Abstract
The P8 proteoglycolipid complex (P8 PGLC) is a glyconjugate expressed by Leishmania mexicana complex parasites. We previously have shown that vaccination with P8 PGLC provides protection against cutaneous leishmaniasis in susceptible BALB/c mice. However, the biological importance of this complex remains unknown. Here we show that P8 PGLC localizes to the surface of Leishmania pifanoi amastigotes and that upon exposure to macrophages, P8 PGLC binds and induces inflammatory cytokine and chemokine mRNAs such as tumor necrosis factor alpha and RANTES early after stimulation. Our studies indicate that cytokine and chemokine induction is dependent upon Toll-like receptor 4 (TLR4). Interestingly, key inflammatory cytokines and chemokines (such as interleukin-6 [IL-6], macrophage inflammatory protein 1β, and beta interferon [IFN-β]) that can be induced through TLR4 activation were not induced or only slightly upregulated by P8 PGLC. Activation by P8 PGLC does not occur in the presence of TLR4 alone and requires both CD14 and myeloid differentiation protein 2 for signaling; this requirement may be responsible for the limited TLR4 response. This is the first characterization of a TLR4 ligand for Leishmania. In vitro experiments indicate that L. pifanoi amastigotes induce lower levels of cytokines in macrophages in the absence of TLR4; however, notably higher IL-10/IFN-γ ratios were found for TLR4-deficient mice than for BALB/c mice. Further, increased levels of parasites persist in BALB/c mice deficient in TLR4. Taken together, these results suggest that TLR4 recognition of Leishmania pifanoi amastigotes is important for the control of infection and that this is mediated, in part, through the P8 PGLC.
Cutaneous leishmaniasis is a dermatropic disease with more than 1.5 million new cases each year (33). Several species of Leishmania which are responsible for this disease have been identified in the New World, including the L. mexicana complex, which is composed of L. mexicana, L. amazonensis, and L. pifanoi (29). The disease presentations caused by members of this complex can be characterized as simple or diffuse; the former is typically a localized ulcerative sore that is often self-healing, while the latter is disseminated, nonulcerative, and characterized by a suppression of the cell-mediated immune response (20, 31, 33).
Host-pathogen interactions mediated by microbial components have been shown to determine the outcome of microbial infection. Indeed, parasite-expressed molecules have been shown to play important roles in mediating Leishmania infection. Historically, these studies have focused primarily on the contribution of promastigote-stage glyconjugates, such as lipophosphoglycan (LPG), GP63, and GP46, to disease (29, 30, 40-42, 48). However, given the fact that the amastigote form of the parasite is responsible for the disease pathology, it is important to understand more fully the contributions of antigenic components of this stage to the host-parasite interaction and in disease pathogenesis. Such studies could provide a more comprehensive understanding of cutaneous leishmaniasis and potentially be useful in the development of effective vaccines or immunotherapeutic approaches for control.
The availability of axenic amastigotes of several Leishmania species allows for detailed studies of the biological and biochemical functions of amastigote-derived antigens. One such antigen, the P8 proteoglycolipid complex (P8 PGLC), is expressed by amastigotes. P8 PGLC is composed of a cysteine and serine metalloproteinase, host-derived ApoE, and four glycolipids (9). Initial characterization of this complex suggests that it is biochemically distinct from other well-characterized amastigote and promastigote glyconjugates (9). Previous studies have shown that vaccination with P8 PGLC is able to confer significant protection against L. pifanoi and L. amazonensis in susceptible BALB/c mice and that protection requires both CD4 and CD8 T cells (8, 29, 47). Further, in vitro studies have shown that peripheral blood mononuclear cells of cutaneous leishmaniasis patients are able to proliferate and produce a Th1-like response to P8 PGLC, suggesting that this PGLC could be a potential vaccine candidate (10).
Since P8 PGLC is highly expressed by Leishmania mexicana complex species, it is plausible that it could play a role in the interaction of the parasite with its primary host cell, the macrophage. Other leishmania-derived antigenic components, such as LPG and glycoinositolphospholipids, have been shown to modulate the macrophage immune response, leading to persistence or clearance within the host (5, 12, 13, 35). Consequently, P8 PGLC could interact with immune receptors on or within the macrophage, leading to activation or downregulation of subsequent inflammatory responses.
Toll-like receptors (TLRs) play an important role in the clearance of microbial pathogens by recognition of pattern-associated molecular patterns (PAMPs) and are found on or within many cells, such as macrophages, dendritic cells, and epithelial cells. All TLRs share a common adaptor, MyD88, which is essential for effective downstream signaling and activation of inflammatory cytokines (25). Numerous studies have characterized the many bacterial and viral PAMPs that can signal through TLRs (50). However, studies have only recently begun to explore the importance of TLRs in parasite clearance. Studies of Trypanosoma, Plasmodium, and Toxoplasma species have shown that the lack of one or more TLRs can lead to decreased inflammatory responses and subsequent parasite persistence (6, 7, 11). TLRs and their associated molecules have also been shown to be important in the recognition of Leishmania species as well. Both TLR2 and TLR4 have been implicated in the recognition of L. major promastigotes; TLR2 has been shown to recognize the promastigote-associated molecule LPG (4, 14, 27). However, no studies to date have focused on understanding importance of TLRs in the interaction with Leishmania amastigotes or their derived molecules.
Here we show that P8 PGLC is expressed on the surface of L. pifanoi amastigotes and interacts with TLR4 on the macrophage surface to induce expression of a distinct subset of inflammatory cytokines and chemokines. Further, recognition of L. pifanoi amastigotes is in part TLR4 dependent, as TLR4−/− mice develop exacerbated disease following infection with L. pifanoi amastigotes. Together these results suggest the importance of TLR4 in the host-pathogen interaction of this infectious stage with its host.
MATERIALS AND METHODS
Mice.
Female C57BL/6 and BALB/c mice were purchased from NCI. C.C3-TLR4Lps-d/J mice (TLR4−/− mice on a BALB/c background) were purchased from The Jackson Laboratory. TLR4−/− and TLR2−/− mice (kindly provided by Richard Flavell and Fayyaz Sutterwala) had been backcrossed to a C57BL/6 background for nine generations. MyD88−/− mice were initially obtained from R. Medizhtov (with the permission of S. Akira); mice were backcrossed for nine generations to a C57BL/6 background. All animals were housed in the Yale University School of Medicine Association and Accreditation of Laboratory Animal Care-approved animal facility. All mice used were between 5 and 12 weeks of age.
Reagents and antibodies.
Purified recombinant mouse gamma interferon (IFN-γ) and interleukin-10 (IL-10) and biotinylated rat anti-mouse IFN-γ and IL-10 were purchased from Pharmingen. Ultrapure lipopolysaccharide (LPS) (Escherichia coli 0111:B4 strain) and Pam3CSK4 and peptidoglycan (PGN) (Staphylococcus aureus) were purchased from InvivoGen. Tumor necrosis factor alpha (TNF-α), IL-12p40 (eBioscience), and IFN-β (Biosource) enzyme-linked immunosorbent assays (ELISAs) were employed according to manufacturers' specifications.
Parasites.
L. pifanoi (MHOM/VE/60/Ltrod) and L. amazonensis (MHOM/BR/77/LTB0016) promastigotes were cultured in Schneider's Drosophila medium (supplemented with 15% fetal bovine serum) at 22 to 23°C. L. pifanoi (MHOM/VE/60/Ltrod) amastigotes were cultured at 31°C in F29 medium, as described previously (47). Parasites were passaged frequently through mice to maintain infectivity. Soluble leishmania antigen (SLA) was made from L. pifanoi and L. amazonensis metacyclic promastigotes that underwent repeated freeze-thaw cycles. For infections, 2 × 106 L. pifanoi amastigotes were injected subcutaneously into the right hind feet of mice. At various time points, the mice were sacrificed and parasite burdens were determined via limiting-dilution assay (47).
P8 PGLC purification and glycolipid extraction.
Membrane preparations of washed L. pifanoi amastigotes were isolated using nitrogen cavitation and differential centrifugation. Membranes were solublized in 1% decanoyl-N-methylglucamide (Mega-10; Sigma-Aldrich), reduced, alkylated, and then fractionated by Sephadex G25 (GE-HealthCare Biosciences AB) gel exclusion chromatography, as previously described (9). The solubilized membranes were then fractionated using anti-P8 immunoaffinity chromatography. The fractions were assessed for protein by measuring the absorbance at 260 and 280 nm. Protein fractions were then pooled, concentrated, and stored at −80°C. Samples were then assayed for endotoxin activity via the Limulus amebocyte lysate assay (Cambrex). All P8 PGLC concentrations (1 to 10 μg/ml) used in the experiments tested below the endotoxin detection levels (≤0.01 endotoxin unit). Glycolipid extraction was done as previously described (9).
Cells.
293 HEK cells were obtained from the American Type Culture Collection (Manassas, VA). Bone marrow-derived macrophages (BMMs) were harvested from the femurs and tibiae and generated in vitro by culturing in L-cell conditioned medium (RPMI 1640 supplemented with 30% L929 cell supernatant, 20% heat-inactivated fetal bovine serum [HIFBS], and 1% penicillin/streptomycin) for 4 days. On the fourth day, cells were fed again with L-cell conditioned medium; on day 6, BMMs were harvested, counted, and used for the experiments described. Elicited peritoneal macrophages were harvested from the peritoneal cavity at 6 days after injection of 4% thioglycolate broth (Difco Laboratories). Resident peritoneal macrophages were obtained by flushing the peritoneal cavity with RPMI 10.
Indirect immunofluorescence microscopy.
L. pifanoi amastigotes were applied in a thin layer at a concentration of 108 cells per ml to spot slides (Cel-line Associates) and rapidly air dried at room temperature. Slides were blocked for 1 h with phosphate-buffered saline (PBS) plus 1% bovine serum albumin (Sigma). Slides were subsequently incubated for 1 hour with P8 PGLC monoclonal antibody-containing hybridoma supernatant and then washed with PBS-1% bovine serum albumin. L. pifanoi amastigotes were incubated with supernatant from a nonsecreting hybridoma cell line as a negative control. The slides then were incubated for 1 h with rhodamine-anti-mouse immunoglobulin G (Jackson Immunoresearch) and examined with a Leitz Orthoplan 2 fluorescence microscope.
Binding assay of immunopurified P8 PGLC.
All binding assays were performed at 4°C. Initially, to visualize the interaction between P8 PGLC and macrophages, an immunofluorescence binding assay was developed. Peritoneal macrophages (adhered to coverslips) were incubated with P8 PGLC for 2 h; cells were subsequently fixed with 1% paraformaldehyde and washed with PBS containing 5% HIFBS. The presence of bound P8 PGLC was detected by incubating the coverslips at room temperature first with a rabbit polyclonal antibody against whole P8 PGLC for 45 min. The P8 PGLC was then visualized by subsequently adding an anti-rabbit rhodamine-labeled antibody. To further assess the nature of the binding of P8 PGLC to macrophages (saturation and qualitative features), an ELISA was developed. For this assay, peritoneal macrophages (105/well) were cultured (37°C) in 96-well microtiter plates overnight. The macrophages were then placed at 4°C and incubated with various concentrations of P8 PGLC for 1 h. After 1 h, the reaction was developed by using a polyclonal rabbit anti-P8 PGLC antibody followed by an anti-rabbit horseradish peroxidase-conjugated antibody and then incubating with the TMB substrate (ImmunoPure TMB [3,3′,5,5′-tetramethylbenzidine] substrate kit; Pierce). After the reaction was stopped, the absorbance at 405 nm was measured using an ELISA plate reader. The specificity of binding of P8 PGLC to macrophages was determined by examining the binding of the anti-P8 PGLC rabbit antibody in the absence of the isolated amastigote P8 PGLC.
Cytokine analysis.
In vitro analysis of cytokines was performed using draining lymph node cells prepared at 1 week postinfection in RPMI 10. Cells were plated at 2.5 × 106 cells/ml and stimulated with concanavalin A or soluble leishmanial antigen (50 μg or 25 μg), cultured with L. pifanoi axenic amastigotes (10:1 or 8:1 ratio), or left untreated. After 72 h, supernatants were harvested. IL-10 and IFN-γ ELISAs were performed using antibodies from BD Pharmingen. In vitro analyses of cytokine responses of peritoneal macrophages or BMMs treated with either P8 PGLC, PGN, LPS, ΙFN-γ, or L. pifanoi axenic amastigotes were performed on cultural supernatants after 48 h of stimulation. IFN-β (Biosource), TNF-α, and IL-12p40 ELISAs were performed according to the manufacturer's recommendations (eBioscience). The IFN-β ELISA sensitivity ranged from 15.6 pg/ml to 1,000 pg/ml; TNF-α and IL-12p40 ELISA sensitivities ranged from 8 pg/ml to 1,000pg/ml.
RNase protection assay and real-time PCR.
Resident peritoneal macrophages were plated into 24-well plates. The macrophages were stimulated for the indicated times with whole P8 PGLC (10 μg protein/well) or extracted P8 PGLC glycolipids (isolated from a comparable amount of protein). RNA was then extracted with Trizol (Invitrogen) and used to perform RNase protection assays with mouse-specific probes (RiboQuant; BD-Bioscience). For real-time PCR analyses, BMMs were stimulated for various times with P8 PGLC (5 μg/ml), LPS(1 μg/ml), or PGN (l0 μg/ml) or left unstimulated. RNA was then extracted with Trizol, and cDNA was synthesized. In other cases, RNA was extracted from L. pifanoi amastigote-infected wild-type (WT) and TLR4−/− (C.C3TLR4Lpsd/j) mouse feet. QuantiTect Primer assays for inducible nitric oxide synthase, IFN-β, arginase, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were used in conjunction with QuantiTect Sybr green kits (Qiagen). Real Time PCR was carried out on a Bio-Rad light cycler. Data were then analyzed using the comparative ΔΔ threshold cycle method.
Luciferase assay.
Mouse plasmids encoding TLR4, CD14, and myeloid differentiation protein 2 (MD-2) as well as plasmids encoding pIIXB (firefly) and Renilla luciferase were kind gifts from the laboratories of R. Medzhitov and R. Baron (Yale University School of Medicine). HEK cells maintained in Dulbecco's medium supplemented with 10% HIFBS were transiently transfected with various combinations of plasmids using FuGENE 6 (Roche); total DNA was maintained with pcDNA3 (Invitrogen). The cells were allowed to rest for 24 h and then stimulated with ultrapure LPS from an E. coli 0111:B4 strain (TLR4 ligand), P8 PGLC, or Pam3CSK4 (TLR2 ligand). After 24 h, the HEK cells were lysed and were analyzed using the dual luciferase assay reporter system (Promega) according to the manufacturer's instructions.
Statistical analysis.
Data were analyzed by using Student's t test to compare two groups. The t tests were performed with the statistical software GraphPad Prism 5 (Graph Pad Software, San Diego, CA). P values of ≤0.05 were considered significant.
RESULTS
P8 PGLC induces inflammatory cytokine expression in macrophages.
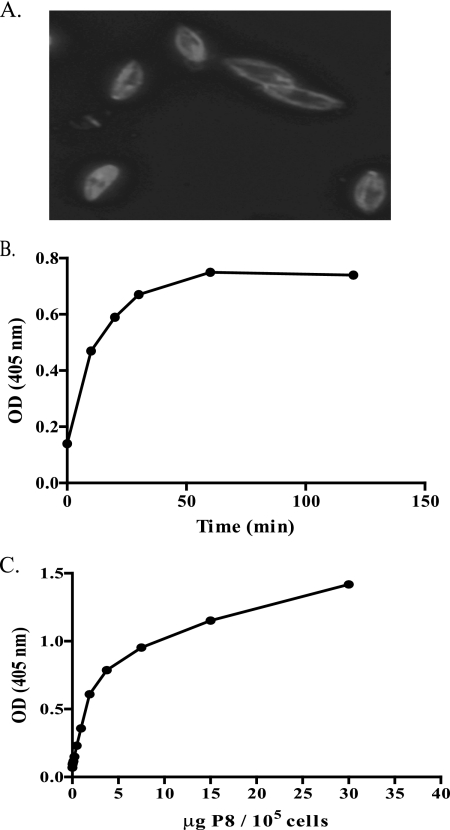
We have previously shown that vaccination with P8 PGLC has the ability to provide genetically unrestricted protection to otherwise susceptible mice (9, 47). Furthermore, since P8 is highly expressed by L. pifanoi amastigotes, it was interest to determine the biological function of P8 PGLC. Indirect immunofluorescence studies were first undertaken to determine where P8 PGLC localized on L. pifanoi amastigotes. These analyses as well as earlier studies (reference 16 and unpublished results) revealed that P8 PGLC localized to the surface and flagellar pocket of L. pifanoi amastigotes (Fig. 1A). This finding was consistent with the vaccine antigenic potential of P8 PGLC (9, 47) and suggested that P8 PGLC might directly interact with its host cell, the macrophage.
FIG. 1.
P8 PGLC is on the surface of L. pifanoi amastigotes and binds to murine macrophages in a concentration- and time-dependent manner. (A) Indirect immunofluorescence of P8 PGLC staining on L. pifanoi amastigotes (see Materials and Methods). (B and C) Resident peritoneal macrophages were plated at 105 cells/well and incubated with 1 μg/ml P8 PGLC over a period of time (B) or with increasing concentrations of P8 PGLC at 4°C for 1 h (C). The level of binding was determined as indicated in Materials and Methods. Data are representative of three independent experiments. OD, optical density.
Consequently, we sought to determine whether the P8 PGLC bound to macrophages. Initially, we observed using indirect immunofluorescence with a polyclonal anti-P8 PGLC rabbit antibody that the P8 PGLC appeared to bind to the surface of BALB/c peritoneal macrophages in what appeared to be a clustered organization (data not shown). To further investigate the P8 PGLC-macrophage interaction, an ELISA was developed using BALB/c peritoneal macrophages plated overnight in 96-well plates and then incubated at 4°C with immunopurified P8 PGLC. An examination of the kinetics of the interaction of P8 PGLC with its receptor in the macrophage membrane showed a high initial rate and saturation after 1 hour (Fig. 1B). The binding of P8 PGLC to resident peritoneal macrophages is dose dependent and appeared to reach saturation at 5 μg/ml of P8 PGLC per 105 macrophages (Fig. 1C). These results are consistent with a receptor-ligand interaction between the P8 PGLC and the macrophage.
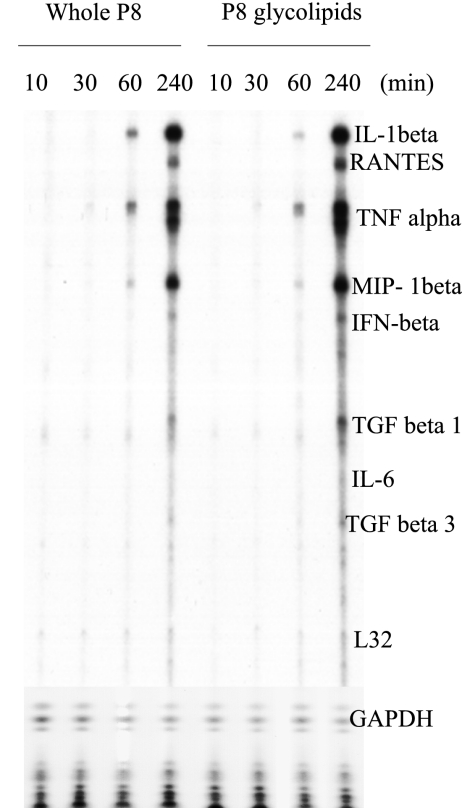
Given that P8 PGLC bound murine macrophages through a receptor-ligand-mediated process, we then examined whether this interaction resulted in specific cytokine responses (inflammatory/anti-inflammatory). The expression of a range of cytokine mRNAs in P8 PGLC-stimulated peritoneal macrophages was examined using an RNase protection assay. These experiments revealed that stimulation with either P8 PGLC alone or its isolated glycolipids led to the induction of inflammatory cytokine mRNAs such as those of IL-1β and TNF-α as early as 60 min poststimulation (Fig. 2). There was a further upregulation of inflammatory cytokines and chemokines at 4 h poststimulation (Fig. 2); additionally, an upregulation of RANTES, and macrophage inflammatory protein 1β was evident after 4 h of stimulation. Notably, IFN-β mRNA was not upregulated significantly. This was confirmed via real-time PCR; further, ELISA (data not shown; sensitivity, 15.6 pg/ml) analyses indicated that IFN-β protein was not produced in response to P8 PGLC stimulation.
FIG. 2.
P8 PGLC induces the induction of inflammatory cytokine mRNA. Resident peritoneal macrophages were stimulated for the indicated times with P8 PGLC (5 μg/ml) PGLC and total RNA extracted. RNA was then used to perform RNase protection assays as described in Materials and Methods. Data are representative of three independent experiments with similar results. TGF, transforming growth factor.
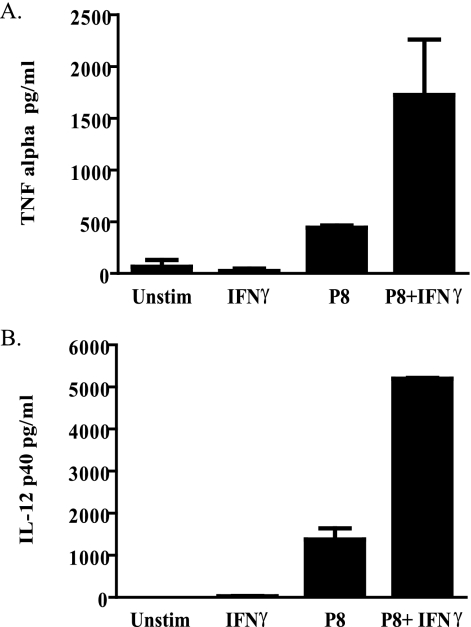
We then confirmed that P8 PGLC could induce inflammatory cytokine protein as well. Levels of TNF-α and IL-12p40 were evaluated, as these are known to have an impact on the ongoing host immune response to leishmanial infection (18, 49). Macrophages were stimulated with IFN-γ, P8 PGLC alone, or P8 PGLC plus IFN-γ or left untreated (Fig. 3). P8 PGLC alone was able to induce TNF-α and IL-12p40 cytokine production, and this response was further augmented in the presence of IFN-γ. These results clearly indicate that P8 PGLC in the presence or absence of IFN-γ is able to induce inflammatory cytokine responses.
FIG. 3.
Macrophages stimulated with P8 PGLC produce TNF-α and IL-12p40. Thioglycolate-elicited C57BL/6 macrophages were stimulated with either P8 PGLC (1 μg/ml), IFN-γ (100 ng), or a combination of both, or left unstimulated, for 48 h. TNF-α (A) and IL-12p40 (B) levels in the supernatant were then measured. ELISAs were assayed in duplicate, and data are representative of three independent experiments with similar results and are expressed as the mean and standard error.
P8 PGLC induces inflammatory cytokines through TLR4.
The ability of P8 PGLC to bind to macrophages in a receptor-ligand-specific fashion and to induce inflammatory cytokines early after stimulation suggested a role of innate immune receptors. Innate receptors such as TLRs have recently been shown to be important in the establishment of parasite infection. Recent studies have shown that Leishmania and Trypanosoma cruzi, a related kinetoplastid parasite, can be recognized by several TLRs (6, 17, 27, 34). Both TLR2 and TLR4 have been previously shown to be involved in the recognition of PAMPs from Leishmania as well as other protozoan parasite molecules (4, 14, 26, 34). Given the biochemical composition of the P8 PGLC (9), initial studies were carried out to elucidate the importance of TLRs in the recognition of P8 PGLC, focusing on TLR2 and TLR4.
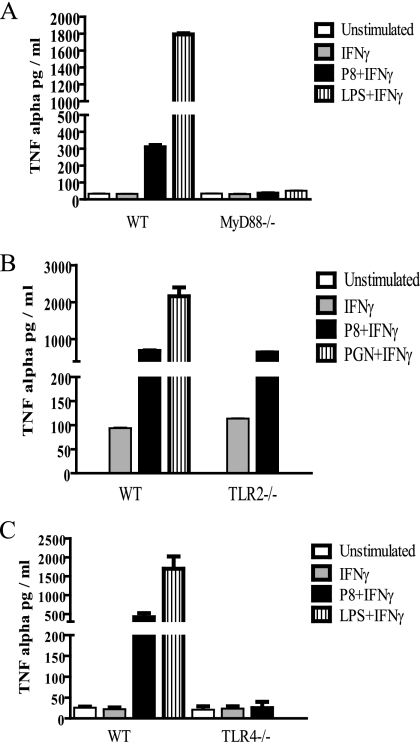
To evaluate whether TLRs were indeed important for P8 PGLC recognition, we first conducted in vitro studies using WT and MyD88−/− (C57BL/6) peritoneal macrophages exposed to IFN-γ alone or a combination of IFN-γ and P8 PGLC. Although treatment of WT macrophages with P8 PGLC and IFN-γ induced TNF-α production, TNF-α production was not induced in MyD88−/− macrophages (Fig. 4A). These data indicate a role for MyD88-dependent signaling in the inflammatory P8 PGLC response and suggest that a TLR could be involved in P8 PGLC recognition.
FIG. 4.
P8 PGLC induces the induction of inflammatory cytokines through TLR. MyD88−/− (A), TLR2−/− (B), TLR4−/− (C) and WT (A, B, and C) (all C57BL/6 background) thioglycolate elicited-macrophages were stimulated with either P8 PGLC (1 μg/ml), LPS (1 μg/ml), PGN (5 μg/ml) in combination with IFN-γ, or IFN-γ alone or left unstimulated. After 48 h, supernatants were harvested and subsequently used to determine TNF-α levels. ELISAs were performed in duplicate, and data are representative of two independent experiments with similar results and are expressed as the mean and standard error.
We then examined whether either TLR2 or TLR4 was important in the MyD88-dependent response that we observed. As shown in Fig. 4B, TLR2−/− (C57BL/6) macrophages produced TNF-α at levels similar to those in WT C57BL/6 mice, suggesting that TLR2 is not important in the recognition of P8 PGLC. In contrast, TLR4−/− (C57BL/6) macrophages did not produce TNF-α when stimulated with P8 PGLC compared to their WT (C57BL/6) counterparts (Fig. 4C). The response of TLR4−/− macrophages was comparable to that of MyD88−/− cells (Fig. 4A). Further, the macrophage responses observed were clearly due to P8 PGLC, as LPS contamination was undetectable when examined using the Limulus amebocyte lysate assay (data not shown). These results suggest that TLR4 is the receptor that is important in the recognition of P8 PGLC.
P8 PGLC requires both CD14 and MD-2 for signaling through TLR4.
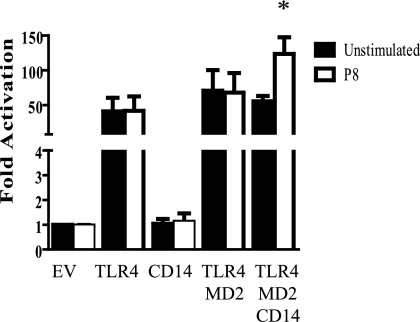
Both CD14 and MD-2 have the ability to associate with TLR4 on the macrophage and can play an important role in the specificity and intensity of TLR4 signaling and the consequent cytokines and chemokines produced (1, 19, 22, 53). For this reason, studies were undertaken to understand what role MD-2 and CD14 may play in the interaction of P8 PGLC with TLR4. HEK293 cells were transfected with various combinations of TLR4, CD14, and/or MD-2 and then stimulated with P8 PGLC. Luciferase assays revealed that cells transfected with TLR4, CD14, MD2, or TLR4-CD14 or TLR4-MD2 combinations could not induce NF-κB activation following P8 PGLC stimulation (Fig. 5 and data not shown). However, when cells were transfected with TLR4, MD2, and CD14 and stimulated with P8 PGLC, there was a significant increase (P ≤ 0.05) in NF-κB activation (Fig. 5). This is unlike the case for LPS, which has the ability to induce NF-κB expression with TLR4-MD2 alone (46). This suggests that both CD14 and MD-2 must be present in order for TLR4 to induce signal transduction. These results further confirmed the importance of TLR4 in the recognition of P8 PGLC.
FIG. 5.
P8 PGLC requires both CD14 and MD-2 for signaling through TLR4. HEK293 cells were transiently transfected with combinations of TLR4, CD14, and MD-2 as described in Materials and Methods. The HEK293 cells were subsequently stimulated with P8 PGLC (1 μg) for 24 h and then assayed for luciferase activity. Data presented are the averages from three independently conducted experiments and are expressed as the mean and standard error. *, P ≤ 0.05 compared to unstimulated TLR4/MD2-CD14. EV, empty vector (pcDNA3).
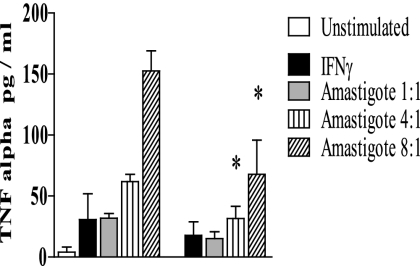
TLR4 is important for the recognition of L. pifanoi amastigotes in vitro.
To understand whether TLR4 was important for the recognition of P8 PGLC-expressing Leishmania amastigotes, IFN-γ-primed BMMs were infected with L. pifanoi amastigotes and the TNF-α response examined. WT (BALB/c) and TLR4−/− (C.C3-TLR4Lps-d/J) BMMs were primed with IFN-γ for 12 h and then subsequently incubated with various ratios of L. pifanoi amastigotes for 48 h; culture supernatants were then assayed for TNF-α. In both strains of mice, TNF-α production was observed; however, the TNF-α production in the infected TLR4−/− BMMs was significantly lower than that in their WT counterparts (P ≤ 0.05) (Fig. 6). These results indicate that TLR4−/− is important in the recognition of L. pifanoi amastigotes, consistent with the interaction of P8 PGLC and TLR4 during the parasite's uptake and entry into the macrophage.
FIG. 6.
TLR4 is important for the recognition of L. pifanoi amastigotes in vitro. WT and TLR4−/− (C.C3-TLR4Lps-d/J) BMMs were primed with IFN-γ (100 ng/ml) for 12 h and then stimulated with L. pifanoi axenic amastigotes at different parasite/macrophage ratios. After 48 h, the supernatants were assessed for TNF-α production via ELISA. The experimental data presented represent the averages from four independently conducted experiments and are expressed as the mean and standard error. *, P ≤ 0.05 compared with WT L. pifanoi-infected macrophages at the same amastigote/macrophage ratio.
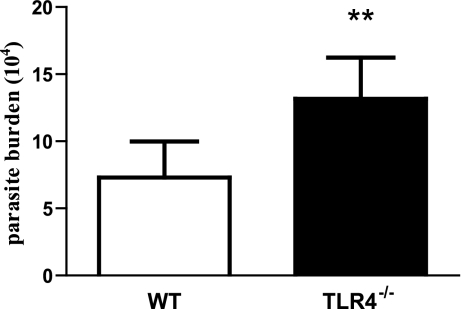
TLR4 is important for the clearance of L. pifanoi amastigotes in vivo.
Since differences were observed in the cytokine responses of WT and TLR4−/− BMMs after infection with L. pifanoi amastigotes, the actual importance of TLR4 in vivo was investigated. For these experiments BALB/c mice were employed, which are known to be highly susceptible to Leishmania. Both TLR4−/− (C.C3-TLR4Lps-d/J) and WT BALB/c mice were infected with L. pifanoi amastigotes; 1 week later, parasite levels were determined. The results presented in Fig. 7 show that 1 week after infection, there were significantly higher numbers of parasites present in the footpad lesions of TLR4−/− mice than in those of their WT counterparts (P = 0.01). These results suggest that TLR4 is important for the recognition and clearance of L. pifanoi amastigotes and that lack of TLR4 may cause a defect in the immune response to the parasite.
FIG. 7.
TLR4 is important for L. pifanoi amastigote clearance in vivo. WT (BALB/c) and TLR4−/− (C.C3-TLR4Lps-d/J) mice were infected with 2 × 106 L. pifanoi amastigotes in the right hind foot. At 1 week postinfection, parasite burden analysis was carried out. These data are average from two experiments (n = 10) and are expressed as the mean and standard error. The difference in parasite loads is statistically significant (**, P ≤ 0.01).
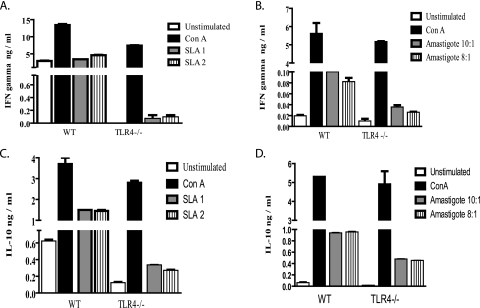
In order to determine if there was a defective immune response in the infected TLR4−/− mice, the levels of the cytokines IFN-γ and IL-10 were examined, as these cytokines are known to induce or dampen, respectively, macrophage and T-cell responses (24, 54) that are critical for parasite control. At 1 week postinfection, cells from the draining lymph nodes of WT and TLR4−/− infected mice were stimulated with SLA or L. pifanoi amastigotes at a 10:1 or 8:1 ratio, and then the levels of IFN-γ and IL-10 were determined. Interestingly, TLR4−/− mice consistently produced lower levels of both IFN-γ and IL-10 than WT mice (Fig. 8). When stimulated with SLA, the overall IL-10/IFN-γ ratios ranged from 2.6 to 3.5 in the TLR4−/− mice and from 0.44 to 0.32 in the WT mice. When stimulated with L. pifanoi amastigotes, the overall IL-10/IFN-γ ratios ranged from 13.8 to 17.9 in the TLR4−/− mice and 9.4 to 11.8 in the WT mice. Regardless of the stimulus, whether promastigote or amastigote derived, the TLR4−/− mice consistently produced higher levels of IL-10 than of IFN-γ. Indeed, given the role of IL-10 in disease exacerbation of L. mexicana complex parasites (24, 54), the higher levels of IL-10 in the TLR4−/− mice may indicate a mechanism contributing to disease exacerbation in the absence of TLR4. These results suggest that the higher IL-10/IFN-γ cytokine ratios may be important in the ineffective control of infection with L. pifanoi amastigotes in the TLR4−/− mice at 1 week postinfection.
FIG. 8.
In vitro cytokine analysis of L. pifanoi-infected WT and TLR4−/− mice. Infected draining lymph nodes from WT and TLR4−/− mice (n = 5, 1 week postinfection) were stimulated with SLA1 (50 μg/ml), SLA2 (25 μg/ml), L. pifanoi axenic amastigotes (10:1 or 8:1), or concanavalin A (1 μg/ml), or left unstimulated, for 72 h in vitro. The supernatants were then assayed for IFN-γ (A and B) and IL-10 (C and D) levels. The results presented are representative of three independent experiments with similar results and are expressed as the mean an standard error.
DISCUSSION
Macrophages are crucial to the innate immune responses to pathogens. Further, the ability of macrophages to recognize and respond to Leishmania is critical to the outcome of the disease. Several antigenic components of Leishmania, such as phosphoglycans, glycoinositolphospholipids, and LPG, have been shown to have immunomodulatory effects on the macrophage (13, 38, 39). Therefore, it would be in the best interest of the parasite to manipulate macrophage responses through stimulation or inhibition of different macrophage functions. In our studies, macrophage exposure to P8 PGLC led to the upregulation of a selective subset of inflammatory cytokines and chemokines, including TNF-α, IL-1β, RANTES, and macrophage inflammatory protein 1β. Interestingly, the amplifying cytokine IFN-β was only slightly induced. Recently, it has been shown that IFN-β induces the upregulation of inducible nitric oxide synthase, which subsequently leads to Leishmania clearance (15). In accordance with this study, it has been shown that IFN-β−/− mice are more susceptible to Leishmania (28). Hence, minimal IFN-β induction could be potentially beneficial to the parasite.
Early induction of inflammatory mRNA as a consequence of the macrophage exposure indicated the importance of the innate immune response. In an attempt to define the receptor(s) involved in P8 PGLC induction of cytokines in macrophages, we focused on TLRs. Our data clearly provide evidence that TLR4 is important in the recognition of the amastigote-derived P8 PGLC and requires CD14 and MD-2 for NF-κB signaling. Interestingly, mounting evidence has shown that the requirements for optimal TLR4 signaling are variable and dependent on numerous factors, including the presence of coreceptors (37, 51, 52). For example, recent studies demonstrated that the TLR4/MD-2 complex can distinguish between rough and smooth forms of LPS. Rough-form LPS signals through TLR4/MD-2 in the absence of CD14, while smooth-form LPS requires the presence of CD14 (21, 22). The difference in requirements of forms of LPS for CD14 leads to the selective induction of cytokines (21, 22). Furthermore, LPS analogs have been shown to induce different receptor clusters to the signaling site, which is thought to be structure specific (52). The data presented here demonstrate that P8 PGLC, like smooth LPS, requires the TLR4/MD-2 complex and CD14 to induce signaling. P8 PGLC is a glycolipid complex consisting of four glycolipid moieties. However, whether these four glycolipids each interact with TLR4 or whether specific structural features are critical to the requirement for TLR4/MD2 and CD14 remains to be determined. Studies have also suggested that the optimal signaling induced by TLR4 ligands can require multiple receptors and surface-associated molecules, such as CD11b/CD18 and HSP90 (37). In addition, given the biochemical complexity of P8 PGLC, it is possible that other macrophage receptors may be involved. Consequently, it is tempting to speculate that P8 PGLC interacts with these associate molecules or other receptors in coordination, leading to a modulation of the TLR4 inflammatory responses.
Unlike the case for P8 PGLC, interaction with L. pifanoi amastigotes did not lead to the full abrogation of the TNF-α response in TLR4−/− macrophages. This could be due to the fact that Leishmania spp. are complex parasites and likely have other surface and/or internal components that could serve as TLR ligands. The TLR4 response could collaborate with other TLR or PAMP receptors to induce a full response to Leishmania. This hypothesis has been supported by the observation that TLR2, TLR3, TLR4, and TLR9 can recognize various Leishmania species and potentially activate in a synergistic manner to induce potent immune responses (4, 17, 27, 45). Additional support for this hypothesis comes from studies with Trypanosoma, Toxoplasma, and Mycobacterium, which have a requirement for two TLRs to coordinate immune responses for full pathogen recognition and clearance (2, 3, 11).
In vivo studies have suggested the importance of TLR4 in the recognition of L. major promastigotes (27). Increased parasite burdens in L. major-infected TLR4−/− mice were shown to be associated with an overall increase in Th1- and Th2-like cytokine production in vitro and increased levels of arginase at the site of infection (27). In contrast, we observed an overall decrease in both Th1- and Th2-like cytokines (IFN-γ and IL-10) and no difference in the levels of arginase at the site of infection between L. pifanoi-infected WT and TLR4−/− mice (Fig. 8 and data not shown). Both IFN-γ and IL-10 are well documented to play a role in the course of Leishmania infection (36, 43). IL-10 has been shown to inhibit a multitude of cytokines such as IL-12 and TNF-α, leading to macrophage deactivation and Leishmania persistence (24, 32). On the other hand, the Th1 cytokine IFN-γ is responsible for the activation of microbicidial mechanisms for Leishmania control (44). In the L. pifanoi-infected TLR4−/− mice, we observed increased parasite burdens and higher ratios of IL-10 to IFN-γ compared to those in WT mice. It is possible that the higher relative levels of IL-10 to IFN γ could lead to macrophage deactivation and, consequently, higher parasite burdens. Further, it has been shown in L. mexicana complex infections that IL-10 deficiency leads to decreased parasite numbers at the site of infection (36). Together these data suggest a role for IL-10 in the enhanced susceptibility to infection of TLR4−/− mice; however, it is possible that other cytokines mediators may also be involved (23, 36).
The identification of specific molecules involved in the cross talk between parasites and host cells could provide novel candidate targets for vaccine and chemotherapy development. We investigated the biological role of P8 PGLC, namely, its ability to interact with macrophages and its importance in leishmanial pathogenesis. Our results indicate that P8 PGLC induces inflammatory cytokines and chemokines by signaling through TLR4. We have shown that P8 PGLC recognition by TLR4 depends on the presence of both CD14 and MD-2. Furthermore, the absence of TLR4 leads to decreased TNF-α production in response to L. pifanoi in vitro. In addition, TLR4−/− mice exhibited exacerbated parasite burdens when infected with L. pifanoi amastigotes. Collectively, our results suggest an important role for P8 PGLC signaling through TLR4 in leishmanial pathogenesis. Most notably, this is the first report identifying an amastigote-derived ligand that can induce macrophage inflammatory responses through TLR4.
Future work will focus on detailed studies of the P8 PGLC structure and the role of TLR in P8 PGLC-induced protection.
Acknowledgments
We thank Fayyaz Sutterwala, Jonathan Kagan, Glenn Rowe, and Danielle Drayton for their technical assistance and gifts of reagents, plasmids, and mice and for helpful discussions.
This work was supported by an NIH grant (AI 27811) to D.M.-P. and NIH predoctoral fellowships (T32 AI07404 and F31 GM69181) to S.M.W.
The authors have no financial conflict of interest.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 25 February 2008.
REFERENCES
- 1.Akashi, S., R. Shimazu, H. Ogata, Y. Nagai, K. Takeda, M. Kimoto, and K. Miyake. 2000. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 1643471-3475. [DOI] [PubMed] [Google Scholar]
- 2.Bafica, A., H. C. Santiago, R. Goldszmid, C. Ropert, R. T. Gazzinelli, and A. Sher. 2006. Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J. Immunol. 1773515-3519. [DOI] [PubMed] [Google Scholar]
- 3.Bafica, A., C. A. Scanga, C. G. Feng, C. Leifer, A. Cheever, and A. Sher. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 2021715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, I., N. Salaiza, M. Aguirre, J. Delgado, N. Carrillo-Carrasco, L. G. Kobeh, A. Ruiz, R. Cervantes, A. P. Torres, N. Cabrera, A. Gonzalez, C. Maldonado, and A. Isibasi. 2003. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol. Biochem. Parasitol. 13065-74. [DOI] [PubMed] [Google Scholar]
- 5.Bekker, L. G., S. Freeman, P. J. Murray, B. Ryffel, and G. Kaplan. 2001. TNF-alpha controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J. Immunol. 1666728-6734. [DOI] [PubMed] [Google Scholar]
- 6.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167416-423. [DOI] [PubMed] [Google Scholar]
- 7.Coban, C., K. J. Ishii, T. Kawai, H. Hemmi, S. Sato, S. Uematsu, M. Yamamoto, O. Takeuchi, S. Itagaki, N. Kumar, T. Horii, and S. Akira. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 20119-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colmenares, M., P. E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 713172-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colmenares, M., M. Tiemeyer, P. Kima, and D. McMahon-Pratt. 2001. Biochemical and biological characterization of the protective Leishmania pifanoi amastigote antigen P-8. Infect. Immun. 696776-6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho, S. G., M. P. Oliveira, A. M. Da-Cruz, P. M. De Luca, S. C. Mendonca, A. L. Bertho, L. Soong, and D. McMahon-Pratt. 1996. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp. Parasitol. 84144-155. [DOI] [PubMed] [Google Scholar]
- 11.Debierre-Grockiego, F., M. A. Campos, N. Azzouz, J. Schmidt, U. Bieker, M. G. Resende, D. S. Mansur, R. Weingart, R. R. Schmidt, D. T. Golenbock, R. T. Gazzinelli, and R. T. Schwarz. 2007. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 1791129-1137. [DOI] [PubMed] [Google Scholar]
- 12.Descoteaux, A., and S. J. Turco. 1993. The lipophosphoglycan of Leishmania and macrophage protein kinase C. Parasitol. Today 9468-471. [DOI] [PubMed] [Google Scholar]
- 13.Descoteaux, A., S. J. Turco, D. L. Sacks, and G. Matlashewski. 1991. Leishmania donovani lipophosphoglycan selectively inhibits signal transduction in macrophages. J. Immunol. 1462747-2753. [PubMed] [Google Scholar]
- 14.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 332822-2831. [DOI] [PubMed] [Google Scholar]
- 15.Diefenbach, A., H. Schindler, N. Donhauser, E. Lorenz, T. Laskay, J. MacMicking, M. Röllinghoff, I. Gresser, and C. Bogdan. 1998. Type 1 interferon (IFN[alpha]/[beta]) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity 877-87. [DOI] [PubMed] [Google Scholar]
- 16.Duboise, S. M. 1994. Developmentally regulated antigens of Leishmania pifanoi amastigotes: characterization, patterns of expression, and immunoprophylactic potential. Yale University, New Haven, CT.
- 17.Flandin, J. F., F. Chano, and A. Descoteaux. 2006. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur. J. Immunol. 36411-420. [DOI] [PubMed] [Google Scholar]
- 18.Fonseca, S. G., P. R. Romao, F. Figueiredo, R. H. Morais, H. C. Lima, S. H. Ferreira, and F. Q. Cunha. 2003. TNF-alpha mediates the induction of nitric oxide synthase in macrophages but not in neutrophils in experimental cutaneous leishmaniasis. Eur. J. Immunol. 332297-2306. [DOI] [PubMed] [Google Scholar]
- 19.Gangloff, M., and N. J. Gay. 2004. MD-2: the Toll ‘gatekeeper’ in endotoxin signalling. Trends Biochem. Sci. 29294-300. [DOI] [PubMed] [Google Scholar]
- 20.Handman, E. 2001. Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber, M., C. Kalis, S. Keck, Z. Jiang, P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, B. Beutler, C. Galanos, and M. A. Freudenberg. 2006. R-form LPS, the master key to the activation of TLR4/MD-2-positive cells. Eur. J. Immunol. 36701-711. [DOI] [PubMed] [Google Scholar]
- 22.Jiang, Z., P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, M. Huber, C. Kalis, S. Keck, C. Galanos, M. Freudenberg, and B. Beutler. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6565-570. [DOI] [PubMed] [Google Scholar]
- 23.Jones, D., M. Ackermann, U. Wille, C. Hunter, and P. Scott. 2002. Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-10-deficient mice does not lead to resolution of infection. Infect. Immun. 702151-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kane, M., and D. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 1661141-1147. [DOI] [PubMed] [Google Scholar]
- 25.Kawai, T., and S. Akira. 2007. TLR signaling. Semin. Immunol. 1924-32. [DOI] [PubMed] [Google Scholar]
- 26.Krishnegowda, G., A. M. Hajjar, J. Zhu, E. J. Douglass, S. Uematsu, S. Akira, A. S. Woods, and D. C. Gowda. 2005. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J. Biol. Chem. 2808606-8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kropf, P., M. A. Freudenberg, M. Modolell, H. P. Price, S. Herath, S. Antoniazi, C. Galanos, D. F. Smith, and I. Muller. 2004. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect. Immun. 721920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattner, J., A. Wandersee-Steinhauser, A. Pahl, M. Rollinghoff, G. R. Majeau, P. S. Hochman, and C. Bogdan. 2004. Protection against progressive leishmaniasis by IFN-beta. J. Immunol. 1727574-7582. [DOI] [PubMed] [Google Scholar]
- 29.McMahon-Pratt, D., and J. Alexander. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201206-224. [DOI] [PubMed] [Google Scholar]
- 30.McMaster, W. R., C. J. Morrison, M. H. MacDonald, and P. B. Joshi. 1994. Mutational and functional analysis of the Leishmania surface metalloproteinase GP63: similarities to matrix metalloproteinases. Parasitology 108(Suppl) S29-S36. [DOI] [PubMed] [Google Scholar]
- 31.Melby, P. C. 2002. Recent developments in leishmaniasis. Curr. Opin. Infect. Dis. 15485-490. [DOI] [PubMed] [Google Scholar]
- 32.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19683-765. [DOI] [PubMed] [Google Scholar]
- 33.Murray, H., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 3661561-1577. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, A. C., J. R. Peixoto, L. B. de Arruda, M. A. Campos, R. T. Gazzinelli, D. T. Golenbock, S. Akira, J. O. Previato, L. Mendonca-Previato, A. Nobrega, and M. Bellio. 2004. Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinositolphospholipids and higher resistance to infection with T. cruzi. J. Immunol. 1735688-5696. [DOI] [PubMed] [Google Scholar]
- 35.Olivier, M., D. J. Gregory, and G. Forget. 2005. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18293-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padigel, U., J. Alexander, and J. Farrell. 2003. The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis. J. Immunol. 1713705-3710. [DOI] [PubMed] [Google Scholar]
- 37.Perera, P. Y., T. N. Mayadas, O. Takeuchi, S. Akira, M. Zaks-Zilberman, S. M. Goyert, and S. N. Vogel. 2001. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166574-581. [DOI] [PubMed] [Google Scholar]
- 38.Piani, A., T. Ilg, A. G. Elefanty, J. Curtis, and E. Handman. 1999. Leishmania major proteophosphoglycan is expressed by amastigotes and has an immunomodulatory effect on macrophage function. Microbes Infect. 1589-599. [DOI] [PubMed] [Google Scholar]
- 39.Proudfoot, L., C. A. O'Donnell, and F. Y. Liew. 1995. Glycoinositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur. J. Immunol. 25745-750. [DOI] [PubMed] [Google Scholar]
- 40.Rivas, L., L. Kahl, K. Manson, and D. McMahon-Pratt. 1991. Biochemical characterization of the protective membrane glycoprotein GP46/M-2 of Leishmania amazonensis. Mol. Biochem. Parasitol. 47235-243. [DOI] [PubMed] [Google Scholar]
- 41.Russell, D. G., and H. Wilhelm. 1986. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J. Immunol. 1362613-2620. [PubMed] [Google Scholar]
- 42.Russo, D. M., J. M. Burns, Jr., E. M. Carvalho, R. J. Armitage, K. H. Grabstein, L. L. Button, W. R. McMaster, and S. G. Reed. 1991. Human T cell responses to gp63, a surface antigen of Leishmania. J. Immunol. 1473575-3580. [PubMed] [Google Scholar]
- 43.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2845-858. [DOI] [PubMed] [Google Scholar]
- 44.Scharton-Kersten, T., and P. Scott. 1995. The role of the innate immune response in Th1 cell development following Leishmania major infection. J. Leukoc. Biol. 57515-522. [DOI] [PubMed] [Google Scholar]
- 45.Schleicher, U., J. Liese, I. Knippertz, C. Kurzmann, A. Hesse, A. Heit, J. A. Fischer, S. Weiss, U. Kalinke, S. Kunz, and C. Bogdan. 2007. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J. Exp. Med. 204893-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 1891777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 633559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spath, G. F., L. A. Garraway, S. J. Turco, and S. M. Beverley. 2003. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. USA 1009536-9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 1771797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeuchi, O., and S. Akira. 2007. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 19185-191. [DOI] [PubMed] [Google Scholar]
- 51.Triantafilou, K., M. Triantafilou, and R. L. Dedrick. 2001. A CD14-independent LPS receptor cluster. Nat. Immunol. 2338-345. [DOI] [PubMed] [Google Scholar]
- 52.Triantafilou, M., K. Brandenburg, S. Kusumoto, K. Fukase, A. Mackie, U. Seydel, and K. Triantafilou. 2004. Combinational clustering of receptors following stimulation by bacterial products determines lipopolysaccharide responses. Biochem. J. 381527-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viriyakosol, S., T. Kirkland, K. Soldau, and P. Tobias. 2000. MD-2 binds to bacterial lipopolysaccharide. J. Endotoxin Res. 6489-491. [PubMed] [Google Scholar]
- 54.Wyler, D., D. Beller, and J. Sypek. 1987. Macrophage activation for antileishmanial defense by an apparantly novel mechanism. J. Immunol. 1381246-1249. [PubMed] [Google Scholar]