Abstract
Proteus mirabilis, a human pathogen that frequently causes urinary tract infections, is intrinsically highly resistant to cationic antimicrobial peptides, such as polymyxin B (PB). To explore the mechanisms underlying P. mirabilis resistance to PB, a mutant which displayed increased (>160-fold) sensitivity to PB was identified by transposon mutagenesis. This mutant was found to have Tn5 inserted into a novel gene, rppA. Sequence analysis indicated that rppA may encode a response regulator of the two-component system and is located upstream of the rppB gene, which may encode a membrane sensor kinase. An rppA knockout mutant of P. mirabilis had an altered lipopolysaccharide (LPS) profile. The LPS purified from the rppA knockout mutant could bind more PB than the LPS purified from the wild type. These properties of the rppA knockout mutant may contribute to its PB-sensitive phenotype. The rppA knockout mutant exhibited greater swarming motility and cytotoxic activity and expressed higher levels of flagellin and hemolysin than the wild type, suggesting that RppA negatively regulates swarming, hemolysin expression, and cytotoxic activity in P. mirabilis. PB could modulate LPS synthesis and modification, swarming, hemolysin expression, and cytotoxic activity in P. mirabilis through an RppA-dependent pathway, suggesting that PB could serve as a signal to regulate RppA activity. Finally, we demonstrated that the expression of rppA was up-regulated by a low concentration of PB and down-regulated by a high concentration of Mg2+. Together, these data highlight the essential role of RppA in regulating PB susceptibility and virulence functions in P. mirabilis.
Cationic antimicrobial polypeptides (CAPs), which are constitutively present in macrophages and neutrophils and are inducibly produced by epithelial cells at mucosal surfaces, play an important role in host defense against microbial infection and are key effectors of the host innate immune response (26). In gram-negative bacteria, CAPs, which have a net positive charge and an amphipathic structure, bind to the negatively charged residues of lipopolysaccharide (LPS) of the outer membrane and then can alter bacterial membrane integrity by solubilization or pore formation (25, 45). Microbial pathogens have evolved distinct mechanisms to resist killing by CAPs, including expelling CAPs through pumps and cleaving CAPs with proteases (45). One of the important mechanisms of resistance to CAPs in gram-negative bacteria involves modification of LPS with positively charged substituents, which leads to the repulsion of CAPs (45).
Polymyxin B (PB), a kind of CAP, contains a fatty acid side chain attached to a seven-member ring structure composed mainly of diaminobutyric acid (4). In a large number of bacterial species, the genes conferring resistance to CAPs, including PB, are regulated by bacterial two-component systems (30, 36, 38, 40, 41, 43). In Salmonella enterica serovar Typhimurium, evasion of CAP killing is regulated in part by the PmrA-PmrB two-component regulatory system (21, 22). PmrA-PmrB confers resistance to CAP by up-regulating genes involved in covalent modifications of the LPS (21, 22). The LPS modifications reduce the negative charge of LPS and consequently decrease attraction and binding of CAP to the outer membrane. The PhoP-PhoQ two-component system, a master regulator of S. enterica serovar Typhimurium virulence functions, also has been shown to be involved in regulating resistance to CAP (18). PhoQ is an inner membrane sensor kinase composed of a periplasmic sensor domain and a cytoplasmic kinase domain that phosphorylates its cognate response regulator, PhoP, upon perception of specific environmental signals. The PhoP-PhoQ system is repressed by millimolar concentrations of magnesium and is activated by micromolar concentrations of magnesium (18, 19). The activation of PhoP-PhoQ increases the expression of PmrD (30), which in turn leads to the activation of PmrA (28), resulting in modification of LPS. More recently, studies have shown that the transcription of PhoP-activated genes is also up-regulated by sublethal concentration of CAPs (5, 11) and that CAPs can bind to and activate the PhoQ sensor directly (6). Modulation of resistance to CAPs by the PhoP-PhoQ and PmrA-PmrB two-component systems has also been observed in Pseudomonas aeruginosa (36, 40).
Proteus mirabilis is an important pathogen of the urinary tract, especially in patients with indwelling urinary catheters (55). In order to develop and maintain a successful urinary tract infection, P. mirabilis needs to overcome the primary bladder defenses, which include physical barriers, such as bulk flow of urine, as well as low pH and high concentration of salts, urea, and organic acids. If the bacteria bypass these defenses, a second line of host responses, the innate host defenses, becomes involved. One of the important innate host defenses is the production of CAPs. P. mirabilis is known to be highly resistant to the action of CAPs, such as PB (9, 27). Although the detailed mechanisms underlying P. mirabilis resistance to PB are not clear, studies have shown that modification of LPS plays an important role in modulating CAP resistance in P. mirabilis (9, 27).
P. mirabilis exhibits a form of multicellular behavior known as swarming migration. It is believed that the ability of P. mirabilis to colonize the urinary tract is associated with its swarming motility. The swarming behavior of P. mirabilis is under the control of a complex regulatory network that may include bacterial two-component systems (33, 35, 54). For instance, we have identified a gene, rsbA, which may encode a histidine-containing phosphotransmitter of the bacterial two-component system and act as a repressor of swarming and virulence factor expression in P. mirabilis (8, 33, 35). It has been shown that LPS plays a critical role in swarming (39, 52) and that LPS modification affects both swarming and PB resistance in P. mirabilis (39). Moreover, activation of the PhoP-PhoQ two-component system, which is known to enhance CAP resistance, can lead to inhibition of swarming through repression of the expression of flagellin in S. enterica serovar Typhimurium (1). Together, these results suggest that swarming and CAP resistance may be coregulated and that it should be possible to isolate mutants with altered sensitivity to CAP through characterization of swarming mutants. In this study, we used a Tn5 transposon mutagenesis approach to isolate superswarming mutants of P. mirabilis. By characterizing these mutants, we identified a mutant with increased sensitivity to PB. This mutant was found to have Tn5 inserted into the rppA gene, a gene which may encode a response regulator of the two-component system. To our knowledge, this is the first report describing a two-component system that can regulate PB resistance in P. mirabilis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth condition.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) medium. A medium referred to as LSW− agar, which prevents the phenotypic expression of swarming motility, was prepared and used to select Tn5-mutagenized clones (7).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant phenotype | Source or reference |
|---|---|---|
| Proteus mirabilis strains | ||
| N2 | Wild type; Tcr | Clinical isolate |
| sw8 | N2 derivative; Tn5-mutagenized rppA mutant; PBs | This study |
| dA10 | N2 derivative; rppA knockout mutant; PBs Kmr | This study |
| dA10c | dA10 containing pACYC184-rppA; rppA-complemented strain; Cmr | This study |
| E. coli strains | ||
| TOP10 | F′ mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen |
| S17-1 λ pir | λ pir lysogen of S17-1 [thi pro hsdR hsdM+recA RP4 2-Tc::Mu-Km::Tn7 (Tpr Smr)]; permissive host able to transfer suicide plasmids requiring the Pir protein by conjugation to recipient cells | 14 |
| Plasmids | ||
| pGEM-T Easy | High-copy-number cloning vector; Ampr | Promega |
| pUT/mini-Tn5(Km) | Suicide plasmid requiring the Pir protein for replication and containing a mini-Tn5 cassette containing a Kmr gene | 14 |
| pACYC184 | Low-copy-number cloning vector, P15A replicon; Cmr Tetr | 13 |
| pACYC184-rppA | pACYC184 containing intact rppA sequence, including its promoter; Cmr | This study |
Transposon mutagenesis and identification of the mutated gene.
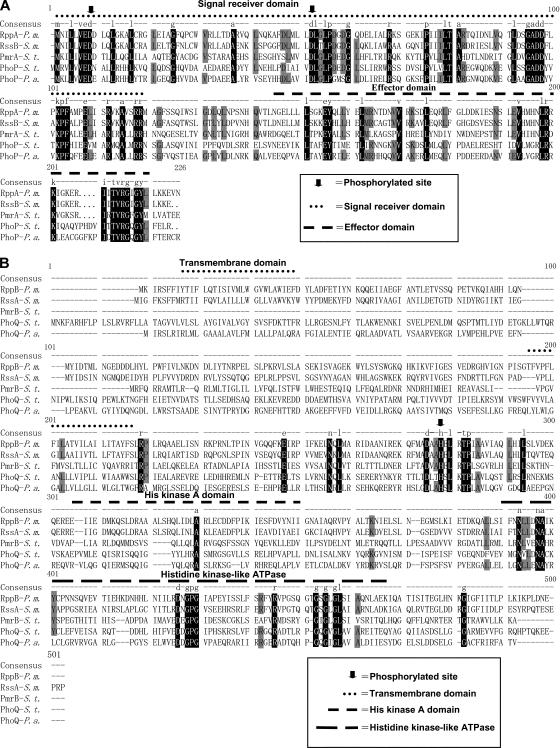
P. mirabilis mutants with aberrant swarming were isolated by mini-Tn5 Cm mutagenesis as described previously (33), except that LSW− agar plates were used to pick the clones with aberrant swarming. Chromosomal DNA was extracted from the mutants and partially digested with AluI, and fragments more than 4 kb long were cloned into EcoRV-digested pZErO-2.1 (Invitrogen, United States). Following transformation of Escherichia coli TOP10, chloramphenicol-resistant Tn5 Cm-containing clones were selected. The nucleotide sequences of the cloned DNA fragments were determined and subjected to a BLAST analysis (http://www.ncbi.nlm.nih.gov/). We then searched for the sequence in the released genome sequence of P. mirabilis (http://www.sanger.ac.uk/) and cloned the rppA and rppB genes, including their promoter, by PCR-TA cloning with primers dA-1F and rppB-overR (Table 2). The nucleotide sequence was determined step by step using a 373A DNA sequencer (Applied Biosystems, United States). Alignment of RppA and RppB with other two-component proteins was performed using the DNAMAN software (version 4.15). Signal receiver, effector, histidine kinase A, and histidine kinase-like ATPase domains were predicted using SBASE (http://hydra.icgeb.trieste.it/sbase/). Putative phosphorylation sites and transmembrane domains were predicted using PredictProtein (http://www.predictprotein.org/).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ to 3′) | Description |
|---|---|---|
| rppAF | GAATATTTTATTAGTTGAAG | Sequence check of rppA; used with rppAR |
| rppAR | AGTTCACTTCTTTTTTTAAG | Sequence check, complementation, and knockout of rppA; used with rppAF or dA-1F |
| rppB-overR | CGTTGGATAGCCACTTTGTG | Cloning of full-length rppA-rppB; used with dA-1F |
| dA-1F | GTGAAATGCTCCCTGAGGAG | Knockout and complementation of rppA; used with rppAR |
| rppA RT-F | CGCTCTGTCGTGGTCTAGAAATT | Real-time PCR measurement of rppA mRNA; used with rppA RT-R |
| rppA RT-R | CACGTGCATCTGTAAGCAATCTT | Real-time PCR measurement of rppA mRNA; used with rppA RT-F |
| hpm RT-F | ACACAAGGTGATGTCGTCATTGA | Real-time PCR measurement of hpmA mRNA; used with hpm RT-R |
| hpm RT-R | CATCGGAAATGAGTTCACTACCTGTA | Real-time PCR measurement of hpmA mRNA; used with hpm RT-F |
| flhdc RT-F | CGCACATCAGCCTGCAAGT | Real-time PCR measurement of flhDC mRNA; used with flhdc RT-R |
| flhdc RT-R | GCAGGATTGGCGGAAAGTT | Real-time PCR measurement of flhDC mRNA; used with flhdc RT-F |
| 16sRNA RT-F | CACGCAGGCGGTCAATTAA | Real-time PCR measurement of 16S rRNA; used with 16sRNA RT-R |
| 16sRNA RT-R | GCCAACCAGTTTCAGATGCA | Real-time PCR measurement of 16S rRNA; used with 16sRNA RT-F |
Gene knockout by homologous recombination.
Full-length rppA, including its promoter region, was amplified by PCR using primers dA-1F and rppAR (Table 2) and cloned into pGEM-T Easy (Promega) to generate pGrppA. The pGrppA plasmid was digested with XbalI and ligated with an XbalI-digested Ω(Kmr) gene cassette (46) to generate pGrppA-km, in which a Kmr cassette was inserted into the rppA gene. The DNA fragment containing the rppA gene with the Kmr cassette inserted was cleaved from pGrppA-km and ligated into SalI/SphI-digested pUT/mini-Tn5(Km) to generate pUTrppA-km. For gene inactivation mutagenesis by homologous recombination, the pUTrppA-km plasmid was transferred from the permissive host strain E. coli S17-1 λ pir to P. mirabilis N2 by conjugation, and the transconjugants were spread on LSW− agar plates containing kanamycin (100 μg/ml) and tetracycline (13 μg/ml). The kanamycin- and tetracycline-resistant colonies were screened for mutants with double-crossover events by PCR screening. Southern hybridization using the PCR-amplified rppA sequence as the probe was performed to confirm the rppA knockout genotypes (data not shown).
Construction of the RppA-complemented strain.
A DNA fragment containing the full-length rppA gene and its promoter region was excised from pGrppA (see above) with SalI and SphI. The DNA fragment was ligated into SalI/SphI-digested low-copy-number plasmid pACYC184 to generate the rppA complementation plasmid pACYC184-rppA. The pACYC184-rppA plasmid was then transformed into the P. mirabilis rppA knockout mutant to generate an RppA-complemented strain.
Real-time RT-PCR.
To study the effects of PB and Mg2+ on the expression of rppA mRNA, an overnight LB medium culture was washed with N-minimal medium [5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, 0.1 mM Tris-HCl, 0.2% glucose, 0.01% Casamino Acids; pH 7.4], diluted to obtain an optical density at 600 nm (OD600) of 0.3 to 0.4, resuspended in N-minimal medium with or without 1 μg/ml PB or 10 mM Mg2+, and grown for 5 h at 37°C. Total RNA was extracted from cells using an RNA-Bee kit (Tel-Test, United Kingdom). cDNA was obtained using Superscript II reverse transcriptase (RT) according to the instructions provided by the manufacturer (Invitrogen, United States). The cDNA was then used as a template for real-time PCR using SYBR green PCR MasterMix (Applied Biosystems) and an ABI Prism 7000 (Applied Biosystems, Foster City, CA) to monitor the expression of rppA mRNA. The levels of rppA mRNA were normalized using 16S rRNA. For determination of the levels of hpmA and flhDC mRNA, cells were plated on LB agar plates and incubated for 3, 4, and 5 h. Total RNA was isolated and subjected to real-time RT-PCR as described above.
MIC assay.
The in vitro MIC of PB was determined by the broth microdilution method using the guidelines proposed by the National Committee for Clinical Laboratory Standards (42). Twofold serial dilutions of a stock solution of PB (40,960 μg/ml) prepared in sterile water were added to 96-well microtiter plates, and aliquots of a bacterial culture (5 × 104 CFU) were then dispensed into the wells and incubated for 16 to 18 h. The MIC was defined as the lowest PB concentration at which no visible growth occurred.
Preparation and analysis of LPS.
LPS extraction and analysis were performed as described previously (48), with some modifications. One hundred microliters of an overnight LB medium culture was inoculated onto LB medium plates with or without 1 μg/ml PB and incubated for 6 h at 37°C. Equal amounts of cells (OD600 × volume [in ml], 100) were washed with MOPS [3-(N-morpholino)propanesulfonic acid]-MgSO4 buffer (150 mM NaCl, 20 mM MOPS, 1 mM MgSO4; pH 6.9) and resuspended in 15 ml of the same buffer. An equal volume of MOPS buffer (20 mM MOPS, pH 6.9)-saturated phenol was added to the cell suspension and incubated at 65°C for 30 min with occasional shaking. The mixture was kept on ice for 10 min and centrifuged for 20 min at 15,000 × g. The top aqueous phases were collected, and 4 volumes of chilled ethanol was added. The solution was mixed by inversion 20 times. The precipitated LPS collected by centrifugation was resuspended in 150 mM NaCl-10 mM MgCl2-20 mM MOPS (pH 6.9) and treated with DNase I and RNase A at 37°C for 30 min. The mixture was then centrifuged for 3 h at 100,000 × g. The clear pellets containing LPS were resuspended in 0.1 ml of 150 mM NaCl-20 mM MOPS (pH 6.9) and analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on 12% acrylamide gels. Each gel was stained with silver as described previously (48) to visualize the LPS profiles. For quantification of LPS, the LPS prepared as described above from equal amounts of wild-type and mutant cells (OD600 × volume [in ml], 100) was subjected to the Purpald assay (32) to determine the concentration of purified LPS. Purified LPS from E. coli 055:B5 (Sigma L 2880) was used as the standard.
Binding of PB by LPS.
The experiments to determine binding of PB by LPS were performed as described previously (12), with some modifications. First, 15 mg/ml of purified LPS obtained as described above from the wild type and the rppA knockout mutant was diluted 10-fold. Aliquots (1, 2, 4, and 8 μl) of diluted LPS were then added to a 2 mM HEPES (pH 7.2) solution to obtain a final volume of 100 μl. The LPS suspensions were mixed with 12 μl of a PB stock solution (100 μg/ml) and incubated at 37°C for 30 min. After incubation, the suspensions were centrifuged (12,000 × g, 10 min) three times, and the supernatants, which contained the unbound PB, were collected. The amount of unbound PB was measured by a radial diffusion assay as described below.
The radial diffusion assay was performed as described previously (12). Briefly, the indicator bacterium E. coli TOP10 was grown in LB medium and collected in the exponential phase of growth. An underlay gel that contained 1% (wt/vol) agarose, 2 mM HEPES (pH 7.2), and 0.3 mg of tryptic soy broth powder per ml was equilibrated at 50°C and mixed with the indicator bacteria at a final concentration of 4 × 105 CFU per ml of molten gel. The gel was poured into petri dishes, and after polymerization, small 10-μl wells were carved. Aliquots (5 μl) of the supernatants containing unbound PB obtained previously were added and allowed to diffuse for 3 h at 37°C. After this, a 10-ml overlay gel composed of 1% agarose and 6% tryptic soy broth powder in water was poured on top of the first gel, and the plates were incubated overnight at 37°C. The next day, the diameters of the inhibition halos were measured, and the results were expressed in inhibition units (1 U = 1 mm) after the diameter of the well was subtracted.
Swarming migration assay.
The swarming migration assay was performed as described previously (24, 33). Briefly, an overnight bacterial culture (5 μl) was inoculated centrally onto the surface of dry LB swarming agar (2%, wt/vol) plates with or without PB (1 μg/ml), which were then incubated at 37°C. The swarming migration distance was measured by monitoring the swarm fronts of the bacterial cells and recording the progress at 60-min intervals.
Measurement of cell differentiation, flagellin level, and hemolysin activity.
Overnight LB medium cultures of the wild type and the rppA knockout mutant were inoculated onto the surfaces of dry LB swarming agar plates with or without 1 μg/ml PB, which were then incubated at 37°C. Cells used for cell differentiation, hemolysin and flagellin assays were prepared as described previously (33, 35). Cell morphology was observed after Gram staining and was examined by light microscopy at a magnification of ×1,000 under oil immersion with an Olympus BH2 microscope equipped with a graticule. The flagellin level and cell membrane-associated hemolysin activity were assayed as described previously (33, 35).
Cytotoxicity assay.
The cytotoxicity experiments were performed as described previously (2), with some modifications. To determine the cytotoxic activity of wild-type P. mirabilis and the rppA knockout mutant that were treated or not treated with PB (1 μg/ml), overnight cultures of properly treated bacteria were used to infect human urothelial cell line NTUB1, which was originally derived from a urinary bladder carcinoma and was obtained from the National Taiwan University Hospital. NTUB1 cells were routinely maintained in RPMI 1640 medium (Gibco, United States) supplemented with 10% (vol/vol) fetal bovine serum (Gibco, United States) at 37°C in a humidified 5% CO2 incubator. Twenty-four-well microplates (Corning, United States) were used for microscopic observation of cytotoxicity. Microplate wells containing confluent monolayer NTUB1 cells were washed twice with Hanks balanced salt solution (HBSS) and then infected at 37°C with 1 ml of serially diluted bacteria in an incubation solution containing HBSS, minimal medium (33), and 0.2 M Tris buffer (pH 7.5) (80:10:10, vol/vol/vol) for 1.5 h. Urothelial cells were then washed twice with HBSS and Gram stained, and the number of intact urothelial cells remaining in each well was estimated by light microscopy. The number of bacteria causing ca. 50% cell lysis (LD50) was determined, and the relative cytotoxic activity of the bacteria was calculated by comparing the LD50 of the bacteria treated or not treated with PB (1 μg/ml) with the LD50 of the untreated wild-type cells. All experiments were performed in triplicate.
Nucleotide sequence accession numbers.
The nucleotide sequences of rppA and rppB have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers EF601922 and EF601923.
RESULTS
Isolation of PB-sensitive P. mirabilis mutant.
As described in the Introduction, it is possible to isolate PB-sensitive P. mirabilis mutants by characterizing swarming mutants. Therefore, we performed mini-Tn5 transposon mutagenesis as described previously (33) to isolate superswarming mutants of P. mirabilis. Through characterization of these mutants, we identified a mutant, sw8, which was >160-fold more sensitive to PB than the wild-type strain P. mirabilis N2 (MICs, 256 and >40,960 μg/ml, respectively). Figure 1 shows the superswarming phenotype of the sw8 mutant. Mutant sw8 could swarm faster than P. mirabilis wild-type strain N2 on both an LB swarming agar plate and an LSW− agar plate.
FIG. 1.
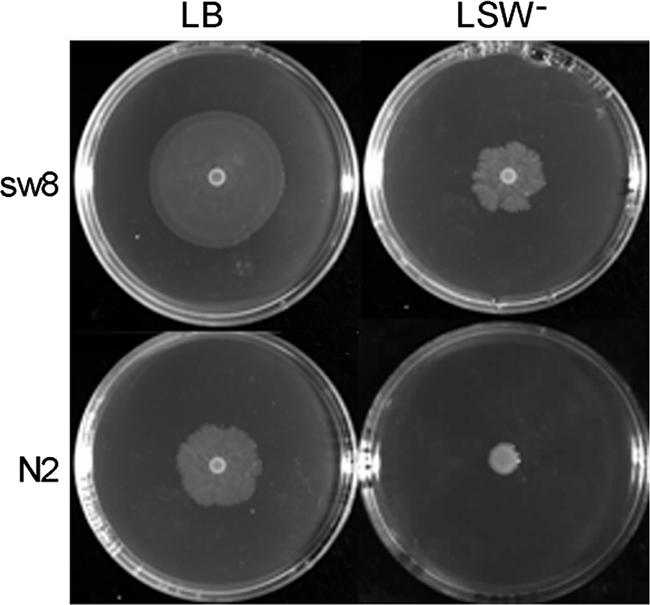
Swarming migration of wild-type P. mirabilis and the rppA Tn5-mutagenized mutant on LB agar swarming plates and LSW− agar plates. Aliquots (5 μl) of overnight cultures were inoculated in the centers of the plates. The plates were incubated at 37°C, and representative pictures were taken after 8 h of incubation. The strains used were N2 (wild type) and sw8 (Tn5-mutagenized rppA mutant).
The nucleotide sequence of the cloned DNA fragment flanking mini-Tn5 in mutant sw8 was obtained. By searching the P. mirabilis released genome sequence (http://www.sanger.ac.uk/) using the sequence that we obtained, we found that Tn5 was inserted into a gene which we designated rppA (regulator of polymyxin B susceptibility in Proteus). The rppA gene and the downstream gene rppB were cloned and sequenced. rppA and rppB were found to be in the same reading frame and to be separated by a stop codon. Promoter sequence analysis using “Prokaryotic promoter analysis using SAK” (http://nostradamus.cs.rhul.ac.uk/∼leo/sak_demo/) and “Prokaryotic Promoter Prediction” (http://bioinformatics.biol.rug.nl/websoftware/ppp/ppp_start.php) indicated that rppA and rppB most likely are in the same operon. The nucleotide sequences of rppA and rppB were found to be 98.8 and 99% identical, respectively, to the corresponding sequences of sequenced P. mirabilis strain HI4320. Analysis of the deduced amino acid sequences encoded by rppA and rppB indicated that these genes may encode a response regulator and a membrane sensor histidine kinase, respectively, of the bacterial two-component signaling system. Figure 2 shows an alignment of the RppA and RppB proteins with other bacterial two-component proteins. RppA is homologous to S. enterica serovar Typhimurium PmrA (40.7% identity and 58.3% similarity), S. enterica serovar Typhimurium PhoP (35.7% identity and 59.3% similarity), Serratia marcescens RssB (75.3% identity and 84.5% similarity), and P. aeruginosa PhoP (36.5% identity and 55.7% similarity), while RppB is homologous to S. enterica serovar Typhimurium PmrB (26.5% identity and 43.7% similarity), S. enterica serovar Typhimurium PhoQ (24.6% identity and 46.0% similarity), S. marcescens RssA (52.0% identity and 72.2% similarity), and P. aeruginosa PhoQ (21.6% identity and 44.1% similarity).
FIG. 2.
Alignment of the RppA (A) and RppB (B) proteins of P. mirabilis (P. m.) with other response regulators and membrane sensor kinases from P. aeruginosa (P. a.), S. enterica serovar Typhimurium (S. t.), and S. marcescens (S. m.). Alignment was performed using the DNAMAN software. Signal receiver, effector, histidine kinase A, and histidine kinase-like ATPase domains were predicted using SBASE (http://hydra.icgeb.trieste.it/sbase/). Putative phosphorylation sites and transmembrane domains were predicted using PredictProtein (http://www.predictprotein.org/). Residues that are shared by all five proteins are indicated by a black background. Residues that are shared by four proteins are indicated by a gray background. The histidine residue conserved among sensor kinases and believed to be the site of autophosphorylation is indicated by an arrow in panel B. The putative phosphorylation sites (aspartate) which are conserved among all five response regulators are indicated by arrows in panel A.
P. mirabilis rppA knockout mutant exhibits increased susceptibility to PB.
To demonstrate the role of RppA in regulating PB susceptibility, we tried to construct a mutant with a mutation in rppA by allelic exchange mutagenesis. An rppA mutant (dA10) was constructed by homologous recombination using plasmid pUT harboring a kanamycin resistance cassette in the rppA internal coding region (see Materials and Methods). Southern blot analysis indicated that the dA10 mutant contained a single disrupted rppA gene and no wild-type rppA allele (data not shown). The MICs of PB for wild-type strain P. mirabilis N2 and the dA10 mutant were determined. While the MIC of PB for the wild-type strain was >40,960 μg/ml, the MIC for the dA10 mutant was about 256 μg/ml. Thus, the dA10 mutant was >160-fold more sensitive to PB than the wild type. To further confirm that RppA can affect PB susceptibility, we constructed an RppA-complemented derivative of dA10, dA10c, by transforming pACYC184-rppA (which is a low-copy-number plasmid carrying a wild-type rppA gene) into the dA10 mutant. We found that the MIC of PB for the dA10c strain was >40,960 μg/ml. Thus, the RppA-complemented strain exhibited resistance to PB similar to that of the wild-type strain, in marked contrast to rppA mutant dA10, which was highly sensitive to PB. Together, these data suggest that RppA may regulate PB susceptibility either directly or indirectly in P. mirabilis.
P. mirabilis rppA knockout mutant has an altered LPS profile.
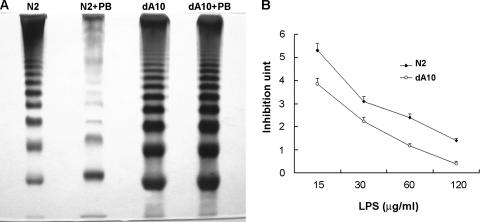
LPS modification plays an important role in PB susceptibility in many gram-negative bacteria, including Salmonella, Yersinia, Pseudomonas, E. coli, and P. mirabilis (39, 40, 47, 53). To investigate the underlying cause of PB sensitivity in the rppA knockout mutant, we compared the LPS profile of the rppA knockout mutant (dA10) with that of the wild-type strain (N2). The LPS was extracted from equal amounts of the wild-type and mutant cells and was subjected to SDS-PAGE analysis. As shown in Fig. 3A, the intensity of the lower bands of the LPS ladder was greater for the rppA mutant than for the wild-type strain (compare lane 1 with lane 3). Moreover, a slight band shift was observed in the LPS ladder of the rppA mutant. These data indicate that the rppA mutant has an altered LPS profile and thus has modified LPS in its outer membrane. To investigate whether the rppA mutant synthesized more LPS than the wild-type strain, the LPS was extracted from equal amounts of the wild-type and mutant cells, and the concentration of LPS was determined (see Materials and Methods). As shown in Table 3, the rppA mutant (dA10) synthesized slightly more LPS than the wild-type strain (N2).
FIG. 3.
(A) LPS profiles of wild-type P. mirabilis and the rppA knockout mutant in the presence and absence of PB (1 μg/ml). Six microliters of LPS purified from the same number of cells (OD600 × volume [in ml], 100) of the wild type and the rppA mutant was subjected to SDS-PAGE analysis. (B) PB-binding ability of LPS purified from a wild-type P. mirabilis strain and an rppA knockout mutant. Various amounts of purified LPS were subjected to the PB-binding assay. The unbound PB was then subjected to the E. coli inhibition assay (see Materials and Methods). The data are the averages and standard deviations of three independent experiments. The wild-type strain used was strain N2, and the rppA knockout mutant used was dA10.
TABLE 3.
Quantitation of LPS produced by P. mirabilis wild-type strain N2 and rppA knockout mutant dA10 in the absence and presence of PB
| Strain | LPS concn (mg/ml)a |
|---|---|
| N2 | 14.7 ± 0.3 |
| dA10 | 16.8 ± 0.2 |
| N2 with PB (1 μg/ml) | 9.2 ± 1.1 |
| dA10 with PB (1 μg/ml) | 17.0 ± 1.3 |
LPS was quantitated as described in Materials and Methods.
It is well known that an alteration in LPS can affect its binding to CAP. We thus tested whether the LPS purified from the rppA mutant and the wild-type strain had different binding activities with PB. Equal amounts of LPS from the rppA mutant (dA10) and the wild-type strain (N2) were incubated with PB, and the unbound fraction was subjected to the E. coli inhibition assay. As shown in Fig. 3B, LPS from the rppA mutant bound a larger amount of PB than LPS from the wild-type strain. Since identical concentrations of LPS were used in the binding assay, these data indicate that there was a qualitative change in the LPS of the rppA mutant and that this change caused the LPS from the rppA mutant to have higher binding activity with PB. The increased PB-binding activity of the rppA mutant may have contributed to its sensitivity to PB.
It is interesting that when the wild-type P. mirabilis strain was incubated in the medium containing a low concentration of PB (1 μg/ml), its LPS profile changed dramatically; the intensity of the LPS ladder decreased, and the bands shifted to a higher molecular weight (Fig. 3A, compare lane 1 with lane 2). In contrast, the LPS profile of the rppA mutant grown in the presence of a low concentration of PB was similar to that of the mutant grown in the absence of PB (Fig. 3A, compare lane 3 with lane 4). Moreover, while the synthesis of LPS in the wild-type strain was inhibited by a low concentration of PB (1 μg/ml), the synthesis of LPS in the rppA mutant was not affected by PB (Table 3). Together, these data suggest that PB can regulate the synthesis and modification of LPS in P. mirabilis and that this regulation is mediated through an RppA-dependent pathway.
Swarming behavior of the P. mirabilis rppA knockout mutant.
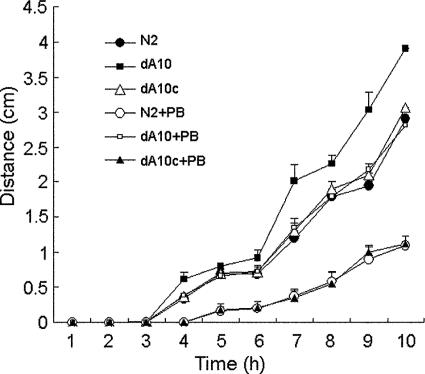
We have shown previously that the sw8 mutant, in which Tn5 is inserted into the rppA gene, had a superswarming phenotype (Fig. 1). To further investigate the role of RppA in regulating swarming, we compared the swarming behaviors of the rppA knockout mutant (dA10), the RppA-complemented strain (dA10c), and the wild-type strain (N2). As shown in Fig. 4, while the rppA knockout mutant migrated faster than the wild-type strain, the RppA-complemented strain exhibited a migration ability similar to that of the wild type. These data indicate that RppA may either directly or indirectly inhibit swarming in P. mirabilis. Thus, since PB seems to be able to serve as a signal to modulate the activity of RppA (Fig. 3A), we tested whether PB could regulate the swarming ability of P. mirabilis. As shown in Fig. 4, while the swarming abilities of the wild-type strain and the RppA-complemented strain were inhibited by a low concentration of PB (1 μg/ml) to similar extents, the swarming ability of the rppA knockout mutant was inhibited less. Together, these data indicate that PB can negatively regulate swarming in P. mirabilis. This regulation by PB may not be mediated solely through RppA, because the swarming ability of the rppA knockout mutant was still inhibited by PB, although to a lesser extent.
FIG. 4.
Swarming migration of a wild-type P. mirabilis strain, an rppA knockout mutant, and an RppA-complemented strain in the presence and absence of PB. Aliquots (5 μl) of overnight cultures were inoculated in the centers of LB swarming plates with or without PB (1 μg/ml). The plates were incubated at 37°C, and the migration distance was measured hourly after inoculation. The data are the averages and standard deviations of three independent experiments. Strain N2 was the wild-type strain used, strain dA10 was the rppA knockout mutant used, and strain dA10c was the RppA-complemented strain used.
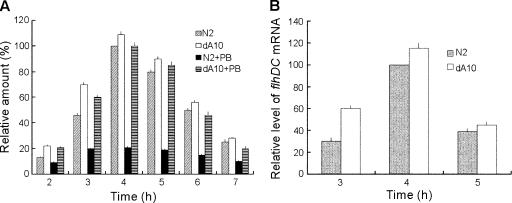
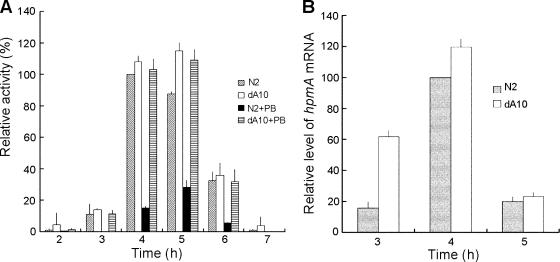
Swarming migration in P. mirabilis involves the coordinated differentiation of short vegetative cells bearing a few peritrichous flagella into long multinucleate swarm cells with a much greater surface density of flagella (3, 34). To further confirm that RppA is involved in the regulation of swarming in P. mirabilis, we measured the amounts of flagellin synthesized in the rppA knockout mutant (dA10) and the wild-type strain (N2) during one differentiation-dedifferentiation cycle of the bacteria. As shown in Fig. 5, the rppA mutant synthesized a higher level of flagellin than the wild-type strain at each time point during the differentiation cycle. Consistent with this, we also found that the rppA mutant synthesized a higher level of flhDC mRNA than the wild-type strain (Fig. 5). Since FlhDC is a master regulator that controls the expression of flagellin genes, the latter result indicates that RppA may negatively regulate flagellin synthesis by down-regulating the expression of flhDC genes. Figure 5A also shows that the synthesis of flagellin was inhibited by PB to a greater extent in the wild-type strain than in the rppA mutant. These data indicate that PB can inhibit flagellin synthesis and that this inhibition is mediated partially through an RppA-dependent pathway.
FIG. 5.
(A) Flagellin levels of a wild-type P. mirabilis strain and an rppA knockout mutant in the presence and absence of 1 μg/ml PB. The flagellin levels were determined at different time points after the wild type and the rppA mutant were seeded on the LB agar plates. The value obtained for the wild-type cells in the absence of PB at 4 h after seeding was defined as 100%, and all other values were expressed relative to this value. The data are the averages and standard deviations of three independent experiments. (B) Expression of flhDC mRNA in a wild-type P. mirabilis strain and an rppA knockout mutant in the absence of PB. Total RNA was isolated from the wild-type and rppA mutant cells at 3, 4, and 5 h after seeding on LB agar plates and was then subjected to real-time RT-PCR for measurement of mRNA. The value obtained for the wild-type cells at 4 h after seeding was defined as100%. The data are the averages and standard deviations of three independent experiments. Strain N2 was the wild-type strain used, and strain dA10 was the rppA knockout mutant used.
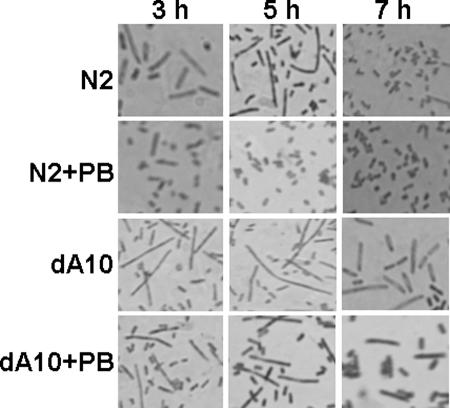
We also tested whether the differentiation of P. mirabilis was regulated by RppA and PB. To this end, we compared the cell length of the rppA knockout mutant (dA10) with that of the wild-type strain (N2) during one differentiation-dedifferentiation cycle of the bacteria both in the absence and in the presence of PB (1 μg/ml). In the absence of PB, the rppA mutant formed longer cells than the wild type formed during the differentiation cycle (Fig. 6), indicating that RppA may negatively regulate swarming differentiation in P. mirabilis. In the presence of PB, the ability to differentiate into long swarm cells was almost completely inhibited in the wild-type strain, while the ability of the rppA mutant to do this was inhibited to a much lesser extent (Fig. 6). This result suggests that PB can negatively regulate the differentiation of P. mirabilis and that this regulation is partially mediated through an RppA-dependent pathway.
FIG. 6.
Microscopic observation of cell differentiation of a wild-type P. mirabilis strain and an rppA knockout mutant in the presence and absence of 1 μg/ml PB. Cells were Gram stained and viewed under oil (magnification, ×1,000). Three independent experiments were performed, and the representative images show cell differentiation at 3, 5, and 7 h after seeding onto LB agar plates. An increase in cell length was considered a sign of cell differentiation. Strain N2 was the wild-type strain used, and strain dA10 was the rppA knockout mutant used.
RppA can regulate hemolysin expression in P. mirabilis.
Previous studies have shown that bacterial two-component systems which regulate susceptibility to CAP, such as the PhoP-PhoQ system of S. enterica serovar Typhimurium, can modulate the expression of virulence genes in a bacterium (18, 19). As shown above, RppA exhibits both functional and amino acid sequence similarity to PhoP. We thus tested whether RppA could also regulate virulence factor expression in P. mirabilis and whether this regulation could be modulated by PB. To this end, the cell membrane-associated hemolysin activities of the rppA knockout mutant (dA10) and the wild-type strain (N2) were assayed during one differentiation-dedifferentiation cycle of the bacteria both in the absence and in the presence of PB (1 μg/ml). As shown in Fig. 7A, in the absence of PB, the rppA mutant expressed higher levels of hemolysin activity than the wild-type strain during the 7-h incubation period. Measurement of hemolysin mRNA (hpmA mRNA) also showed that the rppA mutant expressed higher levels of hemolysin mRNA than the wild-type strain in the absence of PB (Fig. 7B). Together, these data indicate that RppA can negatively regulate the expression of hemolysin in P. mirabilis. In the presence of PB, the hemolysin activity of the wild-type strain was inhibited to a much greater extent than the hemolysin activity of the rppA mutant, suggesting that PB can inhibit the expression of hemolysin in P. mirabilis and that this inhibition is partially mediated through an RppA-dependent pathway.
FIG. 7.
(A) Hemolysin activities of a wild-type P. mirabilis strain and an rppA knockout mutant in the presence and absence of 1 μg/ml PB. Hemolysin activity was determined at different time points after the wild type and the rppA mutant were seeded onto LB agar plates. The value obtained for the wild-type cells in the absence of PB at 4 h after seeding was defined as 100%, and all other values were expressed relative to this value. The data are the averages and standard deviations of three independent experiments. (B) Expression of hemolysin gene (hpmA) mRNA in a wild-type P. mirabilis strain and an rppA knockout mutant in the absence of PB. Total RNA was isolated from the wild-type and rppA mutant cells at 3, 4, and 5 h after seeding onto LB agar plates and was then subjected to real-time RT-PCR for measurement of the mRNA. The value obtained for the wild-type cells at 4 h after seeding was defined as 100%. The data are the averages and standard deviations of three independent experiments. Strain N2 was the wild-type strain used, and strain dA10 was the rppA knockout mutant used.
The cytotoxic activity of P. mirabilis is known to be associated with the hemolysin activity (50). Knowing that the rppA knockout mutant expressed higher levels of hemolysin activity than the wild-type strain, we tested whether the rppA knockout mutant also had higher cytotoxic activity. Indeed, the rppA knockout mutant (dA10) had higher cytotoxic activity against human urothelial NTUB1 cells than the wild-type strain (N2) (Table 4). While the cytotoxic activity of the wild-type strain was inhibited by PB (1 μg/ml), that of the rppA mutant was not (Table 4). Together, these data indicate that RppA can negatively regulate the cytotoxic activity of P. mirabilis and that PB can inhibit the cytotoxic activity of P. mirabilis through an RppA-dependent pathway.
TABLE 4.
Cytotoxic activities of P. mirabilis wild-type strain N2 and rppA knockout mutant dA10 treated and not treated with PB
| Strain | Relative cytotoxicitya |
|---|---|
| N2 | 100b,c |
| N2 with PB (1 μg/ml) | 61 ± 9b |
| dA10 | 305 ± 47c |
| dA10 with PB (1 μg/ml) | 327 ± 75 |
Relative cytotoxicity was calculated as described in Materials and Methods. The LD50 of the wild-type strain not treated with 1 μg/ml PB was defined as 100.
P < 0.05 as determined by Student's t test for a comparison between the cytotoxicities of untreated and PB-treated wild-type cells.
P < 0.01 as determined by Student's t test for a comparison between the cytotoxicities of the wild type and the rppA knockout mutant.
Expression of rppA is regulated by PB and Mg2+.
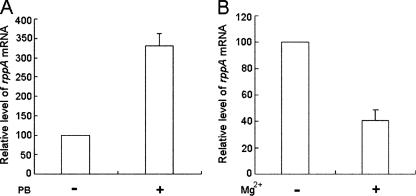
PB and Mg2+ have been shown to be able to regulate the activity of PhoP, which in turn autoregulates the transcription of the phoPQ operon in S. enterica serovar Typhimurium (18, 19, 49). Since RppA shows both functional and sequence similarity to PhoP and can respond to PB (see above), it is possible that the expression of RppA is also regulated by PB and Mg2+. To test this possibility, P. mirabilis was treated or not treated with either PB or a high concentration of Mg2+. The level of rppA mRNA was then measured by real-time RT-PCR. As shown in Fig. 8A, in the presence of PB (1 μg/ml), the expression of rppA mRNA was significantly induced, indicating that PB can up-regulate the expression of rppA. In contrast, in the presence of a high concentration of Mg2+ (10 mM), the expression of rppA was significantly inhibited (Fig. 8B). The regulation of rppA by PB and Mg2+ is reminiscent of the regulation of the phoPQ operon, which is also up-regulated by PB and down-regulated by a high concentration of Mg2+ (18, 19, 49).
FIG. 8.
Effects of PB (1 μg/ml) (A) and Mg2+ (10 mM) (B) on the expression of rppA mRNA in a wild-type P. mirabilis strain. The amount of rppA mRNA was determined by real-time PCR as described in Materials and Methods. The value obtained for cells in the absence of PB or Mg2+ was defined as 100%. The data are the averages and standard deviations of four independent experiments.
DISCUSSION
P. mirabilis is known to be naturally resistant to PB, a CAP often used for treatment of multidrug-resistant gram-negative bacterial infections (16, 17). Although studies have suggested that LPS modification is the elaborate mechanism by which P. mirabilis resists PB (20, 27, 39), the regulatory mechanism underlying PB resistance remains elusive. In this study, we used a novel strategy to isolate PB-sensitive P. mirabilis mutants. Our strategy was based on previous observations that bacterial swarming and resistance to CAP are coregulated (1, 10, 15, 29, 39) and that two-component systems involved in regulating CAP resistance can also regulate swarming (1, 10, 15). By isolating P. mirabilis superswarming mutants using Tn5 mutagenesis, we were able to identify a mutant that is >160 times more sensitive to PB than the wild-type strain. This mutant was found to have Tn5 inserted into the rppA gene. Analysis of the deduced amino acid sequence of rppA indicated that it may encode a protein homologous to PhoP and PmrA, both of which are response regulators of two-component systems, PhoP-PhoQ and PmrA-PmrB, involved in regulation of CAP resistance. RppA most likely acts as a positive regulator of PB resistance and a negative regulator of swarming, because the rppA knockout mutant had increased sensitivity to PB and increased swarming ability compared to the wild-type strain and the rppA knockout mutant complemented with rppA. The regulatory role of RppA in PB resistance and swarming was also supported by the observation that the rppA mutant had an altered LPS profile and expressed higher levels of flhDC mRNA and flagellin. The rppA gene is in an operon that also includes the rppB gene. Sequence analysis indicated that RppB is homologous to the membrane sensor kinases PhoQ and PmrB of the PhoP-PhoQ and PmrA-PmrB two-component systems. Our preliminary data indicated that an rppB knockout mutant also had increased sensitivity to PB and exhibited a superswarming phenotype (data not shown). Together, these data suggest that RppA and RppB may constitute a two-component signaling system regulating PB resistance and swarming in P. mirabilis.
The PhoP-PhoQ two-component system is known to be involved in regulation of CAP resistance, swarming, and virulence functions (1, 10, 15, 18, 19). Several lines of evidence suggest that RppA is the PhoP homologue in P. mirabilis. (i) Previous studies indicated that PhoP mutants of several bacteria show increased sensitivity to PB (15, 18, 38, 43). We found that the rppA knockout mutant of P. mirabilis showed enhanced susceptibility to PB compared to the wild-type strain and the rppA knockout mutant complemented with rppA (Table 3). (ii) The PhoP-PhoQ two-component system has been shown to be involved in regulation of swarming and synthesis of flagellin (1, 10, 15). For instance, the phoP knockout mutant of Photorhabdus luminescens shows increased expression of the flagellin gene fliC and is more motile than the parent strain (15). In S. enterica serovar Typhimurium, activation of the PhoP-PhoQ pathway results in down-regulation of fliC expression, decreased flagellin expression, and reduced cell motility (1). In this study, we found that the rppA knockout mutant of P. mirabilis showed increased flagellin expression (Fig. 5) and could swarm faster than the wild-type strain (Fig. 4). (iii) CAPs, including PB, can serve as signals that activate the PhoP-PhoQ pathway (6, 19). The activation of the PhoP-PhoQ pathway leads to LPS modification, inhibition of swarming, and decreased flagellin expression (1, 18, 51). In this study, we also found that PB could serve as a signal that regulates LPS modification (Fig. 3), repress swarming (Fig. 4), and inhibit flagellin expression (Fig. 5) through an RppA-dependent pathway. (iv) The PhoP-PhoQ pathway has been shown to be activated by PB and repressed by high concentration of Mg2+ (6, 19). Moreover, activated PhoP can bind to the phoPQ promoter and stimulate its transcription (49). We found that the transcription of the rppA gene was activated by PB and repressed by a high concentration of Mg2+ (Fig. 8), suggesting that the transcription of the rppAB operon is regulated in a way similar to the way in which the phoPQ operon is regulated. (v) Sequence analysis indicated that RppA showed sequence homology to S. enterica serovar Typhimurium PhoP (35.7% identity and 59.3% similarity) and P. aeruginosa PhoP (36.5% identity and 55.7% similarity). Together, the evidence described above strongly suggests that RppA is a PhoP homologue in P. mirabilis. However, since RppA also shows sequence homology to S. enterica serovar Typhimurium PmrA and S. marcescens RssB, it is still possible that RppA is a homologue of another response regulator of the two-component systems, such as PmrA, which has been shown to be involved in regulation of CAP susceptibility and virulence functions (18, 19, 21, 22).
The rppA knockout mutant of P. mirabilis was at least 160 times more sensitive to PB than the wild-type strain. Previous studies indicated that LPS modification plays a key role in bacterial resistance to CAPs, including PB (39, 40, 47, 53). Moreover, PB-sensitive P. mirabilis mutants have altered LPS profiles and lack 4-amino-4-deoxy-l-arabinose modification of LPS, which is known to bolster the bacterial resistance to CAP (39). We found that the rppA knockout mutant had an altered LPS profile and that the LPS purified from the rppA knockout mutant had higher binding activity with PB than the LPS purified from the wild-type strain (Fig. 3). We believe that an alteration in LPS confers increased PB sensitivity to the rppA knockout mutant and that RppA is involved in regulation of LPS modification. In this respect, we have started to investigate whether RppA regulates the expression of genes involved in LPS modification. The putative LPS modification genes in P. mirabilis were searched by comparing the genomic DNA sequence of P. mirabilis with the known Salmonella PhoP-activated genes involved in LPS modification. Homologues of pagP, which encodes an outer membrane protein responsible for incorporation of palmitate into the lipid A moiety of the LPS (23), and pmrH, which encodes an aminotransferase involved in 4-amino-4-deoxy-l-arabinose modification of LPS (21, 44), were identified. In Salmonella, the increased expression of pagP and pmrH renders the bacteria resistant to CAPs. Our preliminary data indicated that the levels of transcription of the pagP and pmrH homologous genes were higher in the wild-type P. mirabilis strain than in the rppA knockout mutant (data not shown). These data further support our conclusion that RppA is involved in regulation of LPS modification and that alteration of LPS modification results in an rppA knockout mutant sensitive to PB.
Our data indicated that PB could regulate LPS synthesis and modification (Fig. 3 and Table 3), swarming migration (Fig. 4), flagellin expression (Fig. 5), swarmer cell differentiation (Fig. 6), hemolysin expression (Fig. 7), and cytotoxic activity (Table 4) in P. mirabilis through an RppA-dependent pathway. This suggests that PB could serve as a signal to modulate RppA activity. How does PB regulate RppA activity? PB and other CAPs have been shown to be able to bind the PhoQ sensor kinase and activate its cognate response regulator, PhoP, directly in S. enterica serovar Typhimurium (6). It is possible that PB can also bind the putative sensor kinase RppB, a protein with sequence similarity to PhoQ, and regulate the activity of its putative cognate response regulator, RppA, directly. Alternatively, PB could act on other regulatory systems, which in turn indirectly regulate the activity of RppA. Thus, since a variety of structurally different CAPs can activate PhoQ, it was proposed that the mechanism by which CAPs activate the PhoQ sensor kinase did not involve direct binding but involved alteration of the bacterial membrane (19). It is possible that the putative sensor kinase RppB is activated by sensing the membrane perturbation caused by PB. In this respect, it is worth noting that RppB is highly homologous to S. marcescens RssA (52.0% identity and 72.2% similarity), a sensor kinase that has been suggested to be able to sense the change in membrane fluidity (31). The possibility that the bacterial two-component system can sense and be regulated by an alteration in the membrane has been described previously. For instance, the RcsC-RcsB two-component system is activated by cationic amphipathic molecules that can insert into the lipid bilayer and perturb the bacterial membrane (37).
We found that the swarming ability of the rppA knockout mutant was inhibited by PB, although to a lesser extent than the swarming ability of the wild-type strain (Fig. 4). This suggests that PB can inhibit swarming of P. mirabilis in both RppA-dependent and -independent pathways and that PB may serve as a signal for two-component systems other than RppA-RppB. It is possible that membrane perturbation caused by PB can be sensed by different two-component systems in P. mirabilis. In this respect, we have reported that RcsC-RsbA-RcsB, a putative two-component system involved in regulation of swarming and virulence factor expression in P. mirabilis, is regulated by fatty acids, which has been shown to affect membrane fluidity (31, 35). RsbA (YojN) has been renamed RcsD (37). It would be of interest to study whether the RcsD pathway is also regulated by PB and whether inhibition of swarming by PB in P. mirabilis is also mediated partially through an RsbA-dependent pathway.
We demonstrated that a low concentration of PB (1 μg/ml) can inhibit swarming and the expression of the virulence factor hemolysin in P. mirabilis. We also found that a low concentration of PB can suppress the cytotoxic activity of P. mirabilis (Table 4). Together, these findings suggest that a low concentration of PB and possibly other CAPs can inhibit certain virulence functions of P. mirabilis. In this regard, it is tempting to suggest that CAPs secreted by epithelial cells of the urinary tract may play roles in preventing P. mirabilis infection, even though this bacterium is known to be highly resistant to killing by PB and certain other CAPs.
Acknowledgments
This work was supported by grants from the National Science Council and National Taiwan University Hospital, Taipei, Taiwan.
We thank Yeong-Shiau Pu (National Taiwan University Hospital) for providing the NTUB1 cell line.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Adams, P., R. Fowler, N. Kinsella, G. Howell, M. Farris, P. Coote, and C. D. O'Connor. 2001. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1597-607. [DOI] [PubMed] [Google Scholar]
- 2.Allison, C., N. Coleman, P. L. Jones, and C. Hughes. 1992. Ability of Proteus mirabilis to invade human urothelial cells is coupled to motility and swarming differentiation. Infect. Immun. 604740-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 61583-1591. [DOI] [PubMed] [Google Scholar]
- 4.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47415-433. [DOI] [PubMed] [Google Scholar]
- 5.Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50219-230. [DOI] [PubMed] [Google Scholar]
- 6.Bader, M. W., S. Sanowar, M. E. Daley, A. R. Schneider, U. Cho, W. Xu, R. E. Klevit, H. Le Moual, and S. I. Miller. 2005. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell 122461-472. [DOI] [PubMed] [Google Scholar]
- 7.Belas, R., D. Erskine, and D. Flaherty. 1991. Transposon mutagenesis in Proteus mirabilis. J. Bacteriol. 1736289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belas, R., R. Schneider, and M. Melch. 1998. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J. Bacteriol. 1806126-6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boll, M., J. Radziejewska-Lebrecht, C. Warth, D. Krajewska-Pietrasik, and H. Mayer. 1994. 4-Amino-4-deoxy-l-arabinose in LPS of enterobacterial R-mutants and its possible role for their polymyxin reactivity. FEMS Immunol. Med. Microbiol. 8329-341. [DOI] [PubMed] [Google Scholar]
- 10.Brinkman, F. S., E. L. Macfarlane, P. Warrener, and R. E. Hancock. 2001. Evolutionary relationships among virulence-associated histidine kinases. Infect. Immun. 695207-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodsky, I. E., R. K. Ernst, S. I. Miller, and S. Falkow. 2002. mig-14 is a Salmonella gene that plays a role in bacterial resistance to antimicrobial peptides. J. Bacteriol. 1843203-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos, M. A., M. A. Vargas, V. Regueiro, C. M. Llompart, S. Alberti, and J. A. Bengoechea. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 727107-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 1341141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 1726568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derzelle, S., E. Turlin, E. Duchaud, S. Pages, F. Kunst, A. Givaudan, and A. Danchin. 2004. The PhoP-PhoQ two-component regulatory system of Photorhabdus luminescens is essential for virulence in insects. J. Bacteriol. 1861270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33960-967. [DOI] [PubMed] [Google Scholar]
- 17.Falagas, M. E., and S. K. Kasiakou. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 401333-1341. [DOI] [PubMed] [Google Scholar]
- 18.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 1831835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groisman, E. A., and C. Mouslim. 2006. Sensing by bacterial regulatory systems in host and non-host environments. Nat. Rev. Microbiol. 4705-709. [DOI] [PubMed] [Google Scholar]
- 20.Gunn, J. S. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 757-62. [PubMed] [Google Scholar]
- 21.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 271171-1182. [DOI] [PubMed] [Google Scholar]
- 22.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 686139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95189-198. [DOI] [PubMed] [Google Scholar]
- 24.Gygi, D., M. M. Rahman, H. C. Lai, R. Carlson, J. Guard-Petter, and C. Hughes. 1995. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 171167-1175. [DOI] [PubMed] [Google Scholar]
- 25.Hancock, R. E., and A. Rozek. 2002. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 206143-149. [DOI] [PubMed] [Google Scholar]
- 26.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 978856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaca, W., J. Radziejewska-Lebrecht, and U. R. Bhat. 1990. Effect of polymyxins on the lipopolysaccharide-defective mutants of Proteus mirabilis. Microbios 6123-32. [PubMed] [Google Scholar]
- 28.Kato, A., and E. A. Groisman. 2004. Connecting two-component regulatory systems by a protein that protects a response regulator from dephosphorylation by its cognate sensor. Genes Dev. 182302-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, W., T. Killam, V. Sood, and M. G. Surette. 2003. Swarm-cell differentiation in Salmonella enterica serovar Typhimurium results in elevated resistance to multiple antibiotics. J. Bacteriol. 1853111-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kox, L. F., M. M. Wosten, and E. A. Groisman. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 191861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai, H. C., P. C. Soo, J. R. Wei, W. C. Yi, S. J. Liaw, Y. T. Horng, S. M. Lin, S. W. Ho, S. Swift, and P. Williams. 2005. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J. Bacteriol. 1873407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, C. H., and C. M. Tsai. 1999. Quantification of bacterial lipopolysaccharides by the purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal. Biochem. 267161-168. [DOI] [PubMed] [Google Scholar]
- 33.Liaw, S. J., H. C. Lai, S. W. Ho, K. T. Luh, and W. B. Wang. 2001. Characterisation of p-nitrophenylglycerol-resistant Proteus mirabilis super-swarming mutants. J. Med. Microbiol. 501039-1048. [DOI] [PubMed] [Google Scholar]
- 34.Liaw, S. J., H. C. Lai, S. W. Ho, K. T. Luh, and W. B. Wang. 2003. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J. Med. Microbiol. 5219-28. [DOI] [PubMed] [Google Scholar]
- 35.Liaw, S. J., H. C. Lai, and W. B. Wang. 2004. Modulation of swarming and virulence by fatty acids through the RsbA protein in Proteus mirabilis. Infect. Immun. 726836-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macfarlane, E. L., A. Kwasnicka, M. M. Ochs, and R. E. Hancock. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34305-316. [DOI] [PubMed] [Google Scholar]
- 37.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59379-405. [DOI] [PubMed] [Google Scholar]
- 38.Marceau, M., F. Sebbane, F. Ewann, F. Collyn, B. Lindner, M. A. Campos, J. A. Bengoechea, and M. Simonet. 2004. The pmrF polymyxin-resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP-PhoQ two-component system but not by PmrA-PmrB, and is not required for virulence. Microbiology 1503947-3957. [DOI] [PubMed] [Google Scholar]
- 39.McCoy, A. J., H. Liu, T. J. Falla, and J. S. Gunn. 2001. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 452030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moskowitz, S. M., R. K. Ernst, and S. I. Miller. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss, J. E., P. E. Fisher, B. Vick, E. A. Groisman, and A. Zychlinsky. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell. Microbiol. 2443-452. [DOI] [PubMed] [Google Scholar]
- 42.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 43.Newcombe, J., J. C. Jeynes, E. Mendoza, J. Hinds, G. L. Marsden, R. A. Stabler, M. Marti, and J. J. McFadden. 2005. Phenotypic and transcriptional characterization of the meningococcal PhoPQ system, a magnesium-sensing two-component regulatory system that controls genes involved in remodeling the meningococcal cell surface. J. Bacteriol. 1874967-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noland, B. W., J. M. Newman, J. Hendle, J. Badger, J. A. Christopher, J. Tresser, M. D. Buchanan, T. A. Wright, M. E. Rutter, W. E. Sanderson, H. J. Muller-Dieckmann, K. S. Gajiwala, and S. G. Buchanan. 2002. Structural studies of Salmonella typhimurium ArnB (PmrH) aminotransferase: a 4-amino-4-deoxy-l-arabinose lipopolysaccharide-modifying enzyme. Structure 101569-1580. [DOI] [PubMed] [Google Scholar]
- 45.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10179-186. [DOI] [PubMed] [Google Scholar]
- 46.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29303-313. [DOI] [PubMed] [Google Scholar]
- 47.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 521363-1373. [DOI] [PubMed] [Google Scholar]
- 48.Slauch, J. M., M. J. Mahan, P. Michetti, M. R. Neutra, and J. J. Mekalanos. 1995. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella typhimurium lipopolysaccharide O antigen. Infect. Immun. 63437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soncini, F. C., E. G. Vescovi, and E. A. Groisman. 1995. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 1774364-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swihart, K. G., and R. A. Welch. 1990. Cytotoxic activity of the Proteus hemolysin HpmA. Infect. Immun. 581861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamayo, R., S. S. Ryan, A. J. McCoy, and J. S. Gunn. 2002. Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar Typhimurium. Infect. Immun. 706770-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toguchi, A., M. Siano, M. Burkart, and R. M. Harshey. 2000. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 1826308-6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran, A. X., M. E. Lester, C. M. Stead, C. R. Raetz, D. J. Maskell, S. C. McGrath, R. J. Cotter, and M. S. Trent. 2005. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella typhimurium lipid A. J. Biol. Chem. 28028186-28194. [DOI] [PubMed] [Google Scholar]
- 54.Wang, W. B., H. C. Lai, P. R. Hsueh, R. Y. Chiou, S. B. Lin, and S. J. Liaw. 2006. Inhibition of swarming and virulence factor expression in Proteus mirabilis by resveratrol. J. Med. Microbiol. 551313-1321. [DOI] [PubMed] [Google Scholar]
- 55.Warren, J. W., J. H. Tenney, J. M. Hoopes, H. L. Muncie, and W. C. Anthony. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146719-723. [DOI] [PubMed] [Google Scholar]