J., C. O. Tacket, A. Delehanty, D. R. Maneval, J. Nataro, and J. H. Crabb. 1998. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. J. Infect. Dis. 177662-667. [DOI] [PubMed] [Google Scholar]
- 23.Gustafsson, L., H. O. Hallander, P. Olin, E. Reizenstein, and J. Storsaeter. 1996. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N. Engl. J. Med. 334349-355. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11S45-S53. [DOI] [PubMed] [Google Scholar]
- 25.Hyams, K. C., A. L. Bourgeois, B. R. Merrell, P. Rozmajzl, J. Escamilla, S. A. Thornton, G. M. Wasserman, A. Burke, P. Echeverria, K. Y. Green, et al. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 3251423-1428. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Laboratory Animal Resources. 1996. Guide for the care and use of laboratory animals, 7th ed. National Academy Press, Washington, DC.
- 27.Jiang, Z. D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffen, N. Tornieporth, F. von Sonnenburg, P. Waiyaki, and H. L. DuPont. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185497-502. [DOI] [PubMed] [Google Scholar]
- 28.Jones, T., S. Cyr, F. Allard, N. Bellerose, G. H. Lowell, and D. S. Burt. 2004. Protollin: a novel adjuvant for intranasal vaccines. Vaccine 223691-3697. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, A., K. T. Mountzouros, D. A. Relman, S. Falkow, and J. L. Cowell. 1990. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect. Immun. 587-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirn, T. J., and R. K. Taylor. 2005. TcpF is a soluble colonization factor and protective antigen secreted by El Tor and classical O1 and O139 Vibrio cholerae serogroups. Infect. Immun. 734461-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, L. H., E. Burg III, S. Baqar, A. L. Bourgeois, D. H. Burr, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect. Immun. 675799-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine, M. M., D. R. Nalin, D. L. Hoover, E. J. Bergquist, R. B. Hornick, and C. R. Young. 1979. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 23729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, F. Y., V. A. Ho, H. B. Khiem, D. D. Trach, P. V. Bay, T. C. Thanh, Z. Kossaczka, D. A. Bryla, J. Shiloach, J. B. Robbins, R. Schneerson, and S. C. Szu. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 3441263-1269. [DOI] [PubMed] [Google Scholar]
- 34.Patel, S. K., J. Dotson, K. P. Allen, and J. M. Fleckenstein. 2004. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 721786-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peruski, L. F., Jr., B. A. Kay, R. A. El-Yazeed, S. H. El-Etr, A. Cravioto, T. F. Wierzba, M. Rao, N. El-Ghorab, H. Shaheen, S. B. Khalil, K. Kamal, M. O. Wasfy, A.-M. Svennerholm, J. D. Clemens, and S. J. Savarino. 1999. Phenotypic diversity of enterotoxigenic Escherichia coli strains from a community-based study of pediatric diarrhea in periurban Egypt. J. Clin. Microbiol. 372974-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 862637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sack, R. B. 1990. Traveler's diarrhea: microbiologic bases for prevention and treatment. Rev. Infect. Dis. 12S59-S63. [DOI] [PubMed] [Google Scholar]
- 39.Satterwhite, T. K., D. G. Evans, H. L. DuPont, and D. J. Evans, Jr. 1978. Role of Escherichia coli colonization factor antigen in acute diarrhoea. Lancet ii181-184. [DOI] [PubMed] [Google Scholar]
- 40.Steinsland, H., P. Valentiner-Branth, H. K. Gjessing, P. Aaby, K. Molbak, and H. Sommerfelt. 2003. Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet 362286-291. [DOI] [PubMed] [Google Scholar]
- 41.Tacket, C. O., G. Losonsky, H. Link, Y. Hoang, P. Guesry, H. Hilpert, and M. M. Levine. 1988. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N. Engl. J. Med. 3181240-1243. [DOI] [PubMed] [Google Scholar]
- 42.Walker, R. I., D. Steele, and T. Aguado. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 252545-2566. [DOI] [PubMed] [Google Scholar]
- 43.WHO. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec. 8197-104. [PubMed] [Google Scholar]
- 44.WHO. 1999. New frontiers in the development of vaccines against enterotoxinogenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections: part I. Wkly. Epidemiol. Rec. 7498-101. [PubMed] [Google Scholar]
- 45.Zafriri, D., Y. Oron, B. I. Eisenstein, and I. Ofek. 1987. Growth advantage and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by the cells. J. Clin. Investig. 791210-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]